Relationship between Atherogenic Dyslipidaemia and Lipid Triad and Scales That Assess Insulin Resistance
Abstract
1. Introduction
2. Methods
2.1. Inclusion Criteria
- -
- Being over 17 years of age and under 70.
- -
- Working in one of the companies served by the occupational health services participating in the study.
- -
- Accepting the study conditions and their participation in it.
- -
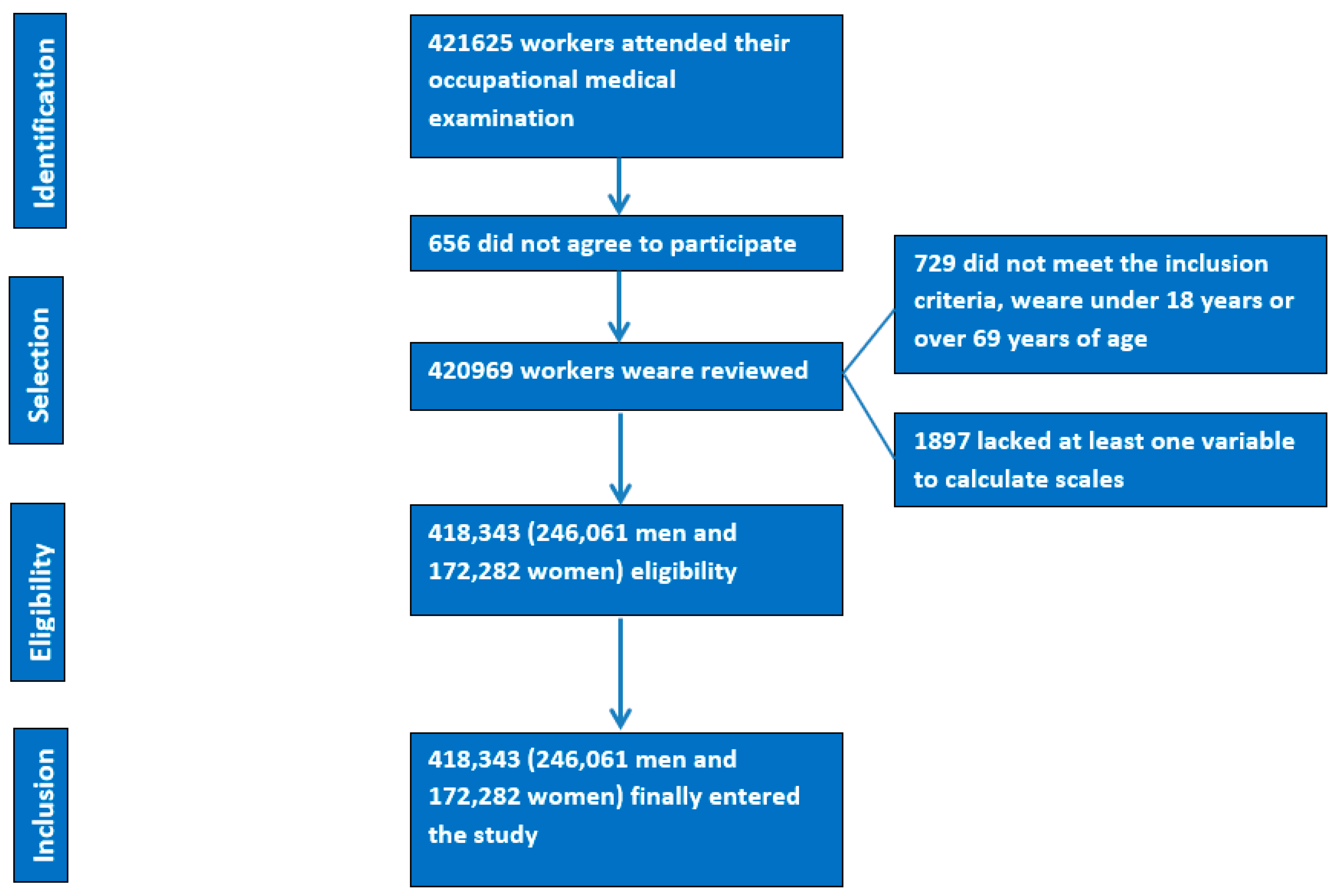
- The PRISMA flow chart is shown in Figure 1.
2.2. Determination of Variables
- Metabolic score of insulin resistance (METS-IR) [32] METS-IR = Ln((2 × Glucose) + Triglycerides) × BMI)/(Ln(HDL-c)). High values are considered as 50 and over.
