Abstract
Middle-aged women belong to a risk group for metabolic dysregulation and menopausal symptoms, mainly due to a dramatic hormonal shift. Supplementation with functional compounds or a single nutrient has been dominantly explored as a nutritional approach for improving aging-related health parameters. However, a meal-based approach might be another strategy for promoting the overall health of the target population. This pilot study aimed to develop a meal-based intervention for middle-aged women and to evaluate its potential health benefits. Considering the nutrient intake status of Korean middle-aged women, diets enriched with four major nutrients (isoflavone, omega-3, fiber, and calcium) were designed and provided to forty-nine women aged 50 to 65 with mild levels of menopausal symptoms for 8 weeks. In the post-intervention phase, they showed reduced body weight and body fat, and improved biochemical metabolic parameters with decreased levels of cholesterol, low-density lipoprotein-cholesterol, ApoB, and fasting insulin. Moreover, bone resorption markers and menopause symptoms were lower in the post-intervention phase. In conclusion, the meal-based intervention might be a prominent strategy for overall health promotion in relatively healthy middle-aged women and further investigation is needed to test its efficacy with a randomized controlled study.
1. Introduction
Middle-aged women are considered a health risk group due to their undergoing a dramatic hormonal shift, so-called menopause, and subsequential metabolic shift [1]. Menopause is the cessation of the menstrual cycle and the loss of ovarian activity influences hormonal changes, including a decline in estrogen, progesterone, and estradiol (E2) and an increase in follicle-stimulating hormone (FSH) [2]. Several accompanying symptoms, including hot flashes, sweating, depression, fatigue, and sleep disorders, have been reported as causes of low quality of life (QoL) in middle-aged women [3]. Moreover, changes in ovarian hormones are associated with reduced bone density, unfavorable alterations in blood lipid parameters, and increased insulin resistance, resulting in an increased risk of developing obesity, metabolic syndrome, and cardiovascular disease (CVD) [4,5]. To prevent complications, it is necessary to improve the health status through various therapeutic strategies, including hormonal therapy, physical activity, lifestyle management, and a healthy diet rich in fiber and antioxidant nutrients [6].
Several reports have suggested the possibility of a nutritional intervention to promote health in middle-aged women. Supplementation with specific nutrients, including isoflavone, fiber, omega-3, and calcium, has been suggested to reduce the risk for chronic metabolic diseases and to alleviate menopausal symptoms in middle-aged women [7,8,9,10,11,12,13,14]. In a prospective study of 60 healthy postmenopausal women, hot flashes and night sweats were reduced after 60 mg of soy isoflavones was administered daily for 12 weeks [7], and isoflavone extracted from soybeans improved mood, vasomotor symptoms, and general menopausal symptoms in menopausal women [8]. In addition, isoflavones contributed to improved lipid profiles in postmenopausal women with mild hypercholesterolemia [9]. Dietary fiber intake is reported to reduce the risk of coronary heart disease in menopausal women through various mechanisms, such as improving blood lipid profiles and reducing blood pressure and insulin resistance [10,11]. Omega-3 supplementation for 6 months improved insulin resistance and reduced inflammatory indicators in postmenopausal women with a moderate risk of metabolic diseases [12]. Increased intake of eicosapentaenoic acid and docosahexaenoic acid for 12 months ameliorated bone resorption and improved lipid profiles with decreases in total cholesterol (TC) and low-density lipoprotein-cholesterol (LDL-C) concentration [13]. Furthermore, a cross-sectional study of menopausal women revealed that a high calcium intake was negatively correlated with CVD risk factors [14].
While the importance of investigations into the health benefits of individual nutrients is well-acknowledged in nutritional epidemiology, the current evidence regarding the supplementation of individual nutrients and health improvement in menopausal women is not conclusive [15,16,17,18,19,20,21]. In particular, the efficacy of dietary intervention on menopausal symptoms is controversial, unlike its effects on metabolic parameters. In a 12-week randomized controlled study, isoflavone-supplemented menopausal women showed alleviated frequency and degree of hot flashes [22]. By contrast, supplementation of dietary isoflavone or soy protein for 12 weeks did not show positive effects; rather, it caused negative side effects, such as abdominal distension [15,16]. In a 4-month intervention study, isoflavone supplementation of 100 mg/day in 80 menopausal women decreased the levels of TC and LDL and menopausal symptoms [23], while isoflavone-rich soy protein supplementation for 6 months did not affect menopausal symptoms [17]. In addition, several randomized controlled trials that examined the effect of omega-3 supplementation on menopausal symptoms reported no improvements in the frequency and severity of hot flashes, insomnia severity, sleep quality, and QoL [18,19,20,21]. These inconsistent results were caused by differences in the concentration, duration, and form of supplementation of individual nutrients, highlighting the importance of the overall intake of crucial nutrients.
Recently, meal-based interventions have emerged as an alternative strategy to reduce risks associated with metabolic dysregulation and menopausal symptoms [6]. Various approaches to meal planning (e.g., modification of the portion of macronutrients or glycemic index) and healthy dietary patterns (e.g., Mediterranean diet) can be effective in achieving healthy metabolic goals [24]. Additionally, the potential synergetic impacts of the complex combinations of individual nutrients or components in food and the possibility of long-term induction of positive interactions between nutrients by consuming meals are strong benefits of the meal-based intervention [25]. In this context, focusing on healthy eating patterns, rather than individual nutrient supplementation, is necessary to ensure menopausal women have adequate nutrient intakes. Despite the distinct benefits of meal-based interventions, few studies have evaluated the effect of meal-based interventions in menopausal women due to the methodological difficulty, including providing an individual with a full meal. Furthermore, most studies have evaluated the effects of dietary intervention in subjects with a disease, resulting in their unverified effects in healthy middle-aged women.
Therefore, this study aimed to develop a specific meal-based intervention targeting isoflavone, omega-3, fiber, and calcium for relatively healthy, low symptomatic middle-aged women and evaluate its effects on health improvement as a pilot study.
