Cyclitols: From Basic Understanding to Their Association with Neurodegeneration
Abstract
1. Introduction
2. Sugar Alcohols
3. Inositol Characteristics
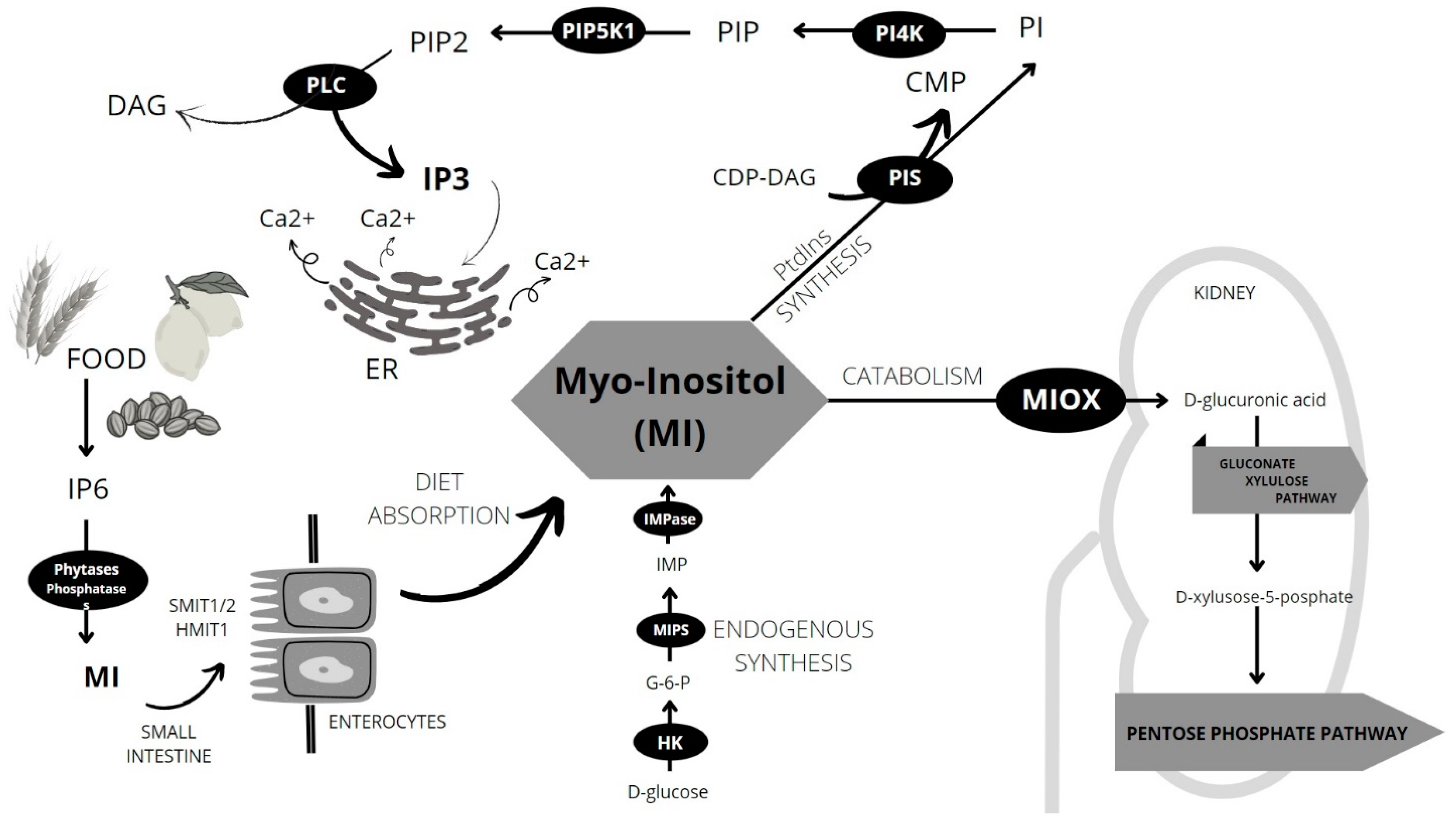
4. Sources and Metabolism of MI
5. Inositol Phosphates
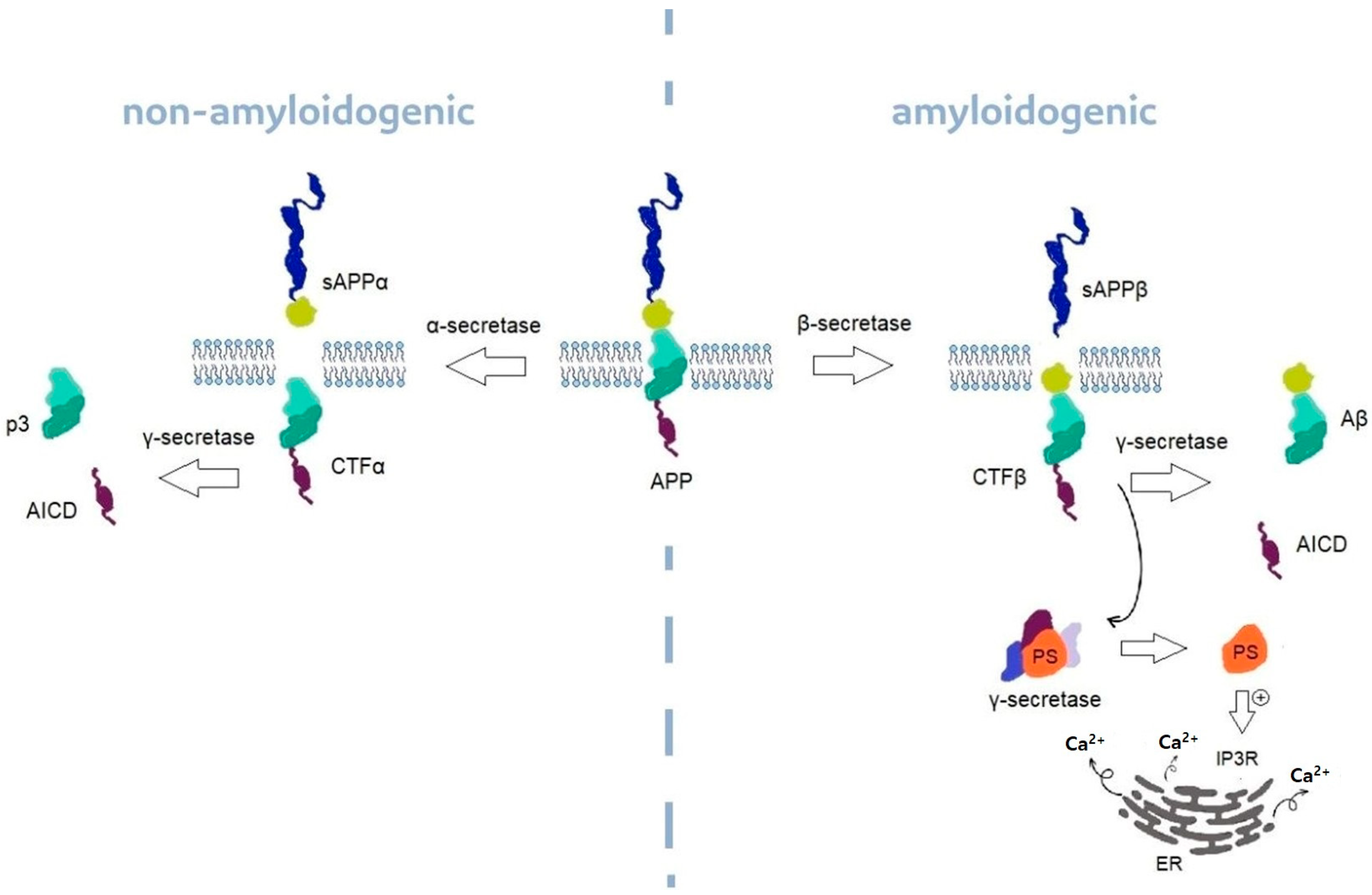
6. Inositol’s Unproper Metabolism Impact on Neurodegenerative Process
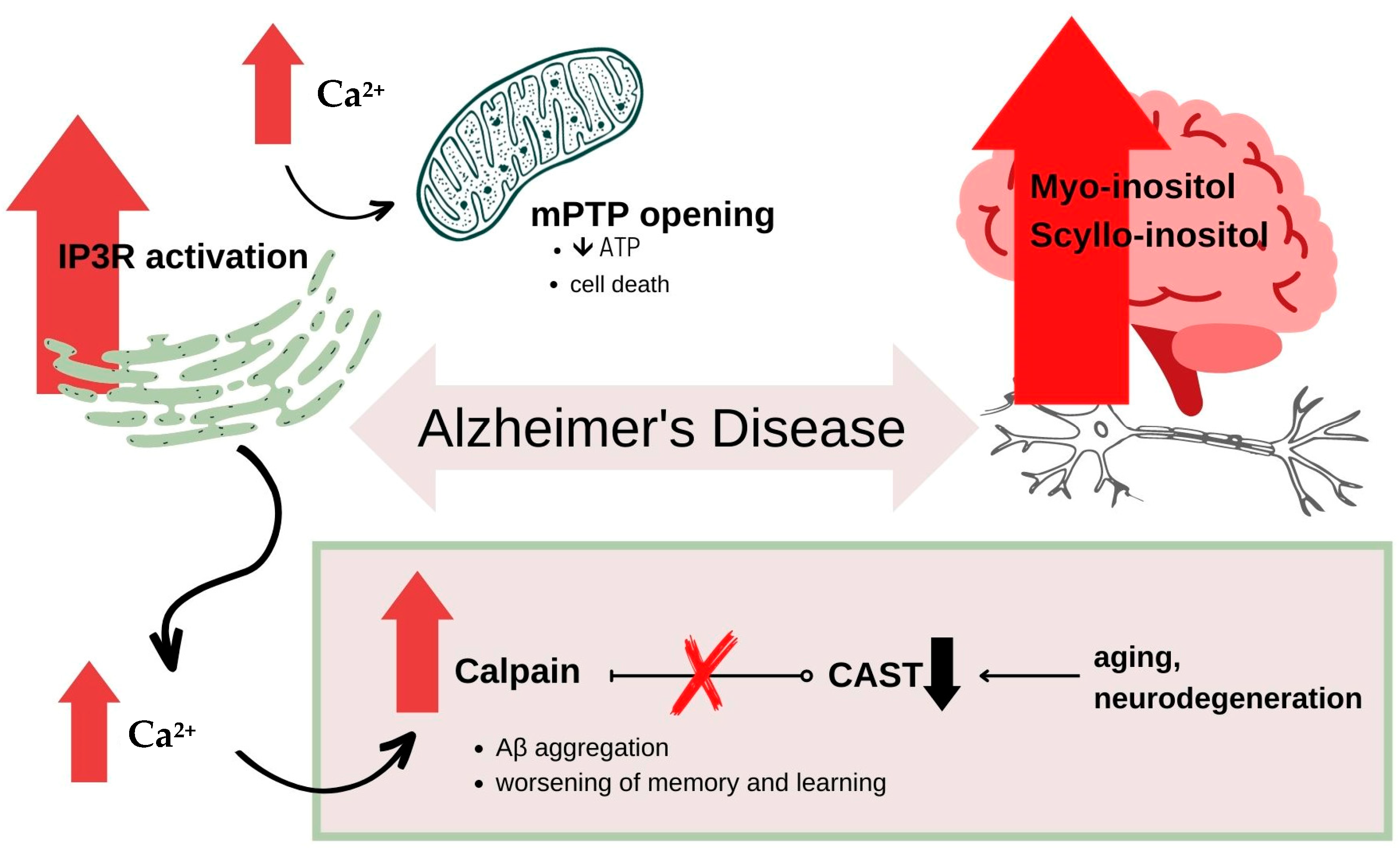
6.1. Alzheimer’s Disease
6.2. Parkinson’s Disease
6.3. Huntington’s Disease
6.4. Spinocerebellar Ataxias
6.5. Other Neurodegenerative Disorders
7. Possible Treatment Ideas
7.1. MI Itself
7.2. Lithium and Valproic Acid
7.3. γ-Secretase Inhibitors
7.4. Other Possibilities
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1H-MRS | Hydrogen 1 proton magnetic resonance spectroscopy |
| 5PP | Inositol 1,4,5-phosphatase enzyme |
| 6-OHDA | 6-hydroxydopamine |
| AD | Alzheimer’s disease |
| AICD | APP intracellular fragment |
| APP | Amyloid precursor protein |
| ATP | Adenosine triphosphate |
| Aβ | Amyloid β |
| CAST | Calpastatin |
| CDP-DAG | Cytidine diphosphate diacylglycerol |
| CFT | APP C-terminal fragment |
| CICR | Calcium induced calcium release |
| CMP | Cytidine Monophosphate |
| CSF | Cerebrospinal fluid |
| DAG | Diacylglycerol |
| ER | Endoplasmic reticulum |
| G-6-P | Glucose-6-Phosphate |
| GPI | Glycosyl-phosphatidyloinositol |
| GSIs | γ-secretase inhibitors |
| HAP1 | Htt-associated protein-1A |
| HD | Huntington’s disease |
| HK | Hexokinase |
| Htt | Huntingtin |
| IICR | Inositol induced Ca-release |
| IMPase | Inositol monophosphatase-1 |
| IP | Inositol phosphate |
| IP3 | Inositol 1,4,5-triphosphate |
| IP3R | Inositol 1,4,5-triphosphate receptor |
| IP6K2 | Hexakisphosphate kinase type 2 |
| IPG | Inositiol-phosphoglycan |
| LTP | Long term potentiation |
| MCI | Mild cognitive impairment |
| MDA | Malondialdehyde |
| mHtt | Mutant huntingtin |
| MI | Myo-inositol |
| MIOX | Myo-inositol Oxygenase |
| MIPS | MI-phosphate synthase |
| mPTP | Mitochondrial permeability transition pore |
| MRS | Magnetic resonance spectroscopy |
| MSNs | medium spiny striatal neurons |
