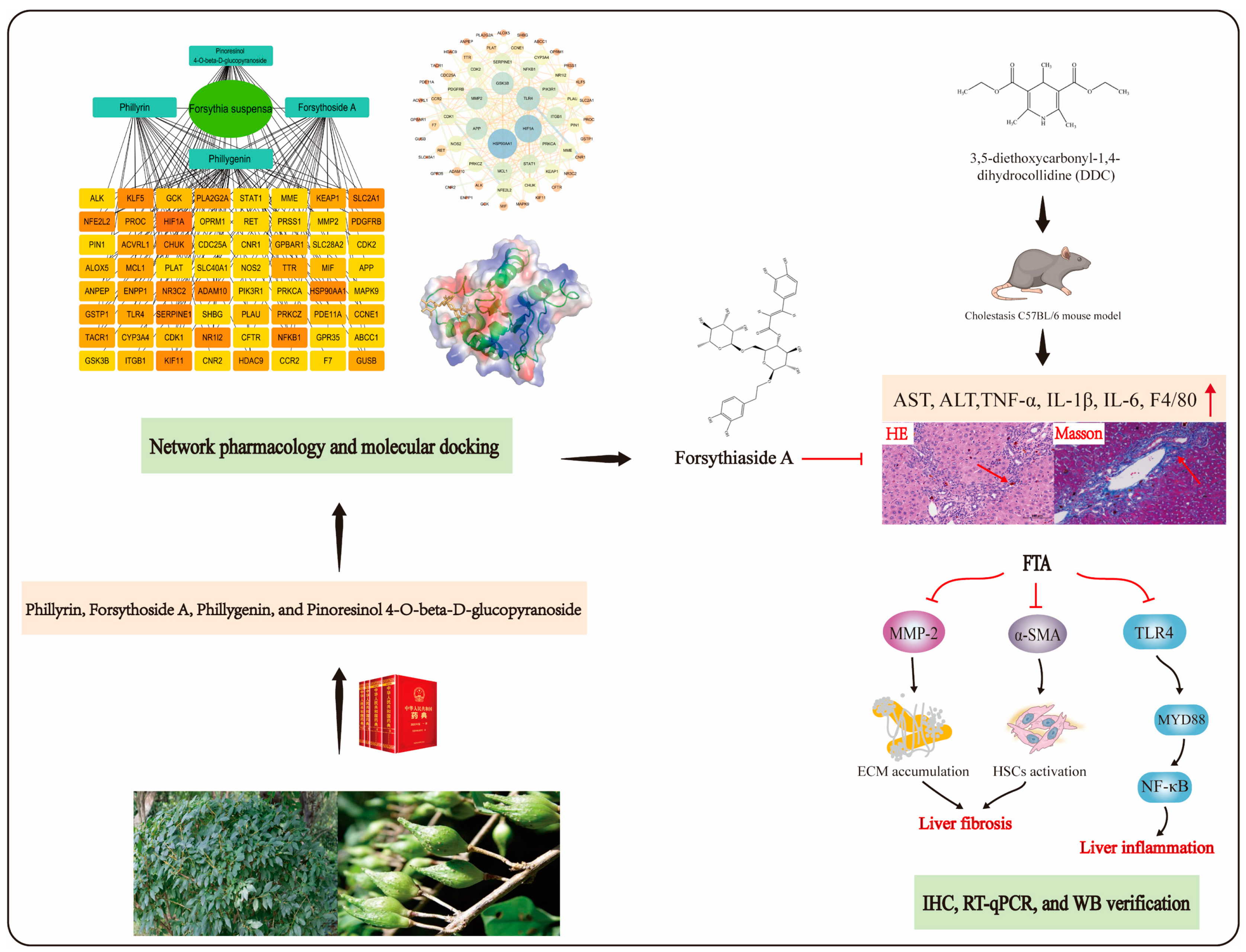
Exploration of the Molecular Basis of Forsythia Fruit in the Prevention and Treatment of Cholestatic Liver Injury through Network Pharmacology and Molecular Docking
Abstract
1. Introduction
2. Materials and Methods
2.1. Network Pharmacology Research
2.1.1. Prediction of Action Targets of FS
2.1.2. Building a “Components-Targets” Network
2.1.3. Building a PPI Network
2.1.4. GO and KEGG Analysis
2.1.5. Screening the Key Component for the Treatment of Cholestasis
2.1.6. Network Pharmacology Research of Key Component
2.2. Molecular Docking Research
2.2.1. Preparation of Ligands and Receptors
2.2.2. The Operation of Molecular Docking
2.3. Experimental Study In Vivo
2.3.1. Reagents and Chemicals
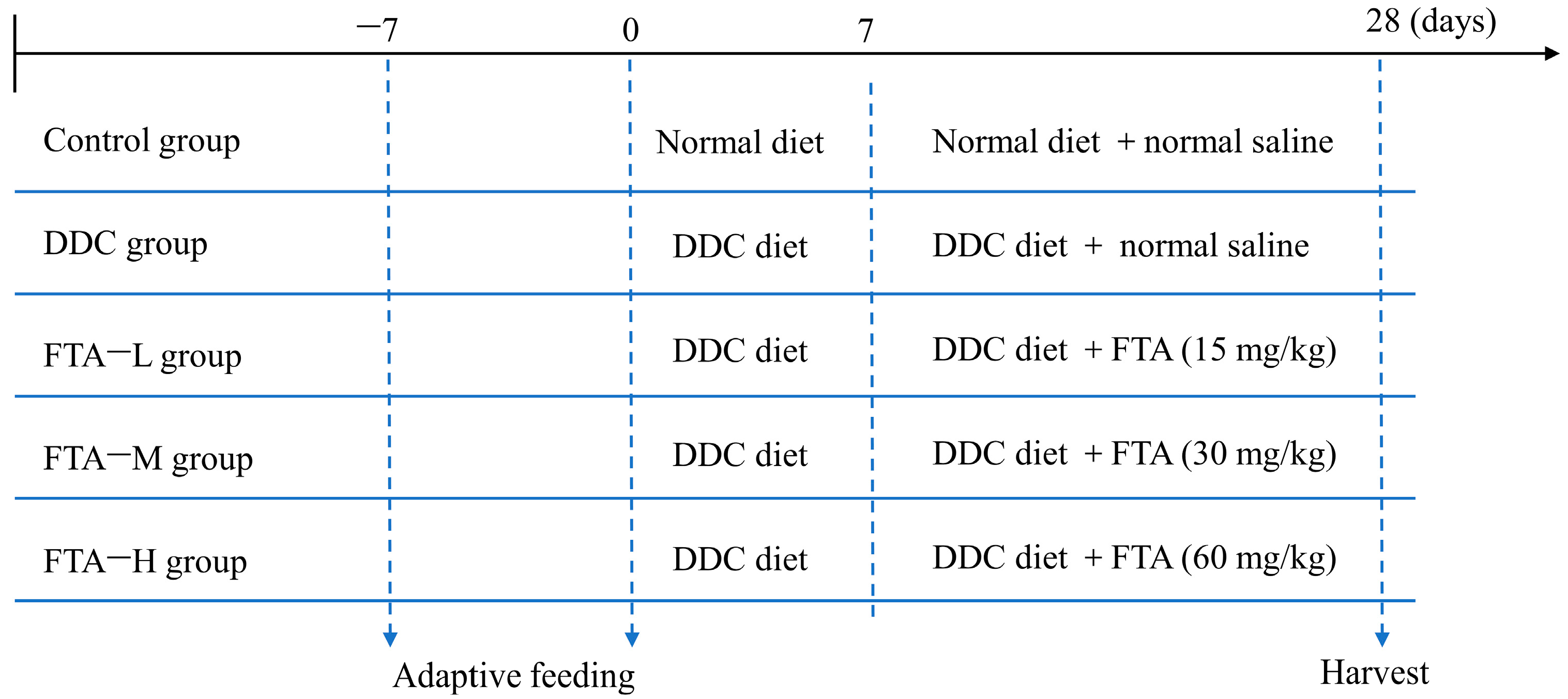
2.3.2. Animals and Treatments
2.3.3. Histological, Biochemical Assessments, and ELISA Analysis
2.3.4. Immunohistochemistry Analysis
2.3.5. Quantitative Real-Time PCR (qPCR) Analysis
2.3.6. Western Blot
2.3.7. Statistical Analysis
3. Results
3.1. The Targets of RAIs of FS in the Treatment of Cholestasis
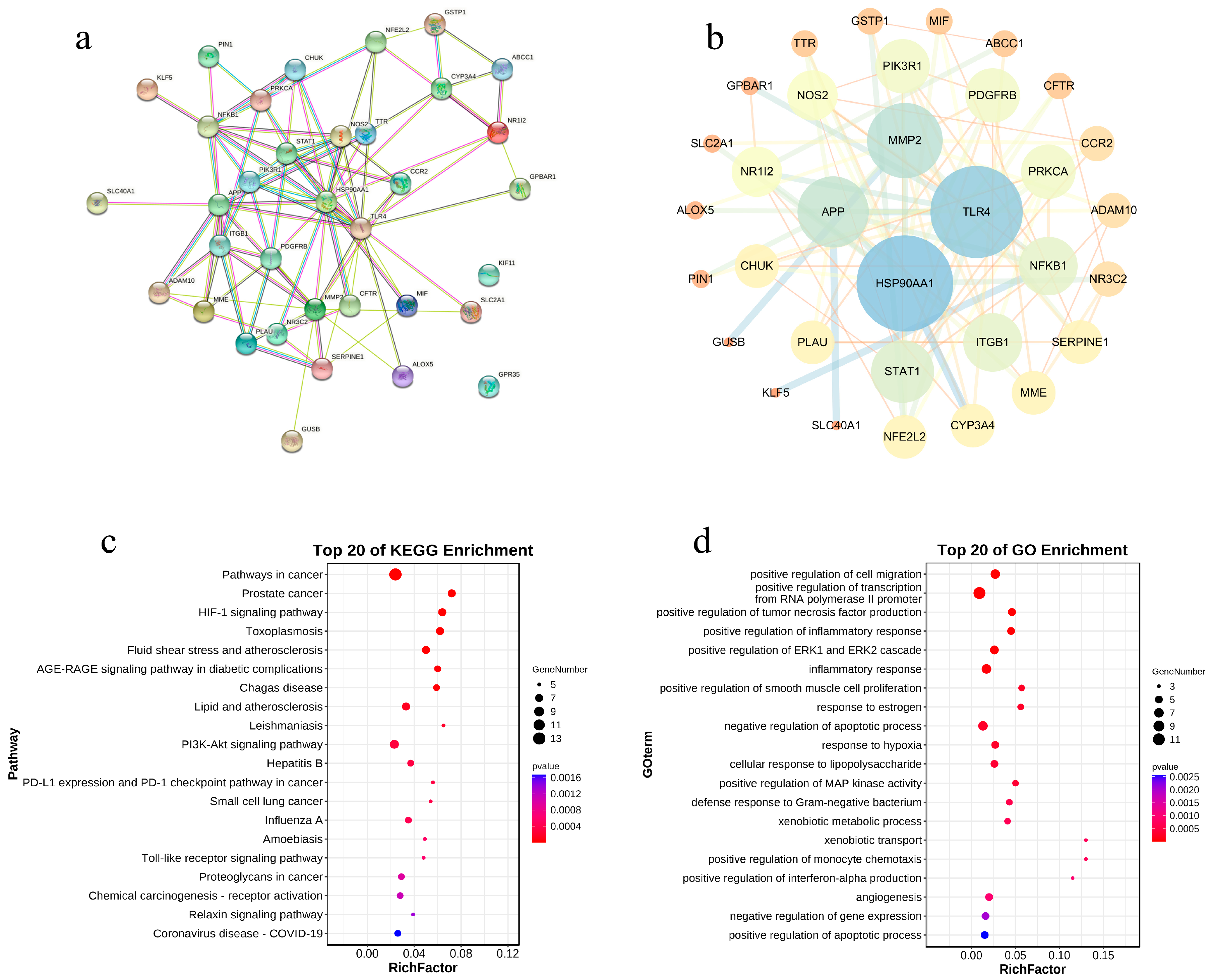
3.2. The Results of the PPI Network
3.3. The Results of GO and KEGG Analysis
3.4. Results of Screening Key Component
3.5. Network Pharmacology Study of Key Component FTA
3.6. Molecular Docking Study of FTA
3.7. Validation of in Vivo Experiment
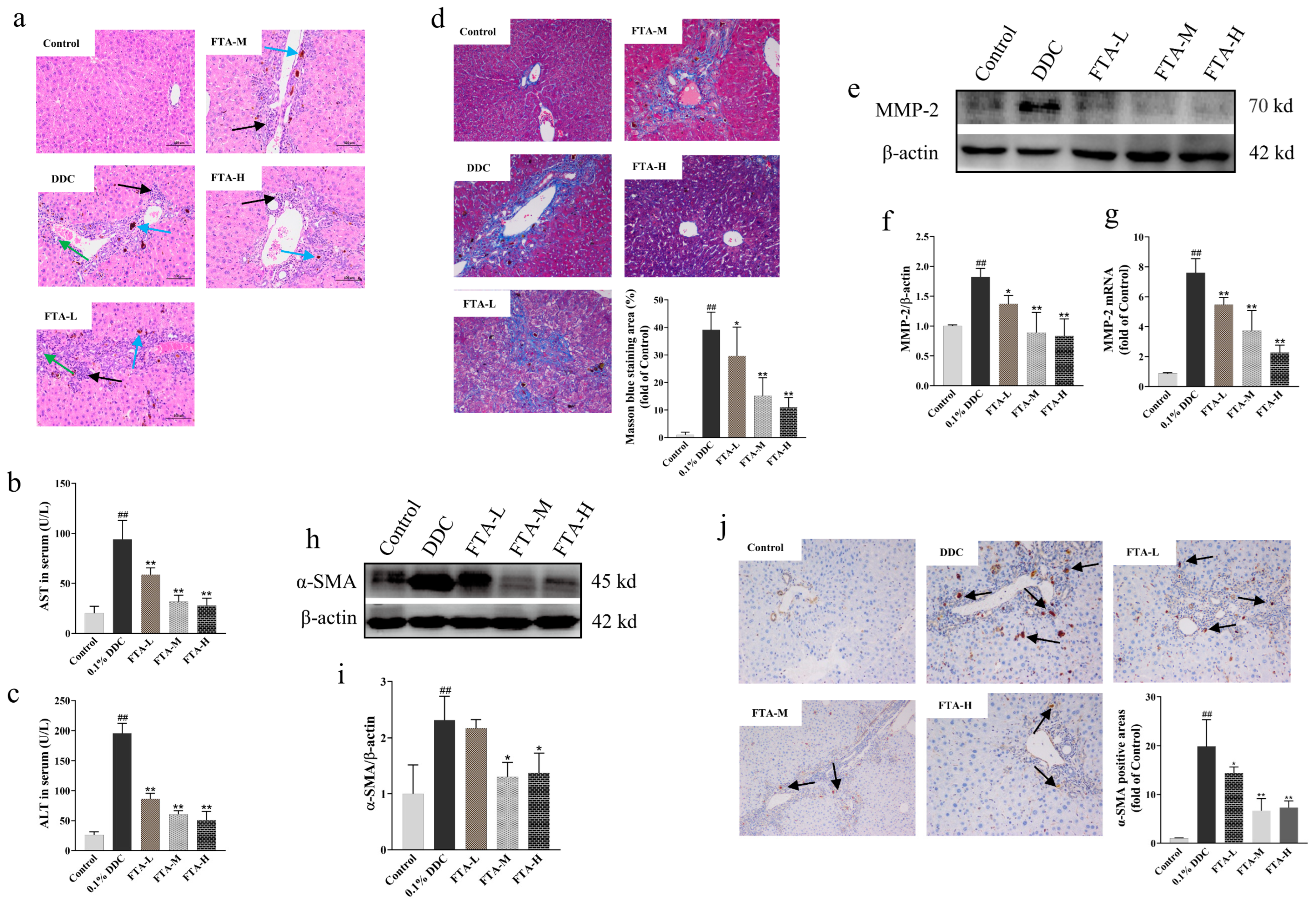
3.7.1. FTA Improved DDC-Induced Liver Injury and Fibrosis
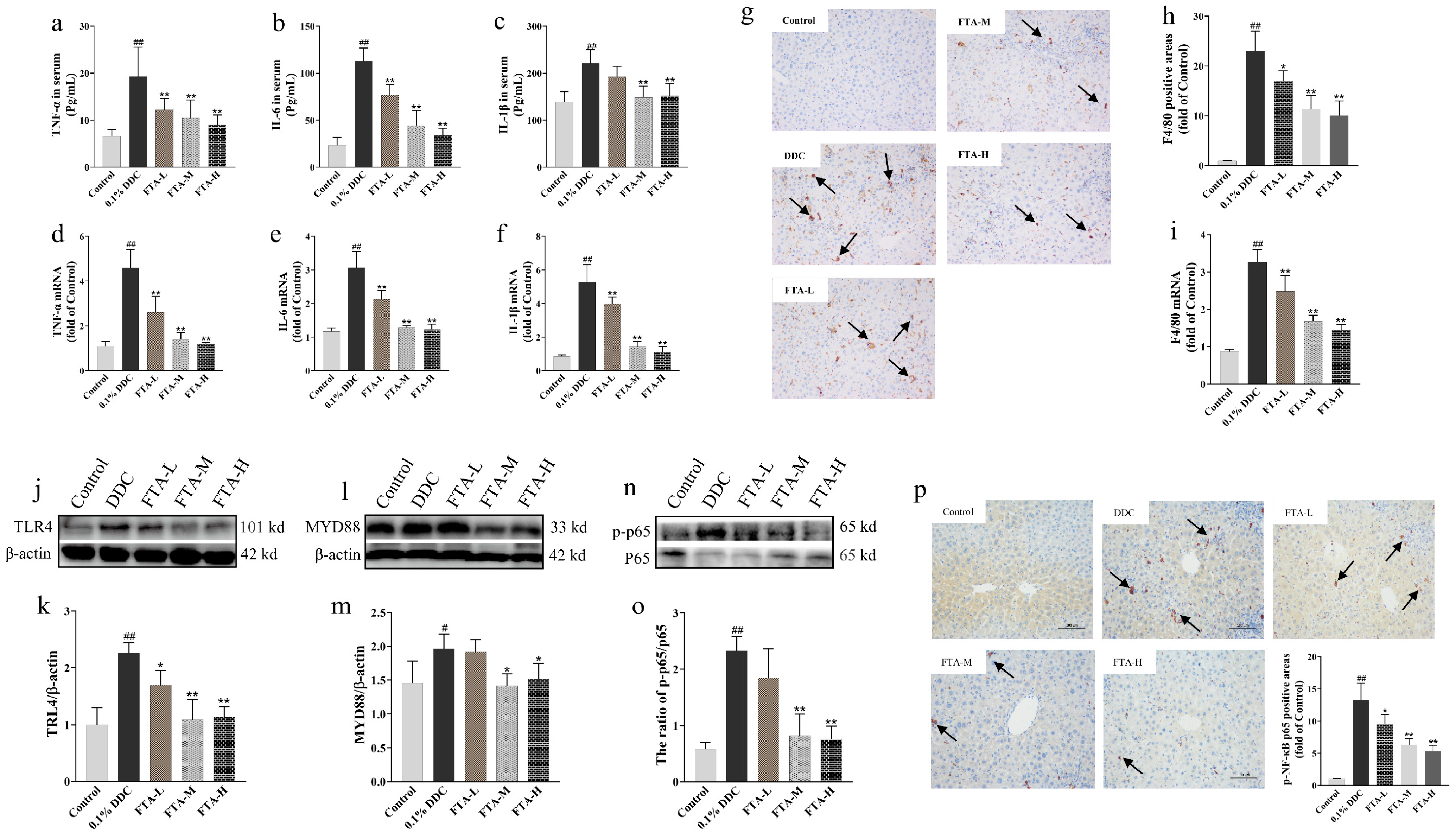
3.7.2. FTA Improved DDC-Induced Cholestatic Liver Injury by Blocking TLR4/MYD88/NF-κB Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hirschfield, G.; Heathcote, E.J.; Gershwin, M.E. Pathogenesis of cholestatic liver disease and therapeutic approaches. Gastroenterology 2010, 139, 1481–1496. [Google Scholar] [CrossRef]
- Chen, H.-L.; Wu, S.-H.; Hsu, S.-H.; Liou, B.-Y.; Chen, H.-L.; Chang, M.-H. Jaundice revisited: Recent advances in the diagnosis and treatment of inherited cholestatic liver diseases. J. Biomed. Sci. 2018, 25, 75. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Yoneda, M.; Kurita, Y.; Nogami, A.; Honda, Y.; Hosono, K.; Nakajima, A. Cholestatic liver disease: Current treatment strategies and new therapeutic agents. Drugs 2021, 81, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Heathcote, E.J. Diagnosis and management of cholestatic liver disease. Clin. Gastroenterol. Hepatol. 2007, 5, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.H.; Kamath, B.M.; Loomes, K.M.; Karpen, S.J. Cholestatic liver diseases of genetic etiology: Advances and controversies. Hepatology 2022, 75, 1627–1646. [Google Scholar] [CrossRef] [PubMed]
