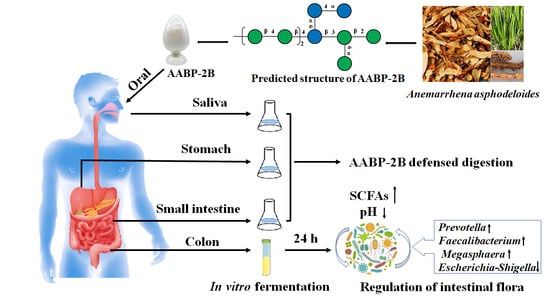
Effects of Simulated In Vitro Digestion on the Structural Characteristics, Inhibitory Activity on α-Glucosidase, and Fermentation Behaviours of a Polysaccharide from Anemarrhena asphodeloides Bunge
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of AABP-2B
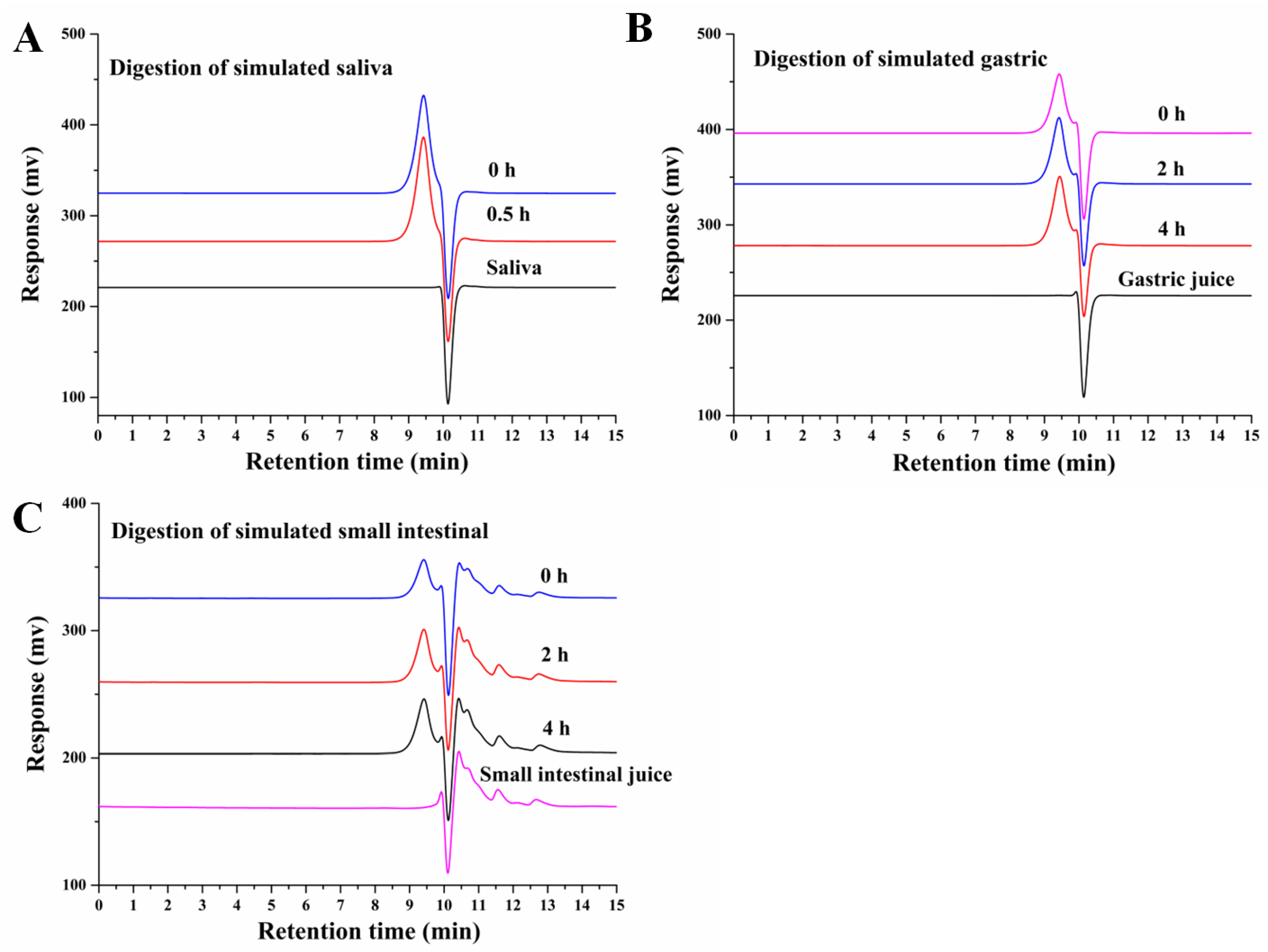
