Aspalathus linearis (Rooibos) Targets Adipocytes and Obesity-Associated Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Aspalathus linearis Extract
2.3. Quantification of Aspalathin Content by HPLC-UV
2.4. Determination of Antioxidant Activity by Ferric-Reducing Antioxidant Power (FRAP) Assay and DPPH Radical-Scavenging Activity
2.5. Blood Cells, Cell Cultures and Reagents
2.5.1. Blood Leukocyte Preparation
2.5.2. PBMC Preparation
2.5.3. Human Monocytic Leukemia Cells
2.5.4. Adipose Cells
- -
- Preadipocyte cells were obtained from patients undergoing surgery for cosmetic purposes without associated pathology in accordance with the Helsinki Declaration, from anonymous healthy donors. Surgical residue was harvested in accordance with French regulations, including a declaration to the Research Ministry (DC no. 2008162) and procurement of written informed consent from the patients. Four different strains were obtained from obese women (BMI > 30). For differentiation into mature adipocytes (Mas), cells were seeded at confluence (33,500 cells/cm2) in a differentiation medium consisting of Dulbecco’s modified Eagle medium (DMEM/F12 (1:1), Gibco) supplemented with 10% FBS, 1% Gln, hydrocortisone (25 mg/mL), insulin (3.5 mg/mL), T3 (6.5 mg/mL), dexamethazon (980 mg/mL), rosiglitazone (1.78 mg/mL), isobutyl-methylxanthine (IBMX) (100 mg/mL, only for the first 3 days), and gentamycin (50 mg/mL). The medium was replaced every two days. Mas were obtained after 8 days of differentiation.
- -
- The human subcutaneous preadipocyte cells were purchased from Lonza Group Ltd. (Basel, Switzerland). The initiation and expansion of the cells used Preadipocyte Growth Medium-2 (Preadipocyte Basal Medium-2 supplemented with 10% FCS, 2 mM Gln, 30 µg/mL genistein and 15 ng/mL ampicillin). To induce differentiation, cells were plated at 10,000 cells/cm2 in Preadipocyte Growth Medium-2. After 24 h, the culture medium was changed to adipocyte differentiation medium, consisting of Preadipocyte Growth Medium-2 supplemented with insulin, dexamethasone, indomethacin and IBMX. Cells were allowed to differentiate for 8 days with no further medium change.