- Nevens, F.; Andreone, P.; Mazzella, G.; Strasser, S.I.; Bowlus, C.; Invernizzi, P.; Drenth, J.P.; Pockros, P.J.; Regula, J.; Beuers, U.; et al. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N. Engl. J. Med. 2016, 375, 631–643. [Google Scholar] [CrossRef]
- Zou, M.; Wang, A.; Wei, J.; Cai, H.; Yu, Z.; Zhang, L.; Wang, X. An insight into the mechanism and molecular basis of dysfunctional immune response involved in cholestasis. Int. Immunopharmacol. 2021, 92, 107328. [Google Scholar] [CrossRef]
- Wagner, M.; Fickert, P. Drug therapies for chronic cholestatic liver diseases. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 503–527. [Google Scholar] [CrossRef]
- Buyel, J. Plants as sources of natural and recombinant anti-cancer agents. Biotechnol. Adv. 2018, 36, 506–520. [Google Scholar] [CrossRef]
- Mukhtar, M.; Arshad, M.; Ahmad, M.; Pomerantz, R.J.; Wigdahl, B.; Parveen, Z. Antiviral potentials of medicinal plants. Virus Res. 2008, 131, 111–120. [Google Scholar] [CrossRef]
- Zhou, Z.-Y.; Liu, X.; Cui, J.-L.; Wang, J.-H.; Wang, M.-L.; Zhang, G. Endophytic fungi and their bioactive secondary metabolites in medicinal leguminosae plants: Nearly untapped medical resources. FEMS Microbiol. Lett. 2022, 369, fnac052. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.; Samanta, S.K. Medicinal plants, human health and biodiversity: A broad review. Adv. Biochem. Eng. Biotechnol. 2015, 147, 59–110. [Google Scholar] [CrossRef] [PubMed]
- Talman, A.M.; Clain, J.; Duval, R.; Ménard, R.; Ariey, F. Artemisinin bioactivity and resistance in malaria parasites. Trends Parasitol. 2019, 35, 953–963. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Hwang, Y.-H.; Kim, T.I.; Oh, Y.-C.; Ma, J.Y. Forsythia fruit prevents fulminant hepatitis in mice and ameliorates inflammation in murine macrophages. Nutrients 2021, 13, 2901. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Guo, C.; Dai, X.; Wang, C.; Gong, L.; Yu, L.; Peng, C.; Li, Y. Forsythiae fructuse water extract attenuates liver fibrosis via TLR4/MyD88/NF-κB and TGF-β/smads signaling pathways. J. Ethnopharmacol. 2020, 262, 113275. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, W.; Han, Y.; Li, Y.; Wan, L.; Guo, C. Study on the antipyretic and cholagogic effects of gardenia and forsythia. World Sci. Technol.—Mod. Tradit. Chin. Med. Mater. Med. 2015, 17, 1486–1491. [Google Scholar]
- Zhang, L.; Yan, S.; Zhou, L.; Xiong, X.; Xiao, C.; Zhang, L.; Qing, H. Effect of the Junchen component of Lidan Mixture on dose-effect relationship of intrahepatic cholestasis in young rats. China J. Tradit. Chin. Med. Pharm. 2019, 34, 3012–3016. [Google Scholar]