| NADPH | Nicotinamide adenine dinucleotide |
| NFTs | Neurofibrillary tangles |
| PD | Parkinson’s disease |
| PI/PtdIns | Phosphatidylinositol |
| PI4K | Phosphatidylinositol 4-kinase |
| PIP | Phosphatidyloinositide |
| PIP2 | Phosphatidylinositol biphosphate |
| PIP5K1 | Phosphatidylinositol phosphate 5-kinase 1 |
| PIS | Phosphatidylinositol synthase |
| PLC | Phospholipase C |
| PPIP5 | inositol pyrophosphate diphosphoinositolpentakisphopshate |
| PS 1,2 | Presenilin 1,2 |
| PSP | Progressive supranuclear palsy |
| ROS | Reactive oxygen species |
| RyR | Ryanodine receptor |
| SCA | Spinocerebellar ataxia |
| SMA | Supplementary Motor Area |
| tCr | Total creatine, creatine + phosphocreatine |
| TG2 | Transglutaminase type 2 |
| tNAA | Total NAA, N-acetylaspartate + N-acetylaspartylglutamate |
| VPA | Valproic acid |
| YAC | Yeast artificial chromosome |
References
- Antonowski, T.; Osowski, A.; Lahuta, L.; Górecki, R.; Rynkiewicz, A.; Wojtkiewicz, J. Health-Promoting Properties of Selected Cyclitols for Metabolic Syndrome and Diabetes. Nutrients 2019, 11, 2314. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, M.; Fuso, A.; Dinicola, S.; Cucina, A.; Bevilacqua, A. Pharmacodynamics and Pharmacokinetics of Inositol(s) in Health and Disease. Expert Opin. Drug Metab. Toxicol. 2016, 12, 1181–1196. [Google Scholar] [CrossRef] [PubMed]
- Owczarczyk-Saczonek, A.; Lahuta, L.B.; Ligor, M.; Placek, W.; Górecki, R.J.; Buszewski, B. The Healing-Promoting Properties of Selected Cyclitols—A Review. Nutrients 2018, 10, 1891. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, K.; Jozwik, M.; Wojtkiewicz, J. Cancer Prevention by Natural Products Introduced into the Diet—Selected Cyclitols. Int. J. Mol. Sci. 2020, 21, 8988. [Google Scholar] [CrossRef]
- Murthy, P.P.N. Biology of Inositols and Phosphoinositides: Subcellular Biochemistry; Majumder, A.L., Biswas, B.B., Eds.; Subcellula; Springer: New York, NY, USA, 2006; ISBN 9780387276007. [Google Scholar]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 892, Inositol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Inositol (accessed on 18 April 2023).