2.6. Kinetics of ROS Production by Blood Leukocytes
2.7. Leukocyte Viability
2.8. Determination of Cytokine Concentrations
2.9. Quantification of Lipid Accumulation
2.10. Evaluation of Gene Expression by Quantitative Real-Time PCR (qRT-PCR)
2.11. Adipospheroid Generation
2.12. Confocal Microscopy
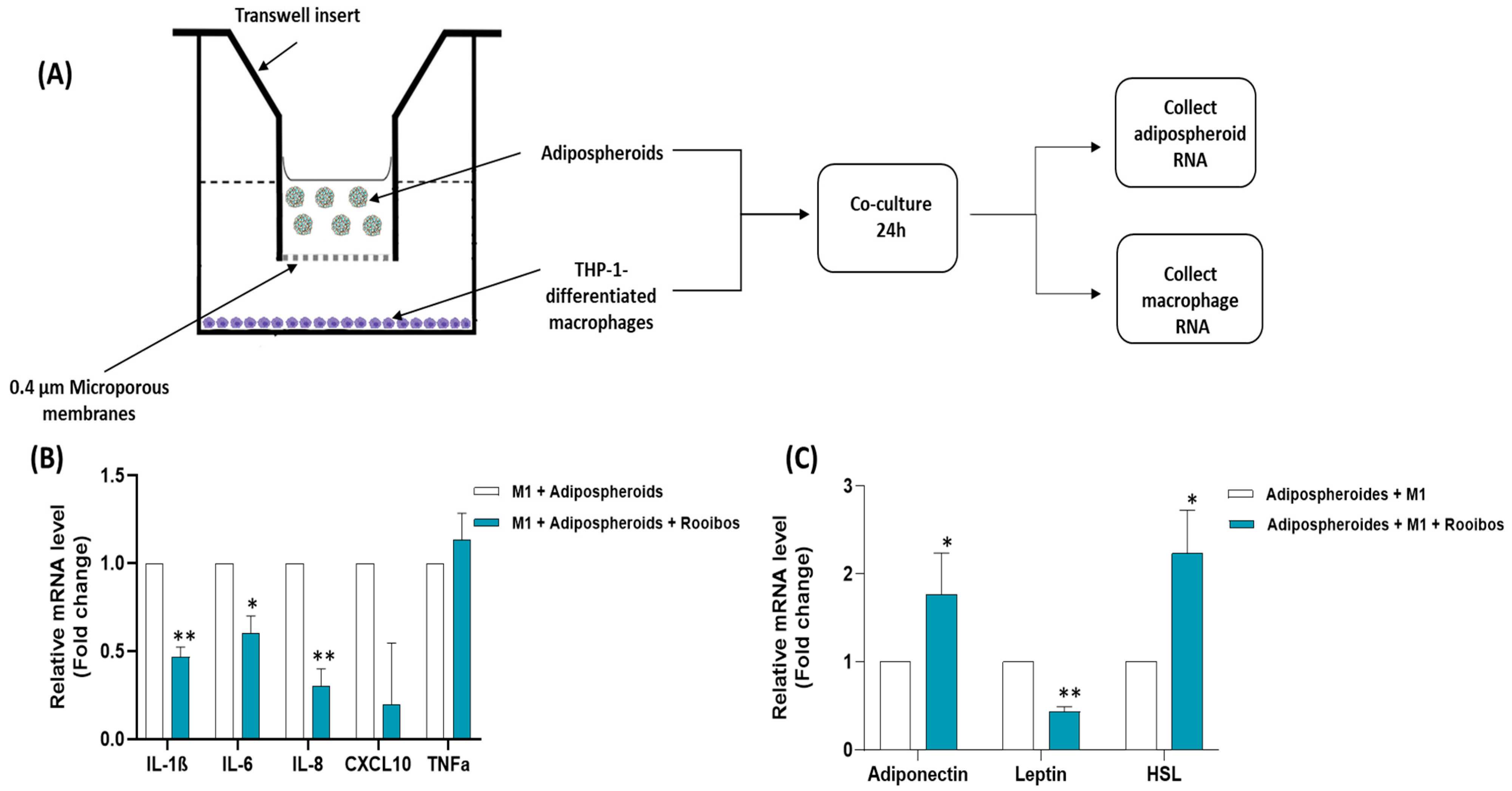
2.13. Co-Culture between Macrophages and Adipospheroids/Evaluation of Cell–Cell Interactions
2.14. Statistical Analysis
3. Results
3.1. Antioxidant and Anti-Inflammatory Impact of Rooibos Extract
3.1.1. Aspalathin Content and Antioxidant Capacity
3.1.2. Rooibos Extract Inhibited ROS Production by Blood Leukocytes
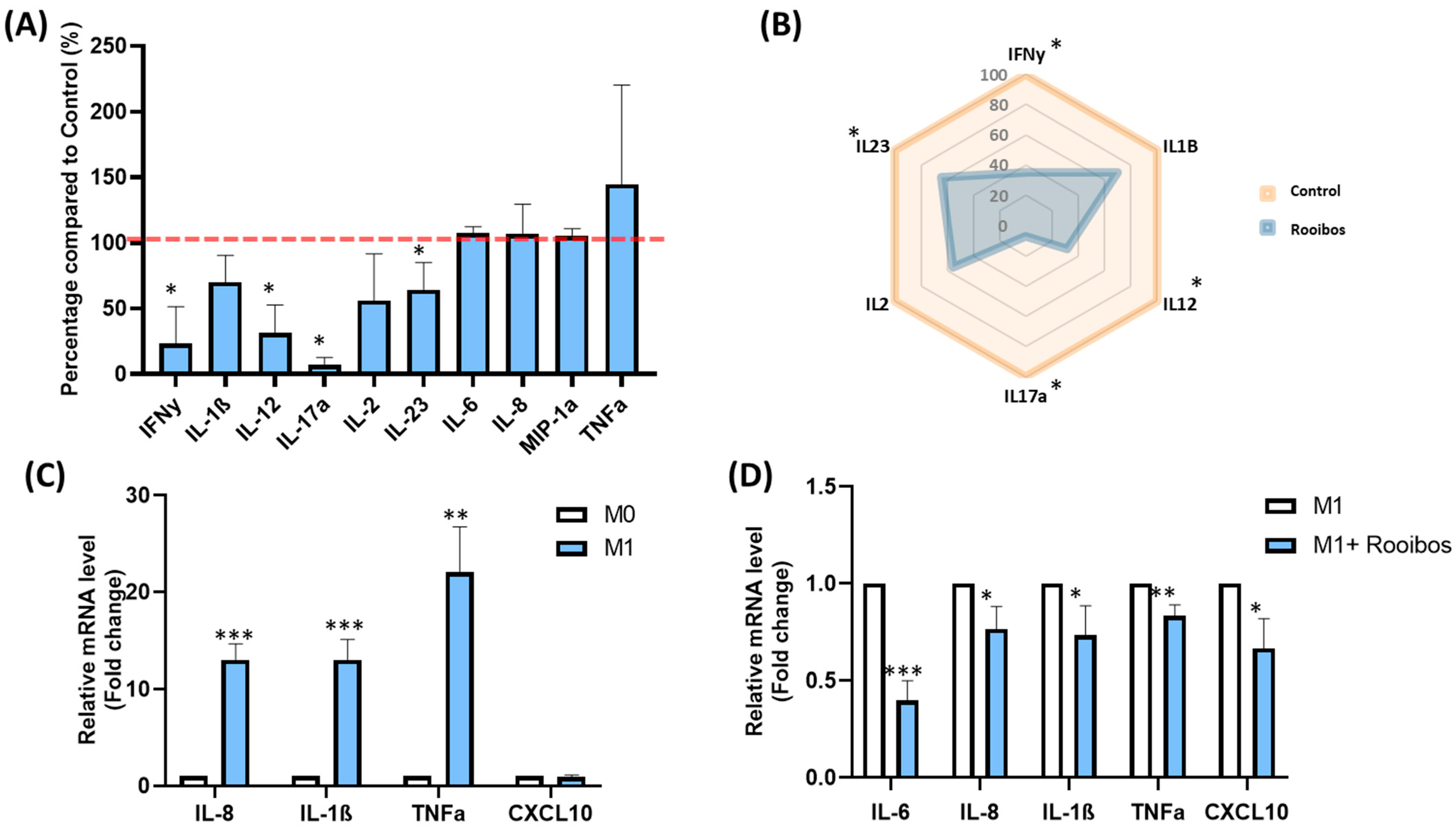
3.1.3. Rooibos Impacted PBMC Cytokine Secretion
3.1.4. Rooibos Modulated the Polarization of Macrophages toward M1-Type
3.2. Impact of Rooibos Extract on Inflammatory State of Adipose Tissue
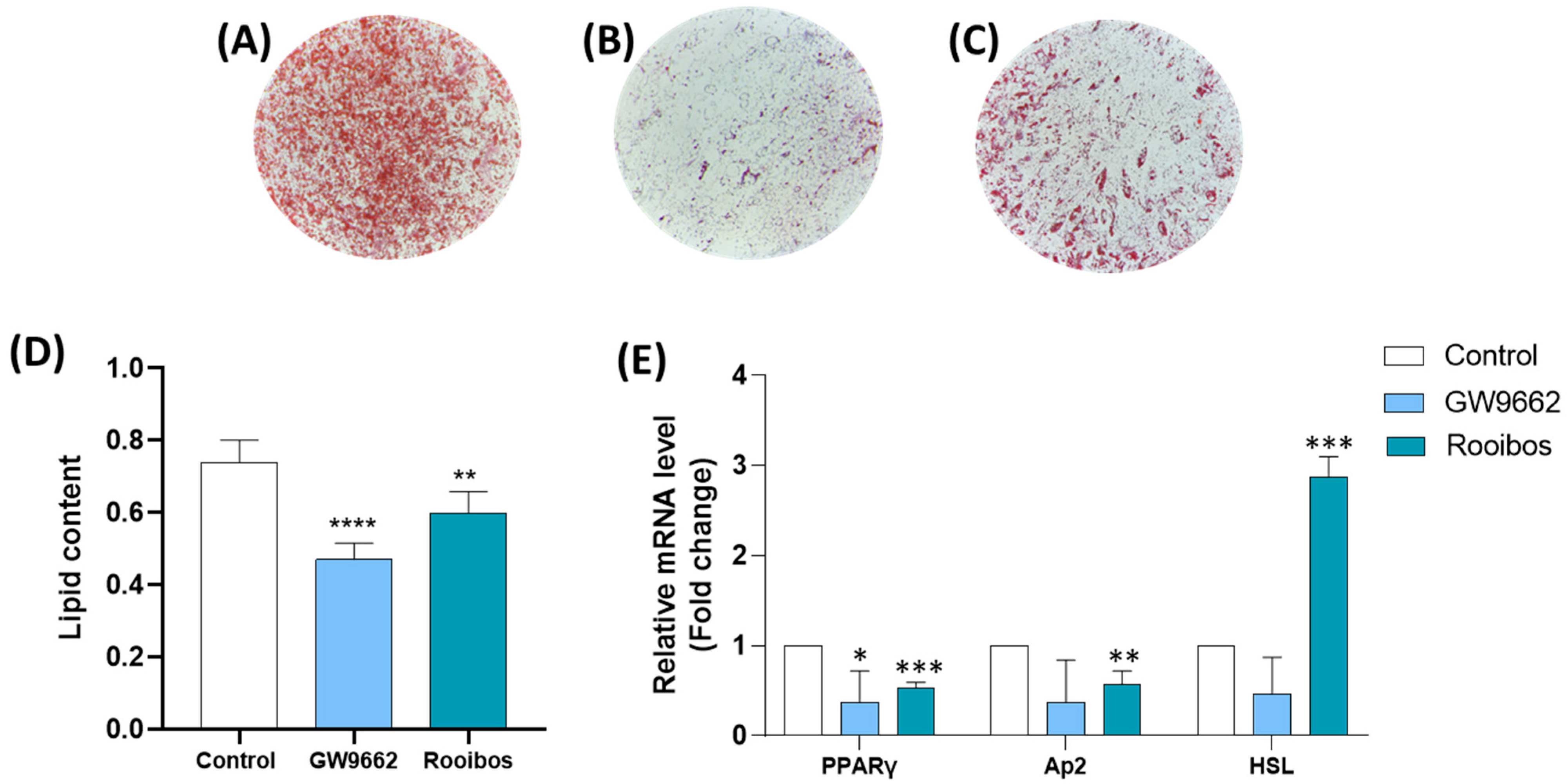
3.2.1. Rooibos Extract Reduced Lipid Accumulation in Differentiated Human Adipocytes
3.2.2. Rooibos Extract Suppressed Adipocyte-Related Gene Expression during Differentiation
3.2.3. Rooibos Extract Affected Adipokine Secretion in Differentiated Human Adipocytes
3.2.4. Rooibos Affected Adipokine and HSL Secretion in Mature Adipocytes
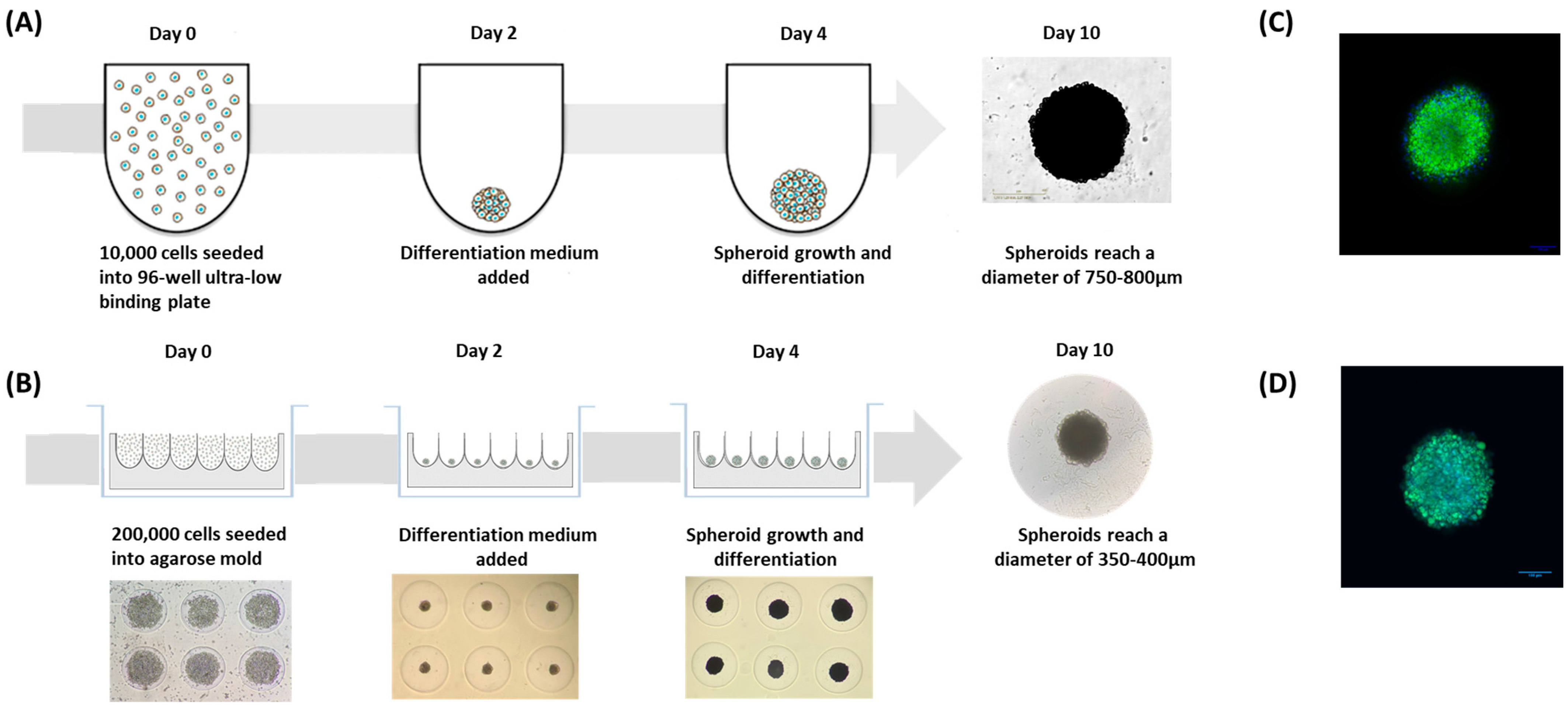
3.2.5. Rooibos Extract Effect on Adipospheroids in the Presence or Absence of Inflammatory Macrophages
- -
- The first method consisted of simply seeding the preadipocyte cells in specific U-shaped ultra-low-binding plates where cell adhesion is almost impossible (Figure 5A).
- -
- The second method consisted of using agarose molds prepared with MicroTissues® 3D Petri Dishes® (Figure 5B). In these molds, 200,000 preadipocyte cells were seeded. After 24 h of incubation, cells present within the suspension spontaneously agglomerated to form loosely adhesive cell spheroids by promoting intercellular adhesion molecules, resulting in the assembly of 81 potential adipospheroids per agarose mold.
