Resveratrol Alleviates Diabetic Periodontitis-Induced Alveolar Osteocyte Ferroptosis Possibly via Regulation of SLC7A11/GPX4
Abstract
1. Introduction
2. Materials and Methods
2.1. Establishment of Diabetes Periodontitis Model and Resveratrol Treatment
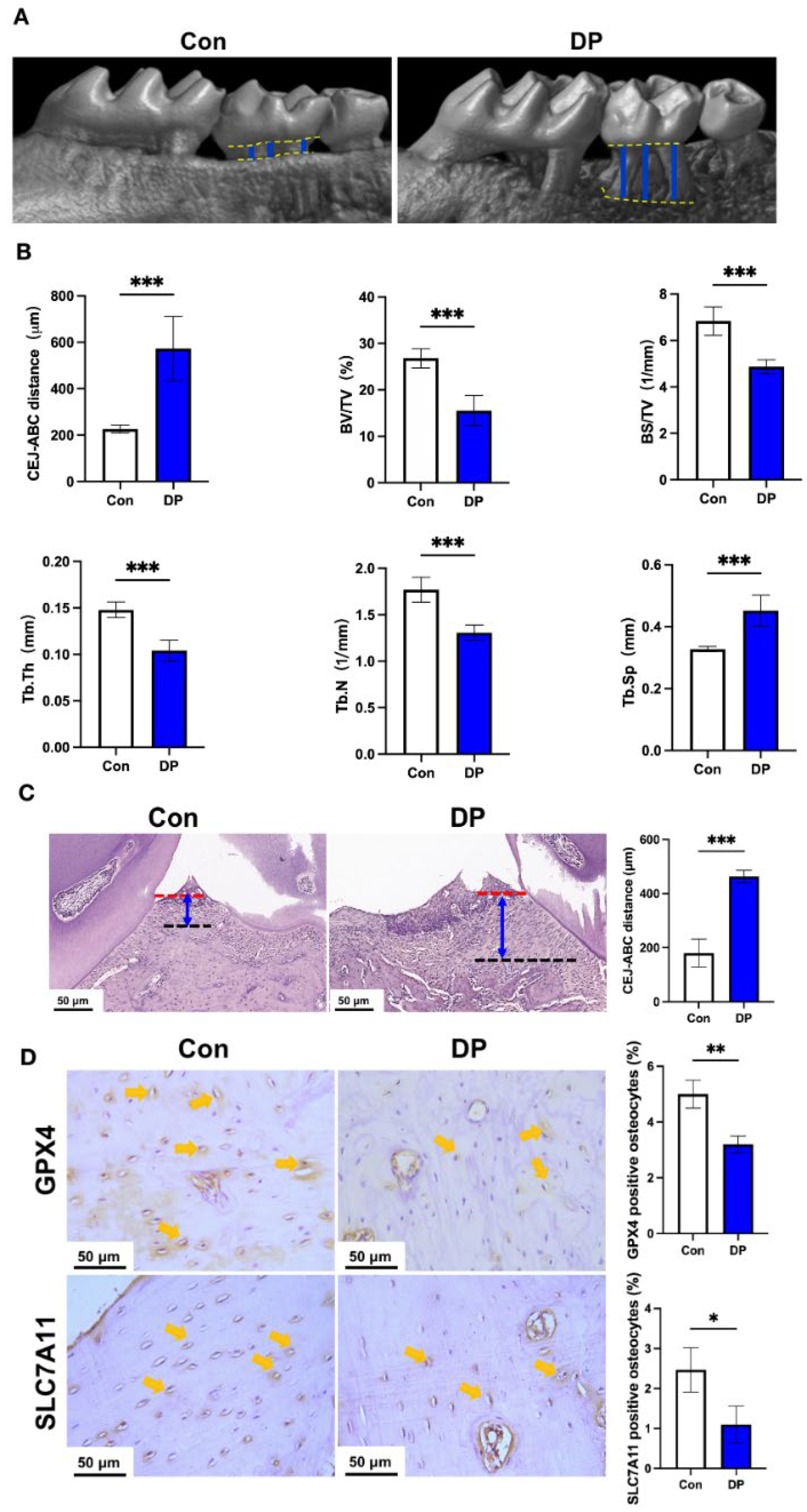
2.2. Micro-Computed Tomography
2.3. Histology and Hematoxylin and Eosin Staining
2.4. Immunohistochemistry
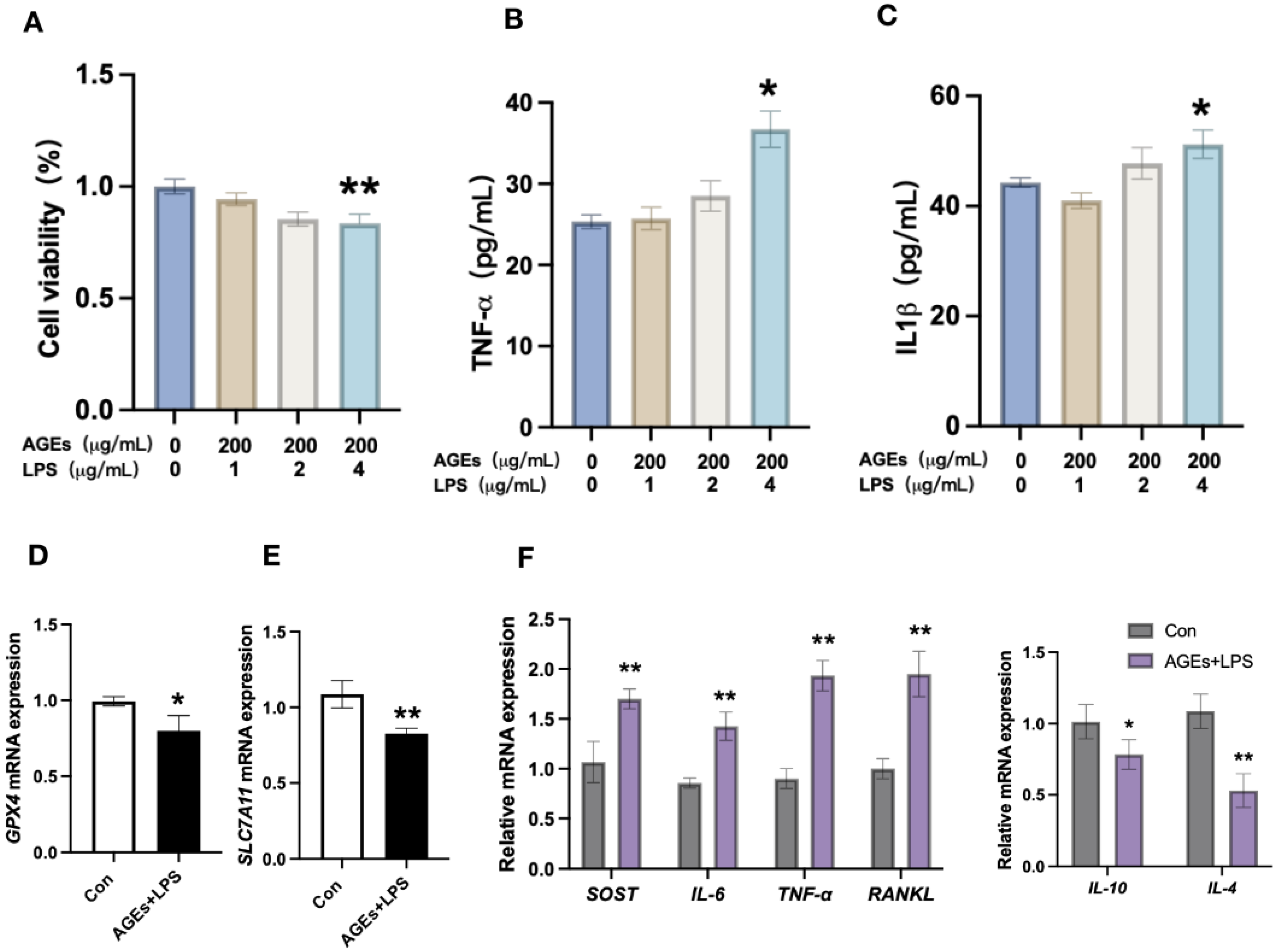
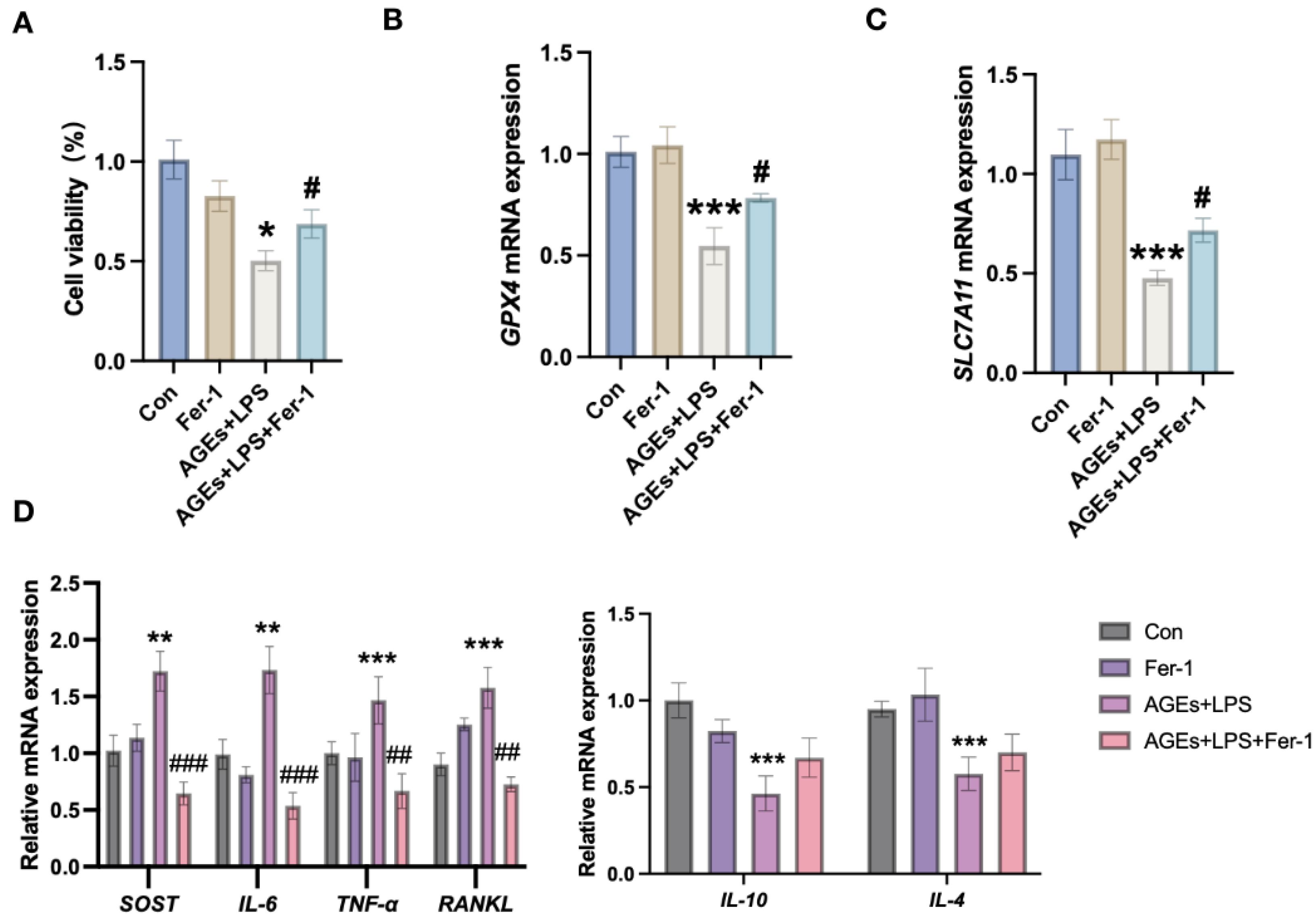
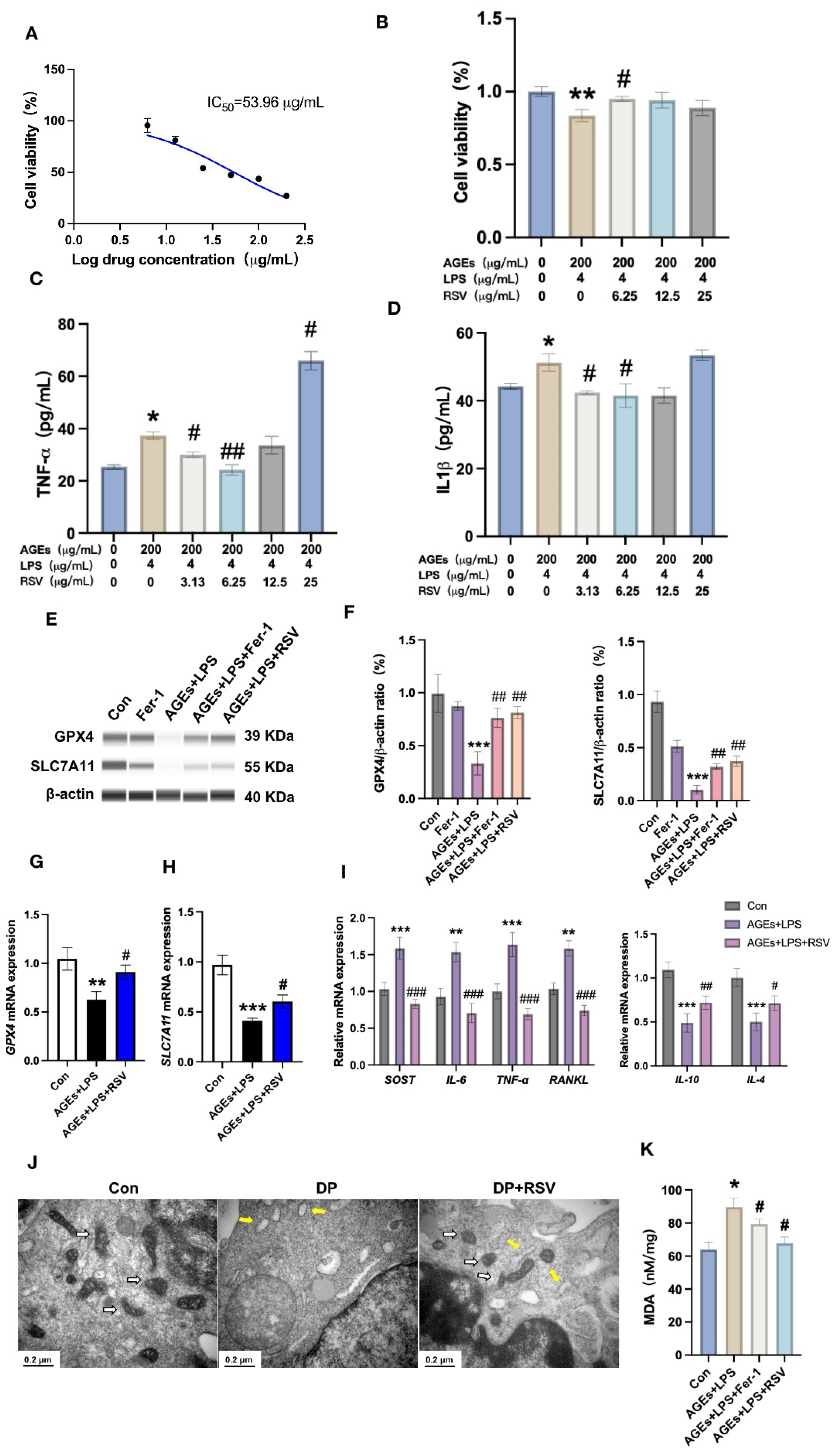
2.5. Osteocyte Culture and Treatment
2.6. RT-qPCR Analysis
2.7. Capillary-Based Immunoassay
2.8. Cell Viability Assay
2.9. Enzyme-Linked Immunosorbent Assay
2.10. Malon-Dialdehyde Assay
2.11. Transmission Electron Microscopy
2.12. mRNA Sequencing
2.13. Statistical Analysis
3. Results
3.1. Diabetic Periodontitis Triggers Alveolar Osteocyte Ferroptosis
3.2. Diabetes Periodontitis Condition In Vitro Induces Osteocyte Death and Modulates the Expression of Ferroptotic Markers and Inflammatory Cytokines
3.3. Inhibition of Ferroptosis Mitigates Diabetic Periodontitis-Induced Osteocyte Cell Death and Inflammation
3.4. Resveratrol Prevents Diabetic Periodontitis-Induced Osteocyte Ferroptosis
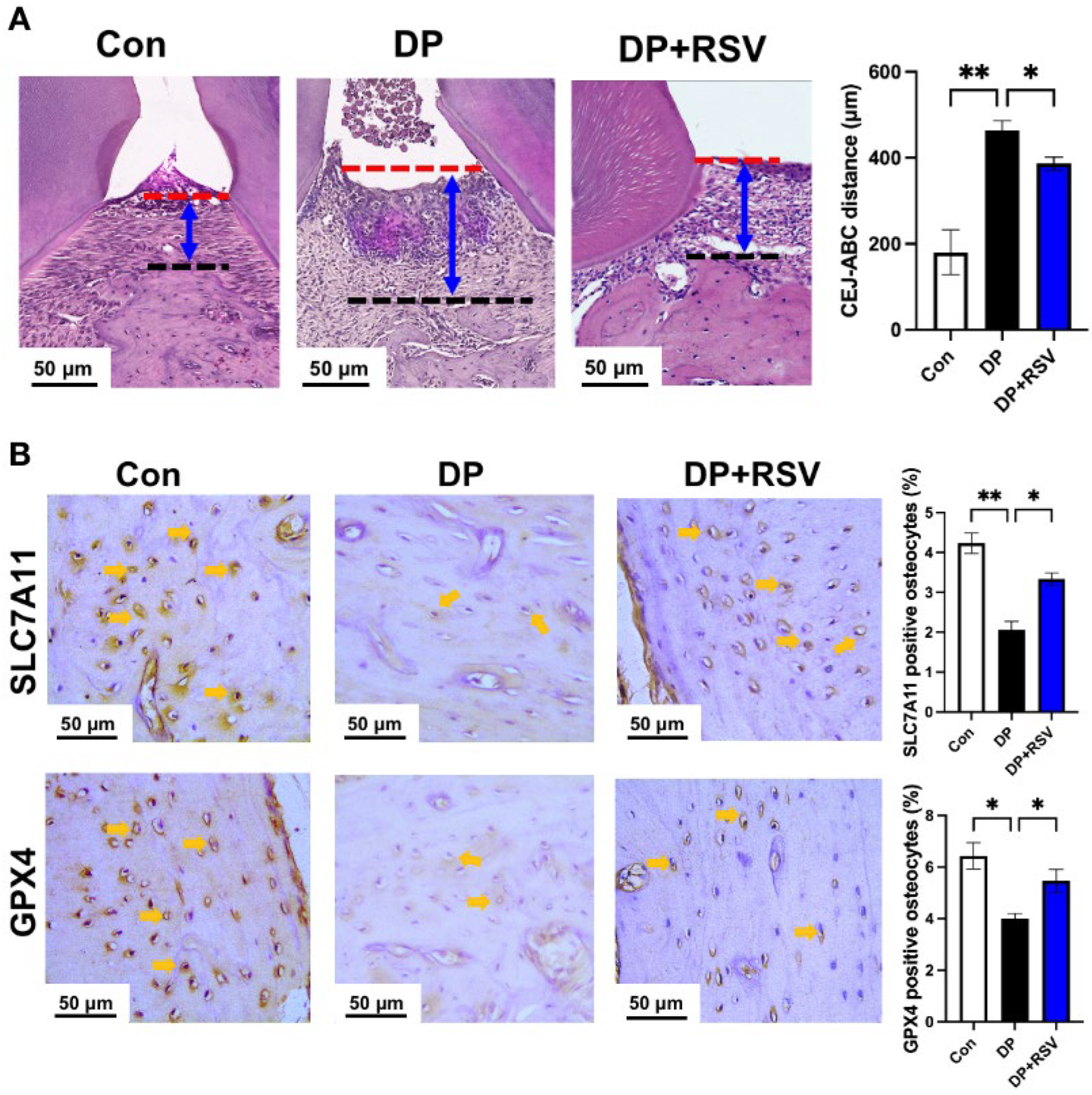
3.5. Resveratrol Alleviates Diabetic Periodontitis-Induced Ferroptosis of Alveolar Osteocytes In Vivo
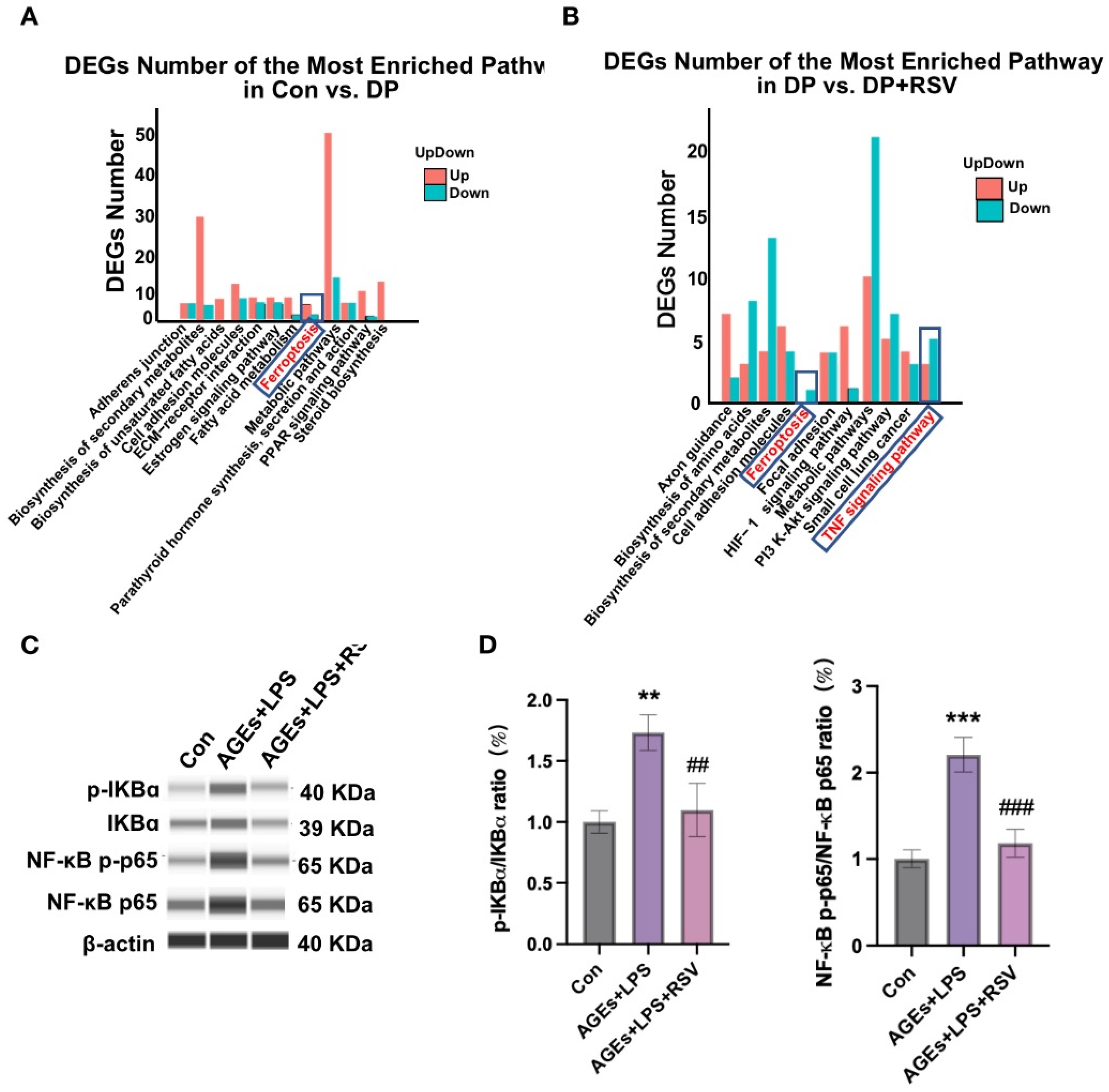
3.6. AGEs+LPS and Resveratrol+AGEs+LPS Treatments Modulate NF-κB Signaling in Osteocytes
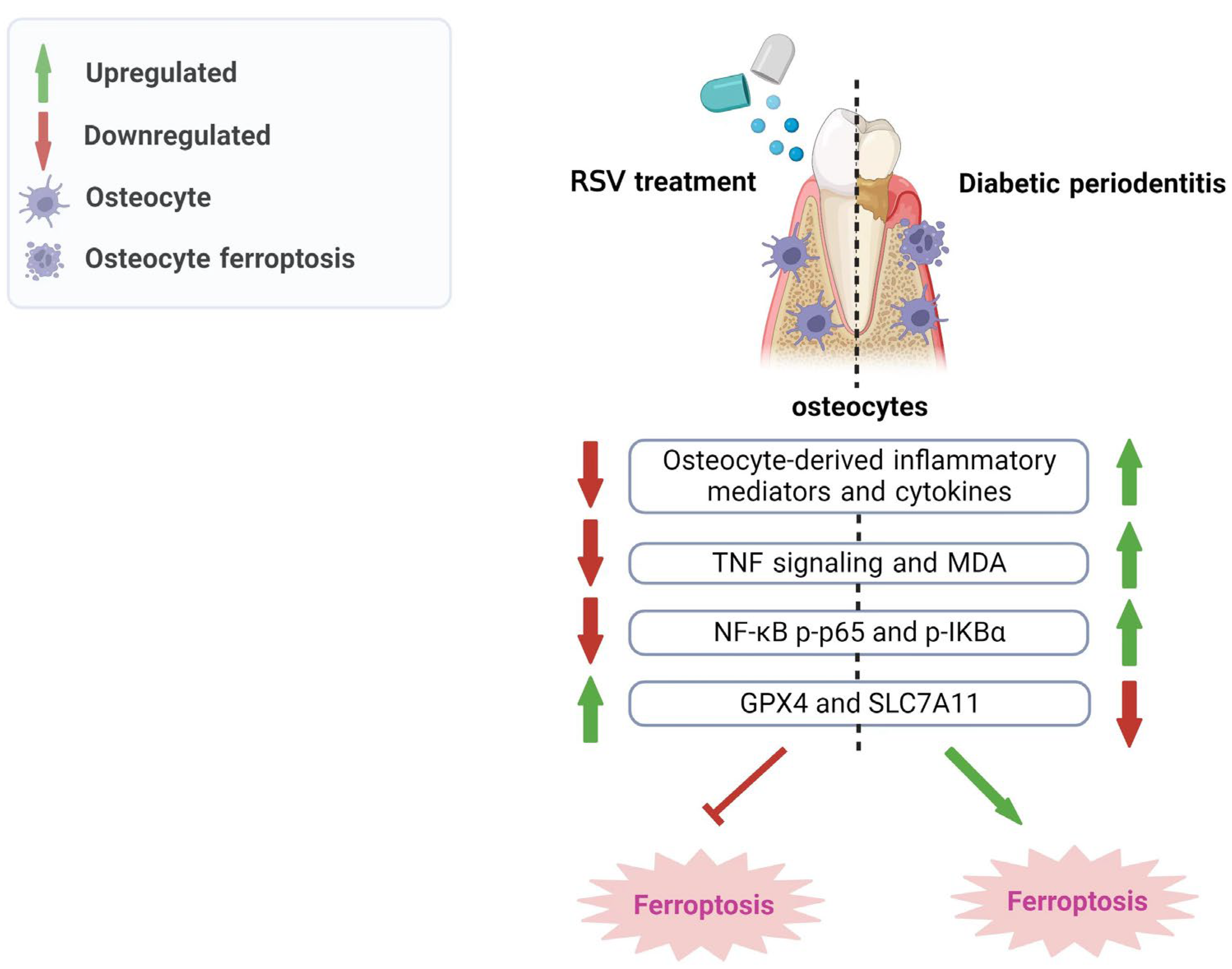
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Prim. 2017, 3, 17038. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Cheng, L.; You, Y.; Tang, C.; Ren, B.; Li, Y.; Xu, X.; Zhou, X. Oral microbiota in human systematic diseases. Int. J. Oral. Sci. 2022, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, L.M.; Bacino, M.; Kapila, Y.L. Periodontal Disease: The Good, The Bad, and The Unknown. Front. Cell Infect Microbiol. 2021, 11, 766944. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.T.; Corrêa, J.D.; Silva, T.A. The Oral Microbiota Is Modified by Systemic Diseases. J. Dent. Res. 2019, 98, 148–156. [Google Scholar] [CrossRef]
- Pathak, J.L.; Yan, Y.; Zhang, Q.; Wang, L.; Ge, L. The role of oral microbiome in respiratory health and diseases. Respir. Med. 2021, 185, 106475. [Google Scholar] [CrossRef]
- Pirih, F.Q.; Monajemzadeh, S.; Singh, N.; Sinacola, R.S.; Shin, J.M.; Chen, T.; Fenno, J.C.; Kamarajan, P.; Rickard, A.H.; Travan, S.; et al. Association between metabolic syndrome and periodontitis: The role of lipids, inflammatory cytokines, altered host response, and the microbiome. Periodontol. 2000 2021, 87, 50–75. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef]
- Bui, F.Q.; Almeida-da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef]