2.3. Oral Digestion Simulation
2.4. Simulation of Gastric Digestion
2.5. Simulation of Small-Intestinal Digestion
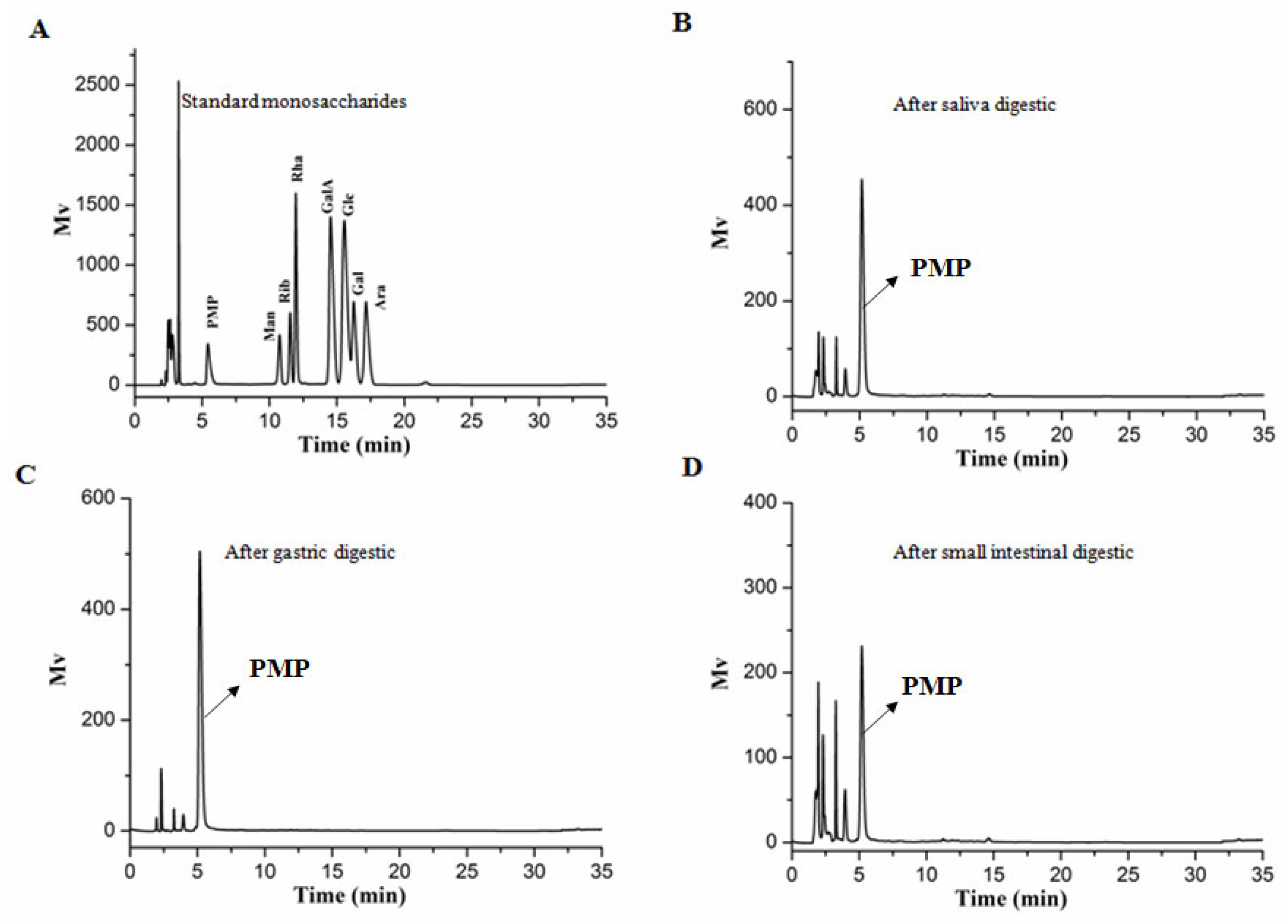
