Relationship of Iron Intake, Ferritin, and Hepcidin with the Transverse Relaxation Rate of Water Protons in the Pancreas
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Assessment of Iron Metabolism
2.3. Imaging Protocol
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Participants
3.2. Associations of R2water in the Health Group
3.3. Associations of R2water in the Acute Pancreatitis Group
3.4. Associations of R2water in the Chronic Pancreatitis Group
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cho, J.; Petrov, M.S. Pancreatitis, pancreatic cancer, and their metabolic sequelae: Projected burden to 2050. Clin. Transl. Gastroenterol. 2020, 11, e00251. [Google Scholar] [CrossRef]
- Petrov, M.S.; Yadav, D. Global epidemiology and holistic prevention of pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Petrov, M.S.; Olesen, S.S. Metabolic sequelae—The pancreatitis zeitgeist of the 21st century. Gastroenterology, 2023; in press. [Google Scholar] [CrossRef]
- Lin, S.Y.; Hsu, W.H.; Lin, C.C.; Lin, C.L.; Tsai, C.H.; Kao, C.H. Effect of acute pancreatitis on the risk of developing osteoporosis: A nationwide cohort study. PLoS ONE 2017, 12, e0179358. [Google Scholar] [CrossRef] [PubMed]
- Duggan, S.N.; Smyth, N.D.; Murphy, A.; MacNaughton, D.; O’Keefe, S.J.; Conlon, K.C. High prevalence of osteoporosis in patients with chronic pancreatitis: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2014, 12, 219–228. [Google Scholar] [CrossRef]
- García-Compeán, D.; Jiménez-Rodríguez, A.R.; Muñoz-Ayala, J.M.; González- González, J.A.; Maldonado-Garza, H.J.; Villarreal-Pérez, J.Z. Post-acute pancreatitis diabetes: A complication waiting for more recognition and understanding. World J. Gastroenterol. 2023, 29, 4405–4415. [Google Scholar] [CrossRef]
- Halefoglu, A.M. Magnetic resonance cholangiopancreatography: A useful tool in the evaluation of pancreatic and biliary disorders. World J. Gastroenterol. 2007, 13, 2529. [Google Scholar] [CrossRef] [PubMed]
- Leyendecker, J.R.; Elsayes, K.M.; Gratz, B.I.; Brown, J.J. MR cholangiopancreatography: Spectrum of pancreatic duct abnormalities. Am. J. Roentgenol. 2002, 179, 1465–1471. [Google Scholar] [CrossRef]
- Vitellas, K.M.; Keogan, M.T.; Spritzer, C.E.; Nelson, R.C. MR cholangiopancreatography of bile and pancreatic duct abnormalities with emphasis on the single-shot fast spin-echo technique. RadioGraphics 2000, 20, 939–957. [Google Scholar] [CrossRef]