- Hegde, R.; Awan, K.H. Effects of periodontal disease on systemic health. Dis. Mon. 2019, 65, 185–192. [Google Scholar] [CrossRef]
- Bendek, M.J.; Canedo-Marroquín, G.; Realini, O.; Retamal, I.N.; Hernández, M.; Hoare, A.; Busso, D.; Monteiro, L.J.; Illanes, S.E.; Chaparro, A. Periodontitis and Gestational Diabetes Mellitus: A Potential Inflammatory Vicious Cycle. Int. J. Mol. Sci. 2021, 22, 11831. [Google Scholar] [CrossRef]
- Mathew, J.E.; Jacob, J.J.; Kalra, S. Periodontitis management in diabetes care. J. Pak. Med. Assoc. 2021, 71, 2097–2099. [Google Scholar]
- Wu, C.Z.; Yuan, Y.H.; Liu, H.H.; Li, S.S.; Zhang, B.W.; Chen, W.; An, Z.J.; Chen, S.Y.; Wu, Y.Z.; Han, B.; et al. Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral Health 2020, 20, 204. [Google Scholar] [CrossRef]
- Li, Y.; Shrestha, A.; Zhang, H.; Li, L.; Li, D.; Fu, T.; Song, J.; Ji, P.; Huang, Y.; Chen, T. Impact of diabetes mellitus simulations on bone cell behavior through in vitro models. J. Bone Miner. Metab. 2020, 38, 607–619. [Google Scholar] [CrossRef]
- Xiao, E.; Mattos, M.; Vieira, G.H.A.; Chen, S.; Corrêa, J.D.; Wu, Y.; Albiero, M.L.; Bittinger, K.; Graves, D.T. Diabetes Enhances IL-17 Expression and Alters the Oral Microbiome to Increase Its Pathogenicity. Cell Host Microbe 2017, 22, 120–128.e124. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, Y.; Yi, J.; Zhao, Z.; Ye, R. Hyperglycemia modulates M1/M2 macrophage polarization via reactive oxygen species overproduction in ligature-induced periodontitis. J. Periodontal. Res. 2021, 56, 991–1005. [Google Scholar] [CrossRef]
- Pathak, J.L.; Bravenboer, N.; Klein-Nulend, J. The Osteocyte as the New Discovery of Therapeutic Options in Rare Bone Diseases. Front. Endocrinol. 2020, 11, 405. [Google Scholar] [CrossRef]
- Shi, C.; Uda, Y.; Dedic, C.; Azab, E.; Sun, N.; Hussein, A.I.; Petty, C.A.; Fulzele, K.; Mitterberger-Vogt, M.C.; Zwerschke, W.; et al. Carbonic anhydrase III protects osteocytes from oxidative stress. FASEB J. 2018, 32, 440–452. [Google Scholar] [CrossRef]
- Liu, Z.; Qian, Z.; Wang, Y.; Wang, H. Necroptosis in pathogenesis of osteoarthritis and its therapeutic implications. Zhejiang Da Xue Xue Bao Yi Xue Ban 2022, 51, 261–265. [Google Scholar] [CrossRef]
- Kitaura, H.; Marahleh, A.; Ohori, F.; Noguchi, T.; Shen, W.R.; Qi, J.; Nara, Y.; Pramusita, A.; Kinjo, R.; Mizoguchi, I. Osteocyte-Related Cytokines Regulate Osteoclast Formation and Bone Resorption. Int. J. Mol. Sci. 2020, 21, 5169. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Jia, X.Y.; Fang, J.Y.; Chai, H.; Huang, Q.; She, C.; Jia, P.; Geng, C.; Xu, W. Reduction of SOST gene promotes bone formation through the Wnt/β-catenin signalling pathway and compensates particle-induced osteolysis. J. Cell. Mol. Med. 2020, 24, 4233–4244. [Google Scholar] [CrossRef]