- Szkodziak, P.; Paszkowski, T. Wpływ Leczenia Mio-Inozytolem Na Insulinooporność u Pacjentek z Zespołem Policystycznych Jajników w Obserwacji 3-Miesięcznej. Available online: https://inofem.pl/wp-content/uploads/2022/03/Inofem-Badanie-kliniczne.pdf (accessed on 5 November 2016).
- Croze, M.L.; Soulage, C.O. Potential Role and Therapeutic Interests of Myo-Inositol in Metabolic Diseases. Biochimie 2013, 95, 1811–1827. [Google Scholar] [CrossRef]
- da Costa, L.L.; Adorian, T.J.; Goulart, F.R.; Leitemperger, J.; do Amaral, A.M.B.; Loro, V.L.; Robalo, S.S.; da Silva, L.P. Phytic Acid in Rhamdia Quelen Nutrition: Antioxidant or Antinutrient? Anim. Feed Sci. Technol. 2021, 276, 114915. [Google Scholar] [CrossRef]
- Pramitha, J.L.; Rana, S.; Aggarwal, P.R.; Ravikesavan, R.; Joel, A.J.; Muthamilarasan, M. Diverse Role of Phytic Acid in Plants and Approaches to Develop Low-Phytate Grains to Enhance Bioavailability of Micronutrients. Adv. Genet. 2021, 107, 89–120. [Google Scholar]
- Schlemmer, U.; Frølich, W.; Prieto, R.M.; Grases, F. Phytate in Foods and Significance for Humans: Food Sources, Intake, Processing, Bioavailability, Protective Role and Analysis. Mol. Nutr. Food Res. 2009, 53, S330–S375. [Google Scholar] [CrossRef]
- Stentz, R.; Osborne, S.; Horn, N.; Li, A.W.H.; Hautefort, I.; Bongaerts, R.; Rouyer, M.; Bailey, P.; Shears, S.B.; Hemmings, A.M.; et al. A Bacterial Homolog of a Eukaryotic Inositol Phosphate Signaling Enzyme Mediates Cross-Kingdom Dialog in the Mammalian Gut. Cell Rep. 2014, 6, 646–656. [Google Scholar] [CrossRef]
- Duhan, A.; Khetarpaul, N.; Bishnoi, S. Content of Phytic Acid and HCl-Extractability of Calcium, Phosphorus and Iron as Affected by Various Domestic Processing and Cooking Methods. Food Chem. 2002, 78, 9–14. [Google Scholar] [CrossRef]
- Beemster, P.; Groenen, P.; Steegers-Theunissen, R. Involvement of Inositol in Reproduction. Nutr. Rev. 2002, 60, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Li, B.; Hertz, L.; Peng, L. Contributions in Astrocytes of SMIT1/2 and HMIT to Myo-Inositol Uptake at Different Concentrations and PH. Neurochem. Int. 2012, 61, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; Yu, H.; Kruse, M.; Traynor-Kaplan, A.; Hille, B. Osmoregulatory Inositol Transporter SMIT1 Modulates Electrical Activity by Adjusting PI(4,5)P2 Levels. Proc. Natl. Acad. Sci. USA 2016, 113, E3290–E3299. [Google Scholar] [CrossRef] [PubMed]
- Kiani, A.K.; Paolacci, S.; Calogero, A.E.; Cannarella, R.; Berardinis, E.D.E.; Giudice, F.D.E.L.; Stuppia, L.; Facchinetti, F. From Myo-Inositol to d-Chiro-Inositol Molecular Pathways. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2390–2402. [Google Scholar]
- Uldry, M.; Ibberson, M.; Horisberger, J.D.; Chatton, J.Y.; Riederer, B.M.; Thorens, B. Identification of a Mammalian H+-Myo-Inositol Symporter Expressed Predominantly in the Brain. EMBO J. 2001, 20, 4467–4477. [Google Scholar] [CrossRef]
- Coldebella, D.; Buzzaccarini, G.; Ferrari, J.; Sleiman, Z.; D’Alterio, M.N.; Della Corte, L.; Cucinella, G.; Gullo, G. Inositols Administration: Further Insights on Their Biological Role. Ital. J. Gynaecol. Obstet. 2023, 35, 30–36. [Google Scholar] [CrossRef]
- Loewus, M.W.; Loewus, F.A.; Brillinger, G.U.; Otsuka, H.; Floss, H.G. Stereochemistry of the Myo-Inositol-1-Phosphate Synthase Reaction. J. Biol. Chem. 1980, 255, 11710–11712. [Google Scholar] [CrossRef]
- Wojciechowska, A.; Osowski, A.; Jóźwik, M.; Górecki, R.; Rynkiewicz, A.; Wojtkiewicz, J. Inositols’ Importance in the Improvement of the Endocrine–Metabolic Profile in PCOS. Int. J. Mol. Sci. 2019, 20, 5787. [Google Scholar] [CrossRef]
- Chang, H.H.; Chao, H.N.; Walker, C.S.; Choong, S.Y.; Phillips, A.; Loomes, K.M. Renal Depletion of Myo-Inositol Is Associated with Its Increased Degradation in Animal Models of Metabolic Disease. Am. J. Physiol. Ren. Physiol. 2015, 309, F755–F763. [Google Scholar] [CrossRef]
- Kruger, N.J.; Von Schaewen, A. The Oxidative Pentose Phosphate Pathway: Structure and Organisation. Curr. Opin. Plant Biol. 2003, 6, 236–246. [Google Scholar] [CrossRef]
- Alfarouk, K.O.; Ahmed, S.B.M.; Elliott, R.L.; Benoit, A.; Alqahtani, S.S.; Ibrahim, M.E.; Bashir, A.H.H.; Alhoufie, S.T.S.; Elhassan, G.O.; Wales, C.C.; et al. The Pentose Phosphate Pathway Dynamics in Cancer and Its Dependency on Intracellular PH. Metabolites 2020, 10, 285. [Google Scholar] [CrossRef] [PubMed]
- Egorova, P.A.; Bezprozvanny, I.B. Inositol 1,4,5-Trisphosphate Receptors and Neurodegenerative Disorders. FEBS J. 2018, 285, 3547–3565. [Google Scholar] [CrossRef] [PubMed]
- Serysheva, I.I. Toward a High-Resolution Structure of IP3R Channel. Cell Calcium 2014, 56, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Briggs, C.A.; Chakroborty, S.; Stutzmann, G.E. Emerging Pathways Driving Early Synaptic Pathology in Alzheimer’s Disease. Biochem. Biophys. Res. Commun. 2017, 483, 988–997. [Google Scholar] [CrossRef]
- Choe, C.; Ehrlich, B.E. (IP 3 R) and Its Regulators: Sometimes Good and sometimes bad teamwork. Sci. STKE 2006, 363, re15. [Google Scholar]
- Takada, S.H.; Ikebara, J.M.; de Sousa, E.; Cardoso, D.S.; Resende, R.R.; Ulrich, H.; Rückl, M.; Rüdiger, S.; Kihara, A.H. Determining the Roles of Inositol Trisphosphate Receptors in Neurodegeneration: Interdisciplinary Perspectives on a Complex Topic. Mol. Neurobiol. 2017, 54, 6870–6884. [Google Scholar] [CrossRef]
- López-Gambero, A.J.; Sanjuan, C.; Serrano-Castro, P.J.; Suárez, J.; De Fonseca, F.R. The Biomedical Uses of Inositols: A Nutraceutical Approach to Metabolic Dysfunction in Aging and Neurodegenerative Diseases. Biomedicines 2020, 8, 295. [Google Scholar] [CrossRef]
- Haris, M.; Cai, K.; Singh, A.; Hariharan, H.; Reddy, R. In Vivo Mapping of Brain Myo-Inositol. Neuroimage 2011, 54, 2079–2085. [Google Scholar] [CrossRef]