2. Materials and Methods
2.1. Study Design and Study Population
This study was designed as a pilot study with pre−post-intervention comparison, in which the 8-week intervention was preceded by a run-in period of 2 weeks. Informed consent was obtained from all subjects involved in the study after an explanation of its purpose and process of the study, and the subjects’ information was recorded in the Case Report Form. This study was reviewed and approved by the Ethics Committee of Ewha Womans University College of Medicine (EUMC 2020-02-047-005, approval date 18 December 2020) and conducted from July 2021 to September 2021 at Ewha Womans University Mok-dong Hospital, Seoul, Korea.
Study participants were recruited as community volunteers through the E.Jo Connection, a recruitment agency for clinical trials, and the community advertisement near the Ewha Clinical Trial Center of Mok-dong Hospital. Women aged over 50 and under 65 were recruited. To evaluate the intervention outcomes in general middle-aged women, disease history or current symptoms were not included in the exclusion criteria. At the screening step, the individual intake amount of four major nutrients was estimated via the dietary survey using 24 h recall on 3 days (2 weekdays and 1 weekend day). According to the nutritional intake evaluation, all subjects showed a low intake level in at least one of the target nutrients below Korean dietary recommendations (75 mg of isoflavone, 2.0 g of omega-3 fatty acid, 18.8 g of fiber, and 600 mg of calcium). Subjects who were hypersensitive to certain foods or ingredients or had difficulty using smartphones were excluded. Among the 65 enrolled subjects, 61 participated in the intervention (Figure 1). A total of 50 completed the intervention (11 subjects withdrew their consent or stopped for personal reasons). According to dietary compliance, a total of 49 subjects who had over 70% of compliance were included in further analyses. The compliance calculation method was as follows: (number of meals consumed/total number of meals provided) × 100.
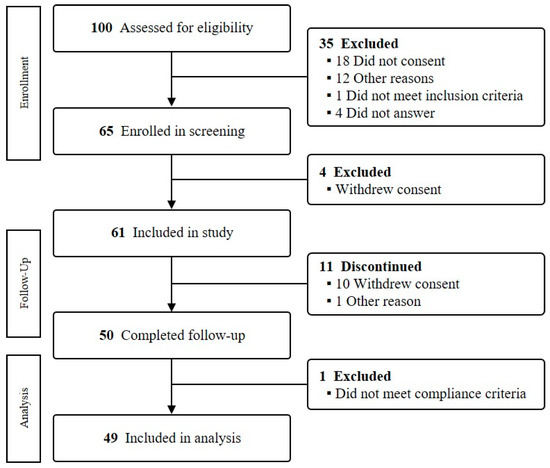
Figure 1.
Flowchart of the study.
2.2. Dietary Intervention and Assessment
Analysis of the current dietary intake status of Korean middle-aged women was conducted using the dietary intake data from the 2016 and 2017 Korean National Health and Nutrition Examination Survey (women aged between 50 to 80 years old, n = 3440; data not shown,; available on request) to screen the main target nutrients for intervention. Low intake nutrients on average, compared to the recommended nutrient intake or adequate intake for the Korean population [26], were identified and narrowed down to four nutrients (calcium, omega-3, fiber, and isoflavone), which are frequently applied for dietary intervention in middle-aged women [27].
Registered dietitians designed the meal plans for two weeks, which were run four times during an 8-week intervention. The meals were served as 2 meals/day (lunch, dinner) and 1 snack under planning to provide approximately 80% of the estimated energy requirement per day and the according macronutrients, and sufficient four target nutrients according to Korean Dietary Reference Intake [26]. The meals were provided in the form of meal kits with recipe card. Participants were instructed to prepare and consume meals at regular times according to each individual’s eating schedule with a simple breakfast meal being recommended.
The usual dietary behavior was surveyed using two different approaches: the Recommended Food Score (RFS); and Meats, Eggs, Dairy, Fried foods, fat In baked foods, Convenience foods, fats added at Table, and Snacks (MEDFICTS). The RFS is a food-based score that assesses diet quality, as suggested by Kant et al. [28], and the RFS modified for the Korean diet was used [29]. MEDFICTS was investigated for dietary fat and cholesterol consumption. At 0 week, before starting the intervention, participants were educated on the dietary and lifestyle guidelines. During the intervention phase, nutritional intake was recorded using the smartphone application E-diary II (Biofood Co., Ltd., Seoul, Republic of Korea) and monitored by researchers every week. Intervention was completed in the range of 82 to 112 meals per person. The average amount of nutrients per day was as follows: 1531.7 kcal of energy, 182.1 mg of isoflavone, 11.9 g of omega-3, 25.3 g of fiber, and 833.4 mg of calcium.
2.3. Measurements
Demographic characteristics, including birth date, residence, age, pregnancy, and menopausal status, were obtained before the intervention. Menopause was defined as at least 12 months of amenorrhea, and a shorter period was designated as perimenopause. Participants visited the hospital after 12 h of fasting in week 0 and week 8 and were examined using the following tests: (1) anthropometric parameters (e.g., height, body weight, body mass index (BMI), waist circumference, hip circumference, systolic blood pressure, and diastolic blood pressure); (2) body composition (e.g., body water, body fat, skeletal muscle, and abdominal fatness); and (3) biochemical parameters, including TC, triglyceride (TG), high-density lipoprotein-cholesterol (HDL-C), LDL-C, lipoprotein(a) (Lp(a)), apoprotein A1 (ApoA1), apoprotein B (ApoB), glucose, insulin, high-sensitivity C-reactive protein (hs-CRP), E2, FSH, C-terminal telopeptide of type I collagen (CTx), and osteocalcin. Body composition was measured using the InBody 720 (Biospace Co., Seoul, Republic of Korea), and the skeletal muscle index (SMI) was calculated using the following formula: [appendicular lean mass (kg)/body weight (kg)] × 100. Most blood parameters were analyzed at the Ewha Womans University Mok-dong Hospital, ApoB was analyzed by GCCL (Yongin, Republic of Korea), CTx was analyzed by EONE Laboratories (Incheon, Republic of Korea), and osteocalcin was analyzed by Seegene, Inc. (Seoul, Republic of Korea). Briefly, the concentrations of glucose and TC were estimated using the COD-POD method, and TG was calculated using the GPO-PAP method. The selective inhibition enzymatic method was used for HDL-C and LDL-C, and turbidimetry was conducted for ApoA1, ApoB, and LP(a) detection. The hormones, including insulin, E2, and osteocalcin, were estimated using electrochemiluminescence microparticle immunoassay, and FSH and CTx were calculated using chemiluminescence microparticle immunoassay and chemiluminescence immunoassay, respectively.