- Steinkohl, E.; Olesen, S.S.; Hansen, T.M.; Drewes, A.M.; Frøkjær, J.B. T1 relaxation times and MR elastography-derived stiffness: New potential imaging biomarkers for the assessment of chronic pancreatitis. Abdom. Radiol. 2021, 46, 5598–5608. [Google Scholar] [CrossRef]
- Tirkes, T.; Yadav, D.; Conwell, D.L.; Territo, P.R.; Zhao, X.; Persohn, S.A.; Dasyam, A.K.; Shah, Z.K.; Venkatesh, S.K.; Takahashi, N.; et al. Quantitative MRI of chronic pancreatitis: Results from a multi-institutional prospective study, magnetic resonance imaging as a non-invasive method for assessment of pancreatic fibrosis (MINIMAP). Abdom. Radiol. 2022, 47, 3792–3805. [Google Scholar] [CrossRef] [PubMed]
- Reeder, S.B.; Hu, H.H.; Sirlin, C.B. Proton density fat-fraction: A standardized MR-based biomarker of tissue fat concentration. J. Magn. Reson. Imaging 2012, 36, 1011–1014. [Google Scholar] [CrossRef] [PubMed]
- Idilman, I.S.; Tuzun, A.; Savas, B.; Elhan, A.H.; Celik, A.; Idilman, R.; Karcaaltincaba, M. Quantification of liver, pancreas, kidney, and vertebral body MRI-PDFF in non-alcoholic fatty liver disease. Abdom. Imaging 2015, 40, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, A.A.; Vilela, G.R.; Araujo, D.B.; Baffa, O. MRI relaxometry: Methods and applications. Braz. J. Phys. 2006, 36, 9–15. [Google Scholar] [CrossRef]
- Cheng, H.-L.; Stikov, N.; Ghugre, N.R.; Wright, G.A. Practical medical applications of quantitative MR relaxometry. J. Magn. Reson. Imaging 2012, 36, 805–824. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Martin, D.R.; Pineda, N.; Xu, Q.; Vos, M.; Anania, F.; Hu, X. Quantitative analysis of T2-correction in single-voxel magnetic resonance spectroscopy of hepatic lipid fraction. J. Magn. Reson. Imaging 2009, 29, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Kühn, J.-P.; Hernando, D.; Mensel, B.; Krüger, P.C.; Ittermann, T.; Mayerle, J.; Hosten, N.; Reeder, S.B. Quantitative chemical shift-encoded MRI is an accurate method to quantify hepatic steatosis. J. Magn. Reson. Imaging 2014, 39, 1494–1501. [Google Scholar] [CrossRef]
- Pineda, N.; Sharma, P.; Xu, Q.; Hu, X.; Vos, M.; Martin, D.R. Measurement of hepatic lipid: High-speed T2-corrected multiecho acquisition at 1H MR spectroscopy: A rapid and accurate technique. Radiology 2009, 252, 568–576. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, W.-K.; Shi, G.-Z.; Gao, M.; Wang, M.-Z.; Shen, J. Liver fat accumulation measured by high-speed T2-corrected multi-echo magnetic resonance spectroscopy can predict risk of cholelithiasis. World J. Gastroenterol. 2020, 26, 4996–5007. [Google Scholar] [CrossRef]