- Thomas, M.P.; Mills, S.J.; Potter, B.V.L. The “Other” Inositols and Their Phosphates: Synthesis, Biology, and Medicine (with Recent Advances in Myo-Inositol Chemistry). Angew. Chem. Int. Ed. 2016, 55, 1614–1650. [Google Scholar] [CrossRef]
- Seaquist, E.R.; Gruetter, R. Identification of a High Concentration of Scyllo-Inositol in the Brain of a Healthy Human Subject Using 1H- and 13C-NMR. Magn. Reson. Med. 1998, 39, 313–316. [Google Scholar] [CrossRef]
- Spector, R.; Lorenzo, A.V. Folate Transport in the Central Nervous System. Am. J. Physiol. 1975, 229, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-K.; Carreras, I.; Dedeoglu, A.; Jenkins, B.G. Detection of Increased Scyllo-Inositol in Brain with Magnetic Resonance Spectroscopy after Dietary Supplementation in Alzheimer’s Disease Mouse Models. Neuropharmacology 2010, 59, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, G.; Morelli, M.; Quattrone, A.; Chiriaco, C.; Vaccaro, M.G.; Gullà, D.; Rocca, F.; Caracciolo, M.; Novellino, F.; Sarica, A.; et al. In Vivo Evidence for Decreased Scyllo-Inositol Levels in the Supplementary Motor Area of Patients with Progressive Supranuclear Palsy: A Proton MR Spectroscopy Study. Park. Relat. Disord. 2019, 62, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L.; Cole, G. Alzheimer Disease. Jama 2002, 287, 2335–2338. [Google Scholar] [CrossRef]
- Gaugler, J.; James, B.; Johnson, T.; Scholz, K.; Weuve, J. 2016 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 2016, 12, 459–509. [Google Scholar] [CrossRef]
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s Disease. Nat. Rev. Dis. Prim. 2015, 1, 15056. [Google Scholar] [CrossRef]
- Harman, D. Alzheimer’s Disease: Role of Aging in Pathogenesis. Ann. N. Y. Acad. Sci. 2002, 959, 384–395. [Google Scholar] [CrossRef]
- Cruts, M.; Hendriks, L.; Van Broeckhoven, C. The Presenilin Genes: A New Gene Family Involved in Alzheimer Disease Pathology. Hum. Mol. Genet. 1996, 5, 1449–1455. [Google Scholar] [CrossRef]
- Cruts, M.; Van Broeckhoven, C. Presenilin Mutations in Alzheimer’s Disease. Hum. Mutat. 1998, 11, 183–190. [Google Scholar] [CrossRef]
- Hutton, M.; Hardy, J. The Presenilins and Alzheimer’s Disease. Hum. Mol. Genet. 1997, 6, 1639–1646. [Google Scholar] [CrossRef]
- Gołaszewska, A.; Bik, W.; Motyl, T.; Orzechowski, A. Bridging the Gap between Alzheimer’s Disease and Alzheimer’s-like Diseases in Animals. Int. J. Mol. Sci. 2019, 20, 1664. [Google Scholar] [CrossRef] [PubMed]
- Multhaup, G.; Huber, O.; Buée, L.; Galas, M.C. Amyloid Precursor Protein (APP) Metabolites APP Intracellular Fragment (AICD), Aβ42, and Tau in Nuclear Roles. J. Biol. Chem. 2015, 290, 23515–23522. [Google Scholar] [CrossRef] [PubMed]
- Hansson, O.; Lehmann, S.; Otto, M.; Zetterberg, H.; Lewczuk, P. Advantages and Disadvantages of the Use of the CSF Amyloid β (Aβ) 42/40 Ratio in the Diagnosis of Alzheimer’s Disease. Alzheimers Res. Ther. 2019, 11, 34. [Google Scholar] [CrossRef]
- O’Neill, C.; Cowburn, R.F.; Bonkale, W.L.; Ohm, T.G.; Fastbom, J.; Carmody, M.; Kelliher, M. Dysfunctional Intracellular Calcium Homoeostasis: A Central Cause of Neurodegeneration in Alzheimer’s Disease. Biochem. Soc. Symp. 2001, 67, 177–194. [Google Scholar] [CrossRef]
- Huang, W.; Alexander, G.E.; Daly, E.M.; Shetty, H.U.; Krasuski, J.S.; Rapoport, S.I.; Schapiro, M.B. High Brain Myo-Inositol Levels in the Predementia Phase of Alzheimer’s Disease in Adults with Down’s Syndrome: A 1H MRS Study. Am. J. Psychiatry 1999, 156, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Q.; Wang, P.J.; Ten, G.J.; Zhan, W.; Li, M.H.; Zang, F.C. Role of Myo-Inositol by Magnetic Resonance Spectroscopy in Early Diagnosis of Alzheimer’s Disease in APP/PS1 Transgenic Mice. Dement. Geriatr. Cogn. Disord. 2009, 28, 558–566. [Google Scholar] [CrossRef]
- Griffith, H.R.; den Hollander, J.A.; Stewart, C.C.; Evanochko, W.T.; Buchthal, S.D.; Harrell, L.E.; Zamrini, E.Y.; Brockington, J.C.; Marson, D.C. Elevated Brain Scyllo-inositol Concentrations in Patients with Alzheimer’s Disease. NMR Biomed. Int. J. Devoted Dev. Appl. Magn. Reson. In Vivo 2007, 20, 709–716. [Google Scholar]
- Firbank, M.J.; Harrison, R.M.; O’Brien, J.T. A Comprehensive Review of Proton Magnetic Resonance Spectroscopy. Dement. Geriatr. Cogn. Disord. 2002, 14, 64–76. [Google Scholar] [CrossRef]
- Jaworska, A.; Dzbek, J.; Styczynska, M.; Kuznicki, J. Analysis of Calcium Homeostasis in Fresh Lymphocytes from Patients with Sporadic Alzheimer’s Disease or Mild Cognitive Impairment. Biochim. Biophys. Acta Mol. Cell Res. 2013, 1833, 1692–1699. [Google Scholar] [CrossRef]
- Ali, F.; Manzoor, U.; Bhattacharya, R.; Bansal, A.K.; Chandrashekharaiah, K.S.; Singh, L.R.; Saraswati, S.M.; Uversky, V.; Dar, T.A. Brain Metabolite, Myo-Inositol, Inhibits Catalase Activity: A Mechanism of the Distortion of the Antioxidant Defense System in Alzheimer’s Disease. ACS Omega 2022, 7, 12690–12700. [Google Scholar] [CrossRef]
- Doraiswamy, P.M.; Charles, H.C.; Krishnan, K.R.R. Prediction of Cognitive Decline in Early Alzheimer’s Disease. Lancet 1998, 352, 1678. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, W. Ca2+ Homeostasis Dysregulation in Alzheimer’s Disease: A Focus on Plasma Membrane and Cell Organelles. FASEB J. 2019, 33, 6697–6712. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.M.; Toral-Barza, L.; Thaler, H.; Tofel-Grehl, B.; Gibson, G.E. Inositol Phosphates and Intracellular Calcium after Bradykinin Stimulation in Fibroblasts from Young, Normal Aged and Alzheimer Donors. Neurobiol. Aging 1991, 12, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. The Inositol Trisphosphate/Calcium Signaling Pathway in Health and Disease. Physiol. Rev. 2016, 96, 1261–1296. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.D.; Enomoto, M.; Ishiyama, N.; Stathopulos, P.B.; Ikura, M. Structural Insights into Endoplasmic Reticulum Stored Calcium Regulation by Inositol 1,4,5-Trisphosphate and Ryanodine Receptors. Biochim. Biophys. Acta Mol. Cell Res. 2014, 1853, 1980–1991. [Google Scholar] [CrossRef]