2.4. Menopausal Index
The Kupperman Index (KI), a self-report method for clinical evaluation of menopausal symptoms in menopausal women [30], was measured to examine the menopausal symptoms between pre- and post-intervention. The modified KI consisting of 12 symptoms (hot flashes/sweating, paresthesia, insomnia, nervousness, depression, vertigo, fatigue, arthralgia/myalgia, headache, palpitation, itching, and vaginal dryness) was used in the study. The severity of each symptom can be selected on a scale between 0 and 3 points, and the total score range was set from 0 to 54 points with 4 times the weight of hot flashes/sweating (0 to 12 points) and multiplied by 2 times the weight of paresthesia, insomnia, and nervousness (0 to 6 points). To measure the improvement in sleep disorders, the Pittsburgh Sleep Quality Index (PSQI) and Insomnia Severity Index (ISI) were examined [31].
2.5. Statistical Analysis
For continuous variables including nutritional intake, menopausal symptoms, body measurement, body composition, and biochemical indicators, the paired t-test was used to compare the parameters in the pre- and post-intervention phases. Additionally, Cohen’s d was calculated for measurement of effect size. Categorical values were analyzed using the chi-square test. All statistical analyses were calculated using SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA) and R software version 4.1.3 (Rstudio Inc., Boston, MA, USA). A p < 0.05 was initially considered to be statistically significant and Bonferroni correction was applied to correct multiple testing (Bonferroni correction p < 0.002).
3. Results
3.1. General Characteristics of Participants
The basic characteristics of the participants are shown in Table 1. The average age of the participants was 58.20 ± 4.24 years, and the average height was 156.34 ± 5.37 cm. Forty-three participants (87.8%) were post-menopausal and 5 participants (10.2%) were perimenopausal. Four of them were under hormonal therapy. The average sleep duration was 6.31 ± 1.16 h, and the ISI score, an index of sleep quality evaluation, was 8.96 ± 5.73 points, indicating subthreshold insomnia, which ranges between 8 and 14 points [31].

Table 1.
General characteristics of participants.
There were no smokers, and 18 participants (36.7%) were current drinkers. The MEDFICTS score was 44.82 ± 24.47 points, which included the Step 1 diet. The average RFS of the participants was 25.80 ± 5.93 points. Regarding drug use, 11 (22.4%) were using hypertension drugs, 15 (30.5%) were using hyperlipidemia drugs, 1 (2%) was using antidepressant drugs, 8 (16.3%) were using sleep-inducing drugs, 1 (2%) was using dietary supplements, and 19 (38.8%) were using drugs for other reasons (e.g., menopausal symptoms, diabetes, and osteoporosis).
3.2. Nutrient Intake Analysis
To compare pre- and post-intervention dietary nutrient intake, the nutrient intake was analyzed (Table 2). The intervention significantly increased the average daily intake of dietary fiber (4.92 ± 6.71 g), resulting in 19.96 ± 4.26 g of fiber intake post-intervention (p < 0.0001). Calcium increased by 354.50 ± 159.20 mg, from 332.61 ± 152.60 mg pre-intervention to 687.11 ± 170.30 mg post-intervention (p < 0.0001), while the intake of sodium, which is involved in renal calcium reabsorption, was not changed by the intervention (p = 0.799). Isoflavone intake increased from 13.14 ± 9.55 mg pre-intervention to 52.81 ± 18.45 mg post-intervention (p < 0.0001). Additionally, among the omega-3 fatty acids, the intake of eicosapentaenoic acid was significantly elevated (46.85 ± 73.13 mg, p < 0.0001). These results indicate that the dietary intervention effectively increased the intake of the target nutrients; in particular, the average dietary fiber intake and calcium intake were higher than the recommended amounts for middle-aged women (18.8 g for dietary fiber and 600.0 mg for calcium).

Table 2.
Daily nutrient and energy intakes at baseline and after 8 weeks of intervention.
Interestingly, the intervention increased the daily energy intake (303.79 ± 416.90 kcal, p < 0.0001), accompanied by an increased intake of macronutrients. Specifically, carbohydrate intake was increased by 23.16 ± 57.35 g, from 192.56 ± 56.09 g pre-intervention to 215.71 ± 38.79 g post-intervention (p = 0.007). Protein intake was increased by 24.06 ± 19.46 g, and fat intake was increased by 12.43 ± 19.36 g (p < 0.0001 for both). The intervention changed the percentages of total energy intake of macronutrients, with a decrease in carbohydrates and an increase in protein and fat (carbohydrate–protein–fat ratio of 60:16:24 pre-intervention and 55:19:26 post-intervention). Average intakes of cholesterol and saturated fatty acid showed no significant changes (p = 0.167 and p = 0.734, respectively).
3.3. Changes in Anthropometric and Body Composition Parameters Induced by Intervention
Despite an increased dietary energy intake, there were favorable changes in anthropometric measurements (Table 3). After 8 weeks, body weight was significantly decreased by −0.52 ± 1.17 kg (p = 0.003); however, its significance was diminished after the Bonferroni correction with small effect size (d = 0.447). BMI and waist circumference were decreased by −0.16 ± 0.49 kg/m2 (p = 0.025) and −1.27 ± 5.00 kg (p = 0.083). There were no differences between the pre- and post-intervention systolic and diastolic blood pressures (p = 0.816 and p = 0.501, respectively). Regarding body composition, the body fat was reduced by −0.30 ± 1.04 kg, from 20.38 ± 4.40 kg pre-intervention to 20.08 ± 4.65 kg post-intervention (p = 0.049), while skeletal muscle mass and SMI were not changed.

Table 3.
Changes in anthropometric measurement and body composition according to intervention.
