Vitamin D in Diabetes: Uncovering the Sunshine Hormone’s Role in Glucose Metabolism and Beyond
Abstract
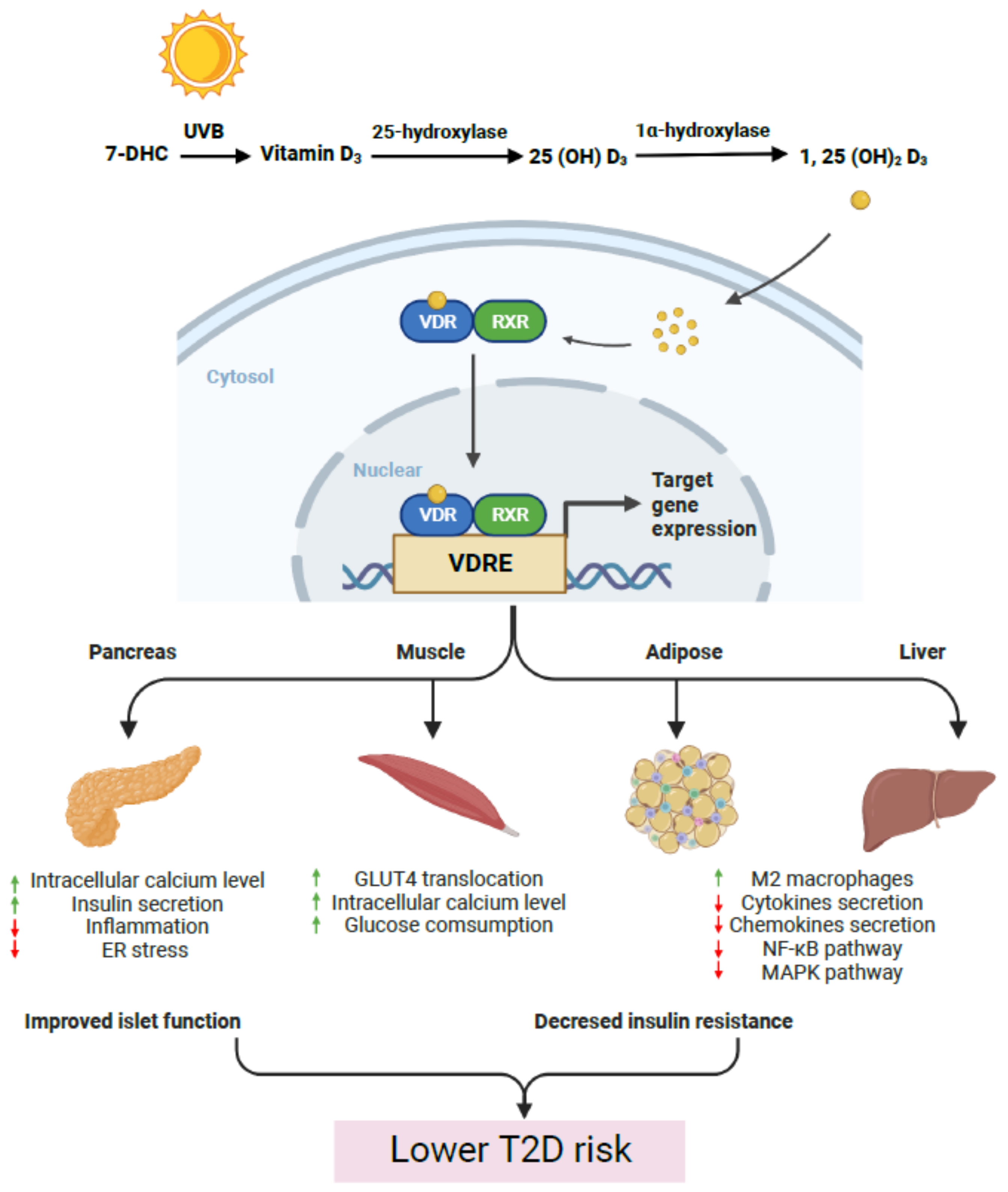
1. Introduction
2. Vitamin D and Islet Dysfunction in T2D Progression
3. Vitamin D and Insulin Sensitivity and Resistance
4. Vitamin D Deficiency and Type 2 Diabetes—Results of Observational and Intervention Studies and Meta-Analyses
5. Vitamin D in T1D Progression
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohammadi, S.; Hajhashemy, Z.; Saneei, P. Serum vitamin D levels in relation to type-2 diabetes and prediabetes in adults: A systematic review and dose-response meta-analysis of epidemiologic studies. Crit. Rev. Food Sci. Nutr. 2022, 62, 8178–8198. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Schnoes, H.K.; DeLuca, H.F.; Suda, T.; Cousins, R.J. Isolation and identification of 1,25-dihydroxycholecalciferol. A metabolite of vitamin D active in intestine. Biochemistry 1971, 10, 2799–2804. [Google Scholar] [CrossRef] [PubMed]
- Talwar, D.; O’Reilly, D.S.J.; McMillan, D.C. Serum 25-hydroxyvitamin D is a reliable indicator of vitamin D status Reply. Am. J. Clin. Nutr. 2011, 94, 620. [Google Scholar] [CrossRef][Green Version]
- Henry, H.L.; Norman, A.W. Vitamin D: Metabolism and biological actions. Annu. Rev. Nutr. 1984, 4, 493–520. [Google Scholar] [CrossRef] [PubMed]
- Umesono, K.; Murakami, K.K.; Thompson, C.C.; Evans, R.M. Direct Repeats as Selective Response Elements for the Thyroid-Hormone, Retinoic Acid, and Vitamin-D3 Receptors. Cell 1991, 65, 1255–1266. [Google Scholar] [CrossRef]
- Hanel, A.; Malmberg, H.-R.; Carlberg, C. Genome-wide effects of chromatin on vitamin D signaling. J. Mol. Endocrinol. 2020, 64, R45–R56. [Google Scholar] [CrossRef]
- Hii, C.S.; Ferrante, A. The Non-Genomic Actions of Vitamin D. Nutrients 2016, 8, 135. [Google Scholar] [CrossRef]
- Cranney, A.; Horsley, T.; O’Donnell, S.; Weiler, H.; Puil, L.; Ooi, D.; Atkinson, S.; Ward, L.; Moher, D.; Hanley, D.; et al. Effectiveness and safety of vitamin D in relation to bone health. Evid. Rep. Technol. Assess. (Full Rep.) 2007, 158, 1–235. [Google Scholar]
- Seshadri, K.; Tamilselvan, B.; Venkatraman, G. Role of vitamin D on the expression of glucose transporters in L6 myotubes. Indian J. Endocrinol. Metab. 2013, 17 (Suppl. S1), S326–S328. [Google Scholar] [CrossRef]
- Manna, P.; Achari, A.E.; Jain, S.K. Vitamin D supplementation inhibits oxidative stress and upregulate SIRT1/AMPK/GLUT4 cascade in high glucose-treated 3T3L1 adipocytes and in adipose tissue of high fat diet-fed diabetic mice. Arch. Biochem. Biophys. 2017, 615, 22–34. [Google Scholar] [CrossRef]
- Benetti, E.; Mastrocola, R.; Chiazza, F.; Nigro, D.; D’antona, G.; Bordano, V.; Fantozzi, R.; Aragno, M.; Collino, M.; Minetto, M.A. Effects of vitamin D on insulin resistance and myosteatosis in diet-induced obese mice. PLoS ONE 2018, 13, e0189707. [Google Scholar] [CrossRef]
- Elseweidy, M.M.; Amin, R.S.; Atteia, H.H.; Ali, M.A. Vitamin D3 intake as regulator of insulin degrading enzyme and insulin receptor phosphorylation in diabetic rats. Biomed. Pharmacother. 2017, 85, 155–159. [Google Scholar] [CrossRef]
- Al-Shoumer, K.A.; Al-Essa, T.M. Is there a relationship between vitamin D with insulin resistance and diabetes mellitus? World J. Diabetes 2015, 6, 1057–1064. [Google Scholar] [CrossRef]
- James, W.P. 22nd Marabou Symposium: The changing faces of vitamin D. Nutr. Rev. 2008, 66, 286–290. [Google Scholar] [CrossRef]
- Ashcroft, F.M.; Rorsman, P. Diabetes mellitus and the beta cell: The last ten years. Cell 2012, 148, 1160–1171. [Google Scholar] [CrossRef]
- Prentki, M.; Peyot, M.L.; Masiello, P.; Madiraju, S.R.M. Nutrient-Induced Metabolic Stress, Adaptation, Detoxification, and Toxicity in the Pancreatic beta-Cell. Diabetes 2020, 69, 279–290. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Inomata, S.; Kadowaki, S.; Yamatani, T.; Fukase, M.; Fujita, T. Effect of 1 alpha (OH)-vitamin D3 on insulin secretion in diabetes mellitus. Bone Miner. 1986, 1, 187–192. [Google Scholar]
- Maestro, B.; Dávila, N.; Carranza, M.; Calle, C. Identification of a Vitamin D response element in the human insulin receptor gene promoter. J. Steroid Biochem. Mol. Biol. 2003, 84, 223–230. [Google Scholar] [CrossRef]
- Norman, A.W.; Frankel, B.J.; Heldt, A.M.; Grodsky, G.M. Vitamin D Deficiency Inhibits Pancreatic Secretion of Insulin. Science 1980, 209, 823–825. [Google Scholar] [CrossRef]
- Cade, C.; Norman, A.W. Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology 1986, 119, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Seino, Y.; Ishida, M.; Yamaoka, K.; Yabuuchi, H.; Ishida, H.; Seino, S.; Seino, Y.; Imura, H. Effect of vitamin D3 on the pancreatic secretion of insulin and somatostatin. Acta. Endocrinol. 1984, 105, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Zeitz, U.; Weber, K.; Soegiarto, D.W.; Wolf, E.; Balling, R.; Erben, R.G. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB J. 2003, 17, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Bourlon, P.M.; Billaudel, B.; Faure-Dussert, A. Influence of vitamin D3 deficiency and 1,25 dihydroxyvitamin D3 on de novo insulin biosynthesis in the islets of the rat endocrine pancreas. J. Endocrinol. 1999, 160, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Al-Sofiani, M.E.; Jammah, A.; Racz, M.; Khawaja, R.A.; Hasanato, R.; El-Fawal, H.A.N.; Mousa, S.A.; Mason, D.L. Effect of Vitamin D Supplementation on Glucose Control and Inflammatory Response in Type II Diabetes: A Double Blind, Randomized Clinical Trial. Int. J. Endocrinol. Metab. 2015, 13, e22604. [Google Scholar] [CrossRef]