- Huang, X.; Xie, M.; Xie, Y.; Mei, F.; Lu, X.; Li, X.; Chen, L. The roles of osteocytes in alveolar bone destruction in periodontitis. J. Transl. Med. 2020, 18, 479. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell. Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Minikes, A.M.; Jiang, X. Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol. Cell. 2022, 82, 2215–2227. [Google Scholar] [CrossRef]
- Pan, S.; Hu, B.; Sun, J.; Yang, Z.; Yu, W.; He, Z.; Gao, X.; Song, J. Identification of cross-talk pathways and ferroptosis-related genes in periodontitis and type 2 diabetes mellitus by bioinformatics analysis and experimental validation. Front. Immunol. 2022, 13, 1015491. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, P.; Zhai, B.; Zhang, M.; Xiang, Y.; Fang, J.; Xu, S.; Gao, Y.; Chen, X.; Sui, X.; et al. The emerging role of ferroptosis in inflammation. Biomed. Pharmacother. 2020, 127, 110108. [Google Scholar] [CrossRef]
- Ma, H.; Wang, X.; Zhang, W.; Li, H.; Zhao, W.; Sun, J.; Yang, M. Melatonin Suppresses Ferroptosis Induced by High Glucose via Activation of the Nrf2/HO-1 Signaling Pathway in Type 2 Diabetic Osteoporosis. Oxid. Med. Cell. Longev. 2020, 2020, 9067610. [Google Scholar] [CrossRef]
- Bhattarai, G.; Poudel, S.B.; Kook, S.H.; Lee, J.C. Resveratrol prevents alveolar bone loss in an experimental rat model of periodontitis. Acta Biomater. 2016, 29, 398–408. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, L.; Chen, H.; Wei, S.; Yao, K.; Sun, X.; Yang, G.; Jiang, L.; Zhang, C.; Wang, N.; et al. Resveratrol protected acrolein-induced ferroptosis and insulin secretion dysfunction via ER-stress- related PERK pathway in MIN6 cells. Toxicology 2022, 465, 153048. [Google Scholar] [CrossRef]
- Pathak, J.L.; Fang, Y.; Chen, Y.; Ye, Z.; Guo, X.; Yan, Y.; Zha, J.; Liang, D.; Ke, X.; Yang, L.; et al. Downregulation of Macrophage-Specific Act-1 Intensifies Periodontitis and Alveolar Bone Loss Possibly via TNF/NF-κB Signaling. Front. Cell. Dev. Biol. 2021, 9, 628139. [Google Scholar] [CrossRef]
- Sajawal Ali, M.; Ciftci Olsen, F.; Franco, R. Primary Cavitary Sarcoidosis: A Diagnostic Challenge for the Clinician. Arch. Bronconeumol. 2018, 54, 387. [Google Scholar] [CrossRef]
- Vijverberg, R.; Ferdinand, R.; Beekman, A.; van Meijel, B. Agreement between patients and mental healthcare providers on unmet care needs in child and adolescent psychiatry. Soc. Psychiatry Psychiatr. Epidemiol. 2021, 56, 2005–2015. [Google Scholar] [CrossRef]
- Fedchenko, N.; Reifenrath, J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue—A review. Diagn. Pathol. 2014, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Feng, Y.; Li, H.; Chen, X.; Wang, G.; Xu, S.; Li, Y.; Zhao, L. Ferrostatin-1 alleviates lipopolysaccharide-induced acute lung injury via inhibiting ferroptosis. Cell. Mol. Biol. Lett. 2020, 25, 10. [Google Scholar] [CrossRef] [PubMed]