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zorena, K.; Jachimowicz-Duda, O.; Ślęzak, D.; Robakowska, M.; Mrugacz, M. Adipokines and Obesity. Potential Link to Metabolic Disorders and Chronic Complications. Int. J. Mol. Sci. 2020, 21, 3570. [Google Scholar] [CrossRef]
- Conway, B.; Rene, A. Obesity as a Disease: No Lightweight Matter. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2004, 5, 145–151. [Google Scholar] [CrossRef]
- World Obesity Atlas. 2022. Available online: https://www.worldobesity.org/resources/resource-library/world-obesity-atlas-2022 (accessed on 10 January 2023).
- Apovian, C.M. Obesity: Definition, Comorbidities, Causes, and Burden. Am. J. Manag. Care 2016, 22, s176–s185. [Google Scholar] [PubMed]
- Milano, W.; De Biasio, V.; Di Munzio, W.; Foggia, G.; Capasso, A. Obesity: The New Global Epidemic Pharmacological Treatment, Opportunities and Limits for Personalized Therapy. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1232–1243. [Google Scholar] [CrossRef]
- Sarjeant, K.; Stephens, J.M. Adipogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008417. [Google Scholar] [CrossRef]
- De Sá, P.M.; Richard, A.J.; Hang, H.; Stephens, J.M. Transcriptional Regulation of Adipogenesis. In Comprehensive Physiology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 635–674. ISBN 978-0-470-65071-4. [Google Scholar]
- De Leeuw, A.J.M.; Oude Luttikhuis, M.A.M.; Wellen, A.C.; Müller, C.; Calkhoven, C.F. Obesity and Its Impact on COVID-19. J. Mol. Med. Berl. Ger. 2021, 99, 899–915. [Google Scholar] [CrossRef] [PubMed]
- Safaei, M.; Sundararajan, E.A.; Driss, M.; Boulila, W.; Shapi’i, A. A Systematic Literature Review on Obesity: Understanding the Causes & Consequences of Obesity and Reviewing Various Machine Learning Approaches Used to Predict Obesity. Comput. Biol. Med. 2021, 136, 104754. [Google Scholar] [CrossRef] [PubMed]