3.4. Alterations in Biochemical Parameters
CTx and osteocalcin, two indicators of osteoporosis risk in postmenopausal women, were decreased by −0.03 ± 0.09 ng/mL (p = 0.037) and −2.08 ± 3.09 ng/mL (p < 0.0001); particularly, the change in osteocalcin was significant even after the Bonferroni correction with medium effect size (d = 0.306), implying a reduced bone loss post intervention (Table 4). Regarding changes in hormone profiles, FSH showed a marginal reduction (p = 0.059), whereas E2 was not affected (p = 0.312). Positive alterations in blood lipid profile were detected, with decreased levels of TC (−10.08 ± 30.51 mg/dL, p = 0.025) and LDL-C (−10.06 ± 26.81 mg/dL, p = 0.012). Moreover, the ratios of LDL-C/HDL-C and TC/HDL-C, which are known as CVD predictive indicators, were reduced post intervention (p = 0.009 and p = 0.025, respectively). ApoA1 and ApoB, which are associated with HDL and LDL, respectively [32], were significantly decreased with medium effect (p < 0.0001, d = 0.673 and p = 0.001, d = 0.502, respectively), whereas there was no significant change in the ratio of ApoB/ApoA1 (p = 0.955). Among the blood glycemic parameters, the level of insulin was significantly reduced (p = 0.040), and the level of glucose was marginally decreased (p = 0.065) post intervention.

Table 4.
Biochemical parameters before and after the 8-week intervention.
3.5. Improvement in KI and PSQI
The severity of menopausal symptoms in the pre-intervention phase belonged to the mild level, with 14.27 ± 8.18 points of KI score. The KI score was significantly decreased by −3.53 ± 5.07 points and the change was significant even after multiple comparison correction with medium effect size (p < 0.0001 and d = 0.697); in addition, the PSQI score was reduced by −0.55 ± 1.78 points (p = 0.018), indicating alleviation of menopausal symptoms and improvement of sleep quality post intervention (Table 5).

Table 5.
Assessment of postmenopausal symptoms at baseline and intervention.
4. Discussion
In the past few years, research on promoting the health status of postmenopausal women has focused on supplementation with functional compounds. In this pilot study, the potential benefits of a meal-based intervention on the health promotion of middle-aged women were investigated by developing a meal plan enriched with target nutrients for menopausal women. Decreases in BMI and body fat, and improvement in menopausal symptoms and sleep quality were detected after dietary intervention. Moreover, parameters for bone metabolism, blood lipid profiles, and glycemic regulation were improved post intervention. Reduction in KI, osteocalcin, and Apo proteins levels was significant, even after adjustment for multiple comparison.
Recently, the focus of nutritional epidemiological investigations has moved beyond individual nutrient approaches to the broader perspective of whole diets [33] due to the complex interactions between nutrients and cumulative effects of food intake with low safety risk [34]. Various studies show that dietary interventions modulating nutrients have beneficial impacts on several metabolic diseases, including obesity, type 2 diabetes, and hypertension, which can cause various complications [35,36,37]. Given that diet directly affects systemic metabolism, including blood glucose and lipid concentrations [38], diet should be considered a prominent strategy. Moreover, as a strong driver of microbiome, diets can modify the gut microbiota composition and lead to changes in the end-products of foods, resulting in favorable health outcomes [39,40]. However, due to the cost, time, and physical limitations of providing an entire meal, most previous studies focusing on menopausal women focused on plant-based supplements or nutrient-enhanced drugs. Eating a meal containing abundant nutrients induces potential synergy by affecting the absorption mechanism and rate of nutrients, whereas approaches that focus on individual nutrients might not be able to consider its effectiveness and relationship with other nutrients [25]. Moreover, dietary intervention could prevent the adverse effects produced by functional components. For instance, with fiber, which is one of the major nutrients of the present study, supplementations with various types of fiber products improved constipation but caused gastrointestinal symptoms including abdominal pain [41]. Furthermore, some studies have showed little-to-no benefits or harmful impacts at high doses of functional components [42,43,44,45]. A cross-over clinical research using two fiber products has revealed that there was an individual range of response to arabinoxylan and a high-dose intake of inulin led to inflammation and liver damage [42]. Most national recommendations, including those in Korea, lack information regarding the consumption of each functional component. These results have shown that types of functional component and dose regime should be considered when functional components are supplemented. Furthermore, recognizing regular eating habits by recording nutritional intake during interventions and improving knowledge of nutrition and health can induce long-term changes toward a desirable diet, leading to health promotion [46,47].
In this study, favorable changes in body weight, BMI, and waist circumference, the improvement of blood lipid, glucose profiles, and bone resorption markers were detected after the intervention. Due to the function of sex hormones in regulating metabolism and sex-specific remodeling of fat cells, the decrease in estrogen in menopausal women induces abdominal central fat gain and body weight gain [48]. In addition, studies have shown that increased FSH induced by ovarian aging positively correlates with body weight [49]. Changes in hormones and body mass in menopause were strongly associated with lipid profile alterations. In post-menopausal women, ApoB, which is involved in LDL metabolism, was increased [50], and the serum TC and LDL levels were positively correlated with FSH [51]. Moreover, high concentrations of free fatty acids, LDL, and ApoB were associated with increased abdominal fat, resulting in an increased risk of CVD [52,53]. These symptoms were alleviated by nutrient supplementation. Omega-3 supplementation of 900 mg/day in 87 postmenopausal women significantly reduced BMI, waist circumference, TG levels, and interleukin-6 concentrations and improved insulin resistance [12]. In addition, calcium intake was negatively related to body weight, body fat, blood glucose, and TG level in middle-aged women [54]. In a two-year randomized controlled study of 500 healthy postmenopausal women, calcium-rich milk effectively attenuated bone resorption and reduced fasting blood glucose, hemoglobin A1c, TC, LDL, and ApoB levels [55]. Providing various plant-derived isoflavone products to menopausal women for 6 months significantly decreased their KI scores and the TC, LDL, and TG levels [56]. One study, which investigated the impacts of 12-week supplementation of isoflavone and γ-linolenic acid compound in Korean menopausal women, reported decreased KI score and oxidative LDL concentration but no improvement in the TC, LDL, and ApoB concentrations [57]. These results suggest that the potential benefits of our dietary intervention on body weight loss and improvement in blood lipid profile in menopausal women might also be a valid program to reduce the risk of CVD.
