Definition of a Dietary Pattern Expressing the Intake of Vegetables and Fruits and Its Association with Intestinal Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Individuals
2.2. Microbiota Analysis
2.3. Dietary Assessment and Dietary Pattern Definition
2.4. Other Measurements
2.5. Statistical Analysis
3. Results
3.1. Summary Statistics of Data
3.2. Definition of Dietary Patterns
3.3. Summary Statistics of Dietary Pattern Quartiles
3.4. Dietary Pattern and β-Diversity
3.5. Dietary Pattern and α-Diversity
3.6. Food Group and α-Diversity
3.7. Food Items and α-Diversity
3.8. Association between Individual Bacterial Genera and Dietary Patterns
3.9. Limitations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the Gut Microbiota in Nutrition and Health. BMJ 2018, 361, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Negi, S.; Das, D.K.; Pahari, S.; Nadeem, S.; Agrewala, J.N. Potential Role of Gut Microbiota in Induction and Regulation of Innate Immune Memory. Front. Immunol. 2019, 10, 2441. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between Microbiota and Immunity in Health and Disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- von Engelhardt, W.; Bartels, J.; Kirschberger, S.; Meyer zu Düttingdorf, H.; Busche, R. Role of Short-Chain Fatty Acids in the Hind Gut. Vet. Q. 1998, 20, S52–S59. [Google Scholar] [CrossRef]
- Oliveira Corrêa, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Aurélio, M.; Vinolo, R. Regulation of Immune Cell Function by Short-Chain Fatty Acids. Clin. Transl. Immunol. 2016, 5, 73. [Google Scholar] [CrossRef]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The Short-Chain Fatty Acid Acetate Reduces Appetite via a Central Homeostatic Mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Generated Metabolites Promote Metabolic Benefits via Gut-Brain Neural Circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef]
- Peterson, D.A.; Frank, D.N.; Pace, N.R.; Gordon, J.I. Metagenomic Approaches for Defining the Pathogenesis of Inflammatory Bowel Diseases. Cell Host Microbe 2008, 3, 417–427. [Google Scholar] [CrossRef]
- Ozato, N.; Saito, S.; Yamaguchi, T.; Katashima, M.; Tokuda, I.; Sawada, K.; Katsuragi, Y.; Kakuta, M.; Imoto, S.; Ihara, K.; et al. Blautia Genus Associated with Visceral Fat Accumulation in Adults 20–76 Years of Age. Npj Biofilms Microbiomes 2019, 5, 28. [Google Scholar] [CrossRef]
- Lach, G.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics 2018, 15, 36–59. [Google Scholar] [CrossRef]
- Conlon, M.A.; Bird, A.R. The Impact of Diet and Lifestyle on Gut Microbiota and Human Health. Nutrients 2015, 7, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Andoh, A.; Kuzuoka, H.; Tsujikawa, T.; Nakamura, S.; Hirai, F.; Suzuki, Y.; Matsui, T.; Fujiyama, Y.; Matsumoto, T. Multicenter Analysis of Fecal Microbiota Profiles in Japanese Patients with Crohn’s Disease. J. Gastroenterol. 2012, 47, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, M.; Fujita, K.; Nonomura, N. Influence of Diet and Nutrition on Prostate Cancer. Int. J. Mol. Sci. 2020, 21, 1447. [Google Scholar] [CrossRef]