- Xu, Z.; Zhuang, L.; Wang, X.; Xu, J.; Li, Q.; Chen, J.; Sang, Y. Clinical study on the treatment of mild intrahepatic cholestasis of pregnancy with artemisia capillaris and ephedra, forsythia and red bean decoction. Chin. J. Fam. Plan. Gynecotokology 2021, 13, 63–67. [Google Scholar]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef]
- Li, S.; Zhang, B. Traditional Chinese medicine network pharmacology: Theory, methodology and application. Chin. J. Nat. Med. 2013, 11, 110–120. [Google Scholar] [CrossRef]
- Nogales, C.; Mamdouh, Z.M.; List, M.; Kiel, C.; Casas, A.I.; Schmidt, H.H. Network pharmacology: Curing causal mechanisms instead of treating symptoms. Trends Pharmacol. Sci. 2021, 43, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, Y.; Dai, E.; Wang, L.; Du, H. Prediction of triptolide targets in rheumatoid arthritis using network pharmacology and molecular docking. Int. Immunopharmacol. 2020, 80, 106179. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Ma, Q.; Cui, H.; Liu, G.; Zhao, X.; Li, W.; Piao, G. How can synergism of traditional medicines benefit from network pharmacology? Molecules 2017, 22, 1135. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Jin, X.; Ma, Y.; Yang, Y.; Li, J.; Liang, L.; Liu, R.; Li, Z. A comprehensive application: Molecular docking and network pharmacology for the prediction of bioactive constituents and elucidation of mechanisms of action in component-based Chinese medicine. Comput. Biol. Chem. 2020, 90, 107402. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Pan, Y.; Leng, T.; Chu, Y.; Zhang, H.; Ma, J.; Ma, X. Progress and prospects of research ideas and methods in the network pharmacology of traditional Chinese medicine. J. Pharm. Pharm. Sci. 2022, 25, 218–226. [Google Scholar] [CrossRef]
- Jiang, N.; Li, H.; Sun, Y.; Zeng, J.; Yang, F.; Kantawong, F.; Wu, J. Network pharmacology and pharmacological evaluation reveals the mechanism of the sanguisorba officinalis in suppressing hepatocellular carcinoma. Front. Pharmacol. 2021, 12, 618522. [Google Scholar] [CrossRef]
- Aihaiti, Y.; Cai, Y.S.; Tuerhong, X.; Ni Yang, Y.; Ma, Y.; Zheng, H.S.; Xu, K.; Xu, P. Therapeutic effects of naringin in rheumatoid arthritis: Network pharmacology and experimental validation. Front. Pharmacol. 2021, 12, 672054. [Google Scholar] [CrossRef]
- Nickel, J.; Gohlke, B.; Erehman, J.; Banerjee, P.; Rong, W.W.; Goede, A.; Dunkel, M.; Preissner, R. SuperPred: Update on drug classification and target prediction. Nucleic Acids Res. 2014, 42, W26–W31. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. Swiss target prediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Fu, K.; Ma, C.; Wang, C.; Zhou, H.; Gong, L.; Zhang, Y.; Li, Y. Forsythiaside A alleviated carbon tetrachloride-induced liver fibrosis by modulating gut microbiota composition to increase short-chain fatty acids and restoring bile acids metabolism disorder. Biomed. Pharmacother. 2022, 151, 113185. [Google Scholar] [CrossRef]