- Plemmenos, G.; Piperi, C. Pathogenic Molecular Mechanisms in Periodontitis and Peri-Implantitis: Role of Advanced Glycation End Products. Life 2022, 12, 218. [Google Scholar] [CrossRef]
- Watanabe, M.; Toyomura, T.; Tomiyama, M.; Wake, H.; Liu, K.; Teshigawara, K.; Takahashi, H.; Nishibori, M.; Mori, S. Advanced glycation end products (AGEs) synergistically potentiated the proinflammatory action of lipopolysaccharide (LPS) and high mobility group box-1 (HMGB1) through their direct interactions. Mol. Biol. Rep. 2020, 47, 7153–7159. [Google Scholar] [CrossRef]
- Holze, C.; Michaudel, C.; Mackowiak, C.; Haas, D.A.; Benda, C.; Hubel, P.; Pennemann, F.L.; Schnepf, D.; Wettmarshausen, J.; Braun, M.; et al. Oxeiptosis, a ROS-induced caspase-independent apoptosis-like cell-death pathway. Nat. Immunol. 2018, 19, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kim, J.Y.; Oh, S.H. Advanced glycation end products (AGEs) promote melanogenesis through receptor for AGEs. Sci. Rep. 2016, 6, 27848. [Google Scholar] [CrossRef]
- Shen, H.; Khan, R.; Wang, X.; Li, Z.; Qu, F. Capillary-based chemiluminescence immunoassay for C-reactive protein with portable imaging device. Anal. Bioanal. Chem. 2018, 410, 7177–7183. [Google Scholar] [CrossRef]
- Caldwell, G.W.; Yan, Z.; Lang, W.; Masucci, J.A. The IC(50) concept revisited. Curr. Top. Med. Chem. 2012, 12, 1282–1290. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Frischholz, S.; Berberich, O.; Böck, T.; Meffert, R.H.; Blunk, T. Resveratrol counteracts IL-1β-mediated impairment of extracellular matrix deposition in 3D articular chondrocyte constructs. J. Tissue Eng. Regen. Med. 2020, 14, 897–908. [Google Scholar] [CrossRef]
- Yuan, B.; Zhao, X.D.; Shen, J.D.; Chen, S.J.; Huang, H.Y.; Zhou, X.M.; Han, Y.L.; Zhou, L.J.; Lu, X.J.; Wu, Q. Activation of SIRT1 Alleviates Ferroptosis in the Early Brain Injury after Subarachnoid Hemorrhage. Oxid. Med. Cell. Longev. 2022, 2022, 9069825. [Google Scholar] [CrossRef]
- Xu, M.; Tao, J.; Yang, Y.; Tan, S.; Liu, H.; Jiang, J.; Zheng, F.; Wu, B. Ferroptosis involves in intestinal epithelial cell death in ulcerative colitis. Cell. Death Dis. 2020, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Toker, H.; Ozdemir, H.; Balcı, H.; Ozer, H. N-acetylcysteine decreases alveolar bone loss on experimental periodontitis in streptozotocin-induced diabetic rats. J. Periodontal. Res. 2012, 47, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Luong, A.; Tawfik, A.N.; Islamoglu, H.; Gobriel, H.S.; Ali, N.; Ansari, P.; Shah, R.; Hung, T.; Patel, T.; Henson, B.; et al. Periodontitis and diabetes mellitus co-morbidity: A molecular dialogue. J. Oral Biosci. 2021, 63, 360–369. [Google Scholar] [CrossRef]
