The Role of the Mediterranean Diet in Breast Cancer Survivorship: A Systematic Review and Meta-Analysis of Observational Studies and Randomised Controlled Trials
Abstract
1. Introduction
2. Methods
2.1. Selection Criteria
2.1.1. Study Design
2.1.2. Participants
2.1.3. Intervention/Exposure
2.1.4. Comparison
2.1.5. Outcomes
2.2. Search Strategy
2.3. Study Records
2.4. Quality Assessment
2.5. Data Analysis
2.6. Sensitivity Analyses
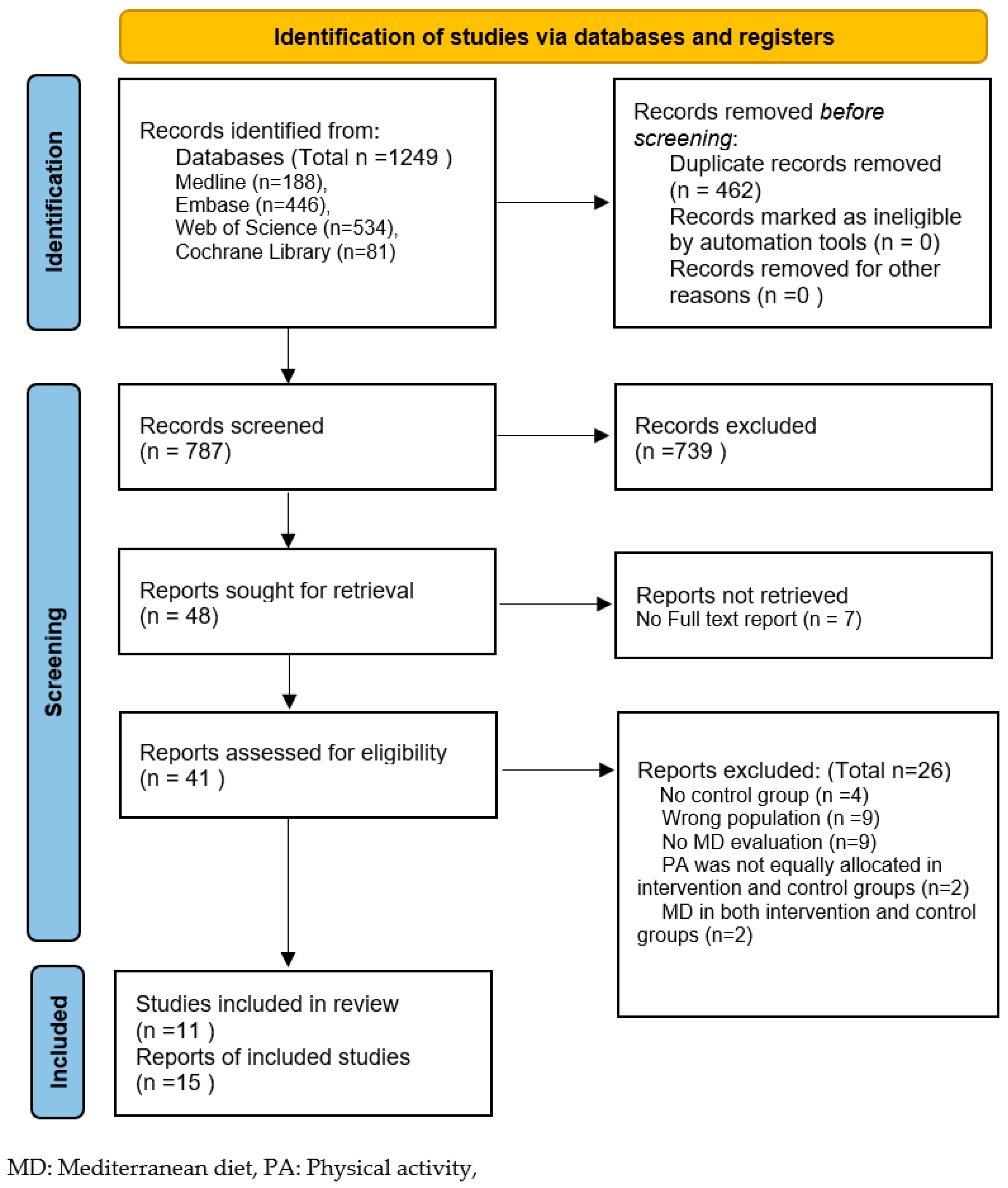
3. Results
3.1. General Characteristics
3.2. Quality Assessment and Strength of Evidence
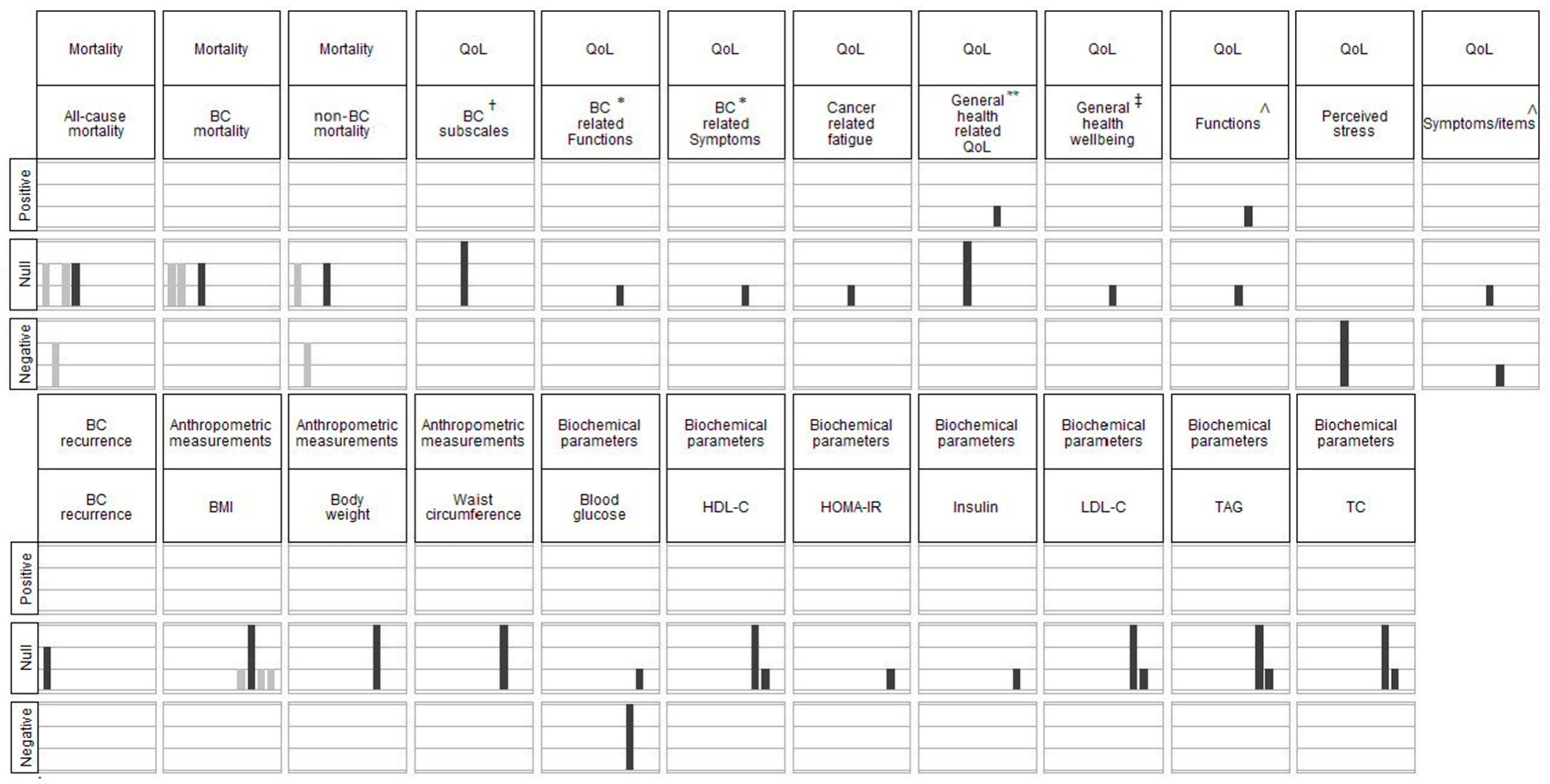
3.3. Main Results
3.3.1. Primary Outcomes
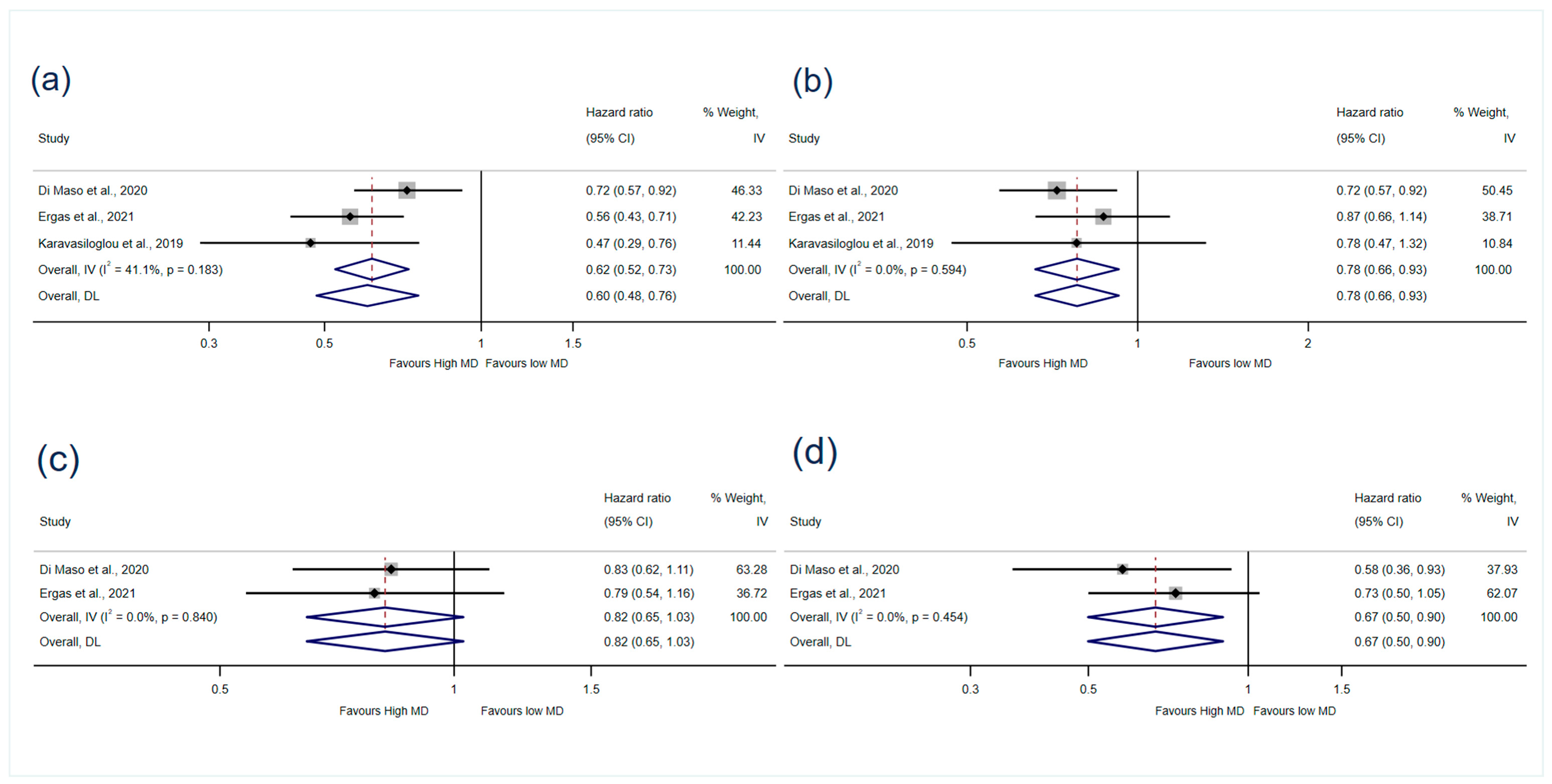
Mortality
BC Recurrence
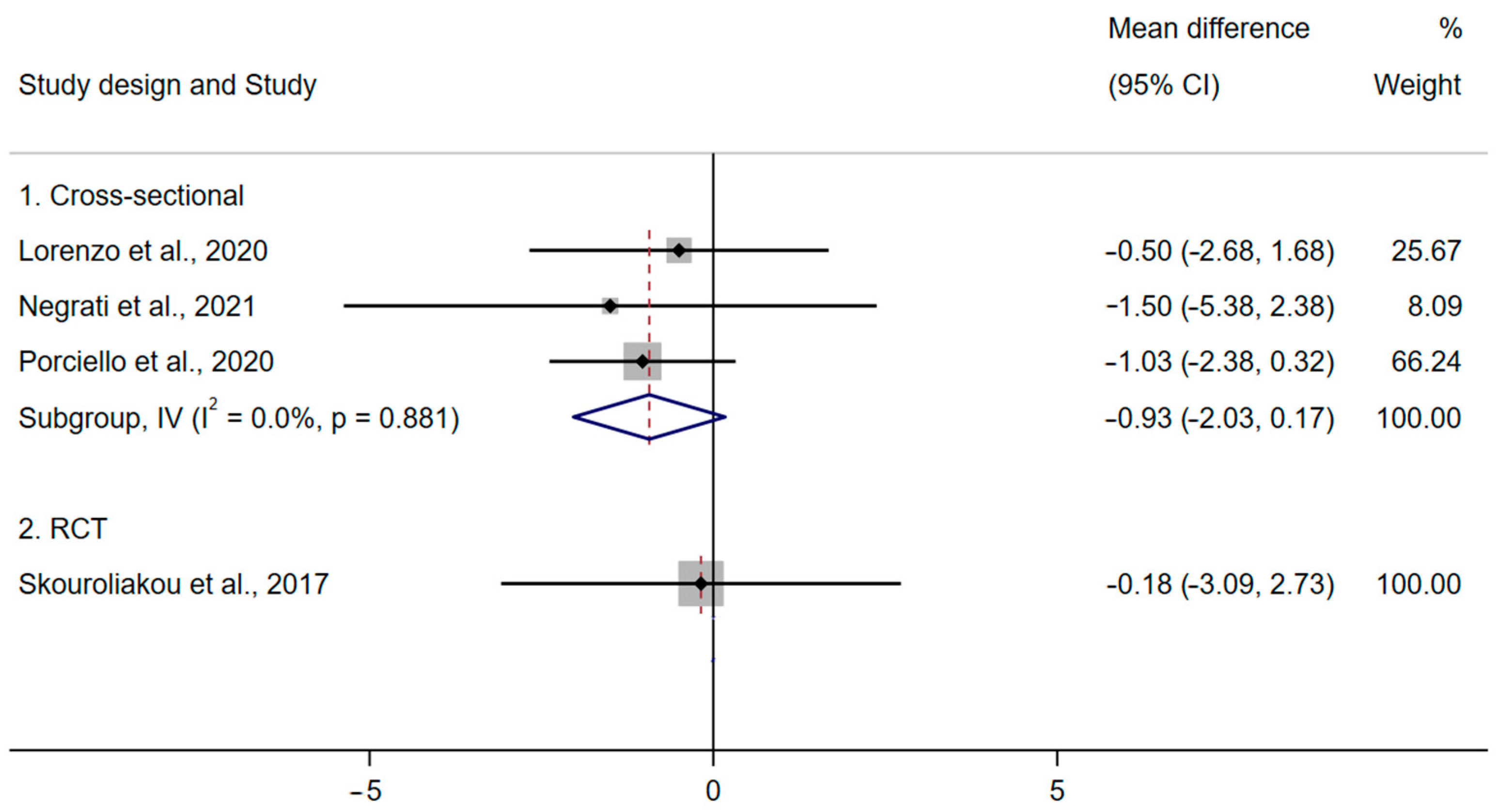
QoL
3.3.2. Secondary Outcomes
Anthropometric Measurements
Biochemical Parameters
3.4. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Systematic Review Registration