- Song, R.; Cohen, A.R.; Song, H.K. Improved transverse relaxation rate measurement techniques for the assessment of hepatic and myocardial iron content. J. Magn. Reson. Imaging 2007, 26, 208–214. [Google Scholar] [CrossRef]
- St. Pierre, T.G.; Clark, P.R.; Chua-anusorn, W.; Fleming, A.J.; Jeffrey, G.P.; Olynyk, J.K.; Pootrakul, P.; Robins, E.; Lindeman, R. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood 2005, 105, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Olsen, S.; Zhang, H.C.; Kannengiesser, S.; Chandarana, H.; Shanbhogue, K.P. Detection of hepatic steatosis and iron content at 3 Tesla: Comparison of two-point Dixon, quantitative multi-echo Dixon, and MR spectroscopy. Abdom. Radiol. 2019, 44, 3040–3048. [Google Scholar] [CrossRef]
- Al-Ani, Z.; Ko, J.; Petrov, M.S. Intra-pancreatic fat deposition across the pancreatitis spectrum and the influence of gut hormones. Dig. Liver Dis. 2023, 55, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Löhr, J.M.; Singer, M.V. The M-ANNHEIM classification of chronic pancreatitis: Introduction of a unifying classification system based on a review of previous classifications of the disease. J. Gastroenterol. 2007, 42, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Uelker, B.; Cushman, I.; Dudek, T.; Herkert, D.M.; Geacintov, D.C.E. High sensitivity hepcidin-25 bioactive ELISAs: Manual and fully automated system for the quantification of hepcidin-25 in human serum and plasma. Blood 2016, 128, 4820. [Google Scholar] [CrossRef]
- Mulligan, A.A.; Luben, R.N.; Bhaniani, A.; Parry-Smith, D.J.; O’Connor, L.; Khawaja, A.P.; Forouhi, N.G.; Khaw, K.-T. A new tool for converting food frequency questionnaire data into nutrient and food group values: FETA research methods and availability. BMJ Open 2014, 4, e004503. [Google Scholar] [CrossRef] [PubMed]
- Kimita, W.; Li, X.; Ko, J.; Bharmal, S.H.; Cameron-Smith, D.; Petrov, M.S. Association between habitual dietary iron intake and glucose metabolism in individuals after acute pancreatitis. Nutrients 2020, 12, 3579. [Google Scholar] [CrossRef]
- Sacks, D.B.; Arnold, M.; Bakris, G.L.; Bruns, D.E.; Horvath, A.R.; Lernmark, Å.; Metzger, B.E.; Nathan, D.M.; Kirkman, M.S. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care 2023, dci230036. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R. Generalized additive models: Some applications. J. Am. Stat. Assoc. 1987, 82, 371. [Google Scholar] [CrossRef]