- Braune, J.; Lindhorst, A.; Fröba, J.; Hobusch, C.; Kovacs, P.; Blüher, M.; Eilers, J.; Bechmann, I.; Gericke, M. Multinucleated Giant Cells in Adipose Tissue Are Specialized in Adipocyte Degradation. Diabetes 2021, 70, 538–548. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose Tissue Inflammation and Metabolic Dysfunction in Obesity. Am. J. Physiol.-Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef] [PubMed]
- Csige, I.; Ujvárosy, D.; Szabó, Z.; Lőrincz, I.; Paragh, G.; Harangi, M.; Somodi, S. The Impact of Obesity on the Cardiovascular System. J. Diabetes Res. 2018, 2018, 3407306. [Google Scholar] [CrossRef]
- Mouton, A.J.; Li, X.; Hall, M.E.; Hall, J.E. Obesity, Hypertension, and Cardiac Dysfunction: Novel Roles of Immunometabolism in Macrophage Activation and Inflammation. Circ. Res. 2020, 126, 789–806. [Google Scholar] [CrossRef] [PubMed]
- Unamuno, X.; Gómez-Ambrosi, J.; Rodríguez, A.; Becerril, S.; Frühbeck, G.; Catalán, V. Adipokine Dysregulation and Adipose Tissue Inflammation in Human Obesity. Eur. J. Clin. Investig. 2018, 48, e12997. [Google Scholar] [CrossRef]
- Velazquez, A.; Apovian, C.M. Updates on Obesity Pharmacotherapy. Ann. N. Y. Acad. Sci. 2018, 1411, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Powell, A. Obesity: Pharmacotherapy. FP Essent. 2020, 492, 25–29. [Google Scholar]
- Ryan, D.H. Drugs for Treating Obesity. Handb. Exp. Pharmacol. 2022, 274, 387–414. [Google Scholar] [CrossRef]
- Tak, Y.J.; Lee, S.Y. Long-Term Efficacy and Safety of Anti-Obesity Treatment: Where Do We Stand? Curr. Obes. Rep. 2021, 10, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Wang, Y.; Hao, Q.; Vandvik, P.O.; Guyatt, G.; Li, J.; Chen, Z.; Xu, S.; Shen, Y.; Ge, L.; et al. Pharmacotherapy for Adults with Overweight and Obesity: A Systematic Review and Network Meta-Analysis of Randomised Controlled Trials. Lancet Lond. Engl. 2022, 399, 259–269. [Google Scholar] [CrossRef]