- Lemieux, P.; Weisnagel, S.J.; Caron, A.Z.; Julien, A.-S.; Morisset, A.-S.; Carreau, A.-M.; Poirier, J.; Tchernof, A.; Robitaille, J.; Bergeron, J.; et al. Effects of 6-month vitamin D supplementation on insulin sensitivity and secretion: A randomised, placebo-controlled trial. Eur. J. Endocrinol. 2019, 181, 287–299. [Google Scholar] [CrossRef]
- Kayaniyil, S.; Vieth, R.; Retnakaran, R.; Knight, J.A.; Qi, Y.; Gerstein, H.C.; Perkins, B.A.; Harris, S.B.; Zinman, B.; Hanley, A.J. Association of vitamin D with insulin resistance and beta-cell dysfunction in subjects at risk for type 2 diabetes. Diabetes Care 2010, 33, 1379–1381. [Google Scholar] [CrossRef]
- Borissova, A.M.; Tankova, T.; Kirilov, G.; Dakovska, L.; Kovacheva, R. The effect of vitamin D3 on insulin secretion and peripheral insulin sensitivity in type 2 diabetic patients. Int. J. Clin. Pr. 2003, 57, 258–261. [Google Scholar]
- Nyomba, B.L.G.; Auwerx, J.; Bormans, V.; Peeters, T.L.; Pelemans, W.; Reynaert, J.; Bouillon, R.; Vantrappen, G.; De Moor, P. Pancreatic secretion in man with subclinical vitamin D deficiency. Diabetologia 1986, 29, 34–38. [Google Scholar] [CrossRef]
- Wolden-Kirk, H.; Overbergh, L.; Gysemans, C.; Brusgaard, K.; Naamane, N.; Van Lommel, L.; Schuit, F.; Eizirik, D.L.; Christesen, H.; Mathieu, C. Unraveling the effects of 1,25OH2D3 on global gene expression in pancreatic islets. J. Steroid Biochem. Mol. Biol. 2013, 136, 68–79. [Google Scholar] [CrossRef]
- Sergeev, I.N.; Rhoten, W.B. 1,25-Dihydroxyvitamin D3 evokes oscillations of intracellular calcium in a pancreatic beta-cell line. Endocrinology 1995, 136, 2852–2861. [Google Scholar] [CrossRef]
- Doyle, M.E.; Egan, J.M. Pharmacological Agents That Directly Modulate Insulin Secretion. Pharmacol. Rev. 2003, 55, 105–131. [Google Scholar] [CrossRef]
- Gilon, P.; Chae, H.Y.; Rutter, G.A.; Ravier, M.A. Calcium signaling in pancreatic beta-cells in health and in Type 2 diabetes. Cell Calcium 2014, 56, 340–361. [Google Scholar] [CrossRef]
- Altieri, B.; Grant, W.B.; Della Casa, S.; Orio, F.; Pontecorvi, A.; Colao, A.; Sarno, G.; Muscogiuri, G. Vitamin D and pancreas: The role of sunshine vitamin in the pathogenesis of diabetes mellitus and pancreatic cancer. Crit. Rev. Food Sci. Nutr. 2017, 57, 3472–3488. [Google Scholar] [CrossRef]
- Kjalarsdottir, L.; Tersey, S.A.; Vishwanath, M.; Chuang, J.-C.; Posner, B.A.; Mirmira, R.G.; Repa, J.J. 1,25-Dihydroxyvitamin D(3) enhances glucose-stimulated insulin secretion in mouse and human islets: A role for transcriptional regulation of voltage-gated calcium channels by the vitamin D receptor. J. Steroid Biochem. Mol. Biol. 2019, 185, 17–26. [Google Scholar] [CrossRef]
- De Boland, A.R.; Boland, R.L. Non-genomic signal transduction pathway of vitamin D in muscle. Cell. Signal. 1994, 6, 717–724. [Google Scholar] [CrossRef]
- Johnson, J.A.; Grande, J.P.; Roche, P.C.; Kumar, R. Immunohistochemical localization of the 1,25(OH)2D3 receptor and calbindin D28k in human and rat pancreas. Am. J. Physiol. 1994, 267 Pt 1, E356–E360. [Google Scholar] [CrossRef]
- Morrissey, R.L.; Bucci, T.J.; Richard, B.; Empson, R.N.; Lufkin, E.G. Calcium-Binding Protein: Its Cellular Localization in Jejunum, Kidney and Pancreas. Exp. Biol. Med. 1975, 149, 56–60. [Google Scholar] [CrossRef]
- Wei, Z.; Yoshihara, E.; He, N.; Hah, N.; Fan, W.; Pinto, A.F.M.; Huddy, T.; Wang, Y.; Ross, B.; Estepa, G.; et al. Vitamin D Switches BAF Complexes to Protect beta Cells. Cell 2018, 173, 1135–1149.e15. [Google Scholar] [CrossRef]
- Chen, C.; Luo, Y.; Su, Y.; Teng, L. The vitamin D receptor (VDR) protects pancreatic beta cells against Forkhead box class O1 (FOXO1)-induced mitochondrial dysfunction and cell apoptosis. Biomed. Pharmacother. 2019, 117, 109170. [Google Scholar] [CrossRef]
- Morró, M.; Vilà, L.; Franckhauser, S.; Mallol, C.; Elias, G.; Ferré, T.; Molas, M.; Casana, E.; Rodó, J.; Pujol, A.; et al. Vitamin D Receptor Overexpression in beta-Cells Ameliorates Diabetes in Mice. Diabetes 2020, 69, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Riek, A.E.; Oh, J.; Sprague, J.E.; Timpson, A.; Fuentes, L.D.L.; Bernal-Mizrachi, L.; Schechtman, K.B.; Bernal-Mizrachi, C. Vitamin D Suppression of Endoplasmic Reticulum Stress Promotes an Antiatherogenic Monocyte/Macrophage Phenotype in Type 2 Diabetic Patients. J. Biol. Chem. 2012, 287, 38482–38494. [Google Scholar] [CrossRef]
- Mathieu, C. Vitamin D and diabetes: Where do we stand? Diabetes Res. Clin. Pr. 2015, 108, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Pittas, A.G.; Lau, J.; Hu, F.B.; Dawson-Hughes, B. The Role of Vitamin D and Calcium in Type 2 Diabetes. A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2007, 92, 2017–2029. [Google Scholar] [CrossRef] [PubMed]
- De Boer, I.H.; Tinker, L.F.; Connelly, S.; Curb, J.D.; Howard, B.V.; Kestenbaum, B.; Larson, J.C.; Manson, J.E.; Margolis, K.L.; Siscovick, D.S.; et al. Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women’s Health Initiative. Diabetes Care 2008, 31, 701–707. [Google Scholar] [CrossRef]