- Wang Erratum: Fruit and Vegetable Consumption and Mortality from All Causes, Cardiovascular Disease, and Cancer: Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. BMJ 2014, 349, 5472. [CrossRef]
- Woodside, J.V.; Young, I.S.; McKinley, M.C. Fruit and Vegetable Intake and Risk of Cardiovascular Disease. Proc. Nutr. Soc. 2013, 72, 399–406. [Google Scholar] [CrossRef]
- Hu, F.B. Plant-Based Foods and Prevention of Cardiovascular Disease: An Overview. Am. J. Clin. Nutr. 2003, 78, 544–551. [Google Scholar] [CrossRef]
- Limon, J.J.; Tang, J.; Li, D.; Wolf, A.J.; Michelsen, K.S.; Funari, V.; Gargus, M.; Nguyen, C.; Sharma, P.; Maymi, V.I.; et al. Malassezia Is Associated with Crohn’s Disease and Exacerbates Colitis in Mouse Models. Cell Host Microbe 2019, 25, 377–388.e6. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-W.; Park, J.G.; Ilhan, Z.E.; Wallstrom, G.; LaBaer, J.; Adams, J.B.; Krajmalnik-Brown, R. Reduced Incidence of Prevotella and Other Fermenters in Intestinal Microflora of Autistic Children. PLoS ONE 2013, 8, e68322. [Google Scholar] [CrossRef] [PubMed]
- Gough, E.K.; Stephens, D.A.; Moodie, E.E.M.; Prendergast, A.J.; Stoltzfus, R.J.; Humphrey, J.H.; Manges, A.R. Linear Growth Faltering in Infants Is Associated with Acidaminococcus Sp. and Community-Level Changes in the Gut Microbiota. Microbiome 2015, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Selle, K.; Klaenhammer, T.R. Genomic and Phenotypic Evidence for Probiotic Influences of Lactobacillus Gasseri on Human Health. FEMS Microbiol. Rev. 2013, 37, 915–935. [Google Scholar] [CrossRef] [PubMed]
- Andoh, A.; Nishida, A.; Takahashi, K.; Inatomi, O.; Imaeda, H.; Bamba, S.; Kito, K.; Sugimoto, M.; Kobayashi, T. Comparison of the gut microbial community between obese and lean peoples using 16S gene sequencing in a Japanese population. J. Clin. Biochem. Nutr. 2016, 59, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.W.; Sanderson, J.D.; Churcher, C.; Parkes, G.C.; Hudspith, B.N.; Rayment, N.; Brostoff, J.; Parkhill, J.; Dougan, G.; Petrovska, L. High-Throughput Clone Library Analysis of the Mucosa-Associated Microbiota Reveals Dysbiosis and Differences between Inflamed and Non-Inflamed Regions of the Intestine in Inflammatory Bowel Disease. BMC Microbiol. 2011, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Kanai, T. The Gut Microbiota and Inflammatory Bowel Disease. Semin. Immunopathol. 2015, 37, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel Disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Manichanh, C.; Rigottier-Gois, L.; Bonnaud, E.; Gloux, K.; Pelletier, E.; Frangeul, L.; Nalin, R.; Jarrin, C.; Chardon, P.; Marteau, P.; et al. Reduced Diversity of Faecal Microbiota in Crohn’s Disease Revealed by a Metagenomic Approach. Gut 2006, 55, 205–211. [Google Scholar] [CrossRef]
- Scher, J.U.; Ubeda, C.; Artacho, A.; Attur, M.; Isaac, S.; Reddy, S.M.; Marmon, S.; Neimann, A.; Brusca, S.; Patel, T.; et al. Decreased Bacterial Diversity Characterizes the Altered Gut Microbiota in Patients with Psoriatic Arthritis, Resembling Dysbiosis in Inflammatory Bowel Disease. Arthritis Rheumatol. 2015, 67, 128–139. [Google Scholar] [CrossRef]