- Hinterberger, M.; Fischer, P. Folate and Alzheimer: When Time Matters. J. Neural Transm. 2013, 120, 211–224. [Google Scholar] [CrossRef]
- Blanchard, B.J.; Thomas, V.L.; Ingram, V.M. Mechanism of Membrane Depolarization Caused by the Alzheimer Aβ1-42 Peptide. Biochem. Biophys. Res. Commun. 2002, 293, 1197–1203. [Google Scholar] [CrossRef]
- LÜckhoff, A.; Clapham, D.E. E. Inositol 1, 3, 4, 5-Tetrakisphosphate Activates an Endothelial Ca2+-Permeable Channel. Nature 1992, 355, 356–358. [Google Scholar] [CrossRef]
- Tsubokawa, H.; Oguro, K.; Robinson, H.P.C.; Masuzawa, T.; Rhee, T.S.G.; Takenawa, T.; Kawai, N. Inositol 1,3,4,5-Tetrakisphosphate as a Mediator of Neuronal Death in Ischemic Hippocampus. Neuroscience 1994, 59, 291–297. [Google Scholar] [CrossRef]
- Haug, L.S.; Østvold, A.C.; Cowburn, R.F.; Winblad, B.; Bogdanovich, N.; Walaas, S.I. Decreased inositol (1, 4, 5)-trisphosphate receptor levels in Alzheimer’s disease cerebral cortex: Selectivity of changes and possible correlation to pathological severity. Neurodegeneration 1996, 5, 169–176. [Google Scholar] [CrossRef]
- Young, L.T.; Kish, S.J.; Li, P.P.; Warsh, J.J. Decreased Brain [3H]Inositol 1,4,5-Trisphosphate Binding in Alzheimer’s Disease. Neurosci. Lett. 1988, 94, 198–202. [Google Scholar] [CrossRef]
- Mak, D.O.D.; Cheung, K.H.; Toglia, P.; Foskett, J.K.; Ullah, G. Analyzing and Quantifying the Gain-of-Function Enhancement of IP3 Receptor Gating by Familial Alzheimer’s Disease-Causing Mutants in Presenilins. PLoS Comput. Biol. 2015, 11, e1004529. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.H.; Mei, L.; Mak, D.O.D.; Hayashi, I.; Iwatsubo, T.; Kang, D.E.; Foskett, J.K. Gain-of-Function Enhancement of IP3 Receptor Modal Gating by Familial Alzheimer’s Disease-Linked Presenilin Mutants in Human Cells and Mouse Neurons. Sci. Signal. 2010, 3, ra22. [Google Scholar] [CrossRef] [PubMed]
- Toglia, P.; Ullah, G. The Gain-of-Function Enhancement of IP3-Receptor Channel Gating by Familial Alzheimer’s Disease-Linked Presenilin Mutants Increases the Open Probability of Mitochondrial Permeability Transition Pore. Cell Calcium 2016, 60, 13–24. [Google Scholar] [CrossRef]
- Elder, G.A.; Sosa, M.A.G.; De Gasperi, R.; Dickstein, D.L.; Hof, P.R. Presenilin Transgenic Mice as Models of Alzheimer’s Disease. Brain Struct. Funct. 2010, 214, 127–143. [Google Scholar] [CrossRef]
- Halestrap, A.P. What Is the Mitochondrial Permeability Transition Pore? J. Mol. Cell. Cardiol. 2009, 46, 821–831. [Google Scholar] [CrossRef]
- Baumgartner, H.K.; Gerasimenko, J.V.; Thorne, C.; Ferdek, P.; Pozzan, T.; Tepikin, A.V.; Petersen, O.H.; Sutton, R.; Watson, A.J.M.; Gerasimenko, O.V. Calcium Elevation in Mitochondria Is the Main Ca2+ Requirement for Mitochondrial Permeability Transition Pore (MPTP) Opening. J. Biol. Chem. 2009, 284, 20796–20803. [Google Scholar] [CrossRef] [PubMed]
- Shilling, D.; Müller, M.; Takano, H.; Mak, D.O.D.; Abel, T.; Coulter, D.A.; Foskett, J.K. Suppression of InsP3 Receptor-Mediated Ca2+ Signaling Alleviates Mutant Presenilin-Linked Familial Alzheimer’s Disease Pathogenesis. J. Neurosci. 2014, 34, 6910–6923. [Google Scholar] [CrossRef] [PubMed]
- Nixon, R.A.; Saito, K.I.; Grynspan, F.; Griffin, W.R.; Katayama, S.; Honda, T.; Mohan, P.S.; Shea, T.B.; Beermann, M. Calcium-Activated Neutral Proteinase (Calpain) System in Aging and Alzheimer’s Disease. Ann. N. Y. Acad. Sci. 1994, 747, 77–91. [Google Scholar] [CrossRef]
- Sorimachi, H.; Hata, S.; Ono, Y. Calpain chronicle—An enzyme family under multidisciplinary characterization. 2011, 87, 287-327. Proc. Jpn. Acad. Ser. B 2011, 87, 287–327. [Google Scholar] [CrossRef]
- Higuchi, M.; Tomioka, M.; Takano, J.; Shirotani, K.; Iwata, N.; Masumoto, H.; Maki, M.; Itohara, S.; Saido, T.C. Distinct Mechanistic Roles of Calpain and Caspase Activation in Neurodegeneration as Revealed in Mice Overexpressing Their Specific Inhibitors. J. Biol. Chem. 2005, 280, 15229–15237. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.V.; Mohan, P.S.; Peterhoff, C.M.; Yang, D.S.; Schmidt, S.D.; Stavrides, P.H.; Campbell, J.; Chen, Y.; Jiang, Y.; Paskevich, P.A.; et al. Marked Calpastatin (CAST) Depletion in Alzheimer’s Disease Accelerates Cytoskeleton Disruption and Neurodegeneration: Neuroprotection by CAST Overexpression. J. Neurosci. 2008, 28, 12241–12254. [Google Scholar] [CrossRef] [PubMed]
- Mahaman, Y.A.R.; Huang, F.; Afewerky, H.K.; Maibouge, T.M.S.; Ghose, B.; Wang, X. Involvement of Calpain in the Neuropathogenesis of Alzheimer’s Disease. Med. Res. Rev. 2019, 39, 608–630. [Google Scholar] [CrossRef] [PubMed]
- Frazier, H.N.; Maimaiti, S.; Anderson, K.L.; Brewer, L.D.; Gant, J.C.; Porter, N.M.; Thibault, O. Calcium’s Role as Nuanced Modulator of Cellular Physiology in the Brain. Biochem. Biophys. Res. Commun. 2017, 483, 981–987. [Google Scholar] [CrossRef]
- Sepasi Tehrani, H.; Moosavi-Movahedi, A.A. Catalase and Its Mysteries. Prog. Biophys. Mol. Biol. 2018, 140, 5–12. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress-and Age-Associated Degenerative Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef]
- Jiang, W.D.; Feng, L.; Liu, Y.; Jiang, J.; Zhou, X.Q. Myo-Inositol Prevents Oxidative Damage, Inhibits Oxygen Radical Generation and Increases Antioxidant Enzyme Activities of Juvenile Jian Carp (Cyprinus Carpio Var. Jian). Aquac. Res. 2009, 40, 1770–1776. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson Disease. Nat. Rev. Dis. Prim. 2017, 3, 17013. [Google Scholar] [CrossRef]
