Effectiveness and Coverage of Severe Acute Malnutrition Treatment with a Simplified Protocol in a Humanitarian Context in Diffa, Niger
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Location
2.2. Socioeconomic Assessment
2.3. Treatment Coverage Assessment
2.4. Data Collection and Analyses
2.5. Ethical Considerations
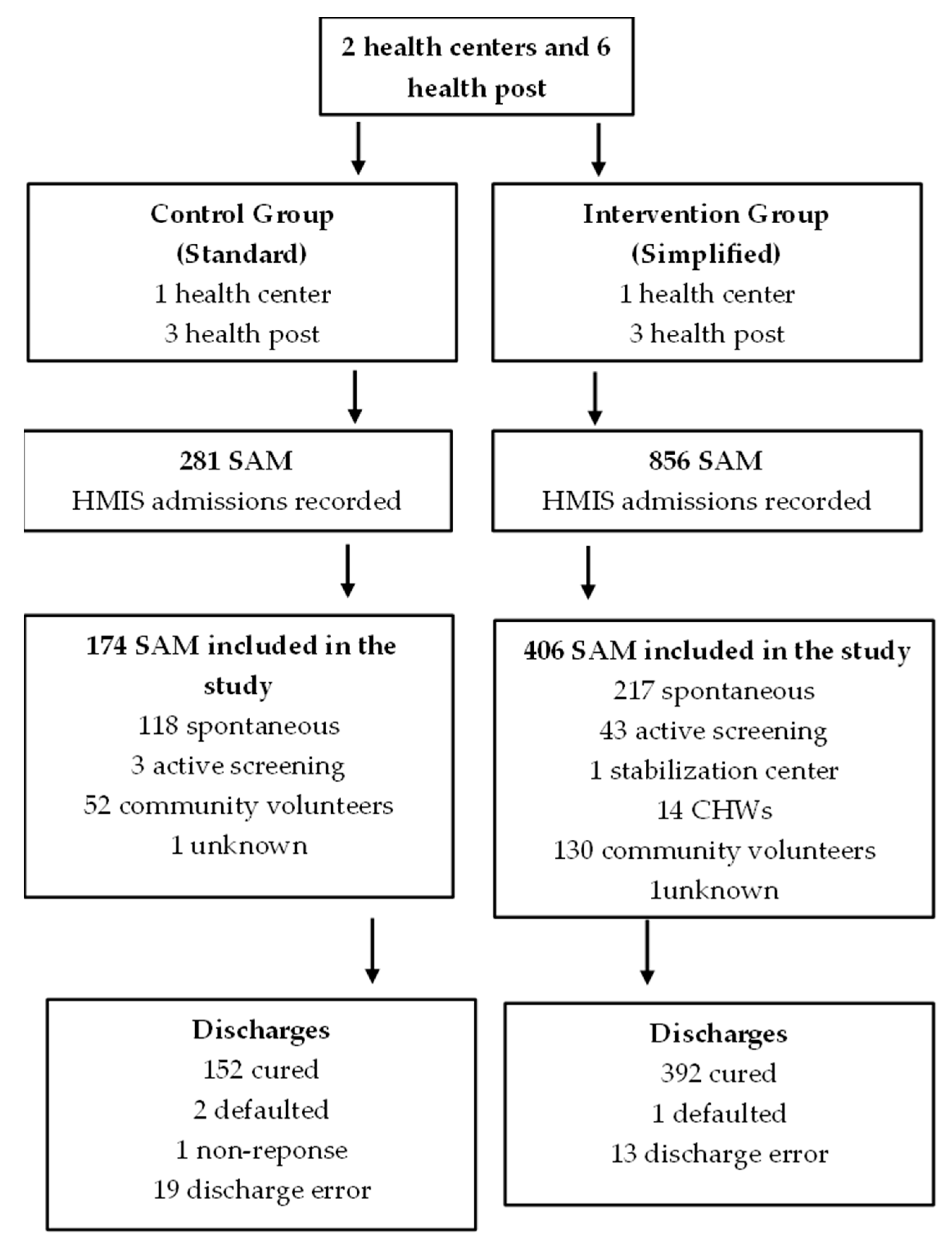
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OCHA. Aperçu des Besoins Humanitaires Niger. 2022. Available online: https://www.unocha.org/niger (accessed on 9 November 2022).