2.3. Ethical Considerations and Aspects
2.4. Statistical Analysis
3. Results
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Russo, G.; Piscitelli, P.; Giandalia, A.; Viazzi, F.; Pontremoli, R.; Fioretto, P.; De Cosmo, S. Atherogenic dyslipidemia and diabetic nephropathy. J. Nephrol. 2020, 33, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Berneis, K. Lipid triad or atherogenic lipoprotein phenotype: A role in cardiovascular prevention? J. Atheroscler. Thromb. 2005, 12, 237–239. [Google Scholar] [CrossRef]
- Lorenzatti, A.J.; Toth, P.P. New Perspectives on Atherogenic Dyslipidaemia and Cardiovascular Disease. Eur. Cardiol. 2020, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.A.; King, M.C.; Vranizan, K.M.; Krauss, R.M. Atherogenic lipoprotein phenotype. A proposed genetic marker for coronary heart disease risk. Circulation 1990, 82, 495–506. [Google Scholar] [CrossRef]
- Ramírez-Manent, J.I.; Tomas-Gil, P.; Martí-Lliteras, P.; Coll-Villalonga, J.L.L.; Martínez-Almoyna Rifá, E.; López-González, Á.A. Dietary Intervention on Overweight and Obesity after Confinement by COVID-19. Nutrients 2023, 15, 912. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Casula, M.; Olmastroni, E.; Norata, G.D.; Catapano, A.L. Global epidemiology of dyslipidaemias. Nat. Rev. Cardiol. 2021, 18, 689–700. [Google Scholar] [CrossRef]
- Akhtar, D.H.; Iqbal, U.; Vazquez-Montesino, L.M.; Dennis, B.B.; Ahmed, A. Pathogenesis of Insulin Resistance and Atherogenic Dyslipidemia in Nonalcoholic Fatty Liver Disease. J. Clin. Transl. Hepatol. 2019, 7, 362–370. [Google Scholar] [CrossRef]
- Grundy, S.M. Atherogenic dyslipidemia associated with metabolic syndrome and insulin resistance. Clin. Cornerstone 2006, 8 (Suppl. 1), S21–S27. [Google Scholar] [CrossRef]
- Hirano, T. Pathophysiology of Diabetic Dyslipidemia. J. Atheroscler. Thromb. 2018, 25, 771–782. [Google Scholar] [CrossRef]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Lale Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Pederiva, C.; Capra, M.E.; Viggiano, C.; Rovelli, V.; Banderali, G.; Biasucci, G. Early Prevention of Atherosclerosis: Detection and Management of Hypercholesterolaemia in Children and Adolescents. Life 2021, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Delgado, F.; Katsiki, N.; Lopez-Miranda, J.; Perez-Martinez, P. Dietary habits, lipoprotein metabolism and cardiovascular disease: From individual foods to dietary patterns. Crit. Rev. Food Sci. Nutr. 2021, 61, 1651–1669. [Google Scholar] [CrossRef]
- Lechner, K.; von Schacky, C.; McKenzie, A.L.; Worm, N.; Nixdorff, U.; Lechner, B.; Kränkel, N.; Halle, M.; Krauss, R.M.; Scherr, J. Lifestyle factors and high-risk atherosclerosis: Pathways and mechanisms beyond traditional risk factors. Eur. J. Prev. Cardiol. 2020, 27, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Ramírez Manent, J.I.; Altisench Jané, B.; Sanchís Cortés, P.; Busquets-Cortés, C.; Arroyo Bote, S.; Masmiquel Comas, L.; López-González, Á.A. Impact of COVID-19 Lockdown on Anthropometric Variables, Blood Pressure, and Glucose and Lipid Profile in Healthy Adults: A before and after Pandemic Lockdown Longitudinal Study. Nutrients 2022, 14, 1237. [Google Scholar] [CrossRef]
- Heeren, J.; Scheja, L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Mol. Metab. 2021, 50, 101238. [Google Scholar] [CrossRef]
- Fernandes Silva, L.; Vangipurapu, J.; Laakso, M. The “Common Soil Hypothesis” Revisited-Risk Factors for Type 2 Diabetes and Cardiovascular Disease. Metabolites 2021, 11, 691. [Google Scholar] [CrossRef]
- Alnami, A.; Bima, A.; Alamoudi, A.; Eldakhakhny, B.; Sakr, H.; Elsamanoudy, A. Modulation of Dyslipidemia Markers Apo B/Apo A and Triglycerides/HDL-Cholesterol Ratios by Low-Carbohydrate High-Fat Diet in a Rat Model of Metabolic Syndrome. Nutrients 2022, 14, 1903. [Google Scholar] [CrossRef] [PubMed]
- Vekic, J.; Vujcic, S.; Bufan, B.; Bojanin, D.; Al-Hashmi, K.; Al-Rasadi; Stoian, A.P.; Zeljkovic, A.; Rizzo, M. The Role of Advanced Glycation End Products on Dyslipidemia. Metabolites 2023, 13, 77. [Google Scholar] [CrossRef]