- Shen, Y.; Guo, S.; Chen, G.; Ding, Y.; Wu, Y.; Tian, W. Hyperglycemia Induces Osteoclastogenesis and Bone Destruction Through the Activation of Ca(2+)/Calmodulin-Dependent Protein Kinase II. Calcif. Tissue Int. 2019, 104, 390–401. [Google Scholar] [CrossRef]
- Wu, X.; Qiu, W.; Hu, Z.; Lian, J.; Liu, Y.; Zhu, X.; Tu, M.; Fang, F.; Yu, Y.; Valverde, P.; et al. An Adiponectin Receptor Agonist Reduces Type 2 Diabetic Periodontitis. J. Dent. Res. 2019, 98, 313–321. [Google Scholar] [CrossRef]
- Graves, D.T.; Alshabab, A.; Albiero, M.L.; Mattos, M.; Corrêa, J.D.; Chen, S.; Yang, Y. Osteocytes play an important role in experimental periodontitis in healthy and diabetic mice through expression of RANKL. J. Clin. Periodontol. 2018, 45, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, A.R.; Choi, Y.H.; Jang, S.; Woo, G.H.; Cha, J.H.; Bak, E.J.; Yoo, Y.J. Tumor necrosis factor-α antagonist diminishes osteocytic RANKL and sclerostin expression in diabetes rats with periodontitis. PLoS ONE 2017, 12, e0189702. [Google Scholar] [CrossRef]
- Jia, X.; Yang, R.; Li, J.; Zhao, L.; Zhou, X.; Xu, X. Gut-Bone Axis: A Non-Negligible Contributor to Periodontitis. Front. Cell. Infect. Microbiol. 2021, 11, 752708. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, G.; So, H.S.; Kieu, T.T.T.; Kook, S.H.; Lee, J.C.; Jeon, Y.M. Astaxanthin Inhibits Diabetes-Triggered Periodontal Destruction, Ameliorates Oxidative Complications in STZ-Injected Mice, and Recovers Nrf2-Dependent Antioxidant System. Nutrients 2021, 13, 3575. [Google Scholar] [CrossRef] [PubMed]
- Polak, D.; Sanui, T.; Nishimura, F.; Shapira, L. Diabetes as a risk factor for periodontal disease-plausible mechanisms. Periodontol. 2000 2020, 83, 46–58. [Google Scholar] [CrossRef]
- Zhou, M.; Graves, D.T. Impact of the host response and osteoblast lineage cells on periodontal disease. Front. Immunol. 2022, 13, 998244. [Google Scholar] [CrossRef]
- Bai, S.; Zhou, J.; Nong, X.; Shi, R.; Yuan, Z.; Ma, C.; Li, J. Mechanism and effects of artesunate on the liver function of rats with type 1 diabetic periodontitis. Can. J. Physiol. Pharmacol. 2022, 100, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.S.; Lee, S.Y.; Kim, J.H.; Choi, Y.H.; Yang, D.W.; Kang, J.H.; Ko, H.M.; Cho, J.H.; Koh, J.T.; Kim, W.J.; et al. Synergistic alveolar bone resorption by diabetic advanced glycation end products and mechanical forces. J. Periodontol. 2019, 90, 1457–1469. [Google Scholar] [CrossRef]
- Tang, L.; Li, T.; Chang, Y.; Wang, Z.; Li, Y.; Wang, F.; Sui, L. Diabetic oxidative stress-induced telomere damage aggravates periodontal bone loss in periodontitis. Biochem. Biophys. Res. Commun. 2022, 614, 22–28. [Google Scholar] [CrossRef]
- Aquino-Martinez, R.; Rowsey, J.L.; Fraser, D.G.; Eckhardt, B.A.; Khosla, S.; Farr, J.N.; Monroe, D.G. LPS-induced premature osteocyte senescence: Implications in inflammatory alveolar bone loss and periodontal disease pathogenesis. Bone 2020, 132, 115220. [Google Scholar] [CrossRef]