- Ibrahim, T.; McLaurin, J.A. α-Synuclein Aggregation, Seeding and Inhibition by Scyllo-Inositol. Biochem. Biophys. Res. Commun. 2016, 469, 529–534. [Google Scholar] [CrossRef]
- Zhang, Z.; Hou, L.; Li, X.; Ju, C.; Zhang, J.; Li, X.; Wang, X.; Liu, C.; Lv, Y.; Wang, Y. Neuroprotection of Inositol Hexaphosphate and Changes of Mitochondrion Mediated Apoptotic Pathway and α-Synuclein Aggregation in 6-OHDA Induced Parkinson’s Disease Cell Model. Brain Res. 2016, 1633, 87–95. [Google Scholar] [CrossRef]
- Kitamura, N.; Hashimoto, T.; Nishino, N.; Tanaka, C. Inositol 1,4,5-Trisphosphate Binding Sites in the Brain: Regional Distribution, Characterization, and Alterations in Brains of Patients with Parkinson’s Disease. J. Mol. Neurosci. 1989, 1, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Novotny, E.; Ashwal, S.; Shevell, M. Proton Magnetic Resonance Spectroscopy: An Emerging Technology in Pediatric Neurology Research. Pediatr. Res. 1998, 44, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mazuel, L.; Chassain, C.; Jean, B.; Pereira, B.; Cladière, A.; Speziale, C.; Durif, F. Proton MR Spectroscopy for Diagnosis and Evaluation of Treatment Efficacy in Parkinson Disease. Radiology 2016, 278, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Ciurleo, R.; Di Lorenzo, G.; Bramanti, P.; Marino, S. Magnetic Resonance Spectroscopy: An in Vivo Molecular Imaging Biomarker for Parkinson’s Disease? Biomed Res. Int. 2014, 2014, 519816. [Google Scholar] [CrossRef]
- Gröger, A.; Chadzynski, G.; Godau, J.; Berg, D.; Klose, U. Three-Dimensional Magnetic Resonance Spectroscopic Imaging in the Substantia Nigra of Healthy Controls and Patients with Parkinson’s Disease. Eur. Radiol. 2011, 21, 1962–1969. [Google Scholar] [CrossRef]
- Morris, H.R. Progressive Supranuclear Palsy. Blue Books Neurol. 2010, 34, 361–374. [Google Scholar] [CrossRef]
- Mishori, A.; Levine, J.; Kahana, E.; Belmaker, R.H. Inositol Is Not Therapeutic in Parkinson’s Disease. Hum. Psychopharmacol. 1999, 14, 271–272. [Google Scholar] [CrossRef]
- Walker, F.O. Huntington’s Disease. Lancet 2007, 369, 218–228. [Google Scholar] [CrossRef]
- Finkbeiner, S. Huntington ’ s Disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a007476. [Google Scholar] [CrossRef]
- Tang, T.S.; Tu, H.; Orban, P.C.; Chan, E.Y.W.; Hayden, M.R.; Bezprozvanny, I. HAP1 Facilitates Effects of Mutant Huntingtin on Inositol 1,4,5-Trisphosphate-Induced Ca2+ Release in Primary Culture of Striatal Medium Spiny Neurons. Eur. J. Neurosci. 2004, 20, 1779–1787. [Google Scholar] [CrossRef]
- Hamada, K.; Terauchi, A.; Nakamura, K.; Higo, T.; Nukina, N.; Matsumoto, N.; Hisatsune, C.; Nakamura, T.; Mikoshiba, K. Aberrant Calcium Signaling by Transglutaminase-Mediated Posttranslational Modification of Inositol 1,4,5-Trisphosphate Receptors. Proc. Natl. Acad. Sci. USA 2014, 111, E3966–E3975. [Google Scholar] [CrossRef] [PubMed]
- Post, J.I.; Leergaard, T.B.; Ratz, V.; Walaas, S.I.; Von Hörsten, S.; Nissen-Meyer, L.S.H. Differential Levels and Phosphorylation of Type 1 Inositol 1,4,5-Trisphosphate Receptor in Four Different Murine Models of Huntington Disease. J. Huntingtons Dis. 2019, 8, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Nagata, E.; Nonaka, T.; Moriya, Y.; Fujii, N.; Okada, Y.; Tsukamoto, H.; Itoh, J.; Okada, C.; Satoh, T.; Arai, T.; et al. Inositol Hexakisphosphate Kinase 2 Promotes Cell Death in Cells with Cytoplasmic TDP-43 Aggregation. Mol. Neurobiol. 2016, 53, 5377–5383. [Google Scholar] [CrossRef] [PubMed]
- Nagata, E.; Saiardi, A.; Tsukamoto, H.; Okada, Y.; Itoh, Y.; Satoh, T.; Itoh, J.; Margolis, R.L.; Takizawa, S.; Sawa, A.; et al. Inositol Hexakisphosphate Kinases Induce Cell Death in Huntington Disease. J. Biol. Chem. 2011, 286, 26680–26686. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.M.; Lu, C.; Wu, Z.Y. Spinocerebellar Ataxia: Relationship between Phenotype and Genotype—A Review. Clin. Genet. 2016, 90, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Kasumu, A.W.; Liang, X.; Egorova, P.; Vorontsova, D.; Bezprozvanny, I. Chronic Suppression of Inositol 1,4,5-Triphosphate Receptor-Mediated Calcium Signaling in Cerebellar Purkinje Cells Alleviates Pathological Phenotype in Spinocerebellar Ataxia 2 Mice. J. Neurosci. 2012, 32, 12786–12796. [Google Scholar] [CrossRef]
- Tipton, P.W.; Guthrie, K.; Strongosky, A.; Reimer, R.; Wszolek, Z.K. Spinocerebellar Ataxia 15: A Phenotypic Review and Expansion. Neurol. Neurochir. Pol. 2017, 51, 86–91. [Google Scholar] [CrossRef]
- Klockgether, T.; Mariotti, C.; Paulson, H.L. Spinocerebellar Ataxia. Nat. Rev. Dis. Prim. 2019, 5, 24. [Google Scholar] [CrossRef]
- Brown, S.A.; Loew, L.M. Integration of Modeling with Experimental and Clinical Findings Synthesizes and Refines the Central Role of Inositol 1,4,5-Trisphosphate Receptor 1 in Spinocerebellar Ataxia. Front. Neurosci. 2015, 8, 453. [Google Scholar] [CrossRef]
- Novak, M.J.U.; Sweeney, M.G.; Li, A.; Treacy, C.; Chandrashekar, H.S.; Giunti, P.; Goold, R.G.; Davis, M.B.; Houlden, H.; Tabrizi, S.J. An ITPR1 Gene Deletion Causes Spinocerebellar Ataxia 15/16: A Genetic, Clinical and Radiological Description. Mov. Disord. 2010, 25, 2176–2182. [Google Scholar] [CrossRef]