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA-Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Rojas, K.; Stuckey, A. Breast Cancer Epidemiology and Risk Factors. Clin. Obstet. Gynecol. 2016, 59, 651–672. [Google Scholar] [CrossRef] [PubMed]
- Maajani, K.; Jalali, A.; Alipour, S.; Khodadost, M.; Tohidinik, H.R.; Yazdani, K. The Global and Regional Survival Rate of Women with Breast Cancer: A Systematic Review and Meta-analysis. Clin. Breast Cancer 2019, 19, 165–177. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics, 2019. CA-Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef]
- Runowicz, C.D.; Leach, C.R.; Henry, N.L.; Henry, K.S.; Mackey, H.T.; Cowens-Alvarado, R.L.; Cannady, R.S.; Pratt-Chapman, M.L.; Edge, S.B.; Jacobs, L.A.; et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline ACS/ASCO Breast Cancer Survivorship Guideline. CA-Cancer J. Clin. 2016, 66, 43–73. [Google Scholar] [CrossRef]
- Cheng, K.K.F.; Lim, Y.T.E.; Koh, Z.M.; Tam, W.W.S. Home-based multidimensional survivorship programmes for breast cancer survivors. Cochrane Database Syst. Rev. 2017, 2017, CD011152. [Google Scholar] [CrossRef]
- Chan, R.J.; Nekhlyudov, L.; Duijts, S.F.A.; Hudson, S.V.; Jones, J.M.; Keogh, J.; Love, B.; Lustberg, M.B.; Mehnert-Theuerkauf, A.; Nathan, P.; et al. Future research in cancer survivorship. J. Cancer Surviv. 2021, 15, 659–667. [Google Scholar] [CrossRef]
- CACA. Breast Cancer Clinical Practice Guidance of China Anti-cancer Association. Chin. Oncol. 2019, 29, 609–680. [Google Scholar]
- NICE. Early and Locally Advanced Breast Cancer: Diagnosis and Management. Available online: https://www.nice.org.uk/guidance/ng101/chapter/Recommendations#lifestyle (accessed on 10 December 2022).
- WCRF/AICR. Diet, Nutrition, Physical Activity and Breast Cancer. Available online: https://www.wcrf.org/wp-content/uploads/2021/02/Breast-cancer-report.pdf (accessed on 10 December 2022).