Menopause is a discontinuation of ovarian activity that causes various symptoms due to changes in sex hormones [1]. The modified KI is a self-rating score that covers 12 aspects of menopausal symptoms. A decrease in the KI score indicates multiple hormonal alterations and physical and metabolic symptoms in menopausal women [58]. In our study, the reinforced dietary intake of target nutrients, such as isoflavone and omega-3, decreased the KI and PSQI, although changes in FSH were not obvious upon intervention. Hot flashes and sweating, two representative symptoms of menopause, are associated with a significantly high FSH level and low E2 level in menopause [52,59,60]. The increase in the level of FSH contributes to vascular calcification and arteriosclerosis by producing foam cells, accumulating lipids in the endothelium of vascular cells, stimulating T cells, and releasing inflammatory cytokines [49,52]. Excessive production of cytokines can induce vascular dysfunction, depression, and fatigue stress in menopausal women [61]. In addition, hormonal changes, such as the lack of estrogen, are considered one of the complex factors of insomnia in menopausal women [62]. Omega-3 fatty acids improved hot flashes and depression in menopausal women [63], and isoflavone supplementation attenuated insomnia symptoms in post-menopausal women [64]. In addition, in a randomized placebo-controlled study, isoflavone or soybean extract supplementation reduced KI scores and the number and severity of facial flushes in menopausal women [23,57,65,66]. However, in some studies, isoflavone supplementation for 12 weeks did not improve the KI score or QoL in menopausal women after 12 weeks [44,45]. It is noteworthy that the changes in the KI score in our study were only from 14 down to 11; however, it was statistically significant, and could therefore not be clinically implied. However, the limited changes might have been due to characteristics of the participants, who were low-symptomatic in the pre-intervention phase with a mild range of KI scores. The results suggested that the meal-based intervention might be an effective strategy to improve overall health parameters as regards middle-aged women and further investigation is required for addressing its efficacy on menopausal symptoms.
In the present pilot study, the results suggested the potential benefits of meal-based intervention. However, there are several points to consider for implementation of the intervention in a larger study. Firstly, the present pilot study adopted a comparison between the pre- and post-intervention phases without a control group or crossover design. The design carried limitations on feasibility, although the effect size was measured to estimate the feasibility and the Bonferroni correction was applied for interpretation of the data. Although subjects were instructed not to change their regular eating schedules and physical activity during the study period, we could not directly control such lifestyle factors. A further larger-scale study including a control group and double-blinded design is needed to confirm the potential effectiveness of our model. Secondly, an improved method of participant recruitment should be sought in order to obtain clearer results. This study was based on the general population regardless of their status of metabolic and menopausal parameters. To test the efficacy of this model, it might be necessary to recruit subjects with certain symptoms and metabolic statuses. Thirdly, meal-based intervention providing meal kits is characterized by cooking the food, which means that the lifestyle of participants should be considered. Although all subjects received instructions on meal intake, the timing of meal intake and meal skipping may be affected by occupational status or the individual’s regular eating schedule. Fourthly, our intervention design showed a relatively high retention rate of participants. Among the 61 participants enrolled, only 1 was excluded due to a dietary compliance issue, suggesting high acceptability of intervention. The others were excluded for personal reasons or difficulty using devices (application and blood glucose meter). These results show that the convenience and good quality of the diet provided in the intervention study led to high compliance, which means that the intervention is feasible for a large population, and consideration of the use of instruments may be necessary for a higher retention. Finally, qualitative data including questionnaires and interviews should be collected to complement this pilot study and additional long-term follow-up data might be useful to demonstrate the sustainability of the intervention.
5. Conclusions
After the meal-based precision intervention, menopausal symptoms, bone metabolism, and blood glucose and lipid profiles were improved, resulting in favorable health outcomes in relatively healthy menopausal women. These results suggested that meal-based interventions specifically designed for middle-aged women might be an effective strategy for health promotion.
Author Contributions
Conceptualization, M.S.C., O.K., Y.K. (Yuri Kim), Y.J.P. and Y.K. (Yangha Kim); investigation, J.S., Y.S., Y.C., Y.K. (Yeri Kim), Y.K. (Yuri Kim), M.S.C., E.H., O.K., Y.K. (Yuri Kim), Y.J.P. and Y.K. (Yangha Kim); data curation, J.S., Y.S., Y.C., Y.K. (Yeri Kim), E.H., Y.K. (Yuri Kim), Y.J.P. and Y.K. (Yangha Kim); writing—original draft preparation, J.S. and Y.S.; writing—review and editing, J.S., Y.K. (Yuri Kim), Y.J.P. and Y.K. (Yangha Kim); funding acquisition, Y.J.P. and Y.K. (Yangha Kim) All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by Ewha Womans University, Dr. Kitchen company, and the BK21 FOUR (Fostering Outstanding Universities for Research), funded by the Ministry of Education (MOE, Republic of Korea) and the National Research Foundation of Korea (NRF-5199990614253, Education Research Center for 4IR-Based Health Care).
Institutional Review Board Statement
The study was approved by the Institutional Review Board Ethics Committee of Ewha Womans University College of Medicine (EUMC 2020-02-047-005, approval date 18 December 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data sharing is not applicable to this article.
Acknowledgments
The authors acknowledge the Dr. Kitchen company, in particular Junghyun Kwon and Hyejin Cho, for technical support and sharing the expertise in developing meal plans for the study. The authors also acknowledge Biofood CRO Co., Ltd. for monitoring the intervention.