- Kulik-Kupka, K.; Jabczyk, M.; Nowak, J.; Jagielski, P.; Hudzik, B.; Zubelewicz-Szkodzińska, B. Fetuin-A and Its Association with Anthropometric, Atherogenic, and Biochemical Parameters and Indices among Women with Polycystic Ovary Syndrome. Nutrients 2022, 14, 4034. [Google Scholar] [CrossRef]
- Kheirollahi, A.; Teimouri, M.; Karimi, M.; Vatannejad, A.; Moradi, N.; Borumandnia, N.; Sadeghi, A. Evaluation of lipid ratios and triglyceride-glucose index as risk markers of insulin resistance in Iranian polycystic ovary syndrome women. Lipids Health Dis. 2020, 19, 235. [Google Scholar] [CrossRef]
- Capomolla, A.S.; Janda, E.; Paone, S.; Parafati, M.; Sawicki, T.; Mollace, R.; Ragusa, S.; Mollace, V. Atherogenic Index Reduction and Weight Loss in Metabolic Syndrome Patients Treated with A Novel Pectin-Enriched Formulation of Bergamot Polyphenols. Nutrients 2019, 11, 1271. [Google Scholar] [CrossRef]
- Cho, K.H. Biomedicinal implications of high-density lipoprotein: Its composition, structure, functions, and clinical applications. BMB Rep. 2009, 42, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Bautista, I.; Mehta, R.; Cabiedes, J.; García-Ulloa, C.; Guillen-Pineda, L.E.; Almeda-Valdés, P.; Cuevas-Ramos, D.; Aguilar-Salinas, C.A. Determinants of VLDL composition and apo B-containing particles in familial combined hyperlipidemia. Clin. Chim. Acta 2015, 438, 160–165. [Google Scholar] [CrossRef]
- Malik, S.A.; Acharya, J.D.; Mehendale, N.K.; Kamat, S.S.; Ghaskadbi, S.S. Pterostilbene reverses palmitic acid mediated insulin resistance in HepG2 cells by reducing oxidative stress and triglyceride accumulation. Free Radic. Res. 2019, 53, 815–827. [Google Scholar] [CrossRef]
- Giglio, R.V.; Carruba, G.; Cicero, A.F.G.; Banach, M.; Patti, A.M.; Nikolic, D.; Cocciadiferro, L.; Zarcone, M.; Montalto, G.; Stoian, A.P.; et al. Pasta Supplemented with Opuntia ficus-indica Extract Improves Metabolic Parameters and Reduces Atherogenic Small Dense Low-Density Lipoproteins in Patients with Risk Factors for the Metabolic Syndrome: A Four-Week Intervention Study. Metabolites 2020, 10, 428. [Google Scholar] [CrossRef]
- Glavinovic, T.; Thanassoulis, G.; de Graaf, J.; Couture, P.; Hegele, R.A.; Sniderman, A.D. Physiological Bases for the Superiority of Apolipoprotein B over Low-Density Lipoprotein Cholesterol and Non-High-Density Lipoprotein Cholesterol as a Marker of Cardiovascular Risk. J. Am. Heart Assoc. 2022, 11, e025858. [Google Scholar] [CrossRef]
- Zheng, D.; Li, H.; Ai, F.; Sun, F.; Singh, M.; Cao, X.; Jiang, J.; He, Y.; Tang, Z.; Guo, X. Association between the triglyceride to high-density lipoprotein cholesterol ratio and the risk of type 2 diabetes mellitus among Chinese elderly: The Beijing Longitudinal Study of Aging. BMJ Open Diabetes Res. Care 2020, 8, e000811. [Google Scholar] [CrossRef] [PubMed]
- Unger, G.; Benozzi, S.F.; Perruzza, F.; Pennacchiotti, G.L. Triglycerides and glucose index: A useful indicator of insulin resistance. Endocrinol. Nutr. 2014, 61, 533–540, (In English, Spanish). [Google Scholar] [CrossRef] [PubMed]
- Selvi, N.M.K.; Nandhini, S.; Sakthivadivel, V.; Lokesh, S.; Srinivasan, A.R.; Sumathi, S. Association of Triglyceride-Glucose Index (TyG index) with HbA1c and Insulin Resistance in Type 2 Diabetes Mellitus. Maedica 2021, 16, 375–381. [Google Scholar] [CrossRef]
- Gu, Q.; Hu, X.; Meng, J.; Ge, J.; Wang, S.J.; Liu, X.Z. Associations of Triglyceride-Glucose Index and Its Derivatives with Hyperuricemia Risk: A Cohort Study in Chinese General Population. Int. J. Endocrinol. 2020, 2020, 3214716. [Google Scholar] [CrossRef] [PubMed]
- Bello-Chavolla, O.Y.; Almeda-Valdes, P.; Gomez-Velasco, D.; Viveros-Ruiz, T.; Cruz-Bautista, I.; Romo-Romo, A.; Sánchez-Lázaro, D.; Meza-Oviedo, D.; Vargas-Vázquez, A.; Campos, O.A.; et al. METS-IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur. J. Endocrinol. 2018, 178, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Busquets-Cortés, C.; López, C.; Paublini, H.; Arroyo Bote, S.; López-González, Á.A.; Ramírez-Manent, J.I. Relationship between Atherogenic Dyslipidaemia and Lipid Triad with Different Scales of Overweight and Obesity in 418,343 Spanish Workers. J. Nutr. Metab. 2022, 2022, 9946255. [Google Scholar] [CrossRef] [PubMed]