- WCRF/AICR. Survivors of Breast and Other Cancers. Available online: https://www.wcrf.org/wp-content/uploads/2021/02/Cancer-Survivors.pdf (accessed on 10 December 2022).
- Jochems, S.H.J.; Van Osch, F.H.M.; Bryan, R.T.; Wesselius, A.; van Schooten, F.J.; Cheng, K.K.; Zeegers, M.P. Impact of dietary patterns and the main food groups on mortality and recurrence in cancer survivors: A systematic review of current epidemiological literature. BMJ Open 2018, 8, e014530. [Google Scholar] [CrossRef]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef]
- Braakhuis, A.; Campion, P.; Bishop, K. The Effects of Dietary Nutrition Education on Weight and Health Biomarkers in Breast Cancer Survivors. Med. Sci. 2017, 5, 12. [Google Scholar] [CrossRef]
- Braakhuis, A.J.; Campion, P.; Bishop, K.S. Reducing Breast Cancer Recurrence: The Role of Dietary Polyphenolics. Nutrients 2016, 8, 547. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to mediterranean diet and risk of cancer: An updated systematic review and meta-analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef]
- Morze, J.; Danielewicz, A.; Przybyłowicz, K.; Zeng, H.; Hoffmann, G.; Schwingshackl, L. An updated systematic review and meta-analysis on adherence to mediterranean diet and risk of cancer. Eur. J. Nutr. 2020, 60, 1561–1586. [Google Scholar] [CrossRef]
- Karavasiloglou, N.; Pestoni, G.; Faeh, D.; Rohrmann, S. Post-diagnostic diet quality and mortality in females with self-reported history of breast or gynecological cancers: Results from the third national health and nutrition examination survey (NHANES III). Nutrients 2019, 11, 2558. [Google Scholar] [CrossRef]
- Papadaki, A.; Nolen-Doerr, E.; Mantzoros, C.S. The Effect of the Mediterranean Diet on Metabolic Health: A Systematic Review and Meta-Analysis of Controlled Trials in Adults. Nutrients 2020, 12, 3342. [Google Scholar] [CrossRef]
- Herber-Gast, G.C.; Mishra, G.D. Fruit, Mediterranean-style, and high-fat and -sugar diets are associated with the risk of night sweats and hot flushes in midlife: Results from a prospective cohort study. Am. J. Clin. Nutr. 2013, 97, 1092–1099. [Google Scholar] [CrossRef]
- Benetou, V.; Orfanos, P.; Feskanich, D.; Michaëlsson, K.; Pettersson-Kymmer, U.; Byberg, L.; Eriksson, S.; Grodstein, F.; Wolk, A.; Jankovic, N.; et al. Mediterranean diet and hip fracture incidence among older adults: The CHANCES project. Osteoporos. Int. 2018, 29, 1591–1599. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Marzorati, C.; Riva, S.; Pravettoni, G. Who Is a Cancer Survivor? A Systematic Review of Published Definitions. J. Cancer Educ. 2017, 32, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean Diet and Survival in a Greek Population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Kouris-Blazos, A.; Wahlqvist, M.L.; Gnardellis, C.; Lagiou, P.; Polychronopoulos, E.; Vassilakou, T.; Lipworth, L.; Trichopoulos, D. Diet and overall survival in elderly people. BMJ 1995, 311, 1457–1460. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- StataCrop. Stata Statistical Software: Release 17; StataCorp LLC: College Station, TX, USA, 2021. [Google Scholar]
- Fletcher, J. What is heterogeneity and is it important? BMJ 2007, 334, 94–96. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.; Altman, D.G. Analysing Data and Undertaking Meta-Analyses. Available online: www.training.cochrane.org/handbook (accessed on 10 December 2022).
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Ogilvie, D.; Fayter, D.; Petticrew, M.; Sowden, A.; Thomas, S.; Whitehead, M.; Worthy, G. The harvest plot: A method for synthesising evidence about the differential effects of interventions. BMC Med. Res. Methodol. 2008, 8, 8. [Google Scholar] [CrossRef]
- Ergas, I.J.; Cespedes Feliciano, E.M.; Bradshaw, P.T.; Roh, J.M.; Kwan, M.L.; Cadenhead, J.; Santiago-Torres, M.; Troeschel, A.N.; Laraia, B.; Madsen, K.; et al. Diet Quality and Breast Cancer Recurrence and Survival: The Pathways Study. JNCI Cancer Spectr. 2021, 5, pkab019. [Google Scholar] [CrossRef]
- Di Maso, M.; Maso, L.D.; Augustin, L.S.A.; Puppo, A.; Falcini, F.; Stocco, C.; Mattioli, V.; Serraino, D.; Polesel, J. Adherence to the Mediterranean Diet and Mortality after Breast Cancer. Nutrients 2020, 12, 3649. [Google Scholar] [CrossRef]
- Kim, E.H.J.; Willett, W.C.; Fung, T.; Rosner, B.; Holmes, M.D. Diet quality indices and postmenopausal breast cancer survival. Nutr. Cancer 2011, 63, 381–388. [Google Scholar] [CrossRef]
- Alvarez-Bustos, A.; de Pedro, C.G.; Romero-Elias, M.; Ramos, J.; Osorio, P.; Cantos, B.; Maximiano, C.; Mendez, M.; Fiuza-Luces, C.; Mendez-Otero, M.; et al. Prevalence and correlates of cancer-related fatigue in breast cancer survivors. Support. Care Cancer 2021, 29, 6523–6534. [Google Scholar] [CrossRef]