Conflicts of Interest
The Kitchen company was involved in the study design. The funder had no role in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Greendale, G.A.; Lee, N.P.; Arriola, E.R. The menopause. Lancet 1999, 353, 571–580. [Google Scholar] [CrossRef]
- Honour, J.W. Biochemistry of the menopause. Ann. Clin. Biochem. 2018, 55, 18–33. [Google Scholar] [CrossRef]
- Pugliese, G.D.; Barrea, L.D.; Laudisio, D.D.; Aprano, S.D.; Castellucci, B.D.; Framondi, L.D.; Di Matteo, R.D.; Savastano, S.P.; Colao, A.P.; Muscogiuri, G.D. Mediterranean diet as tool to manage obesity in menopause: A narrative review. Nutrition 2020, 79-80, 110991. [Google Scholar] [CrossRef] [PubMed]
- Lobo, R.A. Metabolic syndrome after menopause and the role of hormones. Maturitas 2008, 60, 10–18. [Google Scholar] [CrossRef]
- Carr, M.C. The emergence of the metabolic syndrome with menopause. J. Clin. Endocrinol. Metab. 2003, 88, 2404–2411. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Pugliese, G.; Laudisio, D.; Colao, A.; Savastano, S.; Muscogiuri, G. Mediterranean diet as medical prescription in menopausal women with obesity: A practical guide for nutritionists. Crit. Rev. Food Sci. Nutr. 2021, 61, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Wilczek, B.; Warner, M.; Gustafsson, J.-Å.; Landgren, B.-M. Isoflavone treatment for acute menopausal symptoms. Menopause 2007, 14, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Chedraui, P.; San Miguel, G.; Schwager, G. The effect of soy-derived isoflavones over hot flushes, menopausal symptoms and mood in climacteric women with increased body mass index. Gynecol. Endocrinol. 2011, 27, 307–313. [Google Scholar] [CrossRef]
- Wangen, K.E.; Duncan, A.M.; Xu, X.; Kurzer, M.S. Soy isoflavones improve plasma lipids in normocholesterolemic and mildly hypercholesterolemic postmenopausal women. Am. J. Clin. Nutr. 2001, 73, 225–231. [Google Scholar] [CrossRef]
- Pereira, M.A.; O’Reilly, E.; Augustsson, K.; Fraser, G.E.; Goldbourt, U.; Heitmann, B.L.; Hallmans, G.; Knekt, P.; Liu, S.; Pietinen, P. Dietary fiber and risk of coronary heart disease: A pooled analysis of cohort studies. Arch. Intern. Med. 2004, 164, 370–376. [Google Scholar] [CrossRef]
- Juntunen, K.S.; Laaksonen, D.E.; Poutanen, K.S.; Niskanen, L.K.; Mykkänen, H.M. High-fiber rye bread and insulin secretion and sensitivity in healthy postmenopausal women. Am. J. Clin. Nutr. 2003, 77, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Tardivo, A.P.; Nahas-Neto, J.; Orsatti, C.L.; Dias, F.; Poloni, P.; Schmitt, E.; Nahas, E.A. Effects of omega-3 on metabolic markers in postmenopausal women with metabolic syndrome. Climacteric 2015, 18, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Fonolla-Joya, J.; Reyes-García, R.; García-Martín, A.; López-Huertas, E.; Muñoz-Torres, M. Daily intake of milk enriched with n-3 fatty acids, oleic acid, and calcium improves metabolic and bone biomarkers in postmenopausal women. J. Am. Coll. Nutr. 2016, 35, 529–536. [Google Scholar] [CrossRef] [PubMed]
- da Silva Ferreira, T.; Torres, M.R.S.G.; Sanjuliani, A.F. Dietary calcium intake is associated with adiposity, metabolic profile, inflammatory state and blood pressure, but not with erythrocyte intracellular calcium and endothelial function in healthy pre-menopausal women. Br. J. Nutr. 2013, 110, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Van Patten, C.L.; Olivotto, I.A.; Chambers, G.K.; Gelmon, K.A.; Hislop, T.G.; Templeton, E.; Wattie, A.; Prior, J.C. Effect of soy phytoestrogens on hot flashes in postmenopausal women with breast cancer: A randomized, controlled clinical trial. J. Clin. Oncol. 2002, 20, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Murkies, A.; Lombard, C.; Strauss, B.; Wilcox, G.; Burger, H.; Morton, M. Dietary flour supplementation decreases post-menopausal hot flushes: Effect of soy and wheat. Maturitas 1995, 21, 189–195. [Google Scholar] [CrossRef]
- Germain, A.S.; Peterson, C.T.; Robinson, J.G.; Alekel, D.L. Isoflavone-rich or isoflavone-poor soy protein does not reduce menopausal symptoms during 24 weeks of treatment. Menopause 2001, 8, 17–26. [Google Scholar] [CrossRef]
- Mohammady, M.; Janani, L.; Jahanfar, S.; Mousavi, M.S. Effect of omega-3 supplements on vasomotor symptoms in menopausal women: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 228, 295–302. [Google Scholar] [CrossRef]
- Afsane, G.; Azadeh, R.; Ali, K.; Atusa, J. Efficacy of omega-3 on hot flush in perimenopausal women versus placebo. Med. Sci. 2012, 22, 221–225. [Google Scholar]
- Moghadam, R.; Ozgoli, G.; Molayi, B.; Majid, H.; Soori, H.; Ghanati, K. Effect of omega3 on vasomotor disorders in menopausal women. J. Arak Univ. Med. Sci. 2012, 15, 116–126. [Google Scholar]
- Cohen, L.S.; Joffe, H.; Guthrie, K.A.; Ensrud, K.E.; Freeman, M.; Carpenter, J.S.; Learman, L.A.; Newton, K.M.; Reed, S.D.; Manson, J.E. Efficacy of omega-3 treatment for vasomotor symptoms: A randomized controlled trial: Omega-3 treatment for vasomotor symptoms. Menopause 2014, 21, 347. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.M.; Reed, S.D.; LaCroix, A.Z.; Grothaus, L.C.; Ehrlich, K.; Guiltinan, J. Treatment of vasomotor symptoms of menopause with black cohosh, multibotanicals, soy, hormone therapy, or placebo: A randomized trial. Ann. Intern. Med. 2006, 145, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Han, K.K.; Soares, J.M., Jr.; Haidar, M.A.; De Lima, G.R.; Baracat, E.C. Benefits of soy isoflavone therapeutic regimen on menopausal symptoms. Obstet. Gynecol. 2002, 99, 389–394. [Google Scholar] [PubMed]