- Lee, S.; Krüger, B.T.; Ignatius, A.; Tuckermann, J. Distinct Glucocorticoid Receptor Actions in Bone Homeostasis and Bone Diseases. Front. Endocrinol. 2021, 12, 815386. [Google Scholar] [CrossRef]
- Werner, S.L.; Sharma, R.; Woodruff, K.; Horn, D.; Harris, S.E.; Gorin, Y.; Lee, D.Y.; Hua, R.; Gu, S.; Fajardo, R.J.; et al. CSF-1 in Osteocytes Inhibits Nox4-mediated Oxidative Stress and Promotes Normal Bone Homeostasis. JBMR Plus 2020, 4, e10080. [Google Scholar] [CrossRef]
- Yao, S.; Du, Z.; Xiao, L.; Yan, F.; Ivanovski, S.; Xiao, Y. Morphometric Changes of Osteocyte Lacunar in Diabetic Pig Mandibular Cancellous Bone. Biomolecules 2022, 13, 49. [Google Scholar] [CrossRef]
- Li, N.; Wang, W.; Zhou, H.; Wu, Q.; Duan, M.; Liu, C.; Wu, H.; Deng, W.; Shen, D.; Tang, Q. Ferritinophagy-mediated ferroptosis is involved in sepsis-induced cardiac injury. Free Radic. Biol. Med. 2020, 160, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Luo, E.F.; Li, H.X.; Qin, Y.H.; Qiao, Y.; Yan, G.L.; Yao, Y.Y.; Li, L.Q.; Hou, J.T.; Tang, C.C.; Wang, D. Role of ferroptosis in the process of diabetes-induced endothelial dysfunction. World J. Diabetes 2021, 12, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Dogon, G.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. Lipid Peroxidation and Iron Metabolism: Two Corner Stones in the Homeostasis Control of Ferroptosis. Int. J. Mol. Sci. 2022, 24, 449. [Google Scholar] [CrossRef]
- Linkermann, A.; Stockwell, B.R.; Krautwald, S.; Anders, H.J. Regulated cell death and inflammation: An auto-amplification loop causes organ failure. Nat. Rev. Immunol. 2014, 14, 759–767. [Google Scholar] [CrossRef]
- Wang, T.; Yu, X.; He, C. Pro-inflammatory Cytokines: Cellular and Molecular Drug Targets for Glucocorticoid-induced-osteoporosis via Osteocyte. Curr. Drug Targets 2019, 20, 1–15. [Google Scholar] [CrossRef]
- Komatsu, N.; Takayanagi, H. Mechanisms of joint destruction in rheumatoid arthritis—Immune cell-fibroblast-bone interactions. Nat. Rev. Rheumatol. 2022, 18, 415–429. [Google Scholar] [CrossRef]
- Bastidas-Coral, A.P.; Bakker, A.D.; Zandieh-Doulabi, B.; Kleverlaan, C.J.; Bravenboer, N.; Forouzanfar, T.; Klein-Nulend, J. Cytokines TNF-α, IL-6, IL-17F, and IL-4 Differentially Affect Osteogenic Differentiation of Human Adipose Stem Cells. Stem Cells Int. 2016, 2016, 1318256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Chihara, T.; Dong, H.; Kagami, H. Effect of TNF-α and IL-6 on Compact Bone-Derived Cells. Tissue Eng. Regen. Med. 2021, 18, 441–451. [Google Scholar] [CrossRef]
- Adham, A.N.; Abdelfatah, S.; Naqishbandi, A.M.; Mahmoud, N.; Efferth, T. Cytotoxicity of apigenin toward multiple myeloma cell lines and suppression of iNOS and COX-2 expression in STAT1-transfected HEK293 cells. Phytomedicine 2021, 80, 153371. [Google Scholar] [CrossRef]