2.6. Analysis of the Monosaccharide Constituents and Molecular Weight of AABP-2B during Digestion
2.7. Inhibition of α-Glucosidase Activity
2.8. Fermentation of AABP-2B In Vitro
2.9. SCFAs and pH Determination
2.10. Determination of Gut Flora
2.11. Statistical Analysis
3. Results and Discussion
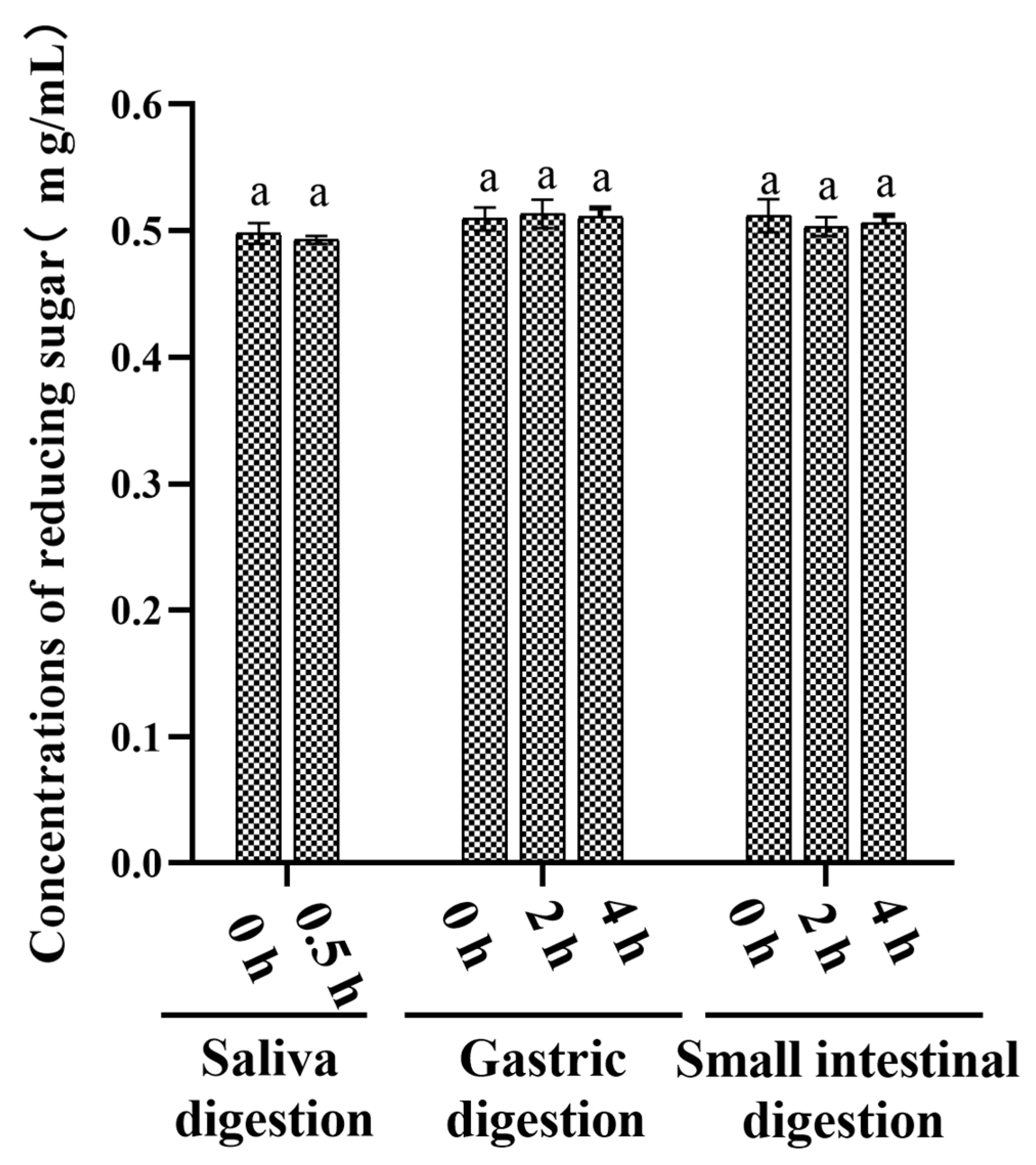