- De Goffau, M.C.; Luopajärvi, K.; Knip, M.; Ilonen, J.; Ruohtula, T.; Härkönen, T.; Orivuori, L.; Hakala, S.; Welling, G.W.; Harmsen, H.J.; et al. Fecal Microbiota Composition Differs between Children with β-Cell Autoimmunity and Those Without. Diabetes 2013, 62, 1238–1244. [Google Scholar] [CrossRef]
- Wang, M.; Karlsson, C.; Olsson, C.; Adlerberth, I.; Wold, A.E.; Strachan, D.P.; Martricardi, P.M.; Åberg, N.; Perkin, M.R.; Tripodi, S.; et al. Reduced Diversity in the Early Fecal Microbiota of Infants with Atopic Eczema. J. Allergy Clin. Immunol. 2008, 121, 129–134. [Google Scholar] [CrossRef]
- Schippa, S.; Iebba, V.; Barbato, M.; Di Nardo, G.; Totino, V.; Checchi, M.P.; Longhi, C.; Maiella, G.; Cucchiara, S.; Conte, M.P. A Distinctive “microbial Signature” in Celiac Pediatric Patients. BMC Microbiol. 2010, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Jason, P.; Egholm, M.; et al. A Core Gut Microbiome between Lean and Obesity Twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Menni, C.; Lin, C.; Cecelja, M.; Mangino, M.; Matey-Hernandez, M.L.; Keehn, L.; Mohney, R.P.; Steves, C.J.; Spector, T.D.; Kuo, C.F.; et al. Gut Microbial Diversity Is Associated with Lower Arterial Stiffness in Women. Eur. Heart J. 2018, 39, 2390a–2397a. [Google Scholar] [CrossRef]
- Opstelten, J.L.; Plassais, J.; Van Mil, S.W.C.; Achouri, E.; Pichaud, M.; Siersema, P.D.; Oldenburg, B.; Cervino, A.C.L. Gut Microbial Diversity Is Reduced in Smokers with Crohn’s Disease. Inflamm. Bowel Dis. 2016, 22, 2070–2077. [Google Scholar] [CrossRef] [PubMed]
- Sani, G.; Manchia, M.; Simonetti, A.; Janiri, D.; Paribello, P.; Pinna, F.; Carpiniello, B. The Role of Gut Microbiota in the High-Risk Construct of Severe Mental Disorders: A Mini Review. Front. Psychiatry 2021, 11, 585769. [Google Scholar] [CrossRef]
- Taylor, B.C.; Weldon, K.C.; Ellis, R.J.; Franklin, D.; Groth, T.; Gentry, E.C.; Tripathi, A.; McDonald, D.; Humphrey, G.; Bryant, M.; et al. Depression in Individuals Coinfected with HIV and HCV Is Associated with Systematic Differences in the Gut Microbiome and Metabolome. mSystems 2020, 5, e00465-20. [Google Scholar] [CrossRef] [PubMed]
- Morinaka, T.; Wozniewicz, M.; Jeszka, J.; Bajerska, J.; Nowaczyk, P.; Sone, Y. Westernization of Dietary Patterns among Young Japanese and Polish Females—A Comparison Study. Ann. Agric. Environ. Med. 2013, 20, 122–130. [Google Scholar]
- Sasaki, S. What Is the Scientific Definition of the Japanese Diet from the Viewpoint of Nutrition and Health? Nutr. Rev. 2020, 78, 18–26. [Google Scholar] [CrossRef]
- Odermatt, A. The Western-Style Diet: A Major Risk Factor for Impaired Kidney Function and Chronic Kidney Disease. Am. J. Physiol. Ren. Physiol. 2011, 301, G919–G928. [Google Scholar] [CrossRef]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean Diet Pyramid Today. Science and Cultural Updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef]