- SMART. Enquête Nutritionnelle et de Mortalité Retrospective au Niger. SMART. 2021. Available online: https://fscluster.org/niger/document/ins-enquete-nutritionnelle-et-de (accessed on 9 November 2022).
- De Onis, M.; Borghi, E.; Arimond, M.; Webb, P.; Croft, T.; Saha, K.; De-Regil, L.M.; Thuita, F.; Heidkamp, R.; Krasevec, J.; et al. Prevalence thresholds for wasting, overweight and stunting in children under 5 years. Public Health Nutr. 2018, 22, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Ministère de la Santé Publique Niger. Plan de Developpement Sanitaire 2017–2021. Available online: https://www.prb.org/wp-content/uploads/2019/06/PLAN-DE-DEVELOPPEMENT-SANITAIRE_2017_2021_Niger.pdf (accessed on 7 December 2022).
- World Health Organization. Universal Health Coverage (UHC). WHO 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/universal-health-coverage-(uhc) (accessed on 11 November 2022).
- United Nations. Transforming our World: The 2030 Agenda for Sustainable Development|Department of Economic and Social Affairs. UUNN 2015. Available online: https://sdgs.un.org/goals (accessed on 11 November 2022).
- Oliphant, N.P.; Ray, N.; Bensaid, K.; Ouedraogo, A.; Gali, A.Y.; Habi, O.; Maazou, I.; Panciera, R.; Muñiz, M.; Sy, Z.; et al. Optimising geographical accessibility to primary health care: A geospatial analysis of community health posts and community health workers in Niger. BMJ Glob. Health 2021, 6, e005238. [Google Scholar] [CrossRef] [PubMed]
- REACH. Informing more Effective Humanitarian Action. Evaluation Multisectorielle des Besoin Niger 2022. Available online: https://reach-info.org/ner/msna2022/ (accessed on 28 January 2022).
- UNICEF. Simplified Approaches. Available online: https://www.simplifiedapproaches.org/es/what-are-simplified-approaches (accessed on 11 November 2022).
- Bailey, J.; Lelijveld, N.; Marron, B.; Onyoo, P.; Ho, L.S.; Manary, M.; Briend, A.; Opondo, C.; Kerac, M. Combined Protocol for Acute Malnutrition Study (ComPAS) in rural South Sudan and urban Kenya: Study protocol for a randomized controlled trial. Trials 2018, 19, 251. [Google Scholar] [CrossRef] [PubMed]
- Kangas, S.T.; Marron, B.; Tausanovitch, Z.; Radin, E.; Andrianarisoa, J.; Dembele, S.; Ouédraogo, C.T.; Coulibaly, I.N.; Biotteau, M.; Ouologuem, B.; et al. Effectiveness of Acute Malnutrition Treatment at Health Center and Community Levels with a Simplified, Combined Protocol in Mali: An Observational Cohort Study. Nutrients 2022, 14, 4923. [Google Scholar] [CrossRef] [PubMed]
- World Food Programme. Vulnerability Analysis and Mapping. Food Consumption Analysis. Calculation and Use of the Food Consumption Score in Food Security Analysis. 2008. Available online: https://documents.wfp.org/stellent/groups/public/documents/manual_guide_proced/wfp197216.pdf (accessed on 27 December 2022).
- Rogers, E.; Myatt, M.; Woodhead, S.; Guerrero, S.; Alvarez, J.L. Coverage of Community-Based Management of Severe Acute Malnutrition Programmes in Twenty-One Countries, 2012–2013. PLoS ONE 2015, 10, e0128666. [Google Scholar] [CrossRef] [PubMed]
- FANTA III USAID; Myatt, M.; Guevarra, E.; Fieschi, L.; Norris, A.; Guerrero, S.; Schofield, L.; Jones, D.; Emru, E.; Sadler, K. Semi-Quantitative Evaluation of Access and Coverage (SQUEAC)/Simplified Lot Quality Assurance Sampling Evaluation of Access and Coverage (SLEAC). 2012. Available online: https://www.fantaproject.org/monitoring-and-evaluation/squeac-sleac (accessed on 27 December 2022).