- Domingo-Salvany, A.; Bacigalupe, A.; Carrasco, J.M.; Espelt, A.; Ferrando, J.; Borrell, C.; del Grupo de Determinantes Sociales de Sociedad Española de Epidemiología. Propuestas de clase social neoweberiana y neomarxista a partir de la Clasificación Nacional de Ocupaciones 2011. Gac. Sanit. 2013, 27, 263–272. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Khan, R.M.M.; Chua, Z.J.Y.; Tan, J.C.; Yang, Y.; Liao, Z.; Zhao, Y. From Pre-Diabetes to Diabetes: Diagnosis, Treatments and Translational Research. Medicina 2019, 55, 546. [Google Scholar] [CrossRef]
- Gittelsohn, J.; Lewis, E.C.; Martin, N.M.; Zhu, S.; Poirier, L.; Van Dongen, E.J.I.; Ross, A.; Sundermeir, S.M.; Labrique, A.B.; Reznar, M.M.; et al. The Baltimore Urban Food Distribution (BUD) App: Study Protocol to Assess the Feasibility of a Food Systems Intervention. Int. J. Environ. Res. Public Health 2022, 19, 9138. [Google Scholar] [CrossRef]
- Dhakal, C.K.; Khadka, S. Heterogeneities in Consumer Diet Quality and Health Outcomes of Consumers by Store Choice and Income. Nutrients 2021, 13, 1046. [Google Scholar] [CrossRef]
- Javed, Z.; Valero-Elizondo, J.; Maqsood, M.H.; Mahajan, S.; Taha, M.B.; Patel, K.V.; Sharma, G.; Hagan, K.; Blaha, M.J.; Blankstein, R.; et al. Social determinants of health and obesity: Findings from a national study of US adults. Obesity 2022, 30, 491–502. [Google Scholar] [CrossRef]
- Ramón-Arbués, E.; Martínez-Abadía, B.; Gracia-Tabuenca, T.; Yuste-Gran, C.; Pellicer-García, B.; Juárez-Vela, R.; Guerrero-Portillo, S.; Sáez-Guinoa, M. Prevalencia de sobrepeso/obesidad y su asociación con diabetes, hipertensión, dislipemia y síndrome metabólico: Estudio transversal de una muestra de trabajadores en Aragón, España [Prevalence of overweight/obesity and its association with diabetes, hypertension, dyslipidemia and metabolic syndrome: A cross-sectional study of a sample of workers in Aragón, Spain]. Nutr. Hosp. 2019, 36, 51–59. (In Spanish) [Google Scholar] [CrossRef]
- Vekic, J.; Zeljkovic, A.; Stefanovic, A.; Jelic-Ivanovic, Z.; Spasojevic-Kalimanovska, V. Obesity and dyslipidemia. Metabolism 2019, 92, 71–81. [Google Scholar] [CrossRef]
- Dikaiakou, E.; Athanasouli, F.; Fotiadou, A.; Kafetzi, M.; Fakiolas, S.; Michalacos, S.; Vlachopapadopoulou, E.A. Hypertriglyceridemic Waist Phenotype and Its Association with Metabolic Syndrome Components, among Greek Children with Excess Body Weight. Metabolites 2023, 13, 230. [Google Scholar] [CrossRef] [PubMed]
- Placzkowska, S.; Pawlik-Sobecka, L.; Kokot, I.; Piwowar, A. Indirect insulin resistance detection: Current clinical trends and laboratory limitations. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech Repub. 2019, 163, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Behiry, E.G.; El Nady, N.M.; AbdEl Haie, O.M.; Mattar, M.K.; Magdy, A. Evaluation of TG-HDL Ratio Instead of HOMA Ratio as Insulin Resistance Marker in Overweight and Children with Obesity. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ye, R.; Yu, C.; Liu, T.; Chen, X. Correlation between Non-insulin-Based Insulin Resistance Indices and Increased Arterial Stiffness Measured by the Cardio-Ankle Vascular Index in Non-hypertensive Chinese Subjects: A Cross-Sectional Study. Front. Cardiovasc. Med. 2022, 9, 903307. [Google Scholar] [CrossRef] [PubMed]
- Ferri, N.; Ruscica, M. Proprotein convertase subtilisin/kexin type 9 (PCSK9) and metabolic syndrome: Insights on insulin resistance, inflammation, and atherogenic dyslipidemia. Endocrine 2016, 54, 588–601, Erratum in Endocrine 2016, 54, 602. [Google Scholar] [CrossRef]
- Toth, P.P. Insulin resistance, small LDL particles, and risk for atherosclerotic disease. Curr. Vasc. Pharmacol. 2014, 12, 653–657. [Google Scholar] [CrossRef]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef] [PubMed]