3.1. An Analysis of the Changes in Molecular Weight (Mw), Monosaccharide Composition, and Reducing Sugar during Saliva–Gastrointestinal Digestion of AABP-2B
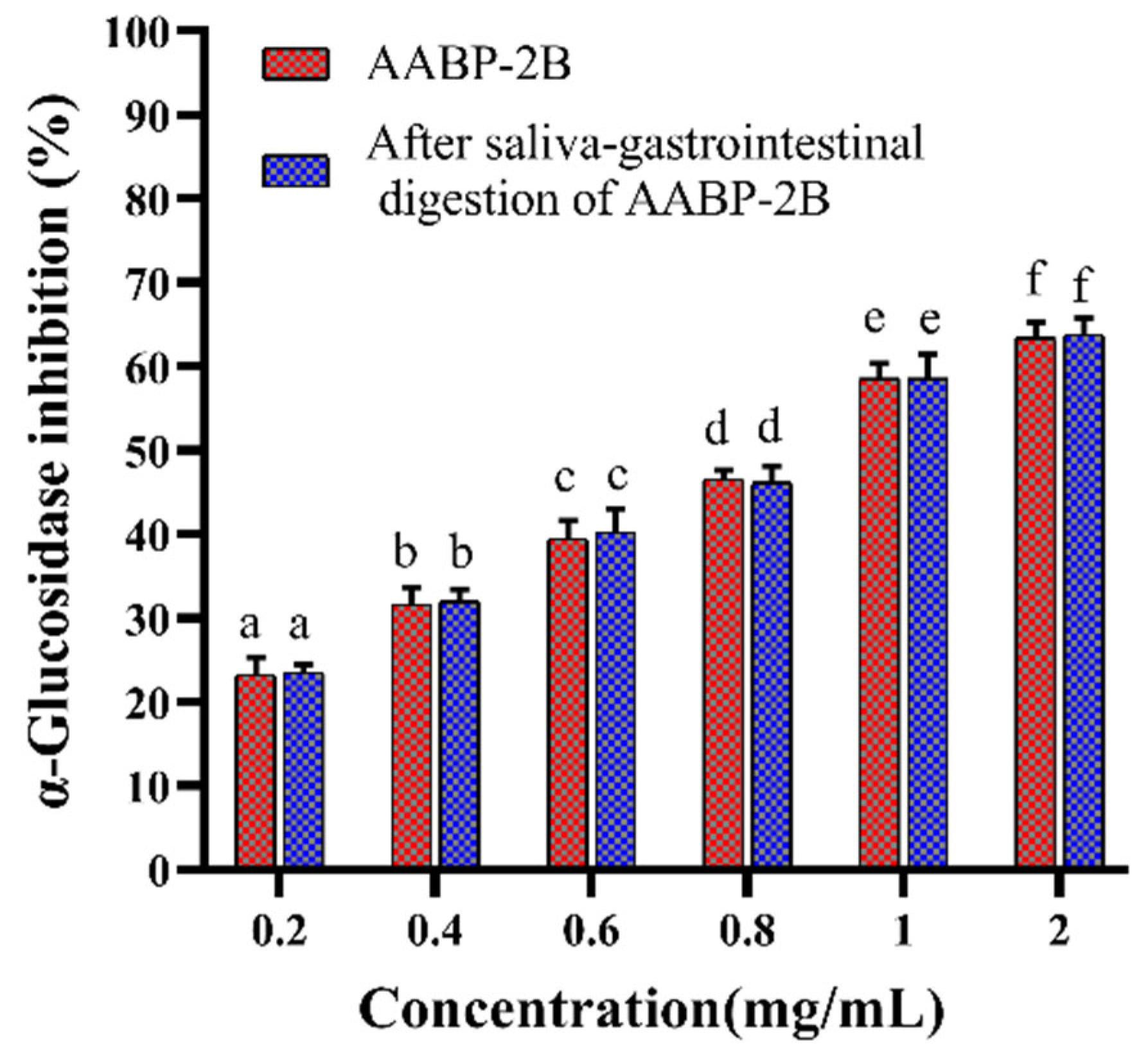
3.2. Analysis of Inhibition α-Glucosidase Activity after In Vitro Simulation of Digestion
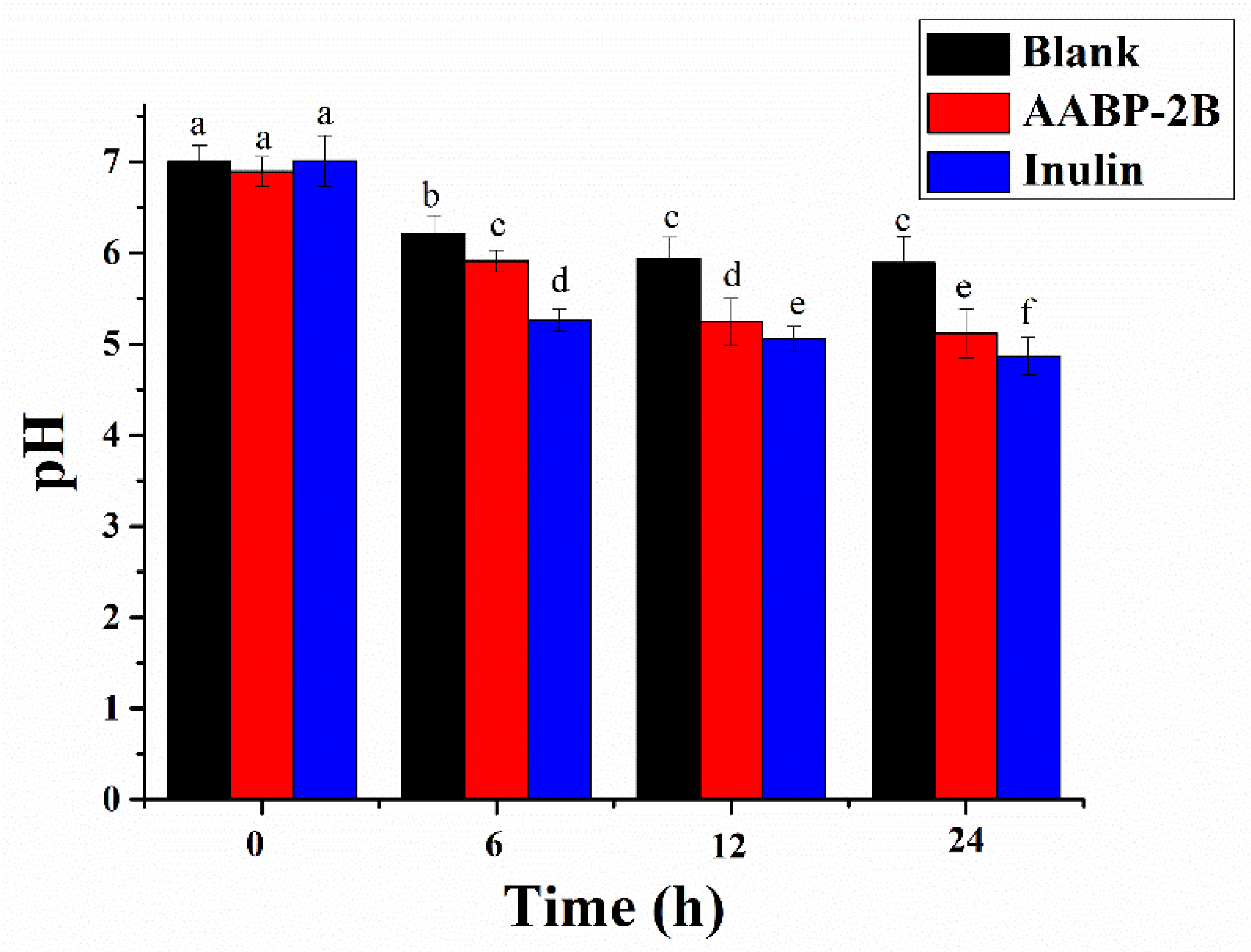
3.3. Change in pH