- Shikany, J.M.; Demmer, R.T.; Johnson, A.J.; Fino, N.F.; Meyer, K.; Ensrud, K.E.; Lane, N.E.; Orwoll, E.S.; Kado, D.M.; Zmuda, J.M.; et al. Association of Dietary Patterns with the Gut Microbiota in Older, Community-Dwelling Men. Am. J. Clin. Nutr. 2019, 110, 1003–1014. [Google Scholar] [CrossRef]
- Rogers, T.S.; Harrison, S.; Judd, S.; Orwoll, E.S.; Marshall, L.M.; Shannon, J.; Langsetmo, L.; Lane, N.E.; Shikany, J.M. Dietary Patterns and Longitudinal Change in Hip Bone Mineral Density among Older Men. Osteoporos. Int. 2018, 29, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Gan, Q.; Xu, P.; Yang, T.; Xu, J.; Cao, W.; Wang, H.; Pan, H.; Ren, Z.; Xiao, H.; et al. Dietary Patterns and Associations with Myopia in Chinese Children. Nutrients 2023, 15, 1946. [Google Scholar] [CrossRef]
- Nakaji, S.; Ihara, K.; Sawada, K.; Parodi, S.; Umeda, T.; Takahashi, I.; Murashita, K.; Kurauchi, S.; Tokuda, I. Social Innovation for Life Expectancy Extension Utilizing a Platform-Centered System Used in the Iwaki Health Promotion Project: A Protocol Paper. SAGE Open Med. 2021, 9, 205031212110026. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a Prokaryotic Universal Primer for Simultaneous Analysis of Bacteria and Archaea Using Next-Generation Sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef]
- Saeidipour, B.; Bakhshi, S. The Relationship between Organizational Culture and Knowledge Management & Their Simultaneous Effects on Customer Relation Management. Adv. Environ. Biol. 2013, 7, 2803–2809. [Google Scholar]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A Versatile Open Source Tool for Metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of RRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Kobayashi, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Comparison of Relative Validity of Food Group Intakes Estimated by Comprehensive and Brief-Type Self-Administered Diet History Questionnaires against 16 d Dietary Records in Japanese Adults. Public Health Nutr. 2011, 14, 1200–1211. [Google Scholar] [CrossRef]
- Kobayashi, S.; Honda, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Both Comprehensive and Brief Self-Administered Diet History Questionnaires Satisfactorily Rank Nutrient Intakes in Japanese Adults. J. Epidemiol. 2012, 22, 151–159. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mantrana, I.; Selma-Royo, M.; Alcantara, C.; Collado, M.C. Shifts on Gut Microbiota Associated to Mediterranean Diet Adherence and Specific Dietary Intakes on General Adult Population. Front. Microbiol. 2018, 9, 890. [Google Scholar] [CrossRef] [PubMed]
- Mitsou, E.K.; Kakali, A.; Antonopoulou, S.; Mountzouris, K.C.; Yannakoulia, M.; Panagiotakos, D.B.; Kyriacou, A. Adherence to the Mediterranean Diet Is Associated with the Gut Microbiota Pattern and Gastrointestinal Characteristics in an Adult Population. Br. J. Nutr. 2017, 117, 1645–1655. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, D.H.; Kim, H.S.; Kim, Y.K. An Exploratory Study on the Effect of Daily Fruits and Vegetable Juice on Human Gut Microbiota. Food Sci. Biotechnol. 2018, 27, 1377–1386. [Google Scholar] [CrossRef]