- Duran, E.K.; Aday, A.W.; Cook, N.R.; Buring, J.E.; Ridker, P.M.; Pradhan, A.D. Triglyceride-Rich Lipoprotein Cholesterol, Small Dense LDL Cholesterol, and Incident Cardiovascular Disease. J. Am. Coll. Cardiol. 2020, 75, 2122–2135. [Google Scholar] [CrossRef]
- Vrdoljak, J.; Kumric, M.; Vilovic, M.; Martinovic, D.; Rogosic, V.; Borovac, J.A.; Ticinovic Kurir, T.; Bozic, J. Can Fasting Curb the Metabolic Syndrome Epidemic? Nutrients 2022, 14, 456. [Google Scholar] [CrossRef]
- Blasco, M.; Ascaso, J.F.; en Representación del Grupo de Dislipidemia Aterogénica de la SEA. Control of the overall lipid profile. Clin. Investig. Arterioscler. 2019, 31 (Suppl. 2), 34–41, (In English, Spanish). [Google Scholar] [CrossRef]
- Aroor, A.R.; Whaley-Connell, A.; Sowers, J.R. Utility of obesity and metabolic dyslipidemia (a non-insulin based determinate of the metabolic syndrome and insulin resistance) in predicting arterial stiffness. J. Clin. Hypertens. 2019, 21, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Kato, K.; Ohkido, I.; Yokoo, T. Role and Treatment of Insulin Resistance in Patients with Chronic Kidney Disease: A Review. Nutrients 2021, 13, 4349. [Google Scholar] [CrossRef]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Young, K.A.; Maturu, A.; Lorenzo, C.; Langefeld, C.D.; Wagenknecht, L.E.; Chen, Y.I.; Taylor, K.D.; Rotter, J.I.; Norris, J.M.; Rasouli, N. The triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio as a predictor of insulin resistance, β-cell function, and diabetes in Hispanics and African Americans. J. Diabetes Complicat. 2019, 33, 118–122. [Google Scholar] [CrossRef]
- Yeh, W.C.; Tsao, Y.C.; Li, W.C.; Tzeng, I.S.; Chen, L.S.; Chen, J.Y. Elevated triglyceride-to-HDL cholesterol ratio is an indicator for insulin resistance in middle-aged and elderly Taiwanese population: A cross-sectional study. Lipids Health Dis. 2019, 18, 176. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, G.; Qin, H.; Cai, Z.; Huang, J.; Chen, H.; Wu, W.; Chen, Z.; Wu, S.; Chen, Y. Higher triglyceride to high-density lipoprotein cholesterol ratio increases cardiovascular risk: 10-year prospective study in a cohort of Chinese adults. J. Diabetes Investig. 2020, 11, 475–481. [Google Scholar] [CrossRef]
- Cho, Y.R.; Ann, S.H.; Won, K.B.; Park, G.M.; Kim, Y.G.; Yang, D.H.; Kang, J.W.; Lim, T.H.; Kim, H.K.; Choe, J.; et al. Association between insulin resistance, hyperglycemia, and coronary artery disease according to the presence of diabetes. Sci. Rep. 2019, 9, 6129. [Google Scholar] [CrossRef] [PubMed]
- Mesut, E.; Cihan, A.; Orhan, G. Is it possible to predict the complexity of peripheral artery disease with atherogenic index? Vascular 2020, 28, 513–519. [Google Scholar] [CrossRef]
- Pantoja-Torres, B.; Toro-Huamanchumo, C.J.; Urrunaga-Pastor, D.; Guarnizo-Poma, M.; Lazaro-Alcantara, H.; Paico-Palacios, S.; del Carmen Ranilla-Seguin, V.; Benites-Zapata, V.A.; Metabolic Syndrome Research Group. High triglycerides to HDL-cholesterol ratio is associated with insulin resistance in normal-weight healthy adults. Diabetes Metab. Syndr. 2019, 13, 382–388. [Google Scholar] [CrossRef]
- Gharipour, M.; Sadeghi, M.; Nezafati, P.; Dianatkhah, M.; Sarrafzadegan, N. Cardiovascular Disease Risk Assessment: Triglyceride/High-Density Lipoprotein versus Metabolic Syndrome Criteria. J. Res. Health Sci. 2019, 19, e00442. [Google Scholar] [CrossRef]
- Uruska, A.; Zozulinska-Ziolkiewicz, D.; Niedzwiecki, P.; Pietrzak, M.; Wierusz-Wysocka, B. TG/HDL-C ratio and visceral adiposity index may be useful in assessment of insulin resistance in adults with type 1 diabetes in clinical practice. J. Clin. Lipidol. 2018, 12, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Cuevas Fernández, F.J.; García Marrero, M.R.; Iglesias Girón, M.J.; Pérez de Armas, A.A.; Cerdeña Rodríguez, E.; Cabrera León, A.; Aguirre-Jaime, A. Effectiveness of the TG/HDL-C ratio to improve GLP-1 prescription in patients with type 2 diabetes in primary care. Medicina de Familia. Semergen 2021, 47, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Yao, X.; Chen, Y.; Lin, J.; Vielhauer, V.; Hu, H. Elevated TG/HDL-C and non-HDL-C/HDL-C ratios predict mortality in peritoneal dialysis patients. BMC Nephrol. 2020, 21, 324. [Google Scholar] [CrossRef]