- Evert, A.B.; Boucher, J.L.; Cypress, M.; Dunbar, S.A.; Franz, M.J.; Mayer-Davis, E.J.; Neumiller, J.J.; Nwankwo, R.; Verdi, C.L.; Urbanski, P. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care 2014, 37 (Suppl. S1), S120–S143. [Google Scholar] [CrossRef]
- Tapsell, L.C.; Neale, E.P.; Satija, A.; Hu, F.B. Foods, nutrients, and dietary patterns: Interconnections and implications for dietary guidelines. Adv. Nutr. 2016, 7, 445–454. [Google Scholar] [CrossRef]
- Ministry of Health and Welfare, The Korean Nutrition Society. Dietary Reference Intakes for Koreans: Energy and Macronutrients; Korean Nutrition Society: Sejong, Republic of Korea, 2020. [Google Scholar]
- Jeong, Y.; Lee, E.; Park, Y.J.; Kim, Y.; Kwon, O.; Kim, Y. A Review of Recent Evidence from Meal-Based Diet Interventions and Clinical Biomarkers for Improvement of Glucose Regulation. Prev. Nutr. Food Sci. 2020, 25, 9–24. [Google Scholar] [CrossRef]
- Kant, A.K.; Schatzkin, A.; Graubard, B.I.; Schairer, C. A prospective study of diet quality and mortality in women. JAMA 2000, 283, 2109–2115. [Google Scholar] [CrossRef]
- Han, K.; Yang, Y.J.; Kim, H.; Kwon, O. A modified recommended food score is inversely associated with high blood pressure in Korean adults. Nutrients 2020, 12, 3479. [Google Scholar] [CrossRef]
- KUPPERMAN, H.S.; BLATT, M.H.; WIESBADER, H.; FILLER, W. Comparative clinical evaluation of estrogenic preparations by the menopausal and amenorrheal indices. J. Clin. Endocrinol. Metab. 1953, 13, 688–703. [Google Scholar] [CrossRef]
- Omachi, T.A. Measuring sleep in rheumatologic diseases: The ESS, FOSQ, ISI, and PSQI. Arthritis Care Res. 2011, 63, S287. [Google Scholar] [CrossRef]
- Walldius, G.; de Faire, U.; Alfredsson, L.; Leander, K.; Westerholm, P.; Malmström, H.; Ivert, T.; Hammar, N. Long-term risk of a major cardiovascular event by apoB, apoA-1, and the apoB/apoA-1 ratio—Experience from the Swedish AMORIS cohort: A cohort study. PLoS Med. 2021, 18, e1003853. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Reedy, J.; Krebs-Smith, S.M.; Miller, P.E.; Liese, A.D.; Kahle, L.L.; Park, Y.; Subar, A.F. Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J. Nutr. 2014, 144, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; El Ghoch, M.; Colao, A.; Hassapidou, M.; Yumuk, V.; Busetto, L. European guidelines for obesity management in adults with a very low-calorie ketogenic diet: A systematic review and meta-analysis. Obes. Facts 2021, 14, 222–245. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.-M.; Knüppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 363–375. [Google Scholar] [CrossRef]
- Filippou, C.D.; Tsioufis, C.P.; Thomopoulos, C.G.; Mihas, C.C.; Dimitriadis, K.S.; Sotiropoulou, L.I.; Chrysochoou, C.A.; Nihoyannopoulos, P.I.; Tousoulis, D.M. Dietary approaches to stop hypertension (DASH) diet and blood pressure reduction in adults with and without hypertension: A systematic review and meta-analysis of randomized controlled trials. Adv. Nutr. 2020, 11, 1150–1160. [Google Scholar] [CrossRef]
- Russell, J.; Flood, V.; Rochtchina, E.; Gopinath, B.; Allman-Farinelli, M.; Bauman, A.; Mitchell, P. Adherence to dietary guidelines and 15-year risk of all-cause mortality. Br. J. Nutr. 2013, 109, 547–555. [Google Scholar] [CrossRef]
- Holmes, E.; Li, J.V.; Athanasiou, T.; Ashrafian, H.; Nicholson, J.K. Understanding the role of gut microbiome—host metabolic signal disruption in health and disease. Trends Microbiol. 2011, 19, 349–359. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- Van Der Schoot, A.; Drysdale, C.; Whelan, K.; Dimidi, E. The effect of fiber supplementation on chronic constipation in adults: An updated systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2022, 116, 953–969. [Google Scholar] [CrossRef]
- Lancaster, S.M.; Lee-McMullen, B.; Abbott, C.W.; Quijada, J.V.; Hornburg, D.; Park, H.; Perelman, D.; Peterson, D.J.; Tang, M.; Robinson, A. Global, distinctive, and personal changes in molecular and microbial profiles by specific fibers in humans. Cell Host Microbe 2022, 30, 848–862.e7. [Google Scholar] [CrossRef] [PubMed]
- Hoving, L.R.; Katiraei, S.; Pronk, A.; Heijink, M.; Vonk, K.K.; Amghar-el Bouazzaoui, F.; Vermeulen, R.; Drinkwaard, L.; Giera, M.; van Harmelen, V. The prebiotic inulin modulates gut microbiota but does not ameliorate atherosclerosis in hypercholesterolemic APOE* 3-Leiden. CETP mice. Sci. Rep. 2018, 8, 16515. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choue, R.; Lim, H. Effect of soy isoflavones supplement on climacteric symptoms, bone biomarkers, and quality of life in Korean postmenopausal women: A randomized clinical trial. Nutr. Res. Pract. 2017, 11, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Nahas, E.P.; Neto, J.N.; De Luca, L.; Traiman, P.; Pontes, A.; Dalben, I. Benefits of soy germ isoflavones in postmenopausal women with contraindication for conventional hormone replacement therapy. Maturitas 2004, 48, 372–380. [Google Scholar] [CrossRef]