- Avenell, A.; Cook, J.A.; MacLennan, G.S.; McPherson, G.C. Vitamin D supplementation and type 2 diabetes: A substudy of a randomised placebo-controlled trial in older people (RECORD trial, ISRCTN 51647438). Age Ageing 2009, 38, 606–609. [Google Scholar] [CrossRef]
- Ying, W.; Lee, Y.S.; Dong, Y.; Seidman, J.S.; Yang, M.; Isaac, R.; Seo, J.B.; Yang, B.H.; Wollam, J.; Riopel, M.; et al. Expansion of Islet-Resident Macrophages Leads to Inflammation Affecting beta Cell Proliferation and Function in Obesity. Cell Metab. 2019, 29, 457–474.e5. [Google Scholar] [CrossRef]
- Viloria, K.; Nasteska, D.; Ast, J.; Hasib, A.; Cuozzo, F.; Heising, S.; Briant, L.J.; Hewison, M.; Hodson, D.J. GC-Globulin/Vitamin D–Binding Protein Is Required for Pancreatic α-Cell Adaptation to Metabolic Stress. Diabetes 2022, 72, 275–289. [Google Scholar] [CrossRef]
- Kuo, T.; Damle, M.; González, B.J.; Egli, D.; Lazar, M.A.; Accili, D. Induction of alpha cell-restricted Gc in dedifferentiating beta cells contributes to stress-induced beta-cell dysfunction. JCI Insight 2019, 5, e128351. [Google Scholar] [CrossRef]
- Maestro, B.; Campion, J.; Dávila, N.; Calle, C. Stimulation by 1,25-Dihydroxyvitamin D3 of Insulin Receptor Expression and Insulin Responsiveness for Glucose Transport in U-937 Human Promonocytic Cells. Endocr. J. 2000, 47, 383–391. [Google Scholar] [CrossRef]
- Maestro, B.; Molero, S.; Bajo, S.; Dávila, N.; Calle, C. Transcriptional activation of the human insulin receptor gene by 1,25-dihydroxyvitamin D(3). Cell Biochem. Funct. 2002, 20, 227–232. [Google Scholar] [CrossRef]
- Dunlop, T.W.; Väisänen, S.; Frank, C.; Molnár, F.; Sinkkonen, L.; Carlberg, C. The human peroxisome proliferator-activated receptor delta gene is a primary target of 1alpha,25-dihydroxyvitamin D3 and its nuclear receptor. J. Mol. Biol. 2005, 349, 248–260. [Google Scholar] [CrossRef]
- Liu, Y.; He, Y.; Wang, Q.; Guo, F.; Huang, F.; Ji, L.; An, T.; Qin, G. Vitamin D(3) supplementation improves testicular function in diabetic rats through peroxisome proliferator-activated receptor-gamma/transforming growth factor-beta 1/nuclear factor-kappa B. J. Diabetes Investig. 2019, 10, 261–271. [Google Scholar] [CrossRef]
- Hoseini, R.; Damirchi, A.; Babaei, P. Vitamin D increases PPARgamma expression and promotes beneficial effects of physical activity in metabolic syndrome. Nutrition 2017, 36, 54–59. [Google Scholar] [CrossRef]
- Zhou, Q.G.; Hou, F.F.; Guo, Z.J.; Liang, M.; Wang, G.B.; Zhang, X. 1,25-Dihydroxyvitamin D improved the free fatty-acid-induced insulin resistance in cultured C2C12 cells. Diabetes/Metab. Res. Rev. 2008, 24, 459–464. [Google Scholar] [CrossRef]
- Wright, D.C.; Hucker, K.A.; Holloszy, J.O.; Han, D.H. Ca2+ and AMPK Both Mediate Stimulation of Glucose Transport by Muscle Contractions. Diabetes 2004, 53, 330–335. [Google Scholar] [CrossRef]
- George, N.; Kumar, T.P.; Antony, S.; Jayanarayanan, S.; Paulose, C.S. Effect of vitamin D3 in reducing metabolic and oxidative stress in the liver of streptozotocin-induced diabetic rats. Br. J. Nutr. 2012, 108, 1410–1418. [Google Scholar] [CrossRef]
- Alkharfy, K.M.; Al-Daghri, N.M.; Yakout, S.M.; Hussain, T.; Mohammed, A.K.; Krishnaswamy, S. Influence of Vitamin D Treatment on Transcriptional Regulation of Insulin-Sensitive Genes. Metab. Syndr. Relat. Disord. 2013, 11, 283–288. [Google Scholar] [CrossRef]
- Dong, B.; Zhou, Y.; Wang, W.; Scott, J.; Kim, K.H.; Sun, Z.; Guo, Q.; Lu, Y.; Gonzales, N.M.; Wu, H.; et al. Vitamin D Receptor Activation in Liver Macrophages Ameliorates Hepatic Inflammation, Steatosis, and Insulin Resistance in Mice. Hepatology 2020, 71, 1559–1574. [Google Scholar] [CrossRef]
- Oh, J.; Riek, A.E.; Darwech, I.; Funai, K.; Shao, J.; Chin, K.; Sierra, O.L.; Carmeliet, G.; Ostlund, R.E.; Bernal-Mizrachi, C. Deletion of Macrophage Vitamin D Receptor Promotes Insulin Resistance and Monocyte Cholesterol Transport to Accelerate Atherosclerosis in Mice. Cell Rep. 2015, 10, 1872–1886. [Google Scholar] [CrossRef]
- Marcotorchino, J.; Gouranton, E.; Romier, B.; Tourniaire, F.; Astier, J.; Malezet, C.; Amiot, M.-J.; Landrier, J.-F. Vitamin D reduces the inflammatory response and restores glucose uptake in adipocytes. Mol. Nutr. Food Res. 2012, 56, 1771–1782. [Google Scholar] [CrossRef] [PubMed]
- Lira, F.S.; Rosa, J.C.; Cunha, C.A.; Ribeiro, E.B.; do Nascimento, C.O.; Oyama, L.M.; Mota, J.F. Supplementing alpha-tocopherol (vitamin E) and vitamin D3 in high fat diet decrease IL-6 production in murine epididymal adipose tissue and 3T3-L1 adipocytes following LPS stimulation. Lipids Health Dis. 2011, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Marziou, A.; Philouze, C.; Couturier, C.; Astier, J.; Obert, P.; Landrier, J.-F.; Riva, C. Vitamin D Supplementation Improves Adipose Tissue Inflammation and Reduces Hepatic Steatosis in Obese C57BL/6J Mice. Nutrients 2020, 12, 342. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef]
- Sadeghi, K.; Wessner, B.; Laggner, U.; Ploder, M.; Tamandl, D.; Friedl, J.; Zügel, U.; Steinmeyer, A.; Pollak, A.; Roth, E.; et al. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur. J. Immunol. 2006, 36, 361–370. [Google Scholar] [CrossRef]