- Chen, Y.; Chang, Z.; Liu, Y.; Zhao, Y.; Fu, J.; Zhang, Y.; Liu, Y.; Zhong, F. Triglyceride to high-density lipoprotein cholesterol ratio and cardiovascular events in the general population: A systematic review and meta-analysis of cohort studies. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 318–329. [Google Scholar] [CrossRef]
- Ouchi, G.; Komiya, I.; Taira, S.; Wakugami, T.; Ohya, Y. Triglyceride/low-density-lipoprotein cholesterol ratio is the most valuable predictor for increased small, dense LDL in type 2 diabetes patients. Lipids Health Dis. 2022, 21, 4. [Google Scholar] [CrossRef]
- Available online: https://www.semfyc.es/wp-content/uploads/2016/05/Guia_Dislipemia_version-extendida.pdf (accessed on 14 April 2023).
- Wang, H.; Zhang, J.; Pu, Y.; Qin, S.; Liu, H.; Tian, Y.; Tang, Z. Comparison of different insulin resistance surrogates to predict hyperuricemia among U.S. non-diabetic adults. Front. Endocrinol. 2022, 13, 1028167. [Google Scholar] [CrossRef]
- Tramunt, B.; Smati, S.; Grandgeorge, N.; Lenfant, F.; Arnal, J.F.; Montagner, A.; Gourdy, V. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 2020, 63, 453–461. [Google Scholar] [CrossRef]
- Lin, C.A.; Li, W.C.; Lin, S.Y.; Chen, Y.C.; Yu, W.; Huang, H.Y.; Xiong, X.J.; Chen, J.Y. Gender differences in the association between insulin resistance and chronic kidney disease in a Chinese population with metabolic syndrome. Diabetol. Metab. Syndr. 2022, 14, 184. [Google Scholar] [CrossRef] [PubMed]
- Trouwborst, I.; Goossens, G.H.; Astrup, A.; Saris, W.H.M.; Blaak, E.E. Sexual Dimorphism in Body Weight Loss, Improvements in Cardiometabolic Risk Factors and Maintenance of Beneficial Effects 6 Months after a Low-Calorie Diet: Results from the Randomized Controlled DiOGenes Trial. Nutrients 2021, 13, 1588. [Google Scholar] [CrossRef]
- Wu, Z.; Cui, H.; Li, W.; Zhang, Y.; Liu, L.; Liu, Z.; Zhang, W.; Zheng, T.; Yang, J. Comparison of three non-insulin-based insulin resistance indexes in predicting the presence and severity of coronary artery disease. Front. Cardiovasc. Med. 2022, 9, 918359. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Lee, J.W.; Kwon, Y.J. Comparison of the triglyceride glucose (TyG) index, triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio, and metabolic score for insulin resistance (METS-IR) associated with periodontitis in Korean adults. Ther. Adv. Chronic. Dis. 2022, 13, 20406223221122671. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R.; Fu, X.; Song, H. Non-insulin-based insulin resistance indexes in predicting severity for coronary artery disease. Diabetol. Metab. Syndr. 2022, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, C.; Ye, R.; Liu, T.; Chen, X. Correlation between non-insulin-based insulin resistance indexes and the risk of prehypertension: A cross-sectional study. J. Clin. Hypertens 2022, 24, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chi, X.; Wang, Y.; Setrerrahmane, S.; Xie, W.; Xu, H. Trends in insulin resistance: Insights into mechanisms and therapeutic strategy. Signal Transduct. Target Ther. 2022, 7, 216. [Google Scholar] [CrossRef]
- Kang, S.Y.; Kim, Y.S. Relationships between fasting glucose levels, lifestyle factors, and metabolic parameters in Korean adults without diagnosis of diabetes mellitus. J. Diabetes 2022, 14, 52–63. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, H.; Chen, M.; Luo, X.; Yao, W.; Liang, Q.; Yang, F.; Wang, X. Association of Triglyceride to high-density lipoprotein cholesterol ratio and incident of diabetes mellitus: A secondary retrospective analysis based on a Chinese cohort study. Lipids Health Dis. 2020, 19, 33. [Google Scholar] [CrossRef] [PubMed]
- Goit, R.; Saddik, S.E.; Dawood, S.N.; Rabih, A.M.; Niaj, A.; Raman, A.; Patsouras, A.; Gravvanis, N.; Antoniou, V.; Litos, A.; et al. Bempedoic Acid’s Use as an Adjunct in Lowering Low-Density Lipoprotein Cholesterol in Patients with Coronary Artery Disease: A Systematic Review. Cureus 2022, 14, e29891. [Google Scholar] [CrossRef]