- Xue, X.; Zhao, X.; Wang, J.; Wang, C.; Ma, C.; Zhang, Y.; Li, Y.; Peng, C. Carthami flos extract against carbon tetrachloride-induced liver fibrosis via alleviating angiogenesis in mice. Phytomedicine 2023, 108, 154517. [Google Scholar] [CrossRef]
- Yuan, T.; Lv, S.; Zhang, W.; Tang, Y.; Chang, H.; Hu, Z.; Fang, L.; Du, J.; Wu, S.; Yang, X.; et al. PF-PLC micelles ameliorate cholestatic liver injury via regulating TLR4/MyD88/NF-κB and PXR/CAR/UGT1A1 signaling pathways in EE-induced rats. Int. J. Pharm. 2022, 615, 121480. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Wu, J.; Fang, S.; Liu, S.; Wang, T.; Li, Y.; Zou, J.; Shi, R.; Wang, Z.; Yang, L.; et al. Cultured bear bile powder ameliorates acute liver injury in cholestatic mice via inhibition of hepatic inflammation and apoptosis. J. Ethnopharmacol. 2022, 284, 114829. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, M.P.; Xu, B.; Fan, F.; Lu, S.F.; Pan, M.; Wu, H.S. A study of regulatory effects of TLR4 and NF-κB on primary biliary cholangitis. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3951–3959. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Y.; Wang, N.; Bai, Y.; Shi, D. Celastrol attenuates intrahepatic cholestasis of pregnancy by inhibiting matrix metalloproteinases-2 and 9. Ann. Hepatol. 2019, 18, 40–47. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, L.; Lu, M.; Shen, Z. Resveratrol reduces matrix metalloproteinases and alleviates intrahepatic cholestasis of pregnancy in rats. Can. J. Physiol. Pharmacol. 2016, 94, 402–407. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, M. Epigallocatechin-3-gallate ameliorates intrahepatic cholestasis of pregnancy by inhibiting matrix metalloproteinase-2 and matrix metalloproteinase-9. Fundam. Clin. Pharmacol. 2017, 31, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Ding, M.; Jia, K.; Ma, S.; Ju, W. Based on network pharmacology and molecular docking method, the research on the active compounds of Dayuan Decoction in the treatment of novel coronavirus pneumonia (COVID-19). Chin. Tradit. Herb. Drugs 2020, 51, 836–844. [Google Scholar]
- Haidl, I.D.; Jefferies, W.A. The macrophage cell surface glycoprotein F4/80 is a highly glycosylated proteoglycan. Eur. J. Immunol. 1996, 26, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Fickert, P.; Thueringer, A.; Moustafa, T.; Silbert, D.; Gumhold, J.; Tsybrovskyy, O.; Lebofsky, M.; Jaeschke, H.; Denk, H.; Trauner, M. The role of osteopontin and tumor necrosis factor alpha receptor-1 in xenobiotic-induced cholangitis and biliary fibrosis in mice. Lab. Investig. 2010, 90, 844–852. [Google Scholar] [CrossRef]