- Candela, M.; Biagi, E.; Soverini, M.; Consolandi, C.; Quercia, S.; Severgnini, M.; Peano, C.; Turroni, S.; Rampelli, S.; Pozzilli, P.; et al. Modulation of Gut Microbiota Dysbioses in Type 2 Diabetic Patients by Macrobiotic Ma-Pi 2 Diet. Br. J. Nutr. 2016, 116, 80–93. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humarán, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium Prausnitzii Is an Anti-Inflammatory Commensal Bacterium Identified by Gut Microbiota Analysis of Crohn Disease Patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef]
- Sandri, M.; Dal Monego, S.; Conte, G.; Sgorlon, S.; Stefanon, B. Raw Meat Based Diet Influences Faecal Microbiome and End Products of Fermentation in Healthy Dogs. BMC Vet. Res. 2017, 13, 65. [Google Scholar] [CrossRef]
- Gao, X.; Jia, R.; Xie, L.; Kuang, L.; Feng, L.; Wan, C. A Study of the Correlation between Obesity and Intestinal Flora in School-Age Children. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Finnicum, C.T.; Doornweerd, S.; Dolan, C.V.; Luningham, J.M.; Beck, J.J.; Willemsen, G.; Ehli, E.A.; Boomsma, D.I.; Ijzerman, R.G.; Davies, G.E.; et al. Metataxonomic Analysis of Individuals at BMI Extremes and Monozygotic Twins Discordant for BMI. Twin Res. Hum. Genet. 2018, 21, 203–213. [Google Scholar] [CrossRef]
- Zhong, X.; Harrington, J.M.; Millar, S.R.; Perry, I.J.; O’toole, P.W.; Phillips, C.M. Gut Microbiota Associations with Metabolic Health and Obesity Status in Older Adults. Nutrients 2020, 12, 2364. [Google Scholar] [CrossRef] [PubMed]

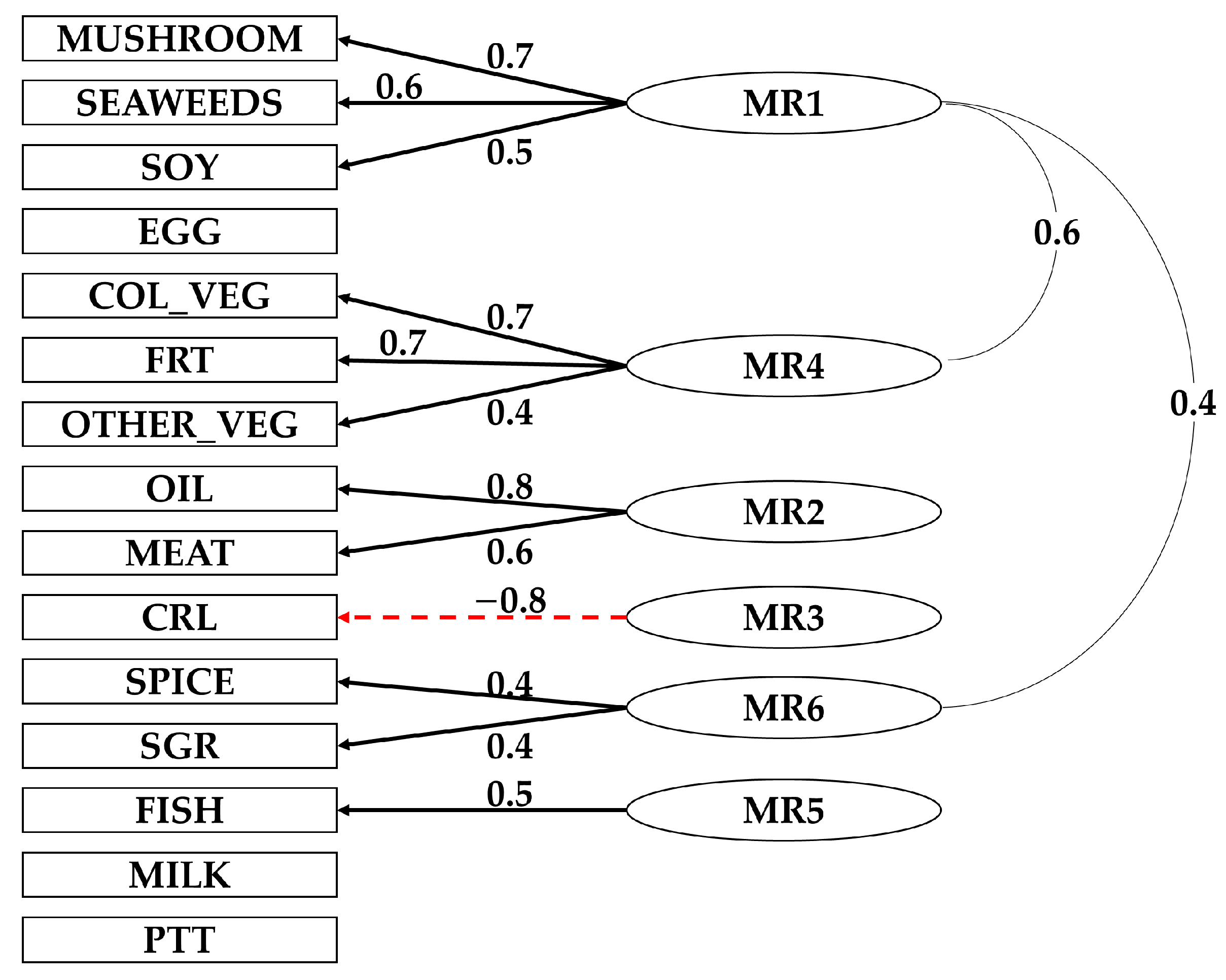
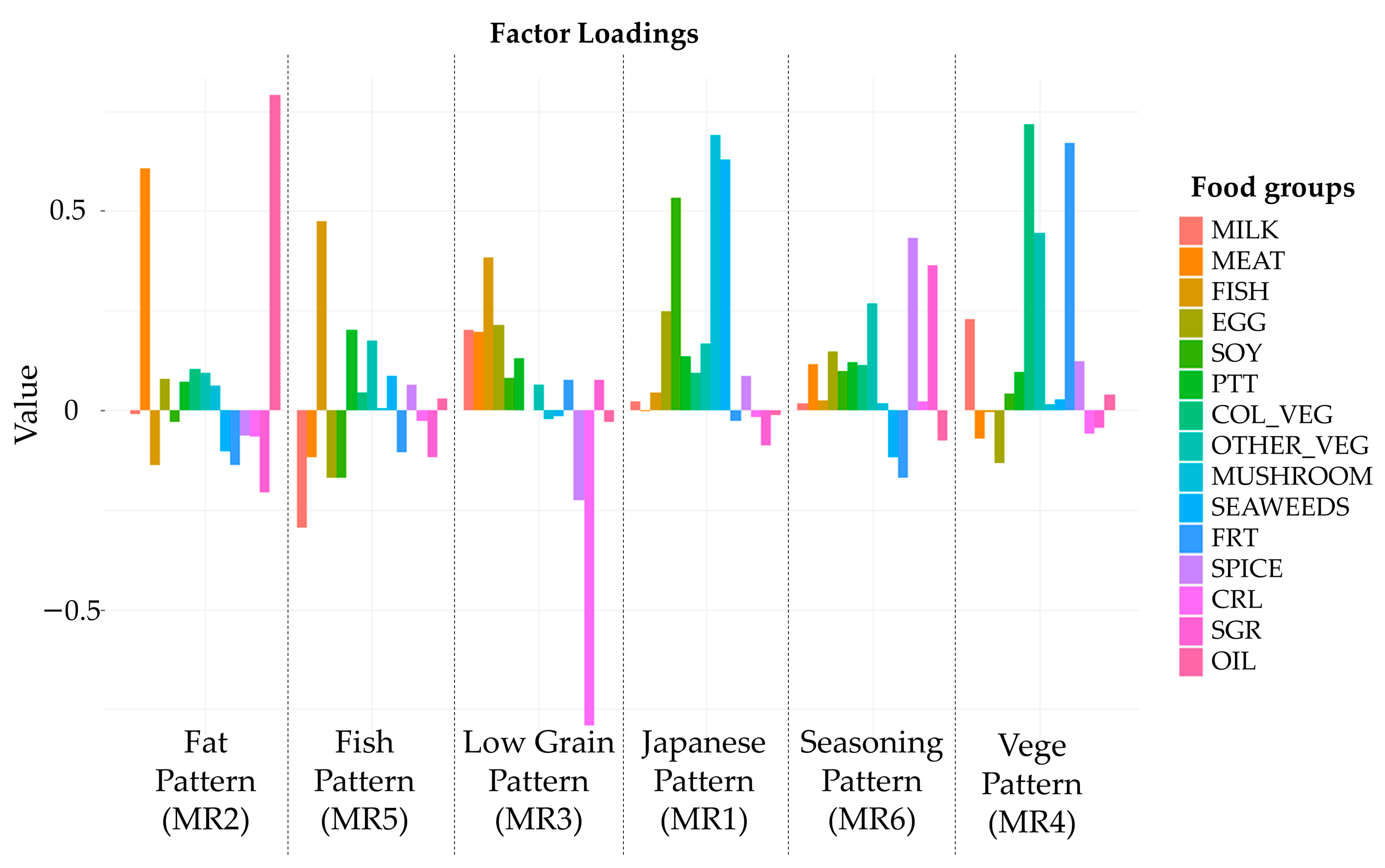

| Food Group | Food Item |
|---|---|
| MUSHROOM | Mushroom |
| SEAWEEDS | Seaweeds |
| SOY | Tofu, Natto |
| EGG | Egg |
| COL_VEG (Colored Vegetables) | Green & Yellow Pickles, Green & Yellow Vegetables, Carrot & Pumpkin, Tomato |
| FRT (Fruits) | Citrus, Persimmon & Strawberry, Other Fruits, Fruit Juice |
| OTHER_VEG (Other Vegetables) | Other Pickles, Raw Lettuce & Cabbage, Cabbage, Radish, Root Vegetables |
| OIL | Cooking Oil |
| MEAT | Chicken, Pork & Beef, Ham, Lever |
| CRL (Cereals) | Bread, Soba, Udon, Ramen, Pasta, Rice |
| SPICE | Mayonnaise, Miso Soup, Noodle Soup, Soy Sauce, Cooking Salt |
| SGR (Sugar) | Sugar, Cooking Sugar |
| FISH | SOSS (Squid, Octopus, Shrimp, Shellfish), Bone Fish, Tuna, Dried Fish, Greasy Fish, Less Fat Fish |
| MILK | Low Fat Milk, Milk, Ice-cream |
| PTT (Potato) | Potato |
| SWT (Sweets) | Pastry, Japanese Sweets, Rice Cracker |
| BEV (Beverage) | Green Tea, Black Tea, Coffee, Coke, Sake, Beer, Shochu, Whiskey, Wine |
| Charasteristics | n | Age | BMI | Sex | Smoking | Drinking | |
|---|---|---|---|---|---|---|---|
| Female (%) | Yes (%) | Yes (%) | |||||
| Overall | 1019 | 54.7 ± 15.1 | 23.1 ± 3.6 | 58.4 | 14.8 | 47.2 | |
| Japanese pattern | 1 (Lowest) | 255 | 51.9 ± 14.4 | 23.7 ± 3.8 | 34.5 | 23.9 | 56.5 |
| 2 | 254 | 53.7 ± 15.0 | 23.0 ± 3.6 | 60.6 | 14.6 | 48.4 | |
| 3 | 255 | 54.5 ± 15.3 | 22.7 ± 3.7 | 69.4 | 11.8 | 43.5 | |
| 4 (Highest) | 255 | 58.9 ± 15.0 | 22.9 ± 3.3 | 69.0 | 9.4 | 40.4 | |