- Taleb, S. Inflammation in atherosclerosis. Arch. Cardiovasc. Dis. 2016, 109, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Domanski, M.J.; Tian, X.; Wu, C.O.; Reis, J.P.; Dey, A.K.; Gu, Y.; Zhao, L.; Bae, S.; Liu, K.; Hasan, A.A.; et al. Time Course of LDL Cholesterol Exposure and Cardiovascular Disease Event Risk. J. Am. Coll. Cardiol. 2020, 76, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Damaskos, C.; Garmpis, N.; Kollia, P.; Mitsiopoulos, G.; Barlampa, D.; Drosos, A.; Patsouras, A.; Gravvanis, N.; Antoniou, V.; Antoniou, V.; et al. Assessing Cardiovascular Risk in Patients with Diabetes: An Update. Curr. Cardiol. Rev. 2020, 16, 266–274. [Google Scholar] [CrossRef]
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; de Bittencourt, P.I., Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016, 473, 4527–4550. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.S.; Brownlee, M. Molecular and Cellular Mechanisms of Cardiovascular Disorders in Diabetes. Circ. Res. 2016, 118, 1808–1829. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Manent, J.I.; Jover, A.M.; Martinez, C.S.; Tomás-Gil, P.; Martí-Lliteras, P.; López-González, Á.A. Waist Circumference Is an Essential Factor in Predicting Insulin Resistance and Early Detection of Metabolic Syndrome in Adults. Nutrients 2023, 15, 257. [Google Scholar] [CrossRef] [PubMed]
| Women n = 172,282 | Men n = 246,061 | Total n = 418,343 | ||
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | p-Value | |
| Age (years) | 39.6 (10.8) | 40.6 (11.1) | 40.2 (11.0) | <0.0001 |
| Height (cm) | 161.8 (6.5) | 174.6 (7.0) | 169.4 (9.3) | <0.0001 |
| Weight (kg) | 66.2 (14.0) | 81.4 (14.7) | 75.1 (16.2) | <0.0001 |
| Waist circumference (cm) | 74.8 (10.6) | 86.2 (11.1) | 81.5 (12.2) | <0.0001 |
| SBP (mmHg) | 117.4 (15.7) | 128.2 (15.5) | 123.7 (16.5) | <0.0001 |
| DBP (mmHg) | 72.6 (10.4) | 77.8 (11.0) | 75.6 (11.0) | <0.0001 |
| Total cholesterol (mg/dL) | 190.6 (35.8) | 192.6 (38.9) | 191.8 (37.7) | <0.0001 |
| HDL-c (mg/dL) | 56.8 (8.7) | 50.3 (8.5) | 53.0 (9.1) | <0.0001 |
| LDL-c (mg/dL) | 116.1 (34.8) | 118.0 (36.7) | 117.2 (35.9) | <0.0001 |
| Triglycerides (mg/dL) | 89.1 (46.2) | 123.7 (86.4) | 109.5 (74.6) | <0.0001 |
| Glycaemia | 87.8 (15.1) | 93.3 (21.3) | 91.0 (19.2) | <0.0001 |
| ALT (U/L) | 20.2 (13.6) | 31.0 (20.2) | 26.6 (18.6) | <0.0001 |
| AST (U/L) | 18.2 (7.9) | 24.4 (13.3) | 21.7 (11.7) | <0.0001 |
| GGT (U/L) | 20.4 (19.7) | 35.8 (39.3) | 29.6 (33.6) | <0.0001 |
| % | % | % | p-value | |
| 18–29 years | 20.7 | 18.8 | 19.6 | <0.0001 |
| 30–39 years | 29.7 | 27.6 | 28.4 | |
| 40–49 years | 29.6 | 30.1 | 29.9 | |
| 50–70 years | 20.0 | 23.6 | 22.2 | |
| Social class I | 6.9 | 4.9 | 5.7 | <0.0001 |
| Social class II | 23.4 | 14.9 | 18.4 | |
| Social class III | 69.7 | 80.3 | 75.9 | |
| Non-smokers | 67.2 | 66.6 | 66.9 | <0.0001 |
| Smokers | 32.8 | 33.4 | 33.2 |
| Women | Men | |||||
| Non AD n = 165,431 | Yes AD n = 6851 | Non AD n = 227,030 | Yes AD n = 19,031 | |||
| Mean (SD) | Mean (SD) | p-Value | Mean (SD) | Mean (SD) | p-Value | |
| Triglycerides/HDL | 1.5 (0.7) | 4.5 (1.9) | <0.0001 | 2.3 (1.6) | 6.5 (3.4) | <0.0001 |
| TyG index | 8.1 (0.4) | 9.2 (0.4) | <0.0001 | 8.4 (0.5) | 9.3 (0.4) | <0.0001 |
| METS-IR | 34.6 (7.9) | 48.2 (9.7) | <0.0001 | 38.1 (7.5) | 52.7 (8.7) | <0.0001 |
| Non LT n = 170,566 | Yes LT n = 1716 | p-value | Non LT n = 240,669 | Yes LT n = 5392 | p-value | |
| Triglycerides/HDL | 1.6 (0.9) | 5.1 (3.1) | <0.0001 | 2.5 (1.8) | 8.0 (5.3) | <0.0001 |
| TyG index | 8.1 (0.5) | 9.2 (0.5) | <0.0001 | 8.5 (0.6) | 9.5 (0.6) | <0.0001 |
| METS-IR | 35.0 (8.3) | 47.6 (9.3) | <0.0001 | 39.0 (8.3) | 53.2 (9.2) | <0.0001 |
| Women | Men | |||||
| Non AD n = 165,431 | Yes AD n = 6851 | Non AD n = 227,030 | Yes AD n = 19,031 | |||
| % (95% CI) | % (95% CI) | p-Value | % (95% CI) | % (95% CI) | p-Value | |
| Triglycerides/HDL high | 14.4 (14.4-4.4) | 100.0 (100.0-100.0) | <0.0001 | 18.8 (18.8-18.9) | 100.0 (100.0-100.0) | <0.0001 |