- Ruiz-Casado, A.; Maximiano, C.; Mendez, M.; Alvarez-Bustos, A.; De Pedro, C.G.; Mendez, M.; Romero-Elias, M.; Cebolla, H.; Ramos, J.; Osorio, P.; et al. Cancer-related fatigue (CRF) in breast cancer survivors after treatment: A focus on physical condition. Am. J. Clin. Oncol. 2020, 38, e24087. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Lio, R.M.S.; Quattrocchi, A.; Degrassi, F.; Catalano, F.; Basile, G.; Agodi, A. The effects of diet and dietary interventions on the quality of life among breast cancer survivors: A cross-sectional analysis and a systematic review of experimental studies. Cancers 2020, 12, 322. [Google Scholar] [CrossRef]
- Porciello, G.; Montagnese, C.; Crispo, A.; Grimaldi, M.; Libra, M.; Vitale, S.; Palumbo, E.; Pica, R.; Calabrese, I.; Cubisino, S.; et al. Mediterranean diet and quality of life in women treated for breast cancer: A baseline analysis of DEDiCa multicentre trial. PLoS ONE 2020, 15, e0239803. [Google Scholar] [CrossRef]
- Porciello, G.; Calabrese, I.; Montagnese, C.; Vitale, S.; Palumbo, E.; Cubisino, S.; Falzone, L.; Crispo, A.; Grimaldi, M.; Libra, M.; et al. Adherence to Mediterranean Diet and Quality of Life in women treated for breast cancer (DEDiCa Study). Nutr. Metab. Cardiovasc. Dis. 2019, 29, 881–882. [Google Scholar] [CrossRef]
- Negrati, M.; Razza, C.; Biasini, C.; Di Nunzio, C.; Vancini, A.; Dall’asta, M.; Lovotti, G.; Trevisi, E.; Rossi, F.; Cavanna, L. Mediterranean diet affects blood circulating lipid-soluble micronutrients and inflammatory biomarkers in a cohort of breast cancer survivors: Results from the seta study. Nutrients 2021, 13, 3482. [Google Scholar] [CrossRef]
- Lorenzo, P.M.; Izquierdo, A.G.; Diaz-Lagares, A.; Carreira, M.C.; Macias-Gonzalez, M.; Sandoval, J.; Cueva, J.; Lopez-Lopez, R.; Casanueva, F.F.; Crujeiras, A.B. ZNF577 Methylation Levels in Leukocytes from Women With Breast Cancer Is Modulated by Adiposity, Menopausal State, and the Mediterranean Diet. Front. Endocrinol. 2020, 11, 245. [Google Scholar] [CrossRef]
- Long Parma, D.A.; Reynolds, G.L.; Muñoz, E.; Ramirez, A.G. Effect of an anti-inflammatory dietary intervention on quality of life among breast cancer survivors. Support. Care Cancer 2022, 30, 5903–5910. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, K.E.; Parma, D.L.; Muñoz, E.; Spaniol, M.; Wargovich, M.; Ramirez, A.G. Dietary intervention among breast cancer survivors increased adherence to a Mediterranean-style, anti-inflammatory dietary pattern: The Rx for Better Breast Health Randomized Controlled Trial. Breast Cancer Res. Treat. 2019, 173, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.G.; Parma, D.L.; Munoz, E.; Mendoza, K.D.; Harb, C.; Holden, A.E.C.; Wargovich, M. An anti-inflammatory dietary intervention to reduce breast cancer recurrence risk: Study design and baseline data. Contemp. Clin. Trials 2017, 57, 1–7. [Google Scholar] [CrossRef]
- Skouroliakou, M.; Grosomanidis, D.; Massara, P.; Kostara, C.; Papandreou, P.; Ntountaniotis, D.; Xepapadakis, G. Serum antioxidant capacity, biochemical profile and body composition of breast cancer survivors in a randomized Mediterranean dietary intervention study. Eur. J. Nutr. 2017, 57, 2133–2145. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; García-Arellano, A.; Toledo, E.; Salas-Salvadó, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schröder, H.; Arós, F.; Gómez-Gracia, E.; et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: The PREDIMED trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Arvaniti, F.; Stefanadis, C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev. Med. 2007, 44, 335–340. [Google Scholar] [CrossRef]
- Cella, D.F.; Tulsky, D.S.; Gray, G.; Sarafian, B.; Linn, E.; Bonomi, A.; Silberman, M.; Yellen, S.B.; Winicour, P.; Brannon, J.; et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J. Clin. Oncol. 1993, 11, 570–579. [Google Scholar] [CrossRef]
- Brady, M.J.; Cella, D.F.; Mo, F.; Bonomi, A.E.; Tulsky, D.S.; Lloyd, S.R.; Deasy, S.; Cobleigh, M.; Shiomoto, G. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J. Clin. Oncol. 1997, 15, 974–986. [Google Scholar] [CrossRef]
- Radloff, L.S. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Andreou, E.; Alexopoulos, E.C.; Lionis, C.; Varvogli, L.; Gnardellis, C.; Chrousos, G.P.; Darviri, C. Perceived Stress Scale: Reliability and validity study in Greece. Int. J. Environ. Res. Public Health 2011, 8, 3287–3298. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Rabin, R.; de Charro, F. EQ-5D: A measure of health status from the EuroQol Group. Ann. Med. 2001, 33, 337–343. [Google Scholar] [CrossRef]
- Becerra-Tomás, N.; Balducci, K.; Abar, L.; Aune, D.; Cariolou, M.; Greenwood, D.C.; Markozannes, G.; Nanu, N.; Vieira, R.; Giovannucci, E.L.; et al. Postdiagnosis dietary factors, supplement use and breast cancer prognosis: Global Cancer Update Programme (CUP Global) systematic literature review and meta-analysis. Int. J. Cancer 2023, 152, 616–634. [Google Scholar] [CrossRef]
- Schwedhelm, C.; Boeing, H.; Hoffmann, G.; Aleksandrova, K.; Schwingshackl, L. Effect of diet on mortality and cancer recurrence among cancer survivors: A systematic review and meta-analysis of cohort studies. Nutr. Rev. 2016, 74, 737–748. [Google Scholar] [CrossRef]
- Lorenzo, P.M.; Crujeiras, A.B. Potential effects of nutrition-based weight loss therapies in reversing obesity-related breast cancer epigenetic marks. Food Funct. 2021, 12, 1402–1414. [Google Scholar] [CrossRef]