- Chen, Y.; Kong, J.; Sun, T.; Li, G.; Szeto, F.L.; Liu, W.; Deb, D.K.; Wang, Y.; Zhao, Q.; Thadhani, R.; et al. 1,25-Dihydroxyvitamin D3 suppresses inflammation-induced expression of plasminogen activator inhibitor-1 by blocking nuclear factor-kappaB activation. Arch. Biochem. Biophys. 2011, 507, 241–247. [Google Scholar] [CrossRef]
- Zhang, Y.; Leung, D.Y.M.; Richers, B.N.; Liu, Y.; Remigio, L.K.; Riches, D.W.; Goleva, E. Vitamin D Inhibits Monocyte/Macrophage Proinflammatory Cytokine Production by Targeting MAPK Phosphatase-1. J. Immunol. 2012, 188, 2127–2135. [Google Scholar] [CrossRef]
- Korhonen, R.; Moilanen, E. Mitogen-Activated Protein Kinase Phosphatase 1 as an Inflammatory Factor and Drug Target. Basic Clin. Pharmacol. Toxicol. 2014, 114, 24–36. [Google Scholar] [CrossRef]
- Giarratana, N.; Penna, G.; Amuchastegui, S.; Mariani, R.; Daniel, K.C.; Adorini, L. A Vitamin D Analog Down-Regulates Proinflammatory Chemokine Production by Pancreatic Islets Inhibiting T Cell Recruitment and Type 1 Diabetes Development. J. Immunol. 2004, 173, 2280–2287. [Google Scholar] [CrossRef]
- Olefsky, J.M.; Glass, C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef]
- Narvaez, C.J.; Simmons, K.M.; Brunton, J.; Salinero, A.; Chittur, S.V.; Welsh, J.E. Induction of STEAP4 correlates with 1,25-dihydroxyvitamin D3 stimulation of adipogenesis in mesenchymal progenitor cells derived from human adipose tissue. J. Cell Physiol 2013, 228, 2024–2036. [Google Scholar] [CrossRef]
- Mahajan, A.; Stahl, C.H. Dihydroxy-cholecalciferol stimulates adipocytic differentiation of porcine mesenchymal stem cells. J. Nutr. Biochem. 2009, 20, 512–520. [Google Scholar] [CrossRef]
- Nimitphong, H.; Holick, M.F.; Fried, S.K.; Lee, M.J. 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 promote the differentiation of human subcutaneous preadipocytes. PLoS ONE 2012, 7, e52171. [Google Scholar] [CrossRef]
- Heaney, R.P.; Horst, R.L.; Cullen, D.M.; Armas, L.A. Vitamin D3 distribution and status in the body. J. Am. Coll. Nutr. 2009, 28, 252–256. [Google Scholar] [CrossRef]
- Cianferotti, L.; Demay, M.B. VDR-mediated inhibition of DKK1 and SFRP2 suppresses adipogenic differentiation of murine bone marrow stromal cells. J. Cell. Biochem. 2007, 101, 80–88. [Google Scholar] [CrossRef]
- Kelly, K.A.; Gimble, J.M. 1,25-Dihydroxy Vitamin D3 Inhibits Adipocyte Differentiation and Gene Expression in Murine Bone Marrow Stromal Cell Clones and Primary Cultures. Endocrinology 1998, 139, 2622–2628. [Google Scholar] [CrossRef]
- Sato, M.; Hiragun, A. Demonstration of 1 alpha,25-dihydroxyvitamin D3 receptor-like molecule in ST 13 and 3T3 L1 preadipocytes and its inhibitory effects on preadipocyte differentiation. J. Cell. Physiol. 1988, 135, 545–550. [Google Scholar] [CrossRef]
- Thomson, B.; Ahrens, J.M.; Ntambi, J.M.; DeLuca, H.F.; Clagett-Dame, M. 2-Methylene-19-nor-1alpha-hydroxyvitamin D3 analogs inhibit adipocyte differentiation and PPARgamma2 gene transcription. Arch. Biochem. Biophys. 2007, 460, 192–201. [Google Scholar] [CrossRef]
- Choi, H.; Myung, K. Vitamin D(3) regulation of body fat, cytokines, and calpain gene expression. J. Sci. Food Agric. 2012, 92, 632–637. [Google Scholar] [CrossRef]
- Duque, G.; Macoritto, M.; Kremer, R. 1,25(OH)2D3 inhibits bone marrow adipogenesis in senescence accelerated mice (SAM-P/6) by decreasing the expression of peroxisome proliferator-activated receptor gamma 2 (PPARgamma2). Exp. Gerontol. 2004, 39, 333–338. [Google Scholar] [CrossRef]
- Narvaez, C.J.; Matthews, D.; Broun, E.; Chan, M.; Welsh, J. Lean Phenotype and Resistance to Diet-Induced Obesity in Vitamin D Receptor Knockout Mice Correlates with Induction of Uncoupling Protein-1 in White Adipose Tissue. Endocrinology 2009, 150, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.; Erben, R.G. Differences in triglyceride and cholesterol metabolism and resistance to obesity in male and female vitamin D receptor knockout mice. J. Anim. Physiol. Anim. Nutr. 2013, 97, 675–683. [Google Scholar] [CrossRef]
- Wong, K.E.; Szeto, F.L.; Zhang, W.; Ye, H.; Kong, J.; Zhang, Z.; Sun, X.J.; Li, Y.C. Involvement of the vitamin D receptor in energy metabolism: Regulation of uncoupling proteins. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E820–E828. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.G.; D’angelo, J.; Drelich, J.; Welsh, J. Adipose-specific Vdr deletion alters body fat and enhances mammary epithelial density. J. Steroid Biochem. Mol. Biol. 2016, 164, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Smogorzewski, M.; Massry, S.G. Effects of parathyroid hormone on cytosolic calcium of rat adipocytes. Endocrinology 1994, 135, 1837–1844. [Google Scholar] [CrossRef]
- Thomas, D.M.; Rogers, S.D.; Sleeman, M.W.; Pasquini, G.M.; Bringhurst, F.R.; Ng, K.W.; Zajac, J.D.; Best, J.D. Modulation of glucose transport by parathyroid hormone and insulin in UMR 106-01, a clonal rat osteogenic sarcoma cell line. J. Mol. Endocrinol. 1995, 14, 263–275. [Google Scholar] [CrossRef]
- Cheng, Q.; Boucher, B.J.; Leung, P.S. Modulation of hypovitaminosis D-induced islet dysfunction and insulin resistance through direct suppression of the pancreatic islet renin–angiotensin system in mice. Diabetologia 2013, 56, 553–562. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Chavez, A.O.; Gastaldelli, A.; Perego, L.; Tripathy, D.; Saad, M.J.; Velloso, L.; Folli, F. The Crosstalk Between Insulin and Renin-Angiotensin-Aldosterone Signaling Systems and its Effect on Glucose Metabolism and Diabetes Prevention. Curr. Vasc. Pharmacol. 2008, 6, 301–312. [Google Scholar] [CrossRef]