| TyG index high | 9.0 (9.0-9.0) | 96.9 (96.2-97.6) | <0.0001 | 21.6 (21.6-21.6) | 95.7 (95.1-96.3) | <0.0001 |
| METS-IR high | 5.1 (5.1-5.1) | 38.6 (37.9-39.3) | <0.0001 | 7.1 (7.1-7.1) | 62.2 (61.8-62.6) | <0.0001 |
| Non LT n = 170,566 | Yes LT n = 1716 | p-value | Non LT n = 240,669 | Yes LT n = 5392 | p-value | |
| Triglycerides/HDL high | 17.0 (17.0-17.0) | 100.0 (100.0-100.0) | <0.0001 | 23.4 (23.4-23.4) | 100.0 (100.0-100.0) | <0.0001 |
| TyG index high | 11.6 (11.6-11.6) | 97.5 (96.0-99.0) | <0.0001 | 25.8 (25.8-25.8) | 96.8 (96.0-97.6) | <0.0001 |
| METS-IR high | 6.1 (6.1-6.2) | 35.1 (33.6-36.6) | <0.0001 | 10.3 (10.3-10.3) | 62.3 (61.5-63.1) | <0.0001 |
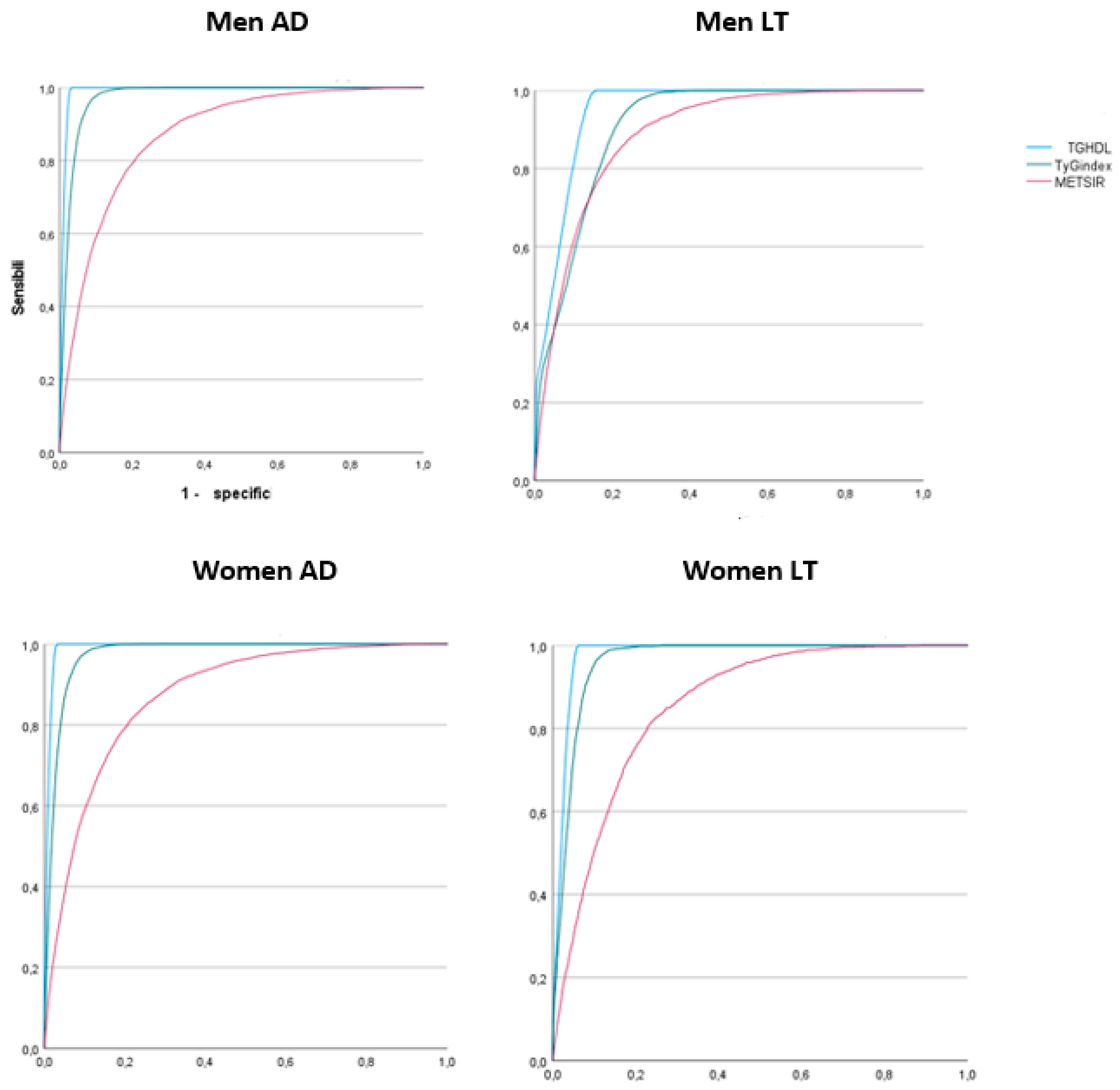
| AD Men | LT Men | |
| AUC-Cutoff-Sensib-Specif-Youden Index | AUC-Cutoff-Sensib-Specif-Youden Index | |
| TG/HDL | 0.964 (0.964-0.965)-4-0.955-0.916-0.871 | 0.947 (0.946-0.948)-4.2-0.898-0.882-0.780 |
| TyG index | 0.916 (0.914-0.917)-8.9-0.894-0.827-0.721 | 0.907 (0.905-0.910)-8.9-0.908-0.799-0.707 |
| METS-IR | 0.905 (0.903-0.907)-44.5-0.839-0.825-0.664 | 0.886 (0.883-0.890)-45-0.826-0.800-0.626 |
| AD Women | LT Women | |
| TG/HDL | 0.991 (0.991-0.991)-3-1.00-0.968-0.968 | 0.979 (0.978-0.980)-3.1-0.993-0.943-0.936 |
| TyG index | 0.974 (0.974-0.975)-8.7-0.969-0.910-0.879 | 0.963 (0.962-0.965)-8.8-0.927-0.913-0.840 |
| METS-IR | 0.872 (0.868-0.876)-40-0.801-0.794-0.595 | 0.856 (0.849-0.863)-40-0.793-0.776-0.569 |
| Women | Men | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Glycaemia | <100 mg/dL | 100–125 mg/dL | ≥126 mg/dL | <100 mg/dL | 100–125 mg/dL | ≥126 mg/dL | ||||
| n | % | % | % | p-Value | n | % | % | % | p-Value | |
| Cholesterol <200 mg/dL | 108,633 | 91.3 | 85.4 | 79.8 | <0.0001 | 147,284 | 81.3 | 73.9 | 67.9 | <0.0001 |
| Cholesterol 200–239 mg/dL | 47,901 | 7.7 | 13.0 | 17.7 | 71,274 | 15.6 | 22.2 | 26.8 | ||
| Cholesterol ≥240 mg/dL | 15,748 | 1.0 | 1.6 | 2.5 | 27,503 | 3.1 | 3.9 | 5.3 | ||
| LDL-c <130 mg/dL | 116,109 | 90.9 | 85.5 | 80.6 | <0.0001 | 155,721 | 80.8 | 74.7 | 68.0 | <0.0001 |
| LDL-c 130–159 mg/dL | 37,234 | 8.0 | 13.0 | 17.2 | 56,236 | 16.0 | 21.8 | 26.7 | ||
| LDL-c ≥160 mg/dL | 18,939 | 1.1 | 1.5 | 2.2 | 34,104 | 3.2 | 3.5 | 5.3 | ||
| Triglycerides <150 mg/dL | 158,532 | 89.8 | 69.7 | 68.6 | <0.0001 | 187,298 | 81.2 | 77.2 | 63.1 | <0.0001 |
| Triglycerides 150–199 mg/dL | 9148 | 9.2 | 24.5 | 22.6 | 30,517 | 16.5 | 18.4 | 27.6 | ||
| Triglycerides ≥200 mg/dL | 4602 | 0.9 | 5.8 | 8.8 | 28,246 | 2.3 | 4.4 | 9.3 | ||
| OR (95% CI) | p-Value | |
|---|---|---|
| <50 years | 1 | <0.001 |
| ≥50 years | 1.64 (1.59-1.69) | |
| Women | 1 | <0.001 |
| Men | 3.03 (2.95-3.12) | |
| TyG normal | 1 | <0.001 |
| TyG high | 1.25 (1.21-1.28) | |
| TG/HDL normal | 1 | <0.001 |
| TG/HDL high | 1.87 (1.80-1.95) | |
| METS-IR normal | 1 | <0.001 |
| METS-IR high | 35.27 (34.15-36.42) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paublini, H.; López González, A.A.; Busquets-Cortés, C.; Tomas-Gil, P.; Riutord-Sbert, P.; Ramírez-Manent, J.I. Relationship between Atherogenic Dyslipidaemia and Lipid Triad and Scales That Assess Insulin Resistance. Nutrients 2023, 15, 2105. https://doi.org/10.3390/nu15092105
Paublini H, López González AA, Busquets-Cortés C, Tomas-Gil P, Riutord-Sbert P, Ramírez-Manent JI. Relationship between Atherogenic Dyslipidaemia and Lipid Triad and Scales That Assess Insulin Resistance. Nutrients. 2023; 15(9):2105. https://doi.org/10.3390/nu15092105
Chicago/Turabian StylePaublini, Hernán, Angel Arturo López González, Carla Busquets-Cortés, Pilar Tomas-Gil, Pere Riutord-Sbert, and José Ignacio Ramírez-Manent. 2023. "Relationship between Atherogenic Dyslipidaemia and Lipid Triad and Scales That Assess Insulin Resistance" Nutrients 15, no. 9: 2105. https://doi.org/10.3390/nu15092105
APA StylePaublini, H., López González, A. A., Busquets-Cortés, C., Tomas-Gil, P., Riutord-Sbert, P., & Ramírez-Manent, J. I. (2023). Relationship between Atherogenic Dyslipidaemia and Lipid Triad and Scales That Assess Insulin Resistance. Nutrients, 15(9), 2105. https://doi.org/10.3390/nu15092105