- Nakamura, T.; Naguro, I.; Ichijo, H. Iron homeostasis and iron-regulated ROS in cell death, senescence and human diseases. Biochim. Biophys. Acta-Gen. Subj. 2019, 1863, 1398–1409. [Google Scholar] [CrossRef]
- Crichton, R. Inorganic Biochemistry of Iron Metabolism, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2001; ISBN 9780471492238. [Google Scholar]
- Lin, H.; Fu, C.; Kannengiesser, S.; Cheng, S.; Shen, J.; Dong, H.; Yan, F. Quantitative analysis of hepatic iron in patients suspected of coexisting iron overload and steatosis using multi-echo single-voxel magnetic resonance spectroscopy: Comparison with fat-saturated multi-echo gradient echo sequence. J. Magn. Reson. Imaging 2018, 48, 205–213. [Google Scholar] [CrossRef]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Nemeth, E. Hepcidin and iron homeostasis. Biochim. Biophys. Acta-Mol. Cell Res. 2012, 1823, 1434–1443. [Google Scholar] [CrossRef]
- Nemeth, E.; Ganz, T. The role of hepcidin in iron metabolism. Acta Haematol. 2009, 122, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Lunova, M.; Schwarz, P.; Nuraldeen, R.; Levada, K.; Kuscuoglu, D.; Stützle, M.; Vujić Spasić, M.; Haybaeck, J.; Ruchala, P.; Jirsa, M.; et al. Hepcidin knockout mice spontaneously develop chronic pancreatitis owing to cytoplasmic iron overload in acinar cells. J. Pathol. 2017, 241, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Ganz, T. Hepcidin and iron in health and disease. Annu. Rev. Med. 2023, 74, 261–277. [Google Scholar] [CrossRef]
- Altamura, S.; Kessler, R.; Gröne, H.J.; Gretz, N.; Hentze, M.W.; Galy, B.; Muckenthaler, M.U. Resistance of ferroportin to hepcidin binding causes exocrine pancreatic failure and fatal iron overload. Cell Metab. 2014, 20, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Kimita, W.; Petrov, M.S. Iron metabolism and the exocrine pancreas. Clin. Chim. Acta 2020, 511, 167–176. [Google Scholar] [CrossRef]
- Tian, C.; Zhao, J.; Xiong, Q.; Yu, H.; Du, H. Secondary iron overload induces chronic pancreatitis and ferroptosis of acinar cells in mice. Int. J. Mol. Med. 2022, 51, 9. [Google Scholar] [CrossRef]
- Arumugam, G.; Padmanaban, M.; Krishnan, D.; Panneerselvam, S.; Rajagopal, S. Influence of copper, iron, zinc and Fe3+ haemoglobin levels on the etiopathogenesis of chronic calcific pancreatitis: A study in patients with pancreatitis. Biol. Trace Elem. Res. 2011, 142, 424–434. [Google Scholar] [CrossRef]
- Carpenter, C.E.; Mahoney, A.W. Contributions of heme and nonheme iron to human nutrition. Crit. Rev. Food Sci. Nutr. 1992, 31, 333–367. [Google Scholar] [CrossRef] [PubMed]