- Wei, Y.; Sowers, J.R.; Clark, S.E.; Li, W.; Ferrario, C.M.; Stump, C.S. Angiotensin II-induced skeletal muscle insulin resistance mediated by NF-kappaB activation via NADPH oxidase. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E345–E351. [Google Scholar] [CrossRef]
- Leung, P.S. The Potential Protective Action of Vitamin D in Hepatic Insulin Resistance and Pancreatic Islet Dysfunction in Type 2 Diabetes Mellitus. Nutrients 2016, 8, 147. [Google Scholar] [CrossRef]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef]
- Chiu, K.C.; Chu, A.; Go, V.L.; Saad, M.F. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am. J. Clin. Nutr. 2004, 79, 820–825. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Ye, Z.; Rickard, A.P.; Khaw, K.T.; Luben, R.; Langenberg, C.; Wareham, N.J. Circulating 25-hydroxyvitamin D concentration and the risk of type 2 diabetes: Results from the European Prospective Investigation into Cancer (EPIC)-Norfolk cohort and updated meta-analysis of prospective studies. Diabetologia 2012, 55, 2173–2182. [Google Scholar] [CrossRef]
- Deleskog, A.; Hilding, A.; Brismar, K.; Hamsten, A.; Efendic, S.; Östenson, C.G. Low serum 25-hydroxyvitamin D level predicts progression to type 2 diabetes in individuals with prediabetes but not with normal glucose tolerance. Diabetologia 2012, 55, 1668–1678. [Google Scholar] [CrossRef]
- Pittas, A.G.; Harris, S.S.; Stark, P.C.; Dawson-Hughes, B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care 2007, 30, 980–986. [Google Scholar] [CrossRef]
- Gedik, O.; Akalin, S. Effects of vitamin D deficiency and repletion on insulin and glucagon secretion in man. Diabetologia 1986, 29, 142–145. [Google Scholar] [CrossRef]
- Ljunghall, S.; Lind, L.; Lithell, H.; Skarfors, E.; Selinus, I.; Sørensen, O.H.; Wide, L. Treatment with One-alpha-hydroxycholecalciferol in Middle-aged Men with Impaired Glucose Tolerance-A Prospective Randomized Double-blind Study. Acta Med. Scand. 1987, 222, 361–367. [Google Scholar] [CrossRef]
- Taylor, A.V.; Wise, P.H. Vitamin D replacement in Asians with diabetes may increase insulin resistance. Postgrad Med. 1998, 74, 365–366. [Google Scholar] [CrossRef]
- Alshahrani, F.; Aljohani, N. Vitamin D: Deficiency, Sufficiency and Toxicity. Nutrients 2013, 5, 3605–3616. [Google Scholar] [CrossRef]
- Jorde, R.; Sneve, M.; Emaus, N.; Figenschau, Y.; Grimnes, G. Cross-sectional and longitudinal relation between serum 25-hydroxyvitamin D and body mass index: The Tromsø study. Eur. J. Nutr. 2010, 49, 401–407. [Google Scholar] [CrossRef]
- Afzal, S.; Bojesen, S.E.; Nordestgaard, B.G. Low 25-Hydroxyvitamin D and Risk of Type 2 Diabetes: A Prospective Cohort Study and Metaanalysis. Clin. Chem. 2013, 59, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Scragg, R.; Sowers, M.; Bell, C. Serum 25-Hydroxyvitamin D, Diabetes, and Ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care 2004, 27, 2813–2818. [Google Scholar] [CrossRef] [PubMed]
- Knekt, P.; Laaksonen, M.; Mattila, C.; Härkänen, T.; Marniemi, J.; Heliövaara, M.; Rissanen, H.; Montonen, J.; Reunanen, A. Serum Vitamin D and Subsequent Occurrence of Type 2 Diabetes. Epidemiology 2008, 19, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Luan, J.; Cooper, A.; Boucher, B.J.; Wareham, N.J. Baseline serum 25-hydroxy vitamin d is predictive of future glycemic status and insulin resistance: The Medical Research Council Ely Prospective Study 1990–2000. Diabetes 2008, 57, 2619–2625. [Google Scholar] [CrossRef] [PubMed]
- Pittas, A.G.; Sun, Q.; Manson, J.E.; Dawson-Hughes, B.; Hu, F.B. Plasma 25-Hydroxyvitamin D Concentration and Risk of Incident Type 2 Diabetes in Women. Diabetes Care 2010, 33, 2021–2023. [Google Scholar] [CrossRef]
- Song, Y.; Wang, L.; Pittas, A.G.; Del Gobbo, L.C.; Zhang, C.; Manson, J.E.; Hu, F.B. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: A meta-analysis of prospective studies. Diabetes Care 2013, 36, 1422–1428. [Google Scholar] [CrossRef]
- Dalgård, C.; Petersen, M.S.; Weihe, P.; Grandjean, P. Vitamin D Status in Relation to Glucose Metabolism and Type 2 Diabetes in Septuagenarians. Diabetes Care 2011, 34, 1284–1288. [Google Scholar] [CrossRef]
- Robinson, J.G.; Manson, J.E.; Larson, J.; Liu, S.; Song, Y.; Howard, B.V.; Phillips, L.; Shikany, J.M.; Allison, M.; Curb, J.D.; et al. Lack of Association Between 25(OH)D Levels and Incident Type 2 Diabetes in Older Women. Diabetes Care 2011, 34, 628–634. [Google Scholar] [CrossRef]
- Pilz, S.; Hurk, K.V.D.; Nijpels, G.; Stehouwer, C.; Riet, E.V.; Kienreich, K.; Tomaschitz, A.; Dekker, J. Vitamin D status, incident diabetes and prospective changes in glucose metabolism in older subjects: The Hoorn study. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 883–889. [Google Scholar] [CrossRef]
- Oosterwerff, M.M.; Eekhoff, E.M.; Van Schoor, N.M.; Boeke, A.J.P.; Nanayakkara, P.; Meijnen, R.; Knol, D.L.; Kramer, M.H.; Lips, P. Effect of moderate-dose vitamin D supplementation on insulin sensitivity in vitamin D–deficient non-Western immigrants in the Netherlands: A randomized placebo-controlled trial. Am. J. Clin. Nutr. 2014, 100, 152–160. [Google Scholar] [CrossRef]