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- The Sphere Handbook. Standards for Quality Humanitarian Response Sphere. 2018. Available online: https://spherestandards.org/es/el-manual/ (accessed on 27 December 2022).
- James, P.T.; Briel, N.V.D.; Rozet, A.; Israël, A.; Fenn, B.; Navarro-Colorado, C. Low-dose RUTF protocol and improved service delivery lead to good programme outcomes in the treatment of uncomplicated SAM: A programme report from Myanmar. Matern. Child Nutr. 2015, 11, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.; Opondo, C.; Lelijveld, N.; Marron, B.; Onyo, P.; Musyoki, E.N.; Adongo, S.W.; Manary, M.; Briend, A.; Kerac, M. A simplified, combined protocol versus standard treatment for acute malnutrition in children 6–59 months (ComPAS trial): A cluster-randomized controlled non-inferiority trial in Kenya and South Sudan. PLoS Med. 2020, 17, e1003192. [Google Scholar] [CrossRef] [PubMed]
- Kangas, S.T.; Salpéteur, C.; Nikièma, V.; Talley, L.; Ritz, C.; Friis, H.; Briend, A.; Kaestel, P. Impact of reduced dose of ready-to-use therapeutic foods in children with uncomplicated severe acute malnutrition: A randomised non-inferiority trial in Burkina Faso. PLoS Med. 2019, 16, e1002887. [Google Scholar] [CrossRef] [PubMed]
- Daures, M.; Phelan, K.; Issoufou, M.; Kouanda, S.; Sawadogo, O.; Issaley, K.; Cazes, C.; Séri, B.; Ouaro, B.; Akpakpo, B.; et al. New approach to simplifying and optimising acute malnutrition treatment in children aged 6–59 months: The OptiMA single-arm proof-of-concept trial in Burkina Faso. Br. J. Nutr. 2019, 123, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Cazes, C.; Phelan, K.; Hubert, V.; Boubacar, H.; Bozama, L.I.; Sakubu, G.T.; Tshiala, B.K.; Tusuku, T.; Alitanou, R.; Kouamé, A.; et al. Simplifying and optimising the management of uncomplicated acute malnutrition in children aged 6–59 months in the Democratic Republic of the Congo (OptiMA-DRC): A non-inferiority, randomised controlled trial. Lancet Glob. Health 2022, 10, e510–e520. [Google Scholar] [CrossRef] [PubMed]
- Dougnon, A.O.; Charle-Cuéllar, P.; Toure, F.; Gado, A.A.; Sanoussi, A.; Lazoumar, R.H.; Tchamba, G.A.; Vargas, A.; Lopez-Ejeda, N. Impact of Integration of Severe Acute Malnutrition Treatment in Primary Health Care Provided by Community Health Workers in Rural Niger. Nutrients 2021, 13, 4067. [Google Scholar] [CrossRef] [PubMed]
- N’Diaye, D.S.; Wassonguema, B.; Nikièma, V.; Kangas, S.T.; Salpéteur, C. Economic evaluation of a reduced dosage of ready-to-use therapeutic foods to treat uncomplicated severe acute malnourished children aged 6–59 months in Burkina Faso. Matern. Child Nutr. 2021, 17, e13118. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Morán, J.L.; Alé, G.B.F.; Charle, P.; Sessions, N.; Doumbia, S.; Guerrero, S. The effectiveness of treatment for Severe Acute Malnutrition (SAM) delivered by community health workers compared to a traditional facility based model. BMC Health Serv. Res. 2018, 18, 207. [Google Scholar] [CrossRef] [PubMed]
- Charle-Cuéllar, P.; Lopez-Ejeda, N.; Souleymane, H.T.; Yacouba, D.; Diagana, M.; Dougnon, A.O.; Vargas, A.; Briend, A. Effectiveness and Coverage of Treatment for Severe Acute Malnutrition Delivered by Community Health Workers in the Guidimakha Region, Mauritania. Children 2021, 8, 1132. [Google Scholar] [CrossRef] [PubMed]
- Wilunda, C.; Mumba, F.G.; Putoto, G.; Maya, G.; Musa, E.; Lorusso, V.; Magige, C.; Leyna, G.; Manenti, F.; Riva, D.D.; et al. Effectiveness of screening and treatment of children with severe acute malnutrition by community health workers in Simiyu region, Tanzania: A quasi-experimental pilot study. Sci. Rep. 2021, 11, 2342. [Google Scholar] [CrossRef] [PubMed]
- Kodish, S.R.; Allen, B.G.; Salou, H.; Schwendler, T.R.; Isanaka, S. Conceptualising factors impacting nutrition services coverage of treatment for acute malnutrition in children: An application of the Three Delays Model in Niger. Public Health Nutr. 2021, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Maust, A.; Koroma, A.S.; Abla, C.; Molokwu, N.; Ryan, K.N.; Singh, L.; Manary, M.J. Severe and Moderate Acute Malnutrition Can Be Successfully Managed with an Integrated Protocol in Sierra Leone. J. Nutr. 2015, 145, 2604–2609. [Google Scholar] [CrossRef] [PubMed]
- UNICEF. Simplified Approaches. Decision-Making in Exceptional Circumstances. Available online: https://www.simplifiedapproaches.org/copy-of-tools-resources (accessed on 27 December 2022).