- Carmody, R.J.; Chen, Y.H. Nuclear factor-kappaB: Activation and regulation during toll-like receptor signaling. Cell Mol. Immunol. 2007, 4, 31–41. [Google Scholar]
- Lawrence, T. The nuclear factor NF-kappa B pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Correia, J.D.S.; Ulevitch, R.J. MD-2 and TLR4 N-linked glycosylations are important for a functional lipopolysaccharide receptor. J. Biol. Chem. 2002, 277, 1845–1854. [Google Scholar] [CrossRef]
- Cernuda-Morollón, E.; Pineda-Molina, E.; Cañada, F.J.; Pérez-Sala, D. 15-deoxy-delta 12,14-prostaglandin J2 inhibition of NF-kappaB-DNA binding through covalent modification of the p50 subunit. J. Biol. Chem. 2001, 276, 35530–35536. [Google Scholar] [CrossRef]
- Matthews, J.R.; Botting, C.H.; Panico, M.; Morris, H.R.; Hay, R.T. Inhibition of NF-kappaB DNA binding by nitric oxide. Nucleic Acids Res. 1996, 24, 2236–2242. [Google Scholar] [CrossRef]
- Ghonem, N.S.; Assis, D.N.; Boyer, J.L. Fibrates and cholestasis. Hepatology 2015, 62, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Yuan, G.; Wu, J.; Wu, Q.; Li, L.; Jiang, P. Prevotella copri ameliorates cholestasis and liver fibrosis in primary sclerosing cholangitis by enhancing the FXR signalling pathway. Biochim. et Biophys. Acta (BBA)-Mol. Basis Dis. 2022, 1868, 166320. [Google Scholar] [CrossRef] [PubMed]
- Hohenester, S.; Kanitz, V.; Kremer, A.E.; Paulusma, C.C.; Wimmer, R.; Kuehn, H.; Denk, G.; Horst, D.; Elferink, R.O.; Beuers, U. Glycochenodeoxycholate promotes liver fibrosis in mice with hepatocellular cholestasis. Cells 2020, 9, 281. [Google Scholar] [CrossRef] [PubMed]
- Parola, M.; Pinzani, M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol. Asp. Med. 2019, 65, 37–55. [Google Scholar] [CrossRef]
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2020, 18, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Rysz, J.; Banach, M.; Stolarek, R.A.; Mikhailidis, D.P.; Cialkowska-Rysz, A.; Pokoca, L.; Piechota, M.; Baj, Z. Serum metalloproteinases MMP-2, MMP-9 and metalloproteinase tissue inhibitors TIMP-1 and TIMP-2 in patients on hemodialysis. Int. Urol. Nephrol. 2011, 43, 491–498. [Google Scholar] [CrossRef]
- Rudra, D.S.; Pal, U.; Chowdhury, N.; Maiti, N.C.; Bagchi, A.; Swarnakar, S. Omeprazole prevents stress induced gastric ulcer by direct inhibition of MMP-2/TIMP-3 interactions. Free. Radic. Biol. Med. 2022, 181, 221–234. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, Q.; Li, J.; Chen, H.; Wu, J.; Song, H. Up-regulation of BSEP and MRP2 by Calculus Bovis administration in 17α-ethynylestradiol-induced cholestasis: Involvement of PI3K/Akt signaling pathway. J. Ethnopharmacol. 2016, 190, 22–32. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Xu, Z.; Shi, S.; Wang, D.; Shi, X.; Wang, Y.; Zeng, B.; Deng, H.; Deng, X.; et al. Melatonin ameliorates ANIT-induced cholestasis by activating Nrf2 through a PI3K/Akt-dependent pathway in rats. Mol. Med. Rep. 2018, 19, 1185–1193. [Google Scholar] [CrossRef]