- Kato, K.; Takahashi, M.; Oh-Hashi, K.; Ando, K.; Hirata, Y. Quercetin and resveratrol inhibit ferroptosis independently of Nrf2-ARE activation in mouse hippocampal HT22 cells. Food Chem. Toxicol. 2023, 172, 113586. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.M.; Dong, X.; Huang, T.; Zhao, J.K.; Zhou, Z.J.; Huang, Y.T.; Xu, Y.L.; Zhao, Q.S.; Wang, Z.S.; Jiang, H.; et al. Inhibition of ferroptosis by icariin treatment attenuates excessive ethanol consumption-induced atrial remodeling and susceptibility to atrial fibrillation, role of SIRT1. Apoptosis 2023, 28, 607–626. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.T.; Cheng, G.Y.; Shih, Y.J.; Lin, C.Y.; Lin, S.J.; Lai, H.Y.; Whang-Peng, J.; Chiu, H.C.; Lee, S.Y.; Fu, E.; et al. Therapeutic applications of resveratrol and its derivatives on periodontitis. Ann. N. Y. Acad. Sci. 2017, 1403, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Lei, X.; Xu, Q.; Ju, Y.; Xu, D.; Mao, L.; Li, J.; Zheng, Y.; Sun, N.; Zhang, X.; et al. Protecting mitochondria via inhibiting VDAC1 oligomerization alleviates ferroptosis in acetaminophen-induced acute liver injury. Cell. Biol. Toxicol. 2022, 38, 505–530. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Dong, H.; Zhang, W.; Wang, N.; Ni, N.; Bai, X.; Liu, N. Lipid Peroxidation, GSH Depletion, and SLC7A11 Inhibition Are Common Causes of EMT and Ferroptosis in A549 Cells, but Different in Specific Mechanisms. DNA Cell. Biol. 2021, 40, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Costa Fernandes, C.J.D.; Zambuzzi, W.F. Fibroblast-secreted trophic factors contribute with ECM remodeling stimulus and upmodulate osteocyte gene markers in osteoblasts. Biochimie 2020, 168, 92–99. [Google Scholar] [CrossRef]
- Feng, X.; Wang, S.; Sun, Z.; Dong, H.; Yu, H.; Huang, M.; Gao, X. Ferroptosis Enhanced Diabetic Renal Tubular Injury via HIF-1α/HO-1 Pathway in db/db Mice. Front. Endocrinol. 2021, 12, 626390. [Google Scholar] [CrossRef]
- Zou, Y.; Palte, M.J.; Deik, A.A.; Li, H.; Eaton, J.K.; Wang, W.; Tseng, Y.Y.; Deasy, R.; Kost-Alimova, M.; Dančík, V.; et al. A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat. Commun. 2019, 10, 1617. [Google Scholar] [CrossRef]
- Li, S.; He, Y.; Chen, K.; Sun, J.; Zhang, L.; He, Y.; Yu, H.; Li, Q. RSL3 Drives Ferroptosis through NF-κB Pathway Activation and GPX4 Depletion in Glioblastoma. Oxid. Med. Cell. Longev. 2021, 2021, 2915019. [Google Scholar] [CrossRef]
- Cao, L.; Sun, H.; Li, Z.; Pan, R.L. Comparison of activities of various species of Rhizoma Cimicifugae and their honey processed products. Zhong Yao Cai 2007, 30, 1561–1563. [Google Scholar]