- Sen, T.; Samanta, S.K. Medicinal Plants, Human Health and Biodiversity: A Broad Review. Adv. Biochem. Eng. Biotechnol. 2015, 147, 59–110. [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef]
- Sprent, J.I.; Odee, D.W.; Dakora, F.D. African Legumes: A Vital but under-Utilized Resource. J. Exp. Bot. 2010, 61, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Sheik Abdul, N.; Marnewick, J.L. Rooibos, a Supportive Role to Play during the COVID-19 Pandemic? J. Funct. Foods 2021, 86, 104684. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.J.F.; Joubert, E.; Chellan, N.; Miura, Y.; Yagasaki, K. New Insights into the Efficacy of Aspalathin and Other Related Phytochemicals in Type 2 Diabetes-A Review. Int. J. Mol. Sci. 2021, 23, 356. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Debón, R.; Rull, A.; Rodríguez-Sanabria, F.; Iswaldi, I.; Herranz-López, M.; Aragonès, G.; Camps, J.; Alonso-Villaverde, C.; Menéndez, J.A.; Micol, V.; et al. Continuous Administration of Polyphenols from Aqueous Rooibos (Aspalathus linearis) Extract Ameliorates Dietary-Induced Metabolic Disturbances in Hyperlipidemic Mice. Phytomedicine Int. J. Phytother. Phytopharm. 2011, 18, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, M.; Mazibuko, S.E.; Joubert, E.; de Beer, D.; Johnson, R.; Pheiffer, C.; Louw, J.; Muller, C.J.F. Effects of Fermented Rooibos (Aspalathus linearis) on Adipocyte Differentiation. Phytomedicine Int. J. Phytother. Phytopharm. 2014, 21, 109–117. [Google Scholar] [CrossRef]
- Cholet, J.; Decombat, C.; Vareille-Delarbre, M.; Gainche, M.; Berry, A.; Senejoux, F.; Ripoche, I.; Delort, L.; Vermerie, M.; Fraisse, D.; et al. In Vitro Anti-Inflammatory and Immunomodulatory Activities of an Extract from the Roots of Bupleurum Rotundifolium. Medicines 2019, 6, 101. [Google Scholar] [CrossRef]