- Geunhee, A. Effectiveness of Exercise Education for the Diabetes Treatment. Korea J. Sport. Sci. 2008, 17, 621–629. [Google Scholar]
- Chung, S.O.; Song, O.K.; Ko, J.M.; Wi, J.H.; Lee, T.H.; Yum, J.H.; Cho, D.K.; Son, J.H.; Nam, H.W.; Yoo, H.J.; et al. The Effects of Teaching Methods on the Dietary Compliance and Hemoglobin A1c, Level in Patients with Diabetes Mellitus. J. Korean Diabetes Assoc. 2000, 24, 560–573. [Google Scholar]
- Lizcano, F.; Guzmán, G. Estrogen Deficiency and the Origin of Obesity during Menopause. BioMed. Res. Int. 2014, 2014, 757461. [Google Scholar] [CrossRef]
- Sowers, M.; Zheng, H.; Tomey, K.; Karvonen-Gutierrez, C.; Jannausch, M.; Li, X.; Yosef, M.; Symons, J. Changes in body composition in women over six years at midlife: Ovarian and chronological aging. J. Clin. Endocrinol. Metab. 2007, 92, 895–901. [Google Scholar] [CrossRef]
- Barrasa, G.R.R.; Cañete, N.G.; Boasi, L.E.V. Age of postmenopause women: Effect of soy isoflavone in lipoprotein and inflammation markers. J. Menopausal Med. 2018, 24, 176–182. [Google Scholar] [CrossRef]
- Chang, C.-J.; Wu, C.-H.; Yao, W.-J.; Yang, Y.-C.; Wu, J.-S.; Lu, F.-H. Relationships of age, menopause and central obesity on cardiovascular disease risk factors in Chinese women. Int. J. Obes. 2000, 24, 1699–1704. [Google Scholar] [CrossRef]
- Zhu, D.; Li, X.; Macrae, V.E.; Simoncini, T.; Fu, X. Extragonadal effects of follicle-stimulating hormone on osteoporosis and cardiovascular disease in women during menopausal transition. Trends Endocrinol. Metab. 2018, 29, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Rebuffé-Scrive, M.; Eldh, J.; Hafström, L.-O.; Björntorp, P. Metabolism of mammary, abdominal, and femoral adipocytes in women before and after menopause. Metabolism 1986, 35, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Villegas, R.; Gao, Y.-T.; Dai, Q.; Yang, G.; Cai, H.; Li, H.; Zheng, W.; Shu, X.O. Dietary calcium and magnesium intakes and the risk of type 2 diabetes: The Shanghai Women’s Health Study. Am. J. Clin. Nutr. 2009, 89, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Garcia, R.; Mendoza, N.; Palacios, S.; Salas, N.; Quesada-Charneco, M.; Garcia-Martin, A.; Fonolla, J.; Lara-Villoslada, F.; Muñoz-Torres, M. Effects of daily intake of calcium and vitamin D-enriched milk in healthy postmenopausal women: A randomized, controlled, double-blind nutritional study. J. Women’s Health 2018, 27, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Cancellieri, F.; De Leo, V.; Genazzani, A.; Nappi, C.; Parenti, G.; Polatti, F.; Ragni, N.; Savoca, S.; Teglio, L.; Finelli, F. Efficacy on menopausal neurovegetative symptoms and some plasma lipids blood levels of an herbal product containing isoflavones and other plant extracts. Maturitas 2007, 56, 249–256. [Google Scholar] [CrossRef]
- Gwak, J.H.; Kim, J.Y.; Kim, H.J.; Shin, D.H.; Lee, J.H. The effect of isoflavone and gamma-linolenic acid supplementation on serum lipids and menopausal symptoms in postmenopausal women. Korean J. Nutr. 2010, 43, 123–131. [Google Scholar] [CrossRef]
- Tao, M.; Shao, H.; Li, C.; Teng, Y. Correlation between the modified Kupperman Index and the Menopause Rating Scale in Chinese women. Patient Prefer. Adherence 2013, 7, 223. [Google Scholar]
- Yun, M.-H.; Yu, S.-J.; Kim, H.-J. A study on relations between hot flush and the Kupperman’s Index, MENQOL, MRS during treatment for hot flush in menopausal women. J. Korean Obstet. Gynecol. 2011, 24, 87–98. [Google Scholar]
- Øverlie, I.; Moen, M.H.; Holte, A.; Finset, A. Androgens and estrogens in relation to hot flushes during the menopausal transition. Maturitas 2002, 41, 69–77. [Google Scholar] [CrossRef]
- Chourbaji, S.; Urani, A.; Inta, I.; Sanchis-Segura, C.; Brandwein, C.; Zink, M.; Schwaninger, M.; Gass, P. IL-6 knockout mice exhibit resistance to stress-induced development of depression-like behaviors. Neurobiol. Dis. 2006, 23, 587–594. [Google Scholar] [CrossRef]
- Lee, J.; Han, Y.; Cho, H.H.; Kim, M.-R. Sleep disorders and menopause. J. Menopausal Med. 2019, 25, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.P.; Hibbeln, J.R.; Silver, M.; Hirschberg, A.M.; Wang, B.; Yule, A.M.; Petrillo, L.F.; Pascuillo, E.; Economou, N.I.; Joffe, H. Omega-3 fatty acids for major depressive disorder associated with the menopausal transition: A preliminary open trial. Menopause 2011, 18, 279. [Google Scholar] [CrossRef] [PubMed]
- Hachul, H.; Brandão, L.C.; D’Almeida, V.; Bittencourt, L.R.A.; Baracat, E.C.; Tufik, S. Isoflavones decrease insomnia in postmenopause. Menopause 2011, 18, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Faure, E.D.; Chantre, P.; Mares, P. Effects of a standardized soy extract on hot flushes: A multicenter, double-blind, randomized, placebo-controlled study. Menopause 2002, 9, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Pruthi, S.; Qin, R.; Terstreip, S.A.; Liu, H.; Loprinzi, C.L.; Shah, T.R.; Tucker, K.F.; Dakhil, S.R.; Bury, M.J.; Carolla, R.L. A phase III, randomized, placebo-controlled, double-blind trial of flaxseed for the treatment of hot flashes: NCCTG N08C7. Menopause 2012, 19, 48. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).