- Fuqua, B.K.; Vulpe, C.D.; Anderson, G.J. Intestinal iron absorption. J. Trace Elem. Med. Biol. 2012, 26, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, L.; Hulthén, L. Prediction of dietary iron absorption: An algorithm for calculating absorption and bioavailability of dietary iron. Am. J. Clin. Nutr. 2000, 71, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- Miret, S.; Simpson, R.J.; McKie, A.T. Physiology and molecular biology of dietary iron absorption. Annu. Rev. Nutr. 2003, 23, 283–301. [Google Scholar] [CrossRef] [PubMed]
- West, A.-R.; Oates, P.-S. Mechanisms of heme iron absorption: Current questions and controversies. World J. Gastroenterol. 2008, 14, 4101–4110. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.; Srai, S.-K. Molecular mechanisms involved in intestinal iron absorption. World J. Gastroenterol. 2007, 13, 4716–4724. [Google Scholar] [CrossRef]
- Kavin, H.; Charlton, R.W.; Jacobs, P.; Green, R.; Torrance, J.D.; Bothwell, T.H. Effect of the exocrine pancreatic secretions on iron absorption. Gut 1967, 8, 556–564. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kulaksiz, H.; Fein, E.; Redecker, P.; Stremmel, W.; Adler, G.; Cetin, Y. Pancreatic β-cells express hepcidin, an iron-uptake regulatory peptide. J. Endocrinol. 2008, 197, 241–249. [Google Scholar] [CrossRef]
- Abunahel, B.M.; Pontre, B.; Kumar, H.; Petrov, M.S. Pancreas image mining: A systematic review of radiomics. Eur. Radiol. 2021, 13, 3447–3467. [Google Scholar] [CrossRef]
- Casà, C.; Piras, A.; D’Aviero, A.; Preziosi, F.; Mariani, S.; Cusumano, D.; Romano, A.; Boskoski, I.; Lenkowicz, J.; Dinapoli, N.; et al. The impact of radiomics in diagnosis and staging of pancreatic cancer. Ther. Adv. Gastrointest. Endosc. 2022, 15, 263177452210815. [Google Scholar] [CrossRef]
- Petrov, M.S. Fatty change of the pancreas: The Pandora’s box of pancreatology. Lancet Gastroenterol. Hepatol. 2023, 8, 671–682. [Google Scholar] [CrossRef]
- Skudder-Hill, L.; Sequeira, I.R.; Cho, J.; Ko, J.; Poppitt, S.D.; Petrov, M.S. Fat distribution within the pancreas according to diabetes status and insulin traits. Diabetes 2022, 71, 1182–1192. [Google Scholar] [CrossRef]
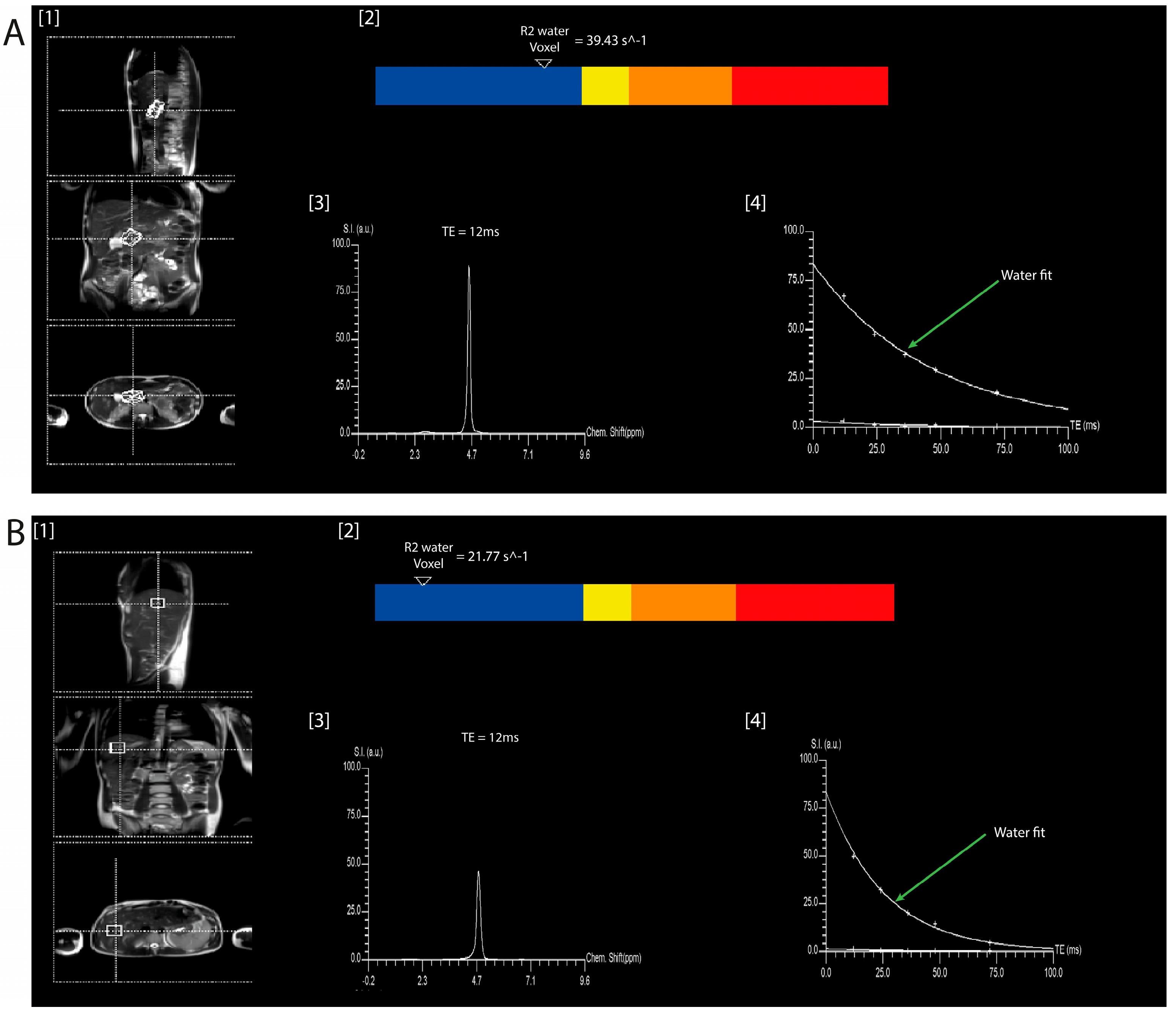
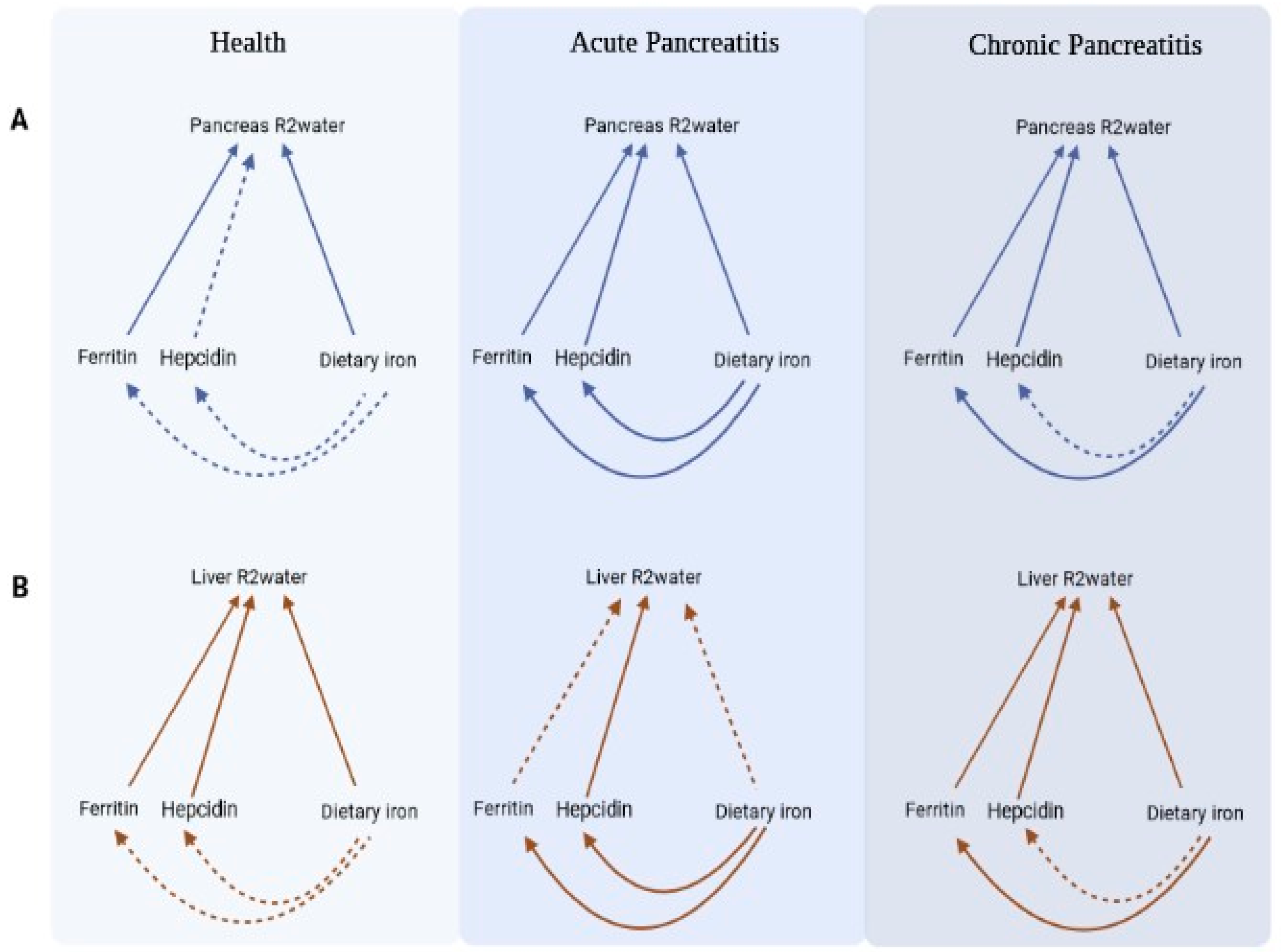
| Characteristic | Health (n = 47) | Acute Pancreatitis (n = 54) | Chronic Pancreatitis (n = 38) | p-Value |
|---|---|---|---|---|
| Age (years) | 55 (32–69) | 58 (46–64) | 58 (49-66) | 0.524 |