- Habanjar, O.; Maurin, A.C.; Vituret, C.; Vachias, C.; Longechamp, L.; Garnier, C.; Decombat, C.; Bourgne, C.; Diab-Assaf, M.; Caldefie-Chezet, F.; et al. A bicellular fluorescent ductal carcinoma in situ (DCIS)-like tumoroid to study the progression of carcinoma: Practical approaches and optimization. Biomater. Sci. 2023. [Google Scholar] [CrossRef]
- Klingelhutz, A.J.; Gourronc, F.A.; Chaly, A.; Wadkins, D.A.; Burand, A.J.; Markan, K.R.; Idiga, S.O.; Wu, M.; Potthoff, M.J.; Ankrum, J.A. Scaffold-Free Generation of Uniform Adipose Spheroids for Metabolism Research and Drug Discovery. Sci. Rep. 2018, 8, 523. [Google Scholar] [CrossRef]
- Miller, N.; De Beer, D.; Joubert, E. Minimising Variation in Aspalathin Content of Aqueous Green Rooibos Extract: Optimising Extraction and Identifying Critical Material Attributes. J. Sci. Food Agric. 2017, 97, 4937–4942. [Google Scholar] [CrossRef]
- Gabuza, K.B.; Buthelezi, N.; Kappo, A.P.; Mabuda, T.I.; Mosa, R.; Louw, J.; Muller, C.J.F. In Vitro and in Vivo Hepatotoxicity Study of AfriplexTM GRT through an Inflammatory Response. Toxicol. Rep. 2022, 9, 1920–1928. [Google Scholar] [CrossRef]
- Huang, M.; du Plessis, J.; du Preez, J.; Hamman, J.; Viljoen, A. Transport of Aspalathin, a Rooibos Tea Flavonoid, across the Skin and Intestinal Epithelium. Phytother. Res. PTR 2008, 22, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Breiter, T.; Laue, C.; Kressel, G.; Gröll, S.; Engelhardt, U.H.; Hahn, A. Bioavailability and Antioxidant Potential of Rooibos Flavonoids in Humans Following the Consumption of Different Rooibos Formulations. Food Chem. 2011, 128, 338–347. [Google Scholar] [CrossRef]
- Courts, F.L.; Williamson, G. The C-Glycosyl Flavonoid, Aspalathin, Is Absorbed, Methylated and Glucuronidated Intact in Humans. Mol. Nutr. Food Res. 2009, 53, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A.; Mullen, W.; Pecorari, M.; Serafini, M.; Crozier, A. Bioavailability of C-Linked Dihydrochalcone and Flavanone Glucosides in Humans Following Ingestion of Unfermented and Fermented Rooibos Teas. J. Agric. Food Chem. 2009, 57, 7104–7111. [Google Scholar] [CrossRef]