- Zhao, Y.-L.; Ma, X.; Zhu, Y.; Chen, Z.; Wang, J.-B.; Li, R.-Y.; Chen, C.; Wei, S.-Z.; Li, J.-Y.; Bing, L.; et al. Paeonia lactiflora Pall. protects against ANIT-induced cholestasis by activating Nrf2 via PI3K/Akt signaling pathway. Drug Des. Dev. Ther. 2015, 9, 5061–5074. [Google Scholar] [CrossRef]
- Burban, A.; Sharanek, A.; Hüe, R.; Gay, M.; Routier, S.; Guillouzo, A.; Guguen-Guillouzo, C. Penicillinase-resistant antibiotics induce non-immune-mediated cholestasis through HSP27 activation associated with PKC/P38 and PI3K/AKT signaling pathways. Sci. Rep. 2017, 7, 1815. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.-K.; Zhang, C.-X.; Zhao, S.-S.; Zhang, S.-H.; Zhang, H.; Cai, S.-Y.; Shao, R.-G.; He, H.-W. The anti-fibrotic effects of epigallocatechin-3-gallate in bile duct-ligated cholestatic rats and human hepatic stellate LX-2 cells are mediated by the PI3K/Akt/Smad pathway. Acta Pharmacol. Sin. 2015, 36, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Zhou, H.; Wang, C.; He, L.; Guo, C.; Peng, C.; Li, Y. Hepatoprotective effect of forsythiaside a against acetaminophen-induced liver injury in zebrafish: Coupling network pharmacology with biochemical pharmacology. J. Ethnopharmacol. 2021, 271, 113890. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-O.; Welch, T.P.; Gonzalez, F.J.; Copple, B.L.; Salhiyyah, K.; Sarathchandra, P.; Latif, N.; Yacoub, M.H.; Chester, A.H.; Manresa, M.C.; et al. Reduced liver fibrosis in hypoxia-inducible factor-1α-deficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G582–G592. [Google Scholar] [CrossRef]
- Quelhas, P.; Breton, M.C.; Oliveira, R.C.; Cipriano, M.A.; Teixeira, P.; Cerski, C.T.; Shivakumar, P.; Vieira, S.M.G.; Kieling, C.O.; Verde, I.; et al. HIF-1alpha-pathway activation in cholangiocytes of patients with biliary atresia: An immunohistochemical/molecular exploratory study. J. Pediatr. Surg. 2022, 58, 587–594. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Z.; Xu, Z.; Tian, P.; Miao, H.; Pan, S.; Song, R.; Sun, X.; Zhao, B.; Wang, D.; et al. Reduction of hepatic fibrosis by overexpression of von Hippel–Lindau protein in experimental models of chronic liver disease. Sci. Rep. 2017, 7, srep41038. [Google Scholar] [CrossRef]
- Abramicheva, P.A.; Balakina, T.A.; Morozov, I.A.; Schelkunova, T.A.; Smirnova, O.V. Prolactin signaling pathways determining its direct effects on kidneys in the cholestasis of pregnancy model. Biochemistry 2019, 84, 1204–1212. [Google Scholar] [CrossRef]