| Sex | 0.002 | |||
| Men | 19 (40%) | 31 (57%) | 30 (79%) | |
| Women | 28 (60%) | 23 (42.6%) | 8 (21%) | |
| BMI (kg/m2) | 24.2 (21.6–28.1) | 27.5 (24.9–33.1) | 28 (24–33) | 0.002 |
| HbA1c (mmol/mol) | 36 (33.0–38.0) | 39 (36–42) | 41 (38–51) | <0.001 |
| Ferritin (ng/mL) | 114 (55–181) | 130 (87–243) | 192 (104–314.5) | 0.050 |
| Hepcidin (ng/mL) | 11.3 (4.4–15.0) | 10.1 (8.0–24.6) | 14.0 (11.7–21.5) | 0.089 |
| Total iron intake (mg/day) | 10.2 (8.2–15.1) | 10.0 (7.1–12.6) | 9.7 (7.4–15.2) | 0.735 |
| Haem iron intake (mg/day) | 0.6 (0.4–1.0) | 0.7 (0.6–1.2) | 0.8 (0.5–1.1) | 0.190 |
| Non-haem iron intake (mg/day) | 9.6 (7.8–14.2) | 8.6 (6.6–11.7) | 9.0 (6.6–13.9) | 0.633 |
| Circulating Marker | Dietary Intake (mg/day) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tissue R2water | Group | Model | Ferritin | Hepcidin | Total Iron | Haem Iron | Non-Haem | |||||
| DF | p-Value | DF | p-Value | DF | p-Value | DF | p-Value | DF | p-Value | |||
| Pancreas R2water | ||||||||||||
| Health | Model 1 | 0.36 | 0.13 | 0.87 | 0.04 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Model 2 | <0.001 | <0.001 | 0.67 | 0.10 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Model 3 | <0.001 | <0.001 | 0.62 | 0.12 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| AP | Model 1 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.06 | 0.07 | <0.001 | <0.001 | |
| Model 2 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.54 | 0.12 | <0.001 | <0.001 | ||
| Model 3 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.58 | 0.12 | <0.001 | <0.001 | ||
| CP | Model 1 | 2.00 | <0.001 | 2.00 | 0.03 | <0.001 | <0.001 | 0.01 | <0.001 | 0.72 | 0.08 | |
| Model 2 | 2.00 | 0.01 | 2.00 | 0.07 | <0.001 | <0.001 | 0.86 | 0.12 | 0.63 | 0.11 | ||
| Model 3 | 2.00 | 0.01 | 2.00 | 0.04 | <0.001 | <0.001 | 0.90 | 0.13 | 0.65 | 0.11 | ||
| Liver R2water | ||||||||||||
| Health | Model 1 | 5.33 | 0.01 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Model 2 | 5.32 | 0.02 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Model 3 | 5.15 | 0.02 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| AP | Model 1 | 0.87 | 0.05 | 0.17 | 0.11 | 0.40 | 0.12 | 0.62 | 0.09 | 0.29 | 0.12 | |
| Model 2 | 0.80 | 0.06 | <0.001 | <0.001 | 0.40 | 0.14 | 0.69 | 0.09 | 0.25 | 0.13 | ||
| Model 3 | 0.84 | 0.05 | <0.001 | <0.001 | 0.51 | 0.13 | 0.76 | 0.09 | 0.37 | 0.14 | ||
| CP | Model 1 | 1.35 | 0.01 | <0.001 | <0.001 | <0.001 | <0.001 | 0.98 | 0.03 | <0.001 | <0.001 | |