| Control Group (Standard) | Intervention Group (Simplified) | |
|---|---|---|
| Treatment providers | Nurses at health facilities and health posts | Nurses and CHWs at health facilities and health post |
| Follow-up frequency | Weekly | Weekly |
| Admission criteria | Edema (+) or WHZ with a <−3 z-score or a MUAC of < 115 mm | Edema (+) or a MUAC of <115 mm |
| Product and dosage | RUTF/weight: 170 Kcal/kg/day | RUTF fixed dosage: 2 sachets/day (= 1000 Kcal/day) * |
| Recovery criteria | No edema and a WHZ of ≥1.5 or a MUAC of ≥125 mm | No edema and a MUAC of ≥125 mm |
| Indicator | Control (Standard) | Intervention (Simplified) | ||||
|---|---|---|---|---|---|---|
| Demographics | N | Mean (SD) or % (n) | N | Mean (SD) or % (n) | p-Value | |
| Number of cohabiting people | 117 | 5.5 (2.4) | 251 | 6.0 (2.8) | 0.110 | |
| Number of children under 5 years of age living with the treated child | 115 | 1.8 (1.3) | 237 | 1.5 (1.1) | 0.066 | |
| Education of primary caregiver (years) | 117 | 0.19 (0.49) | 251 | 0.28 (0.68) | 0.187 | |
| Livelihoods | Type of housing | 117 | 241 | |||
| Property | 88.0% (103) | 58.1% (140) | <0.001 | |||
| For rent | 0.9% (1) | 12.9% (31) | <0.001 | |||
| On loan | 11.1% (13) | 29.0 (70) | <0.001 | |||
| Households with access to safe water | 117 | 38.5% (45) | 251 | 55.8% (140) | 0.003 | |
| Households with access to safe sanitation | 117 | 0.0% (0) | 251 | 1.6% (4) | 0.405 | |
| Households with electricity | 117 | 3.4% (4) | 251 | 15.5% (39) | 0.001 | |
| Households with arable land | 117 | 29.9% (35) | 251 | 4.8% (12) | <0.001 | |
| Households with livestock | 117 | 48.7% (57) | 251 | 53.4% (134) | 0.470 | |
| Number of cows | 57 | 6.7 (9.8) | 134 | 8.4 (13.3) | 0.329 | |
| Number of sheep | 57 | 4.6 (8.5) | 134 | 2.0 (4.3) | 0.030 | |
| Number of goats | 57 | 8.7 (11.2) | 134 | 12.2 (14.8) | 0.070 | |
| Households with a construction floor (concrete, cement, wood, tiles, etc.) | 117 | 0.0% (0) | 251 | 1.2% (3) | 0.572 | |
| Households with a construction roof (concrete, cement, wood, tiles, etc.) | 117 | 0.0% (0) | 251 | 1.2% (3) | 0.572 | |
| Food security | Number of meals per day | 108 | 2.8 (0.5) | 221 | 2.9 (0.4) | 0.819 |
| Lack of food in the last 4 weeks | 116 | 247 | ||||
| Never | 88.8% (103) | 61.1% (151) | <0.001 | |||
| Rarely | 10.3% (12) | 36.0% (89) | <0.001 | |||
| 3–10 times | 0.9% (1) | 2.4% (6) | 0.546 | |||
| More than 10 times | 0.0% (0) | 0.4% (1) | 0.999 | |||
| Food diversity | 117 | 62.3 (21.9) | 251 | 62.8% (20.2) | 0.825 | |
| Poor diet | 7.7% (9) | 2.4% (6) | 0.035 | |||
| Limited diet | 5.1% (6) | 4.8% (12) | 0.999 | |||
| Acceptable diet | 87.2% (102) | 92.8% (233) | 0.116 | |||