- Fidchenko, Y.M.; Kushnareva, N.S.; Smirnova, O.V. Effect of prolactin on the water-salt balance in rat females in the model of cholestasis of pregnancy. Bull. Exp. Biol. Med. 2014, 156, 803–806. [Google Scholar] [CrossRef]
- Kushnareva, N.S.; Smirnova, O.V. Effect of prolactin on excretory function of the liver during the induction and relief of cholestasis in female rats. Bull. Exp. Biol. Med. 2009, 148, 758–761. [Google Scholar] [CrossRef]
- Ezhilarasan, D. Relaxin in hepatic fibrosis: What is known and where to head? Biochimie 2021, 187, 144–151. [Google Scholar] [CrossRef]
- Kaftanovskaya, E.M.; Ng, H.H.; Soula, M.; Rivas, B.; Myhr, C.; Ho, B.A.; Cervantes, B.A.; Shupe, T.D.; Devarasetty, M.; Hu, X.; et al. Therapeutic effects of a small molecule agonist of the relaxin receptor ML290 in liver fibrosis. FASEB J. 2019, 33, 12435–12446. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.G. Relaxin and its role in the development and treatment of fibrosis. Transl. Res. 2009, 154, 1–6. [Google Scholar] [CrossRef] [PubMed]





| Materials | Manufacturer | ID |
|---|---|---|
| FTA (purity above 98%) | Chroma Biotechnology Co., Ltd., Chengdu, China | 79916-77-1 |
| 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) | Xiya Chemical Technology Co., Ltd., Shandong, China | 632-93-9 |
| Aspartate aminotransferase (AST) assay kit | Nanjing Jiancheng Bioengineering Institute, Jiangsu, China | C010-2-1 |
| Alanine aminotransferase (ALT) assay kit | C009-2-1 | |
| Tumor necrosis factor-α (TNF-α) assay kit | Elabscience Biotechnology Co., Ltd., Wuhan, China | E-EL-M0037c |
| Interleukin (IL)-6 assay kit | E-EL-M0044c | |
| IL-1β assay kit | E-EL-M3063 | |
| α-smooth muscle actin (α-SMA) antibody | Affinity, Cincinnati, OH, USA | AF1032 |
| Matrix metalloproteinase 2 (MMP-2) antibody | ABclonal, Wuhan, China | A19080 |
| F4/80 antibody | A18637 | |
| Toll-like receptor 4 (TLR4) antibody | A21626 | |
| Myeloid differentiation primary response 88 (MYD88) antibody | A21905 | |
| Nuclear factor kappa B (NF-κB) p65 antibody | A18210 | |
| p-NF-κB p65 antibody | AP0944 | |
| β-actin antibody | Servicebio, Wuhan, China | GB15001 |
| Goat Anti-Rabbit IgG (H + L) HRP secondary antibody | Multi science, Hangzhou, China | GAR0072 |
| Total RNA isolation kit | Foregene, Chengdu, China | RE-03014 |
| HiScript® IIQ RT SuperMix for qPCR | Vazyme, Nanjing, China | R223-01 |
| ChamQ SYBR qPCR Master Mix | Q311-02 |
| Gene | ID | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|---|
| MMP-2 | 17390 | CCATGTGTCTTCCCCTTCA | CCCCACTTCCGGTCATC |