- Peng, Y.; Gan, R.; Li, H.; Yang, M.; McClements, D.J.; Gao, R.; Sun, Q. Absorption, Metabolism, and Bioactivity of Vitexin: Recent Advances in Understanding the Efficacy of an Important Nutraceutical. Crit. Rev. Food Sci. Nutr. 2021, 61, 1049–1064. [Google Scholar] [CrossRef]
- Hernandez-Quiles, M.; Broekema, M.F.; Kalkhoven, E. PPARgamma in Metabolism, Immunity, and Cancer: Unified and Diverse Mechanisms of Action. Front. Endocrinol. 2021, 12, 624112. [Google Scholar] [CrossRef]
- Recazens, E.; Mouisel, E.; Langin, D. Hormone-Sensitive Lipase: Sixty Years Later. Prog. Lipid Res. 2021, 82, 101084. [Google Scholar] [CrossRef] [PubMed]
- Hundahl, C.; Kotzbeck, P.; Burm, H.B.; Christiansen, S.H.; Torz, L.; Helge, A.W.; Madsen, M.P.; Ratner, C.; Serup, A.K.; Thompson, J.J.; et al. Hypothalamic Hormone-Sensitive Lipase Regulates Appetite and Energy Homeostasis. Mol. Metab. 2021, 47, 101174. [Google Scholar] [CrossRef]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef]
- López-Jaramillo, P.; Gómez-Arbeláez, D.; López-López, J.; López-López, C.; Martínez-Ortega, J.; Gómez-Rodríguez, A.; Triana-Cubillos, S. The Role of Leptin/Adiponectin Ratio in Metabolic Syndrome and Diabetes. Horm. Mol. Biol. Clin. Investig. 2014, 18, 37–45. [Google Scholar] [CrossRef]
- Nehme, R.; Diab-Assaf, M.; Decombat, C.; Delort, L.; Caldefie-Chezet, F. Targeting Adiponectin in Breast Cancer. Biomedicines 2022, 10, 2958. [Google Scholar] [CrossRef] [PubMed]
- Son, M.J.; Minakawa, M.; Miura, Y.; Yagasaki, K. Aspalathin Improves Hyperglycemia and Glucose Intolerance in Obese Diabetic Ob/Ob Mice. Eur. J. Nutr. 2013, 52, 1607–1619. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Judd, R.L. Adiponectin Regulation and Function. Compr. Physiol. 2018, 8, 1031–1063. [Google Scholar] [CrossRef] [PubMed]



| Gene | Species | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|---|
| β-actin | Human | CCTGGCACCCAGCACAAT | GCCGATCCACACGGAGTACT |