| p value | <0.0001 | 0.0150 | <0.0001 | <0.0001 | 0.0018 | ||
| Vege pattern | 1 (Lowest) | 254 | 51.7 ± 14.3 | 23.5 ± 3.7 | 34.6 | 22.8 | 63.4 |
| 2 | 255 | 51.6 ± 15.8 | 22.9 ± 3.8 | 62.0 | 14.5 | 45.5 | |
| 3 | 255 | 55.1 ± 15.1 | 22.9 ± 3.6 | 69.4 | 12.2 | 39.6 | |
| 4 (Highest) | 255 | 60.6 ± 13.6 | 22.9 ± 3.4 | 67.5 | 10.2 | 40.4 | |
| p value | <0.0001 | 0.1450 | <0.0001 | 0.0003 | <0.0001 | ||
| Fat pattern | 1 (Lowest) | 255 | 60.0 ± 14.2 | 23.4 ± 3.5 | 45.9 | 13.7 | 52.9 |
| 2 | 255 | 56.8 ± 14.0 | 22.8 ± 3.3 | 65.5 | 15.3 | 37.3 | |
| 3 | 255 | 52.7 ± 15.4 | 23.0 ± 3.5 | 61.6 | 15.3 | 51.4 | |
| 4 (Highest) | 254 | 49.5 ± 14.8 | 23.1 ± 4.1 | 60.6 | 15.4 | 47.2 | |
| p value | <0.0001 | 0.3630 | <0.0001 | 0.9442 | 0.0016 | ||
| Low grain pattern | 1 (Lowest) | 255 | 53.5 ± 14.7 | 23.7 ± 3.9 | 35.3 | 22.0 | 51.0 |
| 2 | 255 | 54.9 ± 15.4 | 23.0 ± 3.4 | 62.0 | 11.0 | 42.0 | |
| 3 | 254 | 55.0 ± 14.6 | 22.7 ± 3.5 | 71.3 | 10.6 | 42.9 | |
| 4 (Highest) | 255 | 55.6 ± 15.8 | 22.9 ± 3.5 | 65.1 | 16.1 | 52.9 | |
| p value | 0.4740 | 0.0229 | <0.0001 | 0.0007 | 0.0232 | ||
| Seasoning pattern | 1 (Lowest) | 254 | 54.6 ± 14.4 | 23.3 ± 3.9 | 46.1 | 15.7 | 52.8 |
| 2 | 255 | 55.3 ± 14.3 | 23.1 ± 3.4 | 62.7 | 14.9 | 44.3 | |
| 3 | 255 | 53.5 ± 15.5 | 22.6 ± 3.5 | 64.7 | 14.5 | 47.1 | |
| 4 (Highest) | 255 | 55.7 ± 15.3 | 23.3 ± 3.7 | 60.0 | 14.5 | 44.7 | |
| p value | 0.3910 | 0.1110 | <0.0001 | 0.9768 | 0.2004 | ||
| Fish pattern | 1 (Lowest) | 255 | 51.9 ± 14.3 | 23.1 ± 3.9 | 45.1 | 17.6 | 52.9 |
| 2 | 255 | 52.8 ± 15.0 | 23.0 ± 3.5 | 60.8 | 18.0 | 48.6 | |
| 3 | 255 | 54.9 ± 14.6 | 22.9 ± 3.7 | 62.4 | 13.3 | 41.6 | |
| 4 (Highest) | 254 | 59.4 ± 15.7 | 23.3 ± 3.4 | 65.4 | 10.6 | 45.7 | |
| p value | <0.0001 | 0.7400 | <0.0001 | 0.0541 | 0.0699 | ||
| SERVING SIZE | 1 (Lowest) | 255 | 50.9 ± 14.6 | 22.7 ± 3.3 | 46.3 | 23.1 | 65.9 |
| 2 | 254 | 53.0 ± 15.5 | 23.4 ± 3.7 | 51.6 | 13.0 | 55.1 | |
| 3 | 255 | 56.3 ± 15.2 | 23.3 ± 3.7 | 62.0 | 15.3 | 40.4 | |
| 4 (Highest) | 255 | 58.8 ± 14.1 | 23.0 ± 3.7 | 73.7 | 8.2 | 27.5 | |
| p value | <0.0001 | 0.0994 | <0.0001 | <0.0001 | <0.0001 | ||
| Beta Diversity | Japanese Pattern | Vege Pattern | Fat Pattern | Low Grain Pattern | Seasoning Pattern | Fish Pattern | SERVING SIZE | |
|---|---|---|---|---|---|---|---|---|
| p for trend | Bray | 0.001 | 0.027 | 0.001 | 0.028 | 0.086 | 0.106 | 0.001 |
| Jaccard | 0.001 | 0.030 | 0.001 | 0.047 | 0.122 | 0.114 | 0.001 | |
| Alpha Diversity | Shannon | Pielou | Simpson | Invsimpson | |
|---|---|---|---|---|---|
| Japanese Pattern | β coefficient | −0.070 | −0.056 | −0.017 | −0.055 |
| 95% CI | −0.153 | −0.139 | −0.101 | −0.139 | |
| 0.013 | 0.028 | 0.067 | 0.029 | ||
| p for trend | 0.100 | 0.190 | 0.693 | 0.197 | |
| Vege Pattern | β coefficient | 0.046 | 0.103 | 0.056 | 0.084 |
| 95% CI | −0.029 | 0.028 | −0.020 | 0.009 | |
| 0.121 | 0.179 | 0.131 | 0.160 | ||
| p for trend | 0.231 | 0.007 | 0.150 | 0.029 | |
| Fat Pattern | β coefficient | 0.023 | 0.008 | 0.037 | 0.016 |
| 95% CI | −0.040 | −0.055 | −0.026 | −0.047 | |