| TNF-α | 21926 | GACAGTGACCTGGACTGTGG | TGAGACAGAGGCAACCTGAC |
| IL-1β | 16176 | GAAGAAGAGCCCATCCTCTG | TCATCTCGGAGCCTGTAGTG |
| IL-6 | 16193 | CTGCAAGAGACTTCCATCCAG | AGTGGTATAGACAGGTCTGTTGG |
| F4/80 | 13733 | TGGGAGCTACTTCTGCACT | AGGAGCCTGGTACATTGGT |
| Component Name | PubChem CID | Molecular Formula | Canonical SMILES |
|---|---|---|---|
| Phillyrin | 101712 | C27H34O11 | COC1=C(C=C(C=C1)C2C3COC(C3CO2)C4=CC(=C(C=C4)OC5C(C(C(C(O5)CO)O)O)O)OC)OC |
| Forsythoside A | 5281773 | C29H36O15 | CC1C(C(C(C(O1)OCC2C(C(C(C(O2)OCCC3=CC(=C(C=C3)O)O)O)O)OC(=O)C=CC4=CC(=C(C=C4)O)O)O)O)O |
| Phillygenin | 3083590 | C21H24O6 | COC1=C(C=C(C=C1)C2C3COC(C3CO2)C4=CC(=C(C=C4)O)OC)OC |
| Pinoresinol 4-O-beta-D-glucopyranoside | 486614 | C26H32O11 | COC1=C(C=CC(=C1)C2C3COC(C3CO2)C4=CC(=C(C=C4)OC5C(C(C(C(O5)CO)O)O)O)OC)O |
| Gene Symbol | Protein | PDB ID | Resolution | Minimum Binding Free Energy (kcal/mol) | |||
|---|---|---|---|---|---|---|---|
| Forsythoside A | Phillygenin | Phillyrin | Pinoresinol 4-O-Beta-D-Glucopyranoside | ||||
| TLR4 | Toll-like receptor 4 | 4G8A | 2.40 Å | −7.3 | −6.5 | −6.9 | −6.8 |
| NFKB1 | Nuclear factor NF-kappa-B p105 subunit | 1SVC | 2.60 Å | −7.3 | −6.5 | −7.1 | −7.3 |
| Gene Symbol | Protein | PDB ID | Resolution | Minimum Binding Free Energy (kcal/mol) |
|---|---|---|---|---|
| MMP2 | 72 kDa type IV collagenase | 1CK7 | 2.80 Å | −8.7 |
| TLR4 | Toll-like receptor 4 | 4G8A | 2.40 Å | −7.3 |
| MYD88 | Myeloid differentiation primary response protein MyD88 | 7BER | 2.30 Å | −7.4 |
| NFKB1 | Nuclear factor NF-kappa-B p105 subunit | 1SVC | 2.60 Å | −7.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, K.; Li, Y.; Dai, S.; Li, Y. Exploration of the Molecular Basis of Forsythia Fruit in the Prevention and Treatment of Cholestatic Liver Injury through Network Pharmacology and Molecular Docking. Nutrients 2023, 15, 2065. https://doi.org/10.3390/nu15092065
Fu K, Li Y, Dai S, Li Y. Exploration of the Molecular Basis of Forsythia Fruit in the Prevention and Treatment of Cholestatic Liver Injury through Network Pharmacology and Molecular Docking. Nutrients. 2023; 15(9):2065. https://doi.org/10.3390/nu15092065
Chicago/Turabian StyleFu, Ke, Yanzhi Li, Shu Dai, and Yunxia Li. 2023. "Exploration of the Molecular Basis of Forsythia Fruit in the Prevention and Treatment of Cholestatic Liver Injury through Network Pharmacology and Molecular Docking" Nutrients 15, no. 9: 2065. https://doi.org/10.3390/nu15092065
APA StyleFu, K., Li, Y., Dai, S., & Li, Y. (2023). Exploration of the Molecular Basis of Forsythia Fruit in the Prevention and Treatment of Cholestatic Liver Injury through Network Pharmacology and Molecular Docking. Nutrients, 15(9), 2065. https://doi.org/10.3390/nu15092065