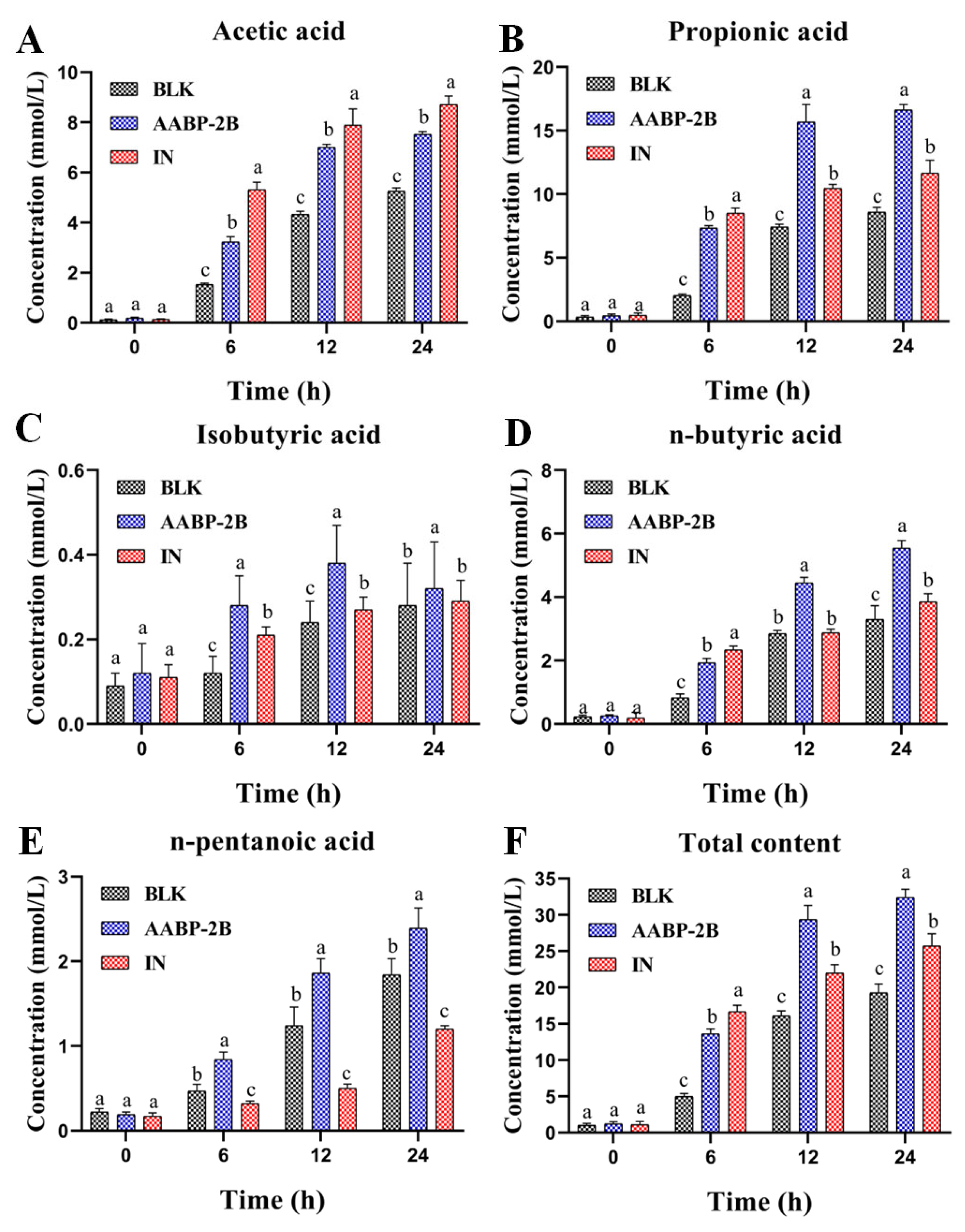
3.4. SCFA Content during Fermentation
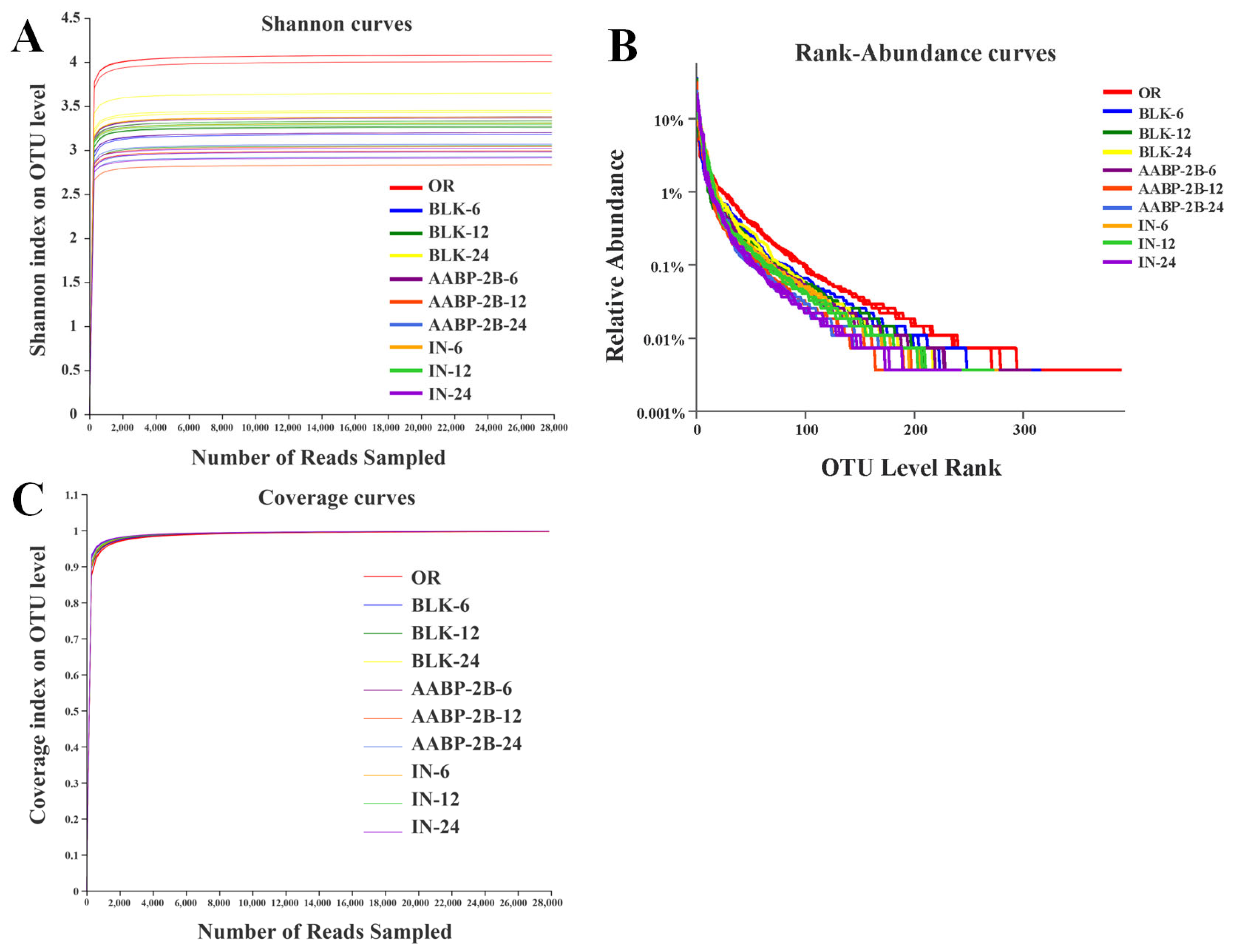
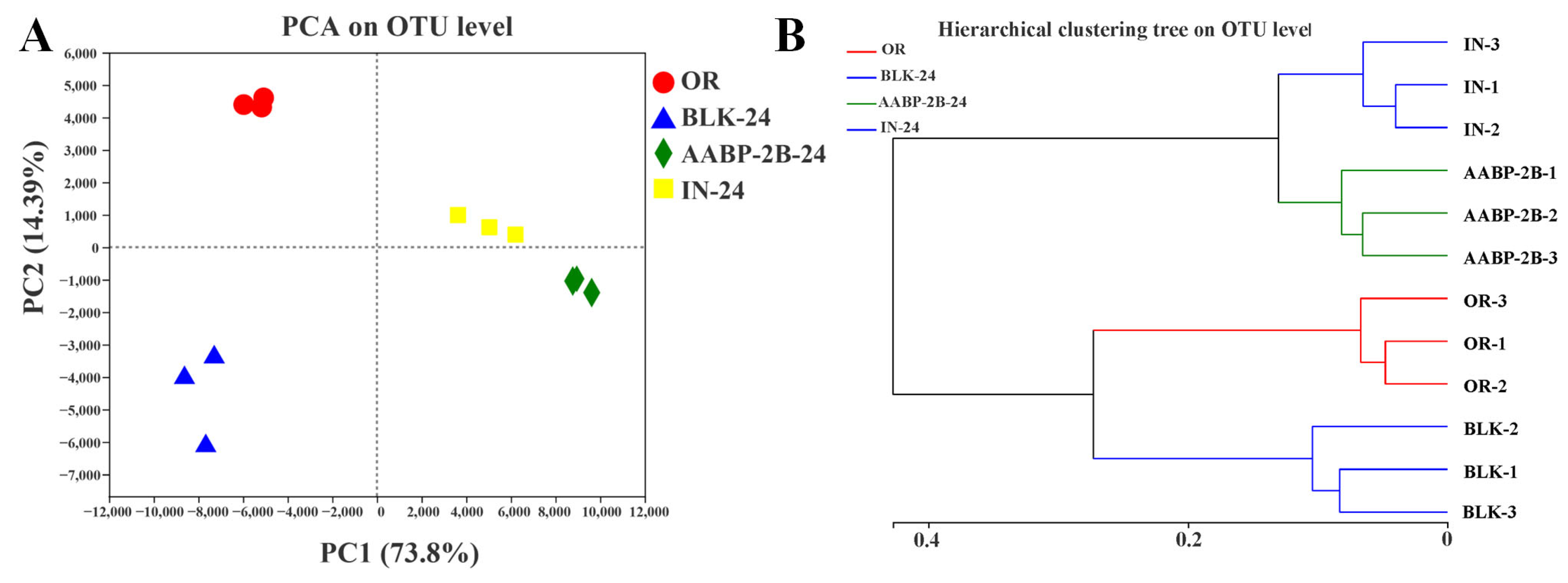