- Pierce, J.P.; Natarajan, L.; Caan, B.J.; Parker, B.A.; Greenberg, E.R.; Flatt, S.W.; Rock, C.L.; Kealey, S.; Al-Delaimy, W.K.; Bardwell, W.A.; et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: The Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA 2007, 298, 289–298. [Google Scholar] [CrossRef]
- Mullan, F. Seasons of survival: Reflections of a physician with cancer. N. Engl. J. Med. 1985, 313, 270–273. [Google Scholar] [CrossRef]
- Levin, K.A. Study design VII. Randomised controlled trials. Evid. Based Dent. 2007, 8, 22–23. [Google Scholar] [CrossRef]
- Smith, G.D.; Ebrahim, S. ‘Mendelian randomization’: Can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 2003, 32, 1–22. [Google Scholar] [CrossRef]
- Haycock, P.C.; Burgess, S.; Wade, K.H.; Bowden, J.; Relton, C.; Davey Smith, G. Best (but oft-forgotten) practices: The design, analysis, and interpretation of Mendelian randomization studies. Am. J. Clin. Nutr. 2016, 103, 965–978. [Google Scholar] [CrossRef]
- Davey Smith, G.; Hemani, G. Mendelian randomization: Genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 2014, 23, R89–R98. [Google Scholar] [CrossRef] [PubMed]
- Higgins Jpt, T.J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; Version 6.3; Updated February 2022; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Kopp, M.; Schweigkofler, H.; Holzner, B.; Nachbaur, D.; Niederwieser, D.; Fleischhacker, W.W.; Kemmler, G.; Sperner-Unterweger, B. EORTC QLQ-C30 and FACT-BMT for the measurement of quality of life in bone marrow transplant recipients: A comparison. Eur. J. Haematol. 2000, 65, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.; Popovic, M.; Chow, E.; Cella, D.; Beaumont, J.L.; Chu, D.; DiGiovanni, J.; Lam, H.; Pulenzas, N.; Bottomley, A. EORTC QLQ-BR23 and FACT-B for the assessment of quality of life in patients with breast cancer: A literature review. J. Comp. Eff. Res. 2015, 4, 157–166. [Google Scholar] [CrossRef] [PubMed]
| Categories of Outcomes | Outcomes Included | |
|---|---|---|
| Primary outcomes | Mortality | All-cause mortality; BC mortality; non-BC mortality. |
| BC recurrence and/or newly diagnosed cancer progression | e.g., local recurrence; metastasis. | |
| Quality of life | Measured by generic or cancer-specific validated scales, (e.g., Functional Assessment of Cancer Therapy—Breast Cancer). | |
| Secondary outcomes | Health-related parameters | Biochemical parameters, (e.g., blood glucose and blood lipids, including total cholesterol, low-density lipoproteins, high-density lipoproteins, triglycerides). |
| Anthropometric measurements, (e.g., body-mass index, waist circumference, body weight). | ||
| Incidence of long-term/late-effect BC treatments | Osteoporosis/osteopenia/fracture; obesity/cardiovascular disease/stroke/diabetes; endometrial cancer/other secondary cancer (primary cancer); cognitive impairment; mortality from cancer treatments. | |
| Adverse events |
| Observational Studies | Assessment Tool: Newcastle–Ottawa Scale (Cohort Studies) | ||||||
|---|---|---|---|---|---|---|---|
| Outcome | Selection (Maximum 4 *) | Comparability (Maximum 2 *) | Outcome (Maximum 3 *) | Overall Stars | Overall Risk | ||
| Ergas et al., 2021 [36] | BC Recurrence | **** | ** | *** | 9/9 | Low | |
| Mortality | **** | ** | *** | 9/9 | Low | ||
| Di Maso et al., 2020 [37] | Mortality | **** | * | *** | 8/9 | Low | |
| Karavasiloglou et al., 2019 [19] | Mortality | **** | * | *** | 8/9 | Low | |
| Kim et al., 2011 [38] | Mortality | ** | * | * | 4/9 | High | |
| Assessment Tool: Newcastle–Ottawa Scale (Adapted for Cross-Sectional Studies) | |||||||
| Selection (Maximum 5 *) | Comparability (Maximum 2 *) | Outcome (Maximum 3 *) | Overall Stars | ||||
| Alvarez-Bustos et al., 2021 [39]; (Ruiz-Casado et al., 2020 [40]) | QoL | ** | ** | 4/10 | High | ||
| Barchitta et al., 2020 [41] | QoL | ** | * | 3/10 | High | ||
| Porciello et al., 2020 [42]; (Porciello et al., 2019 [43]) | QoL | *** | * | ** | 6/10 | Medium | |
| BMI | *** | ** | 5/10 | High | |||
| Negrati et al., 2021 [44] | BMI | ** | *** | 5/10 | High | ||
| Lorenzo et al., 2020 [45] | BMI | ** | *** | 5/10 | High | ||
| RCTs | Assessment Tool: Risk of Bias Version 2 | ||||||
| Outcome | Randomisation Process | Deviations from Intended Interventions | Missing Outcome Data | Measurement of the Outcome | Selection of the Reported Result | Overall Risk | |
| Long Parma et al., 2022 [46]; (Zuniga et al., 2019 [47]; Ramirez et al., 2017 [48]) | QoL | Low | High | low | Some concerns | High | High |
| Skouroliakou et al., 2017 [49] | BMI | High | High | High | Some concerns | Some concerns | High |
| Mortality and BC Recurrence | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Country of Study | Study Design | Sample Size/Number in Analysis | Dietary Assessment and/or MD Adherence Assessment | Duration/ Follow-Up | Exposure | Comparator | Main Result (HR/RR, 95% CI) | Variables Used for Adjustment |