- Sasaki, M.; Ohba, C.; Iai, M.; Hirabayashi, S.; Osaka, H.; Hiraide, T.; Saitsu, H.; Matsumoto, N. Sporadic Infantile-Onset Spinocerebellar Ataxia Caused by Missense Mutations of the Inositol 1,4,5-Triphosphate Receptor Type 1 Gene. J. Neurol. 2015, 262, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, M.; Ishikawa, K.; Izumi, Y.; Takahashi, M.; Niimi, Y.; Sato, N.; Onodera, O.; Kaji, R.; Nishizawa, M.; Mizusawa, H. Prevalence of Inositol 1, 4, 5-Triphosphate Receptor Type 1 Gene Deletion, the Mutation for Spinocerebellar Ataxia Type 15, in Japan Screened by Gene Dosage. J. Hum. Genet. 2012, 57, 202–206. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, T.; Barth, P.; Reneman, L.; Appelhof, B.; Baas, F.; Poll-The, B.T. A de Novo Missense Mutation in the Inositol 1,4,5-Triphosphate Receptor Type 1 Gene Causing Severe Pontine and Cerebellar Hypoplasia: Expanding the Phenotype of ITPR1-Related Spinocerebellar Ataxia’s. Am. J. Med. Genet. Part A 2017, 173, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Weerasekera, A.; Peeters, R.; Sima, D.; Dresselaers, T.; Sunaert, S.; De Vocht, J.; Claeys, K.; Van Huffel, S.; Van Damme, P.; Himmelreich, U. Motor Cortex Metabolite Alterations in Amyotrophic Lateral Sclerosis Assessed in Vivo Using Edited and Non-Edited Magnetic Resonance Spectroscopy. Brain Res. 2019, 1718, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Nagata, E.; Fujii, N.; Kohara, S.; Okada, C.; Satoh, T.; Takekoshi, S.; Takao, M.; Mihara, B.; Takizawa, S. Inositol Hexakisphosphate Kinase 2 Promotes Cell Death of Anterior Horn Cells in the Spinal Cord of Patients with Amyotrophic Lateral Sclerosis. Mol. Biol. Rep. 2020, 47, 6479–6485. [Google Scholar] [CrossRef] [PubMed]
- Cheong, I.; Deelchand, D.K.; Eberly, L.E.; Marjańska, M.; Manousakis, G.; Guliani, G.; Walk, D.; Öz, G. Neurochemical Correlates of Functional Decline in Amyotrophic Lateral Sclerosis. J. Neurol. Neurosurg. Psychiatry 2019, 90, 294–301. [Google Scholar] [CrossRef]
- Srivastava, O.; Hanstock, C.; Chenji, S.; Mah, D.; Eurich, D.; Ta, D.; Seres, P.; Luk, C.; Zinman, L.; Abrahao, A.; et al. Cerebral Degeneration in Amyotrophic Lateral Sclerosis: A Prospective Multicenter Magnetic Resonance Spectroscopy Study. Neurol. Clin. Pract. 2019, 9, 400–407. [Google Scholar] [CrossRef]
- Tiscione, S.A.; Casas, M.; Horvath, J.D.; Lam, V.; Hino, K.; Ory, D.S.; Fernando Santana, L.; Simó, S.; Dixon, R.E.; Dickson, E.J. IP3R-Driven Increases in Mitochondrial Ca2+ Promote Neuronal Death in NPC Disease. Proc. Natl. Acad. Sci. USA 2021, 118, e2110629118. [Google Scholar] [CrossRef]
- Chhetri, D.R. Myo-Inositol and Its Derivatives: Their Emerging Role in the Treatment of Human Diseases. Front. Pharmacol. 2019, 10, 1172. [Google Scholar] [CrossRef]
- Pascente, R.; Frigerio, F.; Rizzi, M.; Porcu, L.; Boido, M.; Davids, J.; Zaben, M.; Tolomeo, D.; Filibian, M.; Gray, W.P.; et al. Cognitive Deficits and Brain Myo-Inositol Are Early Biomarkers of Epileptogenesis in a Rat Model of Epilepsy. Neurobiol. Dis. 2016, 93, 146–155. [Google Scholar] [CrossRef]
- Dubey, A.; Srivastava, K.; Tiwari, M.; Dubey, A. d-Pinitol-A Natural Phytomolecule and Its Pharmacological Effect. Int. J. Pharm. Life Sci. 2020, 11, 6609–6623. [Google Scholar]
- Sánchez-Hidalgo, M.; León-González, A.J.; Gálvez-Peralta, M.; González-Mauraza, N.H.; Martin-Cordero, C. d-Pinitol: A Cyclitol with Versatile Biological and Pharmacological Activities. Phytochem. Rev. 2021, 20, 211–224. [Google Scholar] [CrossRef]
- Barak, Y.; Levine, J.; Glasman, A.; Elizur, A.; Belmaker, R.H. Inositol Treatment of Alzheimer’s Disease: A Double Blind, Cross-over Placebo Controlled Trial. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1996, 20, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Medina-Vera, D.; Navarro, J.A.; Rivera, P.; Rosell-Valle, C.; Gutiérrez-Adán, A.; Sanjuan, C.; López-Gambero, A.J.; Tovar, R.; Suárez, J.; Pavón, F.J.; et al. d-Pinitol Promotes Tau Dephosphorylation through a Cyclin-Dependent Kinase 5 Regulation Mechanism: A New Potential Approach for Tauopathies? Br. J. Pharmacol. 2022, 179, 4655–4672. [Google Scholar] [CrossRef]
- McLaurin, J.A.; Golomb, R.; Jurewicz, A.; Antel, J.P.; Fraser, P.E. Inositol Stereoisomers Stabilize an Oligomeric Aggregate of Alzheimer Amyloid β Peptide and Inhibit Aβ-Induced Toxicity. J. Biol. Chem. 2000, 275, 18495–18502. [Google Scholar] [CrossRef]
- Fenili, D.; Brown, M.; Rappaport, R.; McLaurin, J.A. Properties of Scyllo-Inositol as a Therapeutic Treatment of AD-like Pathology. J. Mol. Med. 2007, 85, 603–611. [Google Scholar] [CrossRef]
- Rafii, M.S.; Skotko, B.G.; McDonough, M.E.; Pulsifer, M.; Evans, C.; Doran, E.; Muranevici, G.; Kesslak, P.; Abushakra, S.; Lott, I.T. A Randomized, Double-Blind, Placebo-Controlled, Phase II Study of Oral ELND005 (Scyllo-Inositol) in Young Adults with Down Syndrome without Dementia. J. Alzheimers Dis. 2017, 58, 401–411. [Google Scholar] [CrossRef]
- Salloway, S.; Sperling, R.; Keren, R.; Porsteinsson, A.P.; Van Dyck, C.H.; Tariot, P.N.; Gilman, S.; Arnold, D.; Abushakra, S.; Hernandez, C.; et al. A Phase 2 Randomized Trial of ELND005, Scyllo-Inositol, in Mild to Moderate Alzheimer Disease. Neurology 2011, 77, 1253–1262. [Google Scholar] [CrossRef]
- Ramp, P.; Lehnert, A.; Matamouros, S.; Wirtz, A.; Baumgart, M.; Bott, M. Metabolic Engineering of Corynebacterium Glutamicum for Production of Scyllo-Inositol, a Drug Candidate against Alzheimer’s Disease. Metab. Eng. 2021, 67, 173–185. [Google Scholar] [CrossRef]
- Michon, C.; Kang, C.M.; Karpenko, S.; Tanaka, K.; Ishikawa, S.; Yoshida, K. ichi A Bacterial Cell Factory Converting Glucose into Scyllo-Inositol, a Therapeutic Agent for Alzheimer’s Disease. Commun. Biol. 2020, 3, 93. [Google Scholar] [CrossRef]
- Sarkar, S.; Rubinsztein, D.C. Inositol and IP3 Levels Regulate Autophagy: Biology and Therapeutic Speculations. Autophagy 2006, 2, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Pietruczuk, K.; Witkowski, J.M. Lithium Salts-Mechanisms of Action|Mechanizmy Działania Soli Litu. Psychiatria 2008, 5, 51–57. [Google Scholar]
- Lazzara, C.A.; Kim, Y.H. Potential Application of Lithium in Parkinson’s and Other Neurodegenerative Diseases. Front. Neurosci. 2015, 9, 403. [Google Scholar] [CrossRef]