| Model 2 | 1.42 | 0.03 | <0.001 | <0.001 | <0.001 | <0.001 | 0.97 | 0.05 | <0.001 | <0.001 | ||
| Model 3 | 1.43 | 0.04 | <0.001 | <0.001 | <0.001 | <0.001 | 1.22 | 0.02 | <0.001 | <0.001 | ||
| Circulating Marker | Model | Total Iron | Haem Iron | Non-Haem Iron | ||||
|---|---|---|---|---|---|---|---|---|
| Group | DF | p-Value | DF | p-Value | DF | p-Value | ||
| Ferritin | ||||||||
| Health | Model 1 | <0.001 | <0.001 | 0.46 | 0.10 | <0.001 | <0.001 | |
| Model 2 | 0.89 | 0.09 | 0.61 | 0.10 | 0.85 | 0.10 | ||
| Model 3 | 0.68 | 0.11 | 0.61 | 0.11 | 0.70 | 0.11 | ||
| AP | Model 1 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Model 2 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Model 3 | <0.001 | <0.001 | 0.07 | 0.08 | <0.001 | <0.001 | ||
| CP | Model 1 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Model 2 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Model 3 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Hepcidin | ||||||||
| Health | Model 1 | 1.99 | 0.08 | <0.001 | <0.001 | 2.02 | 0.05 | |
| Model 2 | 2.02 | 0.09 | 2.61 | 0.05 | 2.02 | 0.09 | ||
| Model 3 | 1.98 | 0.13 | 2.56 | 0.09 | 1.96 | 0.11 | ||
| AP | Model 1 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Model 2 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Model 3 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| CP | Model 1 | 0.46 | 0.11 | <0.001 | <0.001 | 0.51 | 0.11 | |
| Model 2 | 0.71 | 0.09 | <0.001 | <0.001 | 0.71 | 0.09 | ||
| Model 3 | 0.61 | 0.12 | <0.001 | <0.001 | 0.62 | 0.13 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimita, W.; Ko, J.; Petrov, M.S. Relationship of Iron Intake, Ferritin, and Hepcidin with the Transverse Relaxation Rate of Water Protons in the Pancreas. Nutrients 2023, 15, 3727. https://doi.org/10.3390/nu15173727
Kimita W, Ko J, Petrov MS. Relationship of Iron Intake, Ferritin, and Hepcidin with the Transverse Relaxation Rate of Water Protons in the Pancreas. Nutrients. 2023; 15(17):3727. https://doi.org/10.3390/nu15173727
Chicago/Turabian StyleKimita, Wandia, Juyeon Ko, and Maxim S. Petrov. 2023. "Relationship of Iron Intake, Ferritin, and Hepcidin with the Transverse Relaxation Rate of Water Protons in the Pancreas" Nutrients 15, no. 17: 3727. https://doi.org/10.3390/nu15173727
APA StyleKimita, W., Ko, J., & Petrov, M. S. (2023). Relationship of Iron Intake, Ferritin, and Hepcidin with the Transverse Relaxation Rate of Water Protons in the Pancreas. Nutrients, 15(17), 3727. https://doi.org/10.3390/nu15173727