| Di Maso et al., 2020 [37] | Italy | Cohort | 1453/1453 (<5% missing data on BMI and education) | FFQ (2 years before BC diagnosis) and 9-component MD score [26] | Truncated 15 years after diagnosis (cohort follow-up median: 12.6 years; maximum: 16.8 years) | MDS: 6−9 | MDS: 0−3 | All-cause mortality: HR-adjusted: 0.72 (0.32–0.92) Breast-cancer mortality: HR-adjusted: 0.83 (0.62–1.11) Non-breast cancer mortality: HR-adjusted: 0.58 (0.36–0.93) | Age (at diagnosis), total energy intake, years of education, menopausal status, TNM stage, ER/PR status, area of residence and calendar period at diagnosis, (BC and non-BC mortality further adjusted for competing risk according to Fine–Gray model) |
| Ergas et al., 2021 [36] | USA | Cohort | 4505/3660 | FFQ and aMED-diet score (adapted from 9-component MD score) [26] | Recruit 2005–2013, end of follow-up in December 2018, mean: 9.08 years (SD 2.77) | aMDS: 6–9 | aMEDS: 0–2 | Breast-cancer recurrence: HR model 2: 1.08 (0.79–1.47) All-cause mortality: HR model 1: 0.56 (0.43–0.71) HR model 2: 0.79 (0.61–1.03) HR model 3: 0.87(0.66–1.14) Breast-cancer mortality: HR model 2: 0.79 (0.54–1.16) Non-breast cancer mortality: HR model 2: 0.73 (0.5–1.05) | Model 1 (minimally adjusted): age (at diagnosis), total energy intake. Model 2 (medium-adjusted): model 1 + race and ethnicity, education, menopausal status, cancer stage, ER and PR status, physical activity, smoking, HER2 status. Model 3 (maximally adjusted): model 2 + BMI, surgery type, chemotherapy, radiation, HT |
| Karavasiloglou et al., 2019 [19] | Switzerland and USA | Cohort | 110/110 | 24-h dietary recall and 9-component MD score [26] | Recruit 1988–1994, end of follow-up on 31 December 2011, mean 14.2 years (SEM 0.8) | MDS: 5–9 | MDS: 0–4 | All-cause mortality: HR model 1: 0.47 (0.29–0.76) HR model 2: 0.78 (0.47–1.32) | Model 1 (minimally adjusted): age (questionnaire completion), race/ethnicity. Model 2 (maximally adjusted): model 1 + race and ethnicity, total energy intake, BMI, moderate-to-vigorous physical activity, smoking, marital status, socioeconomic status, history of menopausal-hormone-therapy used, period since cancer diagnosis, prevalent chronic diseases |
| Kim et al., 2011 [38] | USA | Cohort | 6367/2729 | FFQ and aMED-diet score (adapted from 9-components MD score, aMEDs) [26] | Recruitment period 1978–1998, end of follow-up in June 2004 | aMEDS Quintile 5 | aMEDS Quintile 1 | All-cause mortality: RR model 1: 0.74 (0.55–0.99) RR model 2: 0.87 (0.64–1.17) Breast-cancer mortality: RR model 1: 1.11 (0.74–1.66) RR model 2: 1.15 (0.74–1.77) Non-breast cancer mortality: RR model 1:0.58 (0.38–0.88) RR model 2: 0.8 (0.5–1.26) | Model 1 (minimally adjusted): age, time since diagnosis. Model 2 (maximally adjusted): model 1 + race and ethnicity, energy, BMI, physical activity, smoking, menopausal status, cancer stage, physical activity, smoking, treatment (chemotherapy, radiation, TAM), oral-contraceptive use, postmenopausal-hormone-therapy use, multivitamin usage at first birth and parity, alcohol intake, weight change |
| QoL | |||||||||
| Study | Country of Study | Study Design | Sample Size/Number in Analysis | Dietary Assessment and/or MD Adherence Assessment | Duration/ Follow-up | Exposure | Comparator | Main result (Mean, SD) | Variables Used for Adjustment |
| Long Parma et al., 2022 [46]; (Zuniga et al., 2019 [47]; Ramirez et al., 2017 [48]) | USA | RCT | I: 76, C: 77/ I: 60, C: 65 | 14-item PREDIMED questionnaire [50] | 6 months/ 12 months | PREDIMED 6-month mean score (SD): 8.7 (0.3) Individualised anti-inflammatory dietary prescriptions and behaviour change, 6-month monthly workshops, 12-month monthly navigation, motivational interviewing and tailored newsletters | PREDIMED 6-month mean score (SD): 7.6 (0.3) Minimal nutritional information and two telephone calls prior to assessment appointments. | FACT-G a: p = 0.41 6-month: I 87.96 (12.48), C 84.47 (15.81) 12-month: I 85.21 (13.38), C 84.57 (16.42) FACT-G subscales: Social Well-Being p = 0.77 6-month: I 20.96 (5.37), C 20.54 (5.94) 12-month: I 20.77 (5.15), C 20.46 (6.17) Emotional Well-Being p = 0.76 6-month: I 20.91 (2.75), C 19.97 (3.67) 12-month: I 20.22 (3.23), C 19.76 (3.98) Functional Well-Being p = 0.98 6-month: I 21.76 (4.29), C 20.71 (5.24) 12-month: I 20.60 (4.68), C 20.78 (5.42) Physical Well-Being p = 0.62 6-month: I 24.13 (3.91), C 23.25 (4.42) 12-month: I 23.60 (4.09), C 23.57 (4.13) BCS b: p = 0.82 6-month: I 25.01 (5.38), C 24.15 (5.86) 12-month: I 24.77 (5.34), C 24.31 (6.37) CES-D c: p = 0.51 6-month: I 2.45 (2.18), C 2.65 (2.39) 12-month: I 2.85 (2.74), C 2.88 (2.70) Perceived Stress Scale d: p = 0.01 Baseline: I 21.77 (7.63), C 19.75 (7.60) 6-month: I 20.64 (7.61), C 20.32 (8.31) 12-month: I 21.59 (7.44), C 20.01 (8.23) (p = 0.019 for main effect in I: reduction between baseline and 6-month) | None |
| Alvarez-Bustos et al., 2021 [39]; (Ruiz-Casado et al., 2020 [40]) | Spain | Cross- sectional | 180/180 | 14-item PREDIMED questionnaire [50] | NA | MDS > 7 | MDS ≤ 7 | Cancer related fatigue: No strong evidence for an association between adherence to the MD and cancer-related fatigue (numerical results were not reported) | None |