- Morlet, É.; Hozer, F.; Costemale-Lacoste, J.F. Neuroprotective Effects of Lithium: What Are the Implications in Humans with Neurodegenerative Disorders? Geriatr. Psychol. Neuropsychiatr. Vieil. 2018, 16, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Schmukler, E.; Pinkas-Kramarski, R. Autophagy Induction in the Treatment of Alzheimer’s Disease. Drug Dev. Res. 2020, 81, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Wang, Z.; Zhang, J.; Feng, F. γ-Secretase Inhibitors for Breast Cancer and Hepatocellular Carcinoma: From Mechanism to Treatment. Life Sci. 2021, 268, 119007. [Google Scholar] [CrossRef]
- Golde, T.E.; Koo, E.H.; Felsenstein, K.M.; Osborne, B.A.; Miele, L. γ-Secretase Inhibitors and Modulators. Biochim. Biophys. Acta-Biomembr. 2013, 1828, 2898–2907. [Google Scholar] [CrossRef]
- Fouka, P.; Alexopoulos, H.; Chatzi, I.; Dedos, S.G.; Samiotaki, M.; Panayotou, G.; Politis, P.; Tzioufas, A.; Dalakas, M.C. Antibodies to Inositol 1,4,5-Triphosphate Receptor 1 in Patients with Cerebellar Disease. Neurol. Neuroimmunol. NeuroInflamm. 2017, 4, e306. [Google Scholar] [CrossRef]
- Saleem, H.; Tovey, S.C.; Molinski, T.F.; Taylor, C.W. Interactions of Antagonists with Subtypes of Inositol 1, 4, 5-trisphosphate (IP 3) Receptor. Br. J. Pharmacol. 2014, 171, 3298–3312. [Google Scholar] [CrossRef]
- Gambardella, J.; Morelli, M.B.; Wang, X.; Castellanos, V.; Mone, P.; Santulli, G. The Discovery and Development of IP3 Receptor Modulators: An Update. Expert Opin. Drug Discov. 2021, 16, 709–718. [Google Scholar] [CrossRef]
- López-Sánchez, J.I.; Moreno, D.A.; García-Viguera, C. d-Pinitol, a Highly Valuable Product from Carob Pods: Health-Promoting Effects and Metabolic Pathways of This Natural Super-Food Ingredient and Its Derivatives. AIMS Agric. Food 2018, 3, 41–63. [Google Scholar] [CrossRef]
- Azab, A. d-Pinitol—Active Natural Product from Carob with Notable Insulin Regulation. Nutrients 2022, 14, 1453. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Thomason, L.A.M.; McLaurin, J.A. Scyllo-Inositol, Preclinical, and Clinical Data for Alzheimer’s Disease; Elsevier Inc.: Amsterdam, The Netherlands, 2012; Volume 64, ISBN 9780123948168. [Google Scholar]
- Liang, C.; Savinov, S.N.; Fejzo, J.; Eyles, S.J.; Chen, J. Modulation of Amyloid-Β42 Conformation by Small Molecules through Nonspecific Binding. J. Chem. Theory Comput. 2019, 15, 5169–5174. [Google Scholar] [CrossRef] [PubMed]
- McLaurin, J.; Franklin, T.; Chakrabartty, A.; Fraser, P.E. Phosphatidylinositol and Inositol Involvement in Alzheimer Amyloid-β Fibril Growth and Arrest. J. Mol. Biol. 1998, 278, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, K.; Byrjalsen, I.; Christiansen, C.; Karsdal, M.A. Relationship between Serum Levels of Tau Fragments and Clinical Progression of Alzheimer’s Disease. J. Alzheimers Dis. 2014, 43, 1331–1341. [Google Scholar] [CrossRef]
- Doody, R.S.; Raman, R.; Sperling, R.A.; Seimers, E.; Sethuraman, G.; Mohs, R.; Farlow, M.; Iwatsubo, T.; Vellas, B.; Sun, X.; et al. Peripheral and Central Effects of γ-Secretase Inhibition by Semagacestat in Alzheimer’s Disease. Alzheimers Res. Ther. 2015, 7, 36. [Google Scholar] [CrossRef]
| Cyclitol 1,2,3,4-cyclohexenetetrol | 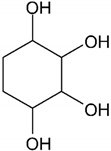 |
| Myo-inositol | 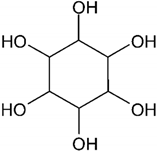 |
| Pinitol | 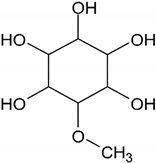 |
| Disorder | Linkage to MI and It’s Derivatives | References |
|---|---|---|
| Amyotrophic Lateral Sclerosis (ALS) |
| [107] |
| [108] | |
| [109,110] | |
| Niemann-Pick Disease type C (NPC) |
| [111] |
| Epilepsy |
| [112] |
| [113] |
| Substance (Drug Name) | Function | Results | Diseases | References |
|---|---|---|---|---|
| d-pinitol | Insulin-sensitizer; Inhibits γ-secretase; Lowers Tau phosphorylation | ↓ Aβ production | AD, tauopathies | [114,117,134,135] |
| Scyllo-inositol (ELND005, AZD-103) | Stabilize Aβ42, neutralize cell derived Aβ trimers, promote low molecular weight Aβ in vivo; Inhibits the aggregation of α-synuclein in Parkinson’s disease | Decreased neuronal toxicity, increased long-term potentiation (LTP) and ablation of cognitive deficits in multiple mouse models of AD | AD, PD | [30,120,122,123,136,137] |
| Epi-inositol | Stabilize Aβ42 | Decreased aggregation of Aβ; Lowers anxiety | AD | [30,138] |
| Lithium; IMP-ase inhibitor (L-690,330) | Inhibits IMP-ase, | ↓ MI ↓ IP3 ↑ clearance of autophagy substrates | HD, PD, SCAs | [112,124] |
| Valproic acid | Inhibits MIPS in human brain, lowers MI level, inhibits y-secretase | inhibits Aβ production | AD | [112] |
| γ-secretase inhibitors (Semagacestat (LY450139), MK 0752, E2012, GSI 136) | Inhibits γ-secretase and lowers β-amyloid in blood and spinal fluid in humans | Semagacestat did not slow disease progression, worsened cognitive functions in patients, study drug was stopped in all studies. | AD | [139,140] |
| Antibodies | Antibodies against IP3 receptor 1 | This antibody may have a direct involvement in neurodegenerative process or can be a marker or cerebellar injury. | Cerebellar ataxia, epilepsy | [131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derkaczew, M.; Martyniuk, P.; Osowski, A.; Wojtkiewicz, J. Cyclitols: From Basic Understanding to Their Association with Neurodegeneration. Nutrients 2023, 15, 2029. https://doi.org/10.3390/nu15092029
Derkaczew M, Martyniuk P, Osowski A, Wojtkiewicz J. Cyclitols: From Basic Understanding to Their Association with Neurodegeneration. Nutrients. 2023; 15(9):2029. https://doi.org/10.3390/nu15092029
Chicago/Turabian StyleDerkaczew, Maria, Piotr Martyniuk, Adam Osowski, and Joanna Wojtkiewicz. 2023. "Cyclitols: From Basic Understanding to Their Association with Neurodegeneration" Nutrients 15, no. 9: 2029. https://doi.org/10.3390/nu15092029
APA StyleDerkaczew, M., Martyniuk, P., Osowski, A., & Wojtkiewicz, J. (2023). Cyclitols: From Basic Understanding to Their Association with Neurodegeneration. Nutrients, 15(9), 2029. https://doi.org/10.3390/nu15092029