| 0.086 | 0.071 | 0.101 | 0.079 | ||
| p for trend | 0.474 | 0.808 | 0.247 | 0.623 | |
| Low Grain Pattern | β coefficient | −0.027 | −0.030 | −0.023 | −0.030 |
| 95% CI | −0.090 | −0.093 | −0.086 | −0.094 | |
| 0.036 | 0.034 | 0.041 | 0.034 | ||
| p for trend | 0.396 | 0.361 | 0.485 | 0.353 | |
| Seasoning Pattern | β coefficient | 0.040 | 0.019 | −0.002 | 0.036 |
| 95% CI | −0.026 | −0.047 | −0.068 | −0.030 | |
| 0.105 | 0.085 | 0.065 | 0.102 | ||
| p for trend | 0.238 | 0.570 | 0.965 | 0.289 | |
| Fish Pattern | β coefficient | 0.053 | 0.022 | 0.015 | 0.025 |
| 95% CI | −0.006 | −0.038 | −0.046 | −0.035 | |
| 0.113 | 0.082 | 0.075 | 0.085 | ||
| p for trend | 0.079 | 0.468 | 0.634 | 0.410 | |
| SERVING SIZE | β coefficient | −0.027 | 0.034 | 0.029 | 0.026 |
| 95% CI | −0.086 | −0.025 | −0.030 | −0.033 | |
| 0.031 | 0.092 | 0.088 | 0.085 | ||
| p for trend | 0.356 | 0.260 | 0.335 | 0.385 | |
| Alpha Diversity | Shannon | Pielou | Simpson | Invsimpson | |
|---|---|---|---|---|---|
| COL_VEG | β coefficient | 0.017 | 0.052 | 0.016 | 0.046 |
| 95% CI | −0.054 | −0.020 | −0.056 | −0.026 | |
| 0.089 | 0.124 | 0.088 | 0.118 | ||
| p for trend | 0.640 | 0.159 | 0.662 | 0.210 | |
| OTHER_ VEG | β coefficient | 0.040 | 0.009 | 0.013 | 0.019 |
| 95% CI | −0.030 | −0.062 | −0.058 | −0.052 | |
| 0.110 | 0.080 | 0.083 | 0.089 | ||
| p for trend | 0.265 | 0.804 | 0.728 | 0.608 | |
| FRT | β coefficient | 0.001 | 0.035 | 0.036 | 0.021 |
| 95% CI | −0.063 | −0.030 | −0.029 | −0.044 | |
| 0.064 | 0.099 | 0.100 | 0.085 | ||
| p for trend | 0.986 | 0.289 | 0.278 | 0.528 | |
| Alpha Diversity | Shannon | Pielou | Simpson | Invsimpson | |
|---|---|---|---|---|---|
| GrYwPick | β coefficient | 0.000 | −0.004 | −0.024 | −0.025 |
| 95% CI | −0.056 | −0.061 | −0.081 | −0.082 | |
| 0.057 | 0.053 | 0.033 | 0.032 | ||
| p for trend | 0.986 | 0.890 | 0.411 | 0.387 | |
| GrYwVege | β coefficient | 0.053 | 0.057 | 0.039 | 0.053 |
| 95% CI | −0.002 | 0.002 | −0.017 | −0.003 | |
| 0.108 | 0.113 | 0.095 | 0.109 | ||
| p for trend | 0.059 | 0.043 | 0.168 | 0.061 | |
| CarPump | β coefficient | −0.035 | −0.015 | −0.019 | −0.025 |
| 95% CI | −0.092 | −0.072 | −0.077 | −0.083 | |
| 0.021 | 0.042 | 0.038 | 0.032 | ||
| p for trend | 0.221 | 0.604 | 0.507 | 0.390 | |
| Tomato | β coefficient | 0.016 | 0.039 | 0.047 | 0.029 |
| 95% CI | −0.040 | −0.017 | −0.008 | −0.027 | |
| 0.071 | 0.095 | 0.103 | 0.085 | ||
| p for trend | 0.577 | 0.169 | 0.096 | 0.312 | |
| OtherPick | β coefficient | 0.055 | 0.019 | 0.030 | 0.024 |
| 95% CI | −0.002 | −0.039 | −0.027 | −0.034 | |
| 0.112 | 0.077 | 0.088 | 0.082 | ||
| p for trend | 0.060 | 0.519 | 0.303 | 0.421 | |
| RawLtCb | β coefficient | 0.029 | 0.020 | 0.031 | 0.041 |
| 95% CI | −0.026 | −0.035 | −0.024 | −0.014 | |
| 0.083 | 0.075 | 0.086 | 0.097 | ||
| p for trend | 0.305 | 0.476 | 0.273 | 0.142 | |
| Cabbage | β coefficient | 0.001 | 0.002 | −0.004 | −0.003 |
| 95% CI | −0.054 | −0.054 | −0.060 | −0.059 | |
| 0.057 | 0.058 | 0.052 | 0.053 | ||
| p for trend | 0.958 | 0.942 | 0.895 | 0.922 | |
| Radish | β coefficient | 0.010 | 0.005 | 0.011 | 0.003 |