- Nazarian, S.; Peter, J.V.S.; Boston, R.C.; Jones, S.A.; Mariash, C.N. Vitamin D3 supplementation improves insulin sensitivity in subjects with impaired fasting glucose. Transl. Res. 2011, 158, 276–281. [Google Scholar] [CrossRef]
- Talaei, A.; Mohamadi, M.; Adgi, Z. The effect of vitamin D on insulin resistance in patients with type 2 diabetes. Diabetol. Metab. Syndr. 2013, 5, 8. [Google Scholar] [CrossRef]
- Hanafy, A.S.; Elkatawy, H.A. Beneficial Effects of Vitamin D on Insulin Sensitivity, Blood Pressure, Abdominal Subcutaneous Fat Thickness, and Weight Loss in Refractory Obesity. Clin. Diabetes 2018, 36, 217–225. [Google Scholar] [CrossRef]
- Asemi, Z.; Karamali, M.; Esmaillzadeh, A. Effects of calcium–vitamin D co-supplementation on glycaemic control, inflammation and oxidative stress in gestational diabetes: A randomised placebo-controlled trial. Diabetologia 2014, 57, 1798–1806. [Google Scholar] [CrossRef]
- Pittas, A.G.; Jorde, R.; Kawahara, T.; Dawson-Hughes, B. Response to Letter to the Editor from Dalan: “Vitamin D Supplementation for Prevention of Type 2 Diabetes Mellitus: To D or Not to D?”. J. Clin. Endocrinol. Metab. 2020, 106, 1928–1929. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, H.; Tang, J.; Li, J.; Chong, W.; Hai, Y.; Feng, Y.; Lunsford, L.D.; Xu, P.; Jia, D.; et al. Effects of Vitamin D Supplementation on Prevention of Type 2 Diabetes in Patients with Prediabetes: A Systematic Review and Meta-analysis. Diabetes Care 2020, 43, 1650–1658. [Google Scholar] [CrossRef]
- Krishnamurthy, B.; Dudek, N.L.; McKenzie, M.D.; Purcell, A.; Brooks, A.; Gellert, S.; Colman, P.G.; Harrison, L.C.; Lew, A.; Thomas, H.E.; et al. Responses against islet antigens in NOD mice are prevented by tolerance to proinsulin but not IGRP. J. Clin. Investig. 2006, 116, 3258–3265. [Google Scholar] [CrossRef]
- Nakayama, M.; Abiru, N.; Moriyama, H.; Babaya, N.; Liu, E.; Miao, D.; Yu, L.; Wegmann, D.R.; Hutton, J.C.; Elliott, J.F.; et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature 2005, 435, 220–223. [Google Scholar] [CrossRef]
- Turley, S.; Poirot, L.; Hattori, M.; Benoist, C.; Mathis, D. Physiological beta cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. J. Exp. Med. 2003, 198, 1527–1537. [Google Scholar] [CrossRef]
- Anderson, M.S.; Bluestone, J.A. The Nod Mouse: A Model of Immune Dysregulation. Annu. Rev. Immunol. 2005, 23, 447–485. [Google Scholar] [CrossRef]
- Lieberman, S.; DiLorenzo, T. A comprehensive guide to antibody and T-cell responses in type 1 diabetes. Tissue Antigens 2003, 62, 359–377. [Google Scholar] [CrossRef] [PubMed]
- André, I.; Gonzalez, A.; Wang, B.; Katz, J.; Benoist, C.; Mathis, D. Checkpoints in the progression of autoimmune disease: Lessons from diabetes models. Proc. Natl. Acad. Sci. USA 1996, 93, 2260–2263. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.D.; Wang, B.; Haskins, K.; Benoist, C.; Mathis, D. Following a diabetogenic T cell from genesis through pathogenesis. Cell 1993, 74, 1089–1100. [Google Scholar] [CrossRef]
- Burton, A.R.; Vincent, E.; Arnold, P.Y.; Lennon, G.P.; Smeltzer, M.; Li, C.-S.; Haskins, K.; Hutton, J.; Tisch, R.M.; Sercarz, E.E.; et al. On the Pathogenicity of Autoantigen-Specific T-Cell Receptors. Diabetes 2008, 57, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Serreze, D.V.; Fleming, S.A.; Chapman, H.D.; Richard, S.D.; Leiter, E.H.; Tisch, R.M. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. J. Immunol. 1998, 161, 3912–3918. [Google Scholar] [CrossRef] [PubMed]
- Greeley, S.A.W.; Katsumata, M.; Yu, L.; Eisenbarth, G.S.; Moore, D.J.; Goodarzi, H.; Barker, C.F.; Naji, A.; Noorchashm, H. Elimination of maternally transmitted autoantibodies prevents diabetes in nonobese diabetic mice. Nat. Med. 2002, 8, 399–402. [Google Scholar] [CrossRef]
- Hu, C.-Y.; Rodriguez-Pinto, D.; Du, W.; Ahuja, A.; Henegariu, O.; Wong, F.S.; Shlomchik, M.J.; Wen, L. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J. Clin. Investig. 2007, 117, 3857–3867. [Google Scholar] [CrossRef]
- Inaba, M.; Nishizawa, Y.; Song, K.; Tanishita, H.; Okuno, S.; Miki, T.; Morii, H. Partial protection of 1α-hydroxyvitamin D3 against the development of diabetes induced by multiple low-dose streptozotocin injection in CD-1 mice. Metabolism 1992, 41, 631–635. [Google Scholar] [CrossRef]
- Mathieu, C.; Laureys, J.; Sobis, H.; Vandeputte, M.; Waer, M.; Bouillon, R. 1,25-Dihydroxyvitamin D3 prevents insulitis in NOD mice. Diabetes 1992, 41, 1491–1495. [Google Scholar] [CrossRef]
- Mathieu, C.; Waer, M.; Laureys, J.; Rutgeerts, O.; Bouillon, R. Prevention of autoimmune diabetes in NOD mice by 1,25 dihydroxyvitamin D3. Diabetologia 1994, 37, 552–558. [Google Scholar] [CrossRef]
- Gregori, S.; Giarratana, N.; Smiroldo, S.; Uskokovic, M.; Adorini, L. A 1α,25-Dihydroxyvitamin D3 Analog Enhances Regulatory T-Cells and Arrests Autoimmune Diabetes in NOD Mice. Diabetes 2002, 51, 1367–1374. [Google Scholar] [CrossRef]
- Hyppönen, E.; Läärä, E.; Reunanen, A.; Järvelin, M.-R.; Virtanen, S.M. Intake of vitamin D and risk of type 1 diabetes: A birth-cohort study. Lancet 2001, 358, 1500–1503. [Google Scholar] [CrossRef]