| Barchitta et al., 2020 [41] | Italy | Cross- sectional | 68/68 | 14-item PREDIMED questionnaire [50] | NA | PREDIMED ≥10 positive items | PREDIMED ≤5 positive items | No strong evidence for an association between MD adherence and overall QoL or QoL subscales (EORTC QLQ-C30 c) (numerical results were not reported) | None |
| Porciello et al., 2020 [42]; (Porciello et al., 2019 [43]) | Italy | Cross- sectional | 309/309 | 14-item PREDIMED questionnaire [50] | NA | PREDIMED > 7 | PREDIMED ≤ 7 | EORTC QLQ-C30 e subscales: Physical functioning: MDH 83.3 (14.5), MDL 78.9 (17.8), p = 0.02 β-model 1: 0.199, p = 0.001 β-model 2: 0.207, p = 0.001 β-model 3: 0.169, p = 0.006 Pain: MDH 23.1 (21.7), MDL 28.5 (24.3), p = 0.04 β-model 1: −0.175, p = 0.002 β-model 2: −0.174, p = 0.005 β-model 3: −0.131, p = 0.027 Dyspnoea: β-model 1: −0.115, p = 0.045 Insomnia: β -model 1: −0.114, p = 0.048 β -model 2: −0.131, p = 0.029 EQ-5D-3L Scale f: MDH 0.87 (0.11), MDL 0.84 (0.12), p = 0.05 β-model 1: 0.167, p = 0.004 β-model 2: 0.190, p = 0.003 (Results in other subscales and EORTC QLQ-B23 e are presented in Supplementary Materials Table S7) | Model 1: age, cancer stag. Model 2: age, cancer stage, BMI, type of surgery, comorbidities, combined therapy. Model 3: age, cancer stage, smoking status, step count, education, civil status (married or single) |
| Health-Related Parameters | |||||||||
| Skouroliakou et al., 2017 [49] | Greece | RCT | I: 35, C: 35/ I: 26, C: 24 | FFQ and 0–9 score (revised to include fish intake) [25] | 6 months | MDS at 6 months mean (SD): 7.65 (0.68) Personalized dietary intervention based on MD and physical-activity recommendations from ACS | MDS at 6 months mean (SD): 4.44 (1.04) Updated American Cancer Society Guidelines | BMI (kg/m2): p = 0.97 I 27.55 (4.69), C 27.73 (5.7) Body weight (kg): p = 0.89 I 72.69 (13.83), C 72.53 (15.61) Waist circumference (cm): p = 0.48 I 94.36 (11.37), C 96.97 (13.06) Blood glucose (mg/dL): p < 0.002 (ANCOVA p = 0.01) I 91.03 (9.96), C 105.95 (21.04) TC (mg/dL): p = 0.62 I 203.83 (44.56), C 209.15 (36.36) LDL-C (mg/dL): p = 0.56 I 123.18 (46.73), C 130.78 (34.39) HDL-C (mg/dL): p = 0.08 I 66.52 (17.56), C 57.36 (13.83) TAG (mg/dL): p = 0.86 I 89 (61.13), C 86.79 (43.74) | Blood-glucose levels adjusted for BMI and estimated weekly MET-mins in ANCOVA analysis |
| Lorenzo et al., 2020 [45] | Spain | Cross- sectional | 90/67 | FFQ and 12 questions from the 14-item PREDIMED questionnaire [50] | NA | PREDIMED (12 questions) >7 | PREDIMED (12 questions) ≤7 | BMI (kg/m2): p ≥ 0.05 MDH 27.8 (3.2), MDL 28.3 (5.7) Body weight (kg): p ≥ 0.05 MDH 68.9 (8.9), MDL 72.3 (14.1) Waist circumference (cm): p ≥ 0.05 MDH 87.8 (9.1), MDL 91.7 (15.3) Hip circumference (cm): p ≥ 0.05 MDH 106.3 (11.7), MDL 104.7 (11.7) Waist to hip ratio: p ≥ 0.05 MDH 0.82 (0.14), MDL 0.87 (0.18) Prevalence of obesity: p ≥ 0.05 MDH 68.9%, MDL 80% | Age and BMI |
| Negrati et al., 2021 [44] | Italy | Cross- sectional | 139/80 | Diet score (range 0–55) [51] | NA | Diet score (range 0–55). Quartile 4: mean 38 | Diet score (range 0–55). Quartile 1: mean 28.5 | BMI (kg/m2): r = −0.110, p ≥ 0.05 MDH 29.3 (6.30), MDL 30.8 (6.20) Blood glucose (mg/dL): r = −0.216, p ≥ 0.05 MDH 85.3 (14.72), MDL 91.2 (17.32) Insulin: r = −0.20, p ≥ 0.05 MDH 8.7 (11.282), MDL 12.8 (4.69) HOMA-IR: r = −0.176, p ≥ 0.05 MDH 1.92 (3.05), MDL 3.06 (1.25) TC (mg/dL): r = −0.024, p ≥ 0.05 MDH 239.1 (31.08), MDL 230 (94.94), LDL-C (mg/dL): r = −0.192, p ≥ 0.05 MDH 132.4 (34.74), MDL 148.8 (33.09) HDL-C (mg/dL): r = −0.02, p ≥ 0.05 MDH 60.3 (10.58), MDL 59.1 (13.97) TAG (mg/dL): r = 0.11, p ≥ 0.05 MDH 143 (62.39), MDL 135.5 (68.44) | None |
| Porciello et al., 2020 [42]; (Porciello et al., 2019 [43]) | Italy | Cross- sectional | 309/309 | 14-item PREDIMED questionnaire [50] | NA | PREDIMED > 7 | PREDIMED ≤ 7 | BMI (kg/m2): MDH 27.21 (6.13), MDL 28.24 (5.97) | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Leary, S.; Niu, J.; Perry, R.; Papadaki, A. The Role of the Mediterranean Diet in Breast Cancer Survivorship: A Systematic Review and Meta-Analysis of Observational Studies and Randomised Controlled Trials. Nutrients 2023, 15, 2099. https://doi.org/10.3390/nu15092099
Chen G, Leary S, Niu J, Perry R, Papadaki A. The Role of the Mediterranean Diet in Breast Cancer Survivorship: A Systematic Review and Meta-Analysis of Observational Studies and Randomised Controlled Trials. Nutrients. 2023; 15(9):2099. https://doi.org/10.3390/nu15092099
Chicago/Turabian StyleChen, Ge, Sam Leary, Jizhao Niu, Rachel Perry, and Angeliki Papadaki. 2023. "The Role of the Mediterranean Diet in Breast Cancer Survivorship: A Systematic Review and Meta-Analysis of Observational Studies and Randomised Controlled Trials" Nutrients 15, no. 9: 2099. https://doi.org/10.3390/nu15092099
APA StyleChen, G., Leary, S., Niu, J., Perry, R., & Papadaki, A. (2023). The Role of the Mediterranean Diet in Breast Cancer Survivorship: A Systematic Review and Meta-Analysis of Observational Studies and Randomised Controlled Trials. Nutrients, 15(9), 2099. https://doi.org/10.3390/nu15092099