| 95% CI | −0.046 | −0.051 | −0.046 | −0.054 | |
| 0.066 | 0.062 | 0.067 | 0.059 | ||
| p for trend | 0.734 | 0.856 | 0.714 | 0.928 | |
| RootVege | β coefficient | 0.018 | 0.018 | 0.017 | 0.014 |
| 95% CI | −0.037 | −0.038 | −0.039 | −0.042 | |
| 0.074 | 0.075 | 0.074 | 0.071 | ||
| p for trend | 0.519 | 0.522 | 0.545 | 0.621 | |
| Citrus | β coefficient | 0.071 | 0.075 | 0.078 | 0.067 |
| 95% CI | 0.015 | 0.019 | 0.021 | 0.010 | |
| 0.127 | 0.132 | 0.134 | 0.124 | ||
| p for trend | 0.013 | 0.009 | 0.007 | 0.020 | |
| PerStr | β coefficient | 0.083 | 0.085 | 0.094 | 0.085 |
| 95% CI | 0.028 | 0.029 | 0.038 | 0.028 | |
| 0.139 | 0.141 | 0.150 | 0.141 | ||
| p for trend | 0.003 | 0.003 | 0.001 | 0.003 | |
| OtherFruits | β coefficient | −0.038 | 0.004 | −0.005 | −0.022 |
| 95% CI | −0.098 | −0.055 | −0.064 | −0.082 | |
| 0.021 | 0.064 | 0.055 | 0.038 | ||
| p for trend | 0.202 | 0.883 | 0.880 | 0.470 | |
| Juice | β coefficient | 0.025 | 0.035 | 0.027 | 0.038 |
| 95% CI | −0.030 | −0.020 | −0.029 | −0.017 | |
| 0.080 | 0.091 | 0.082 | 0.094 | ||
| p for trend | 0.379 | 0.210 | 0.345 | 0.177 | |
| Genus | Vege Pattern | GrYw Vege | Citrus | PerStr | Share Rating (%) | Median Relative Abundance (%) |
|---|---|---|---|---|---|---|
| Neisseria | 〇 | 〇 | 〇 | 3.2 | 0.0046 | |
| Barnesiella | 〇 | 〇 | 〇 | 40.1 | 0.15 | |
| Actinomyces | ● | ● | ● | 97.0 | 0.052 | |
| Faecalibacterium | 〇 | 〇 | 97.4 | 7.3 | ||
| Escherichia.Shigella | ● | ● | 77.9 | 0.037 | ||
| Solobacterium | ● | ● | 33.8 | 0.0071 | ||
| Acinetobacter | ● | ● | 1.7 | 0.0030 | ||
| Succinivibrio | ● | ● | 2.5 | 0.12 | ||
| Gardnerella | ● | ● | 1.4 | 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamauchi, T.; Koyama, N.; Hirai, A.; Suganuma, H.; Suzuki, S.; Murashita, K.; Mikami, T.; Tamada, Y.; Sato, N.; Imoto, S.; et al. Definition of a Dietary Pattern Expressing the Intake of Vegetables and Fruits and Its Association with Intestinal Microbiota. Nutrients 2023, 15, 2104. https://doi.org/10.3390/nu15092104
Yamauchi T, Koyama N, Hirai A, Suganuma H, Suzuki S, Murashita K, Mikami T, Tamada Y, Sato N, Imoto S, et al. Definition of a Dietary Pattern Expressing the Intake of Vegetables and Fruits and Its Association with Intestinal Microbiota. Nutrients. 2023; 15(9):2104. https://doi.org/10.3390/nu15092104
Chicago/Turabian StyleYamauchi, Toshitaka, Naoko Koyama, Ayumi Hirai, Hiroyuki Suganuma, Shigenori Suzuki, Koichi Murashita, Tatsuya Mikami, Yoshinori Tamada, Noriaki Sato, Seiya Imoto, and et al. 2023. "Definition of a Dietary Pattern Expressing the Intake of Vegetables and Fruits and Its Association with Intestinal Microbiota" Nutrients 15, no. 9: 2104. https://doi.org/10.3390/nu15092104
APA StyleYamauchi, T., Koyama, N., Hirai, A., Suganuma, H., Suzuki, S., Murashita, K., Mikami, T., Tamada, Y., Sato, N., Imoto, S., Itoh, K., & Nakaji, S. (2023). Definition of a Dietary Pattern Expressing the Intake of Vegetables and Fruits and Its Association with Intestinal Microbiota. Nutrients, 15(9), 2104. https://doi.org/10.3390/nu15092104