- Fronczak, C.M.; Barón, A.E.; Chase, H.P.; Ross, C.; Brady, H.L.; Hoffman, M.; Eisenbarth, G.S.; Rewers, M.; Norris, J.M. In Utero Dietary Exposures and Risk of Islet Autoimmunity in Children. Diabetes Care 2003, 26, 3237–3242. [Google Scholar] [CrossRef]
- Sørensen, I.M.; Joner, G.; Jenum, P.A.; Eskild, A.; Torjesen, P.A.; Stene, L.C. Maternal serum levels of 25-hydroxy-vitamin D during pregnancy and risk of type 1 diabetes in the offspring. Diabetes 2012, 61, 175–178. [Google Scholar] [CrossRef]
- Zipitis, C.S.; Akobeng, A.K. Vitamin D supplementation in early childhood and risk of type 1 diabetes: A systematic review and meta-analysis. Arch. Dis. Child. 2008, 93, 512–517. [Google Scholar] [CrossRef]
- Vitamin D supplement in early childhood and risk for Type I (insulin-dependent) diabetes mellitus. The EURODIAB Substudy 2 Study Group. Diabetologia 1999, 42, 51–54. [CrossRef]
- Gabbay, M.A.; Sato, M.N.; Finazzo, C.; Duarte, A.J.; Dib, S.A. Effect of cholecalciferol as adjunctive therapy with insulin on protective immunologic profile and decline of residual beta-cell function in new-onset type 1 diabetes mellitus. Arch. Pediatr. Adolesc. Med. 2012, 166, 601–607. [Google Scholar] [CrossRef]
- Walter, M.; Kaupper, T.; Adler, K.; Foersch, J.; Bonifacio, E.; Ziegler, A.G. No effect of the 1alpha,25-dihydroxyvitamin D3 on beta-cell residual function and insulin requirement in adults with new-onset type 1 diabetes. Diabetes Care 2010, 33, 1443–1448. [Google Scholar] [CrossRef]
- Bizzarri, C.; Pitocco, D.; Napoli, N.; Di Stasio, E.; Maggi, D.; Manfrini, S.; Suraci, C.; Cavallo, M.G.; Cappa, M.; Ghirlanda, G.; et al. No protective effect of calcitriol on beta-cell function in recent-onset type 1 diabetes: The IMDIAB XIII trial. Diabetes Care 2010, 33, 1962–1963. [Google Scholar] [CrossRef]
- Provvedini, D.M.; Tsoukas, C.D.; Deftos, L.J.; Manolagas, S.C. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science 1983, 221, 1181–1183. [Google Scholar] [CrossRef]
- Provvedini, D.M.; Tsoukas, C.D.; Deftos, L.J.; Manolagas, S.C. 1 alpha,25-Dihydroxyvitamin D3-binding macromolecules in human B lymphocytes: Effects on immunoglobulin production. J. Immunol. 1986, 136, 2734–2740. [Google Scholar] [CrossRef] [PubMed]
- Veldman, C.M.; Cantorna, M.T.; DeLuca, H.F. Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch. Biochem. Biophys. 2000, 374, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Nakayama, Y.; Horiuchi, H.; Ohta, T.; Komoriya, K.; Ohmori, H.; Kamimura, T. Human neutrophils express messenger RNA of vitamin D receptor and respond to 1alpha,25-dihydroxyvitamin D3. Immunopharmacol. Immunotoxicol. 2002, 24, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Kreutz, M.; Andreesen, R.; Krause, S.W.; Szabo, A.; Ritz, E.; Reichel, H. 1,25-Dihydroxyvitamin-D3 Production and Vitamin-D3 Receptor Expression Are Developmentally-Regulated during Differentiation of Human Monocytes into Macrophages. Blood 1993, 82, 1300–1307. [Google Scholar] [CrossRef]
- Hewison, M.; Freeman, L.; Hughes, S.V.; Evans, K.N.; Bland, R.; Eliopoulos, A.G.; Kilby, M.D.; Moss, P.A.H.; Chakraverty, R. Differential Regulation of Vitamin D Receptor and Its Ligand in Human Monocyte-Derived Dendritic Cells. J. Immunol. 2003, 170, 5382–5390. [Google Scholar] [CrossRef]
- Baeke, F.; Korf, H.; Overbergh, L.; van Etten, E.; Verstuyf, A.; Gysemans, C.; Mathieu, C. Human T lymphocytes are direct targets of 1,25-dihydroxyvitamin D3 in the immune system. J. Steroid Biochem. Mol. Biol. 2010, 121, 221–227. [Google Scholar] [CrossRef]
- Griffin, M.D.; Xing, N.; Kumar, R. Vitamin d and its analogs as regulators of immune activation and antigen presentation. Annu. Rev. Nutr. 2003, 23, 117–145. [Google Scholar] [CrossRef]
- Baeke, F.; Van Etten, E.; Overbergh, L.; Mathieu, C. Vitamin D3 and the immune system: Maintaining the balance in health and disease. Nutr. Res. Rev. 2007, 20, 106–118. [Google Scholar] [CrossRef]
- Xu, H.; Soruri, A.; Gieseler, R.K.H.; Peters, J.H. 1,25-Dihydroxyvitamin D3 Exerts Opposing Effects to IL-4 on MHC Class-II Antigen Expression, Accessory Activity, and Phagocytosis of Human Monocytes. Scand. J. Immunol. 1993, 38, 535–540. [Google Scholar] [CrossRef]
- Almerighi, C.; Sinistro, A.; Cavazza, A.; Ciaprini, C.; Rocchi, G.; Bergamini, A. 1α,25-Dihydroxyvitamin D3 inhibits CD40L-induced pro-inflammatory and immunomodulatory activity in Human Monocytes. Cytokine 2009, 45, 190–197. [Google Scholar] [CrossRef]
- Penna, G.; Adorini, L. 1α,25-Dihydroxyvitamin D3 Inhibits Differentiation, Maturation, Activation, and Survival of Dendritic Cells Leading to Impaired Alloreactive T Cell Activation. J. Immunol. 2000, 164, 2405–2411. [Google Scholar] [CrossRef]
- Berer, A.; Stöckl, J.; Majdic, O.; Wagner, T.; Kollars, M.; Lechner, K.; Geissler, K.; Oehler, L. 1,25-Dihydroxyvitamin D3 inhibits dendritic cell differentiation and maturation in vitro. Exp. Hematol. 2000, 28, 575–583. [Google Scholar] [CrossRef]
- Van Halteren, A.G.S.; van Etten, E.; de Jong, E.C.; Bouillon, R.; Roep, B.O.; Mathieu, C. Redirection of human autoreactive T-cells upon interaction with dendritic cells modulated by TX527, an analog of 1,25 dihydroxyvitamin D-3. Diabetes 2002, 51, 2119–2125. [Google Scholar] [CrossRef]