| Health care access | Behavior if the child is sick | 116 | 251 | |||
| Health post or CHW | 99.1% (115) | 92.8% (233) | 0.022 | |||
| Traditional medicine | 0.0% (0) | 6.8% (17) | 0.009 | |||
| Self-medication | 0.9% (1) | 0.4% (1) | 0.999 | |||
| Households with difficulties in access | 117 | 3.4% (4) | 251 | 1.2% (3) | 0.296 | |
| Time needed to reach treatment | 117 | 251 | ||||
| 30 min or less | 32.5% (57) | 41.3% (104) | 0.230 | |||
| Up to 1 h 30 min | 47.0% (31) | 31.6% (79) | 0.396 | |||
| More than 2 h | 20.5% (24) | 27.1% (68) | 0.219 | |||
| Control (Standard) N = 173 | Intervention (Simplified) N = 405 | p-Value | |
|---|---|---|---|
| Referred by: | % (n) | % (n) | |
| Spontaneous | 68.2% (118) | 53.6% (217) | 0.039 |
| Active screening campaigns | 1.7% (3) | 10.6% (43) | |
| Stabilization centers | 0% (0) | 0.2% (1) | |
| CHWs | 0% (0) | 3.5% (14) | |
| Community volunteer | 30.1% (52) | 32.1% (130) | |
| Immunization up to date | 55.6% (95) | 45.7% (179) | 0.031 |
| Comorbidities in the last 7 days: | % (n) | % (n) | |
| Diarrhea | 3.5% (6) | 14.9% (60) | <0.001 |
| Vomit | 0% (0) | 3.0% (12) | 0.048 |
| Fever | 1.7% (3) | 8.7% (35) | 0.002 |
| Cough | 3.4% (6) | 18.1% (73) | <0.001 |
| Acute respiratory infection | 0.6% (1) | 1.0% (4) | 0.980 |
| Malaria-positive | 0% (0) | 1.2% (5) | 0.327 |
| Pale conjunctiva | 1.4% (3) | 4.7% (19) | 0.089 |
| Edema (mild) | 0% (0) | 0% (0) | -- |
| Anthropometry | |||
| Weight (kg); mean (SD) | 6.2 (1.1) | 6.3 (1.2) | <0.001 |
| Height (cm); mean (SD) | 69.1 (6.5) | 70.2 (6.8) | 0.068 |
| WHZ; mean (SD) | −3.17 (1.02) | −2.67 (1.31) | <0.001 |
| WHZ < −3.47; %(n) * | 30.1% (52) | 21.9% (88) | 0.036 |
| MUAC; mean (SD) | 111.1 (5.2) | 111.3 (4.0) | 0.883 |
| MUAC < 110 mm; %(n) * | 17.2% (30) | 15.0% (61) | 0.501 |
| Treatment Outcomes | Control (Standard) | Intervention (Simplified) | ꭓ2 p-Value | Crude HR (95% CI) | * Adjusted HR (95% CI) |
|---|---|---|---|---|---|
| Cured | 87.4% (152) | 96.6% (392) | <0.001 | 1.242 (1.026–1.504) | 1.300 (1.055–1.601) |
| Defaulted | 1.1% (2) | 0.2% (1) | 0.449 | -- | -- |
| Non-response | 0.6% (1) | 0% (0) | 0.662 | -- | -- |
| Referred | 0% (0) | 0% (0) | -- | -- | -- |
| Death | 0% (0) | 0% (0) | -- | -- | -- |
| Discharge error | 10.9% (19) | 3.2% (13) | <0.001 | 0.298 (0.135–0.656) | 0.283 (0.112–0.713) |
| Control (Standard) | Intervention (Simplified) | p-Value | |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| Length of stay (days) | 35.0 (29.0–47.0) | 35.0 (28.0–42.0) | 0.254 |
| RUTF (sachets) | 95.0 (74.0–120.0) | 70.0 (56.0–77.0) | <0.001 |