| IL-8 | Human | CTGGCCGTGGCTCTCTTG | CCTTGGCAAAACTGCACCTT |
| IL-1β | Human | CCTGTCCTGCGTGTTGAAAGA | GGGAACTGGGCAGACTCAAA |
| IL-6 | Human | GCTGCAGGCACAGAACCA | ACTCCTTAAAGCTGCGCAGAA |
| TNFα | Human | TCTTCTCGAACCCCGAGTGA | GGAGCTGCCCCTCAGCTT |
| CXCL10 | Human | GGAAATCGTGCGTGACATTA | AGGAAGGAAGGCTGGAAGAG |
| PPARγ | Human | GGATTCAGCTGGTCGATATCAC | GTTTCAGAAATGCCTTGCAGT |
| Ap2 | Human | ATCACATCCCCATTCACACT | ACTTGTCTCCAGTGAAAACTTTG |
| HSL | Human | GCCTGGGCTTCCAGTTCAC | CCTGTCTCGTTGCGTTTGTAGT |
| Leptin | Human | CGGAGAGTACAGTGAGCCA | CGGAATCTCGCTCTGTCAT |
| Adiponectin | Human | CCCAAAGAGGAGAGGAA | TCAGAAACAGGACACAAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nehme, R.; Chervet, A.; Decombat, C.; Longechamp, L.; Rossary, A.; Boutin, R.; Rousset, A.; Senejoux, F.; Vachias, C.; Auxenfans, C.; et al. Aspalathus linearis (Rooibos) Targets Adipocytes and Obesity-Associated Inflammation. Nutrients 2023, 15, 1751. https://doi.org/10.3390/nu15071751
Nehme R, Chervet A, Decombat C, Longechamp L, Rossary A, Boutin R, Rousset A, Senejoux F, Vachias C, Auxenfans C, et al. Aspalathus linearis (Rooibos) Targets Adipocytes and Obesity-Associated Inflammation. Nutrients. 2023; 15(7):1751. https://doi.org/10.3390/nu15071751
Chicago/Turabian StyleNehme, Rawan, Arthur Chervet, Caroline Decombat, Lucie Longechamp, Adrien Rossary, Rebecca Boutin, Amandine Rousset, François Senejoux, Caroline Vachias, Céline Auxenfans, and et al. 2023. "Aspalathus linearis (Rooibos) Targets Adipocytes and Obesity-Associated Inflammation" Nutrients 15, no. 7: 1751. https://doi.org/10.3390/nu15071751
APA StyleNehme, R., Chervet, A., Decombat, C., Longechamp, L., Rossary, A., Boutin, R., Rousset, A., Senejoux, F., Vachias, C., Auxenfans, C., Fraisse, D., Guyon, J.-B., Filaire, E., Berthon, J.-Y., Diab-Assaf, M., Delort, L., & Caldefie-Chezet, F. (2023). Aspalathus linearis (Rooibos) Targets Adipocytes and Obesity-Associated Inflammation. Nutrients, 15(7), 1751. https://doi.org/10.3390/nu15071751