- Pedersen, A.W.; Holmstrøm, K.; Jensen, S.S.; Fuchs, D.; Rasmussen, S.; Kvistborg, P.; Claesson, M.H.; Zocca, M.B. Phenotypic and functional markers for 1alpha,25-dihydroxyvitamin D(3)-modified regulatory dendritic cells. Clin. Exp. Immunol. 2009, 157, 48–59. [Google Scholar] [CrossRef]
- Van Halteren, A.G.; Tysma, O.M.; van Etten, E.; Mathieu, C.; Roep, B.O. 1alpha,25-dihydroxyvitamin D3 or analogue treated dendritic cells modulate human autoreactive T cells via the selective induction of apoptosis. J. Autoimmun. 2004, 23, 233–239. [Google Scholar] [CrossRef]
- Adorini, L. Tolerogenic dendritic cells induced by vitamin D receptor ligands enhance regulatory T cells inhibiting autoimmune diabetes. Ann. N. Y. Acad. Sci. 2003, 987, 258–261. [Google Scholar] [CrossRef]
- Penna, G.; Roncari, A.; Amuchastegui, S.; Daniel, K.C.; Berti, E.; Colonna, M.; Adorini, L. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood 2005, 106, 3490–3497. [Google Scholar] [CrossRef]
- Piemonti, L.; Monti, P.; Sironi, M.; Fraticelli, P.; Leone, B.E.; Dal Cin, E.; Allavena, P.; Di Carlo, V. Vitamin D3 Affects Differentiation, Maturation, and Function of Human Monocyte-Derived Dendritic Cells. J. Immunol. 2000, 164, 4443–4451. [Google Scholar] [CrossRef]
- Walker, L.S.K.; von Herrath, M. CD4 T cell differentiation in type 1 diabetes. Clin. Exp. Immunol. 2015, 183, 16–29. [Google Scholar] [CrossRef]
- Emamaullee, J.A.; Davis, J.; Merani, S.; Toso, C.; Elliott, J.F.; Thiesen, A.; Shapiro, A.J. Inhibition of Th17 Cells Regulates Autoimmune Diabetes in NOD Mice. Diabetes 2009, 58, 1302–1311. [Google Scholar] [CrossRef]
- Tian, J.; Lehmann, P.V.; Kaufman, D.L. Determinant Spreading of T Helper Cell 2 (Th2) Responses to Pancreatic Islet Autoantigens. J. Exp. Med. 1997, 186, 2039–2043. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, A.; Barrat, F.J.; Crain, C.; Heath, V.L.; Savelkoul, H.F.J.; O’garra, A. 1α,25-Dihydroxyvitamin D3 Has a Direct Effect on Naive CD4+ T Cells to Enhance the Development of Th2 Cells. J. Immunol. 2001, 167, 4974–4980. [Google Scholar] [CrossRef] [PubMed]
- Mahon, B.D.; Wittke, A.; Weaver, V.; Cantorna, M.T. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J. Cell. Biochem. 2003, 89, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhou, R.; Luger, D.; Zhu, W.; Silver, P.B.; Grajewski, R.S.; Su, S.-B.; Chan, C.-C.; Adorini, L.; Caspi, R.R. Calcitriol Suppresses Antiretinal Autoimmunity through Inhibitory Effects on the Th17 Effector Response. J. Immunol. 2009, 182, 4624–4632. [Google Scholar] [CrossRef]
- Riachy, R.; Vandewalle, B.; Belaich, S.; Kerr-Conte, J.; Gmyr, V.; Zerimech, F.; D’Herbomez, M.; Lefebvre, J.; Pattou, F. Beneficial effect of 1,25 dihydroxyvitamin D3 on cytokine-treated human pancreatic islets. J. Endocrinol. 2001, 169, 161–168. [Google Scholar] [CrossRef]
- Gysemans, C.; Cardozo, A.K.; Callewaert, H.; Giulietti, A.; Hulshagen, L.; Bouillon, R.; Eizirik, D.L.; Mathieu, C. 1,25-Dihydroxyvitamin D3 Modulates Expression of Chemokines and Cytokines in Pancreatic Islets: Implications for Prevention of Diabetes in Nonobese Diabetic Mice. Endocrinology 2005, 146, 1956–1964. [Google Scholar] [CrossRef]
- Brusko, T.M.; Putnam, A.L.; Bluestone, J.A. Human regulatory T cells: Role in autoimmune disease and therapeutic opportunities. Immunol. Rev. 2008, 223, 371–390. [Google Scholar] [CrossRef]
- Liu, W.; Putnam, A.L.; Xu-Yu, Z.; Szot, G.L.; Lee, M.R.; Zhu, S.; Gottlieb, P.A.; Kapranov, P.; Gingeras, T.R.; de St Groth, B.F.; et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 2006, 203, 1701–1711. [Google Scholar] [CrossRef]
- Jeffery, L.E.; Burke, F.; Mura, M.; Zheng, Y.; Qureshi, O.S.; Hewison, M.; Walker, L.S.K.; Lammas, D.A.; Raza, K.; Sansom, D.M. 1,25-Dihydroxyvitamin D3 and IL-2 Combine to Inhibit T Cell Production of Inflammatory Cytokines and Promote Development of Regulatory T Cells Expressing CTLA-4 and FoxP3. J. Immunol. 2009, 183, 5458–5467. [Google Scholar] [CrossRef]
- Chen, S.; Sims, G.P.; Chen, X.X.; Gu, Y.Y.; Chen, S.; Lipsky, P.E. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol 2007, 179, 1634–1647. [Google Scholar] [CrossRef]
- Pieńkowska, A.; Janicka, J.; Duda, M.; Dzwonnik, K.; Lip, K.; Mędza, A.; Szlagatys-Sidorkiewicz, A.; Brzeziński, M. Controversial Impact of Vitamin D Supplementation on Reducing Insulin Resistance and Prevention of Type 2 Diabetes in Patients with Prediabetes: A Systematic Review. Nutrients 2023, 15, 983. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Atkins, A.; Downes, M.; Wei, Z. Vitamin D in Diabetes: Uncovering the Sunshine Hormone’s Role in Glucose Metabolism and Beyond. Nutrients 2023, 15, 1997. https://doi.org/10.3390/nu15081997
Wu J, Atkins A, Downes M, Wei Z. Vitamin D in Diabetes: Uncovering the Sunshine Hormone’s Role in Glucose Metabolism and Beyond. Nutrients. 2023; 15(8):1997. https://doi.org/10.3390/nu15081997
Chicago/Turabian StyleWu, Jie, Annette Atkins, Michael Downes, and Zong Wei. 2023. "Vitamin D in Diabetes: Uncovering the Sunshine Hormone’s Role in Glucose Metabolism and Beyond" Nutrients 15, no. 8: 1997. https://doi.org/10.3390/nu15081997
APA StyleWu, J., Atkins, A., Downes, M., & Wei, Z. (2023). Vitamin D in Diabetes: Uncovering the Sunshine Hormone’s Role in Glucose Metabolism and Beyond. Nutrients, 15(8), 1997. https://doi.org/10.3390/nu15081997