| Weight gain (g/kg/day) | 7.04 (5.03–9.76) | 5.95 (3.62–8.40) | <0.001 |
| MUAC Gain (mm/day) | 0.43 (0.31–0.50) | 0.46 (0.37–0.57) | 0.001 |
| Control (Standard) N = 165 | Intervention (Simplified) N = 379 | p-Value | ||
|---|---|---|---|---|
| WHZ only (<−3 z-score) | Total cases, % (n) | 9.1% (15) | ||
| Cured, % (n) | 80.0% (12) | |||
| Length of stay(days), median (IQR) | 32.0 (21.8–44.3) | |||
| Admission of RUTF to recovery, median (IQR) | 100.0 (60.0–115.0) | |||
| MUAC only (<115 mm) | Total cases, % (n) | 30.9% (51) | 58.6% (222) | |
| Cured, % (n) | 96.1% (49) | 96.4% (214) | 1.000 | |
| Length of stay(days), median (IQR) | 35.0 (29.3–42.8) | 35.0 (28.0–43.0) | 0.803 | |
| Admission of RUTF to recovery, median (IQR) | 95.0 (75.0–120.0) | 70.0 (56.0–84.0) | <0.001 | |
| WHZ and MUAC | Total cases, % (n) | 60.0% (99) | 41.4% (157) | |
| Cured, % (n) | 85.9% (85) | 96.8% (152) | 0.001 | |
| Length of stay (days), median (IQR) | 38.0 (28.0–52.0) | 35.0 (28.0–42.0) | 0.071 | |
| Admission of RUTF to recovery, median (IQR) | 94.0 (59.0–112.5) | 70.0 (56.0–72.0) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charle-Cuéllar, P.; Lopez-Ejeda, N.; Aziz Gado, A.; Dougnon, A.O.; Sanoussi, A.; Ousmane, N.; Hamidou Lazoumar, R.; Sánchez-Martínez, L.J.; Toure, F.; Vargas, A.; et al. Effectiveness and Coverage of Severe Acute Malnutrition Treatment with a Simplified Protocol in a Humanitarian Context in Diffa, Niger. Nutrients 2023, 15, 1975. https://doi.org/10.3390/nu15081975
Charle-Cuéllar P, Lopez-Ejeda N, Aziz Gado A, Dougnon AO, Sanoussi A, Ousmane N, Hamidou Lazoumar R, Sánchez-Martínez LJ, Toure F, Vargas A, et al. Effectiveness and Coverage of Severe Acute Malnutrition Treatment with a Simplified Protocol in a Humanitarian Context in Diffa, Niger. Nutrients. 2023; 15(8):1975. https://doi.org/10.3390/nu15081975
Chicago/Turabian StyleCharle-Cuéllar, Pilar, Noemi Lopez-Ejeda, Abdoul Aziz Gado, Abdias Ogobara Dougnon, Atté Sanoussi, Nassirou Ousmane, Ramatoulaye Hamidou Lazoumar, Luis Javier Sánchez-Martínez, Fanta Toure, Antonio Vargas, and et al. 2023. "Effectiveness and Coverage of Severe Acute Malnutrition Treatment with a Simplified Protocol in a Humanitarian Context in Diffa, Niger" Nutrients 15, no. 8: 1975. https://doi.org/10.3390/nu15081975
APA StyleCharle-Cuéllar, P., Lopez-Ejeda, N., Aziz Gado, A., Dougnon, A. O., Sanoussi, A., Ousmane, N., Hamidou Lazoumar, R., Sánchez-Martínez, L. J., Toure, F., Vargas, A., & Guerrero, S. (2023). Effectiveness and Coverage of Severe Acute Malnutrition Treatment with a Simplified Protocol in a Humanitarian Context in Diffa, Niger. Nutrients, 15(8), 1975. https://doi.org/10.3390/nu15081975