- Ikeda, E.; Tanaka, D.; Glogauer, M.; Tenenbaum, H.C.; Ikeda, Y. Healing effects of monomer and dimer resveratrol in a mouse periodontitis model. BMC Oral Health 2022, 22, 460. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, S.; Xu, W.; Zhou, Y.; Luan, H.; Wang, D. Resveratrol decreases local inflammatory markers and systemic endotoxin in patients with aggressive periodontitis. Medicine 2022, 101, e29393. [Google Scholar] [CrossRef]
- Ramírez-Garza, S.L.; Laveriano-Santos, E.P.; Marhuenda-Muñoz, M.; Storniolo, C.E.; Tresserra-Rimbau, A.; Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Health Effects of Resveratrol: Results from Human Intervention Trials. Nutrients 2018, 10, 1892. [Google Scholar] [CrossRef]
| Gene | Acc. No | Primer Sequence (5’~3’) | Product Length (bp) |
|---|---|---|---|
| Mus GPX4 | NM_001367995.1 | F: GTACAGGAGCCAGGAGTAATC | 134 |
| R: GGCTGGACTTTCATCCATTTC | |||
| Mus SLC7A11 | NM_011990.2 | F: CTTCGATACAAACGCCCAGATA | 119 |
| R: CTGAATGGGTCCGAGTAAGAG | |||
| Mus SOST | NM_001379100.1 | F: ATCTGCCTACTTGTGCACGC | 179 |
| R: TCATAGGGATGGTGGGGAGG | |||
| Mus RANKL | NM_001314054.1 | F: CACAGCCCTCTCTCTTGAGC | 188 |
| R: AGACTGTGACCCCCTTCCAT | |||
| Mus IL-6 | NM_001314054.1 | F: CTGCAAGAGACTTCCATCCA | 131 |
| R: AGTGGTATAGACAGGTCTGTTGG | |||
| Mus IL-1β | NM_008361.4 | F: GAAATGCCACCTTTGACAGTG | 116 |
| R: TGGATGCTCTCATCAGGACAG | |||
| Mus TNF-α | NM_013693.3 | F: TGTCTCAGCCTCTCTCATT | 153 |
| R: TGATCTGAGTGTGTGAGGGTCT | |||
| Mus IL-10 | NM_010548.2 | F: ACTTGGGTTGCCAAGCCTTA | 223 |
| R: GACACCTTGGTCTGGAGCTTA | |||
| Mus IL-4 | NM_021283.2 | F: GGGTCTCAACCCCCAGCTA | 200 |
| R: CGAGCTCACTCTCTGTGTGTT | |||
| Mus GAPDH | NM_001379100.1 | F: GTGAAGGTCGGTGTGAACGG | 227 |
| R: TCCTGGAAGATGGTGATGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Huang, Z.; Pan, S.; Feng, Y.; He, H.; Cheng, S.; Wang, L.; Wang, L.; Pathak, J.L. Resveratrol Alleviates Diabetic Periodontitis-Induced Alveolar Osteocyte Ferroptosis Possibly via Regulation of SLC7A11/GPX4. Nutrients 2023, 15, 2115. https://doi.org/10.3390/nu15092115
Li Y, Huang Z, Pan S, Feng Y, He H, Cheng S, Wang L, Wang L, Pathak JL. Resveratrol Alleviates Diabetic Periodontitis-Induced Alveolar Osteocyte Ferroptosis Possibly via Regulation of SLC7A11/GPX4. Nutrients. 2023; 15(9):2115. https://doi.org/10.3390/nu15092115
Chicago/Turabian StyleLi, Yue, Zhijun Huang, Shuaifei Pan, Yuhui Feng, Haokun He, Shuguang Cheng, Lijing Wang, Liping Wang, and Janak Lal Pathak. 2023. "Resveratrol Alleviates Diabetic Periodontitis-Induced Alveolar Osteocyte Ferroptosis Possibly via Regulation of SLC7A11/GPX4" Nutrients 15, no. 9: 2115. https://doi.org/10.3390/nu15092115
APA StyleLi, Y., Huang, Z., Pan, S., Feng, Y., He, H., Cheng, S., Wang, L., Wang, L., & Pathak, J. L. (2023). Resveratrol Alleviates Diabetic Periodontitis-Induced Alveolar Osteocyte Ferroptosis Possibly via Regulation of SLC7A11/GPX4. Nutrients, 15(9), 2115. https://doi.org/10.3390/nu15092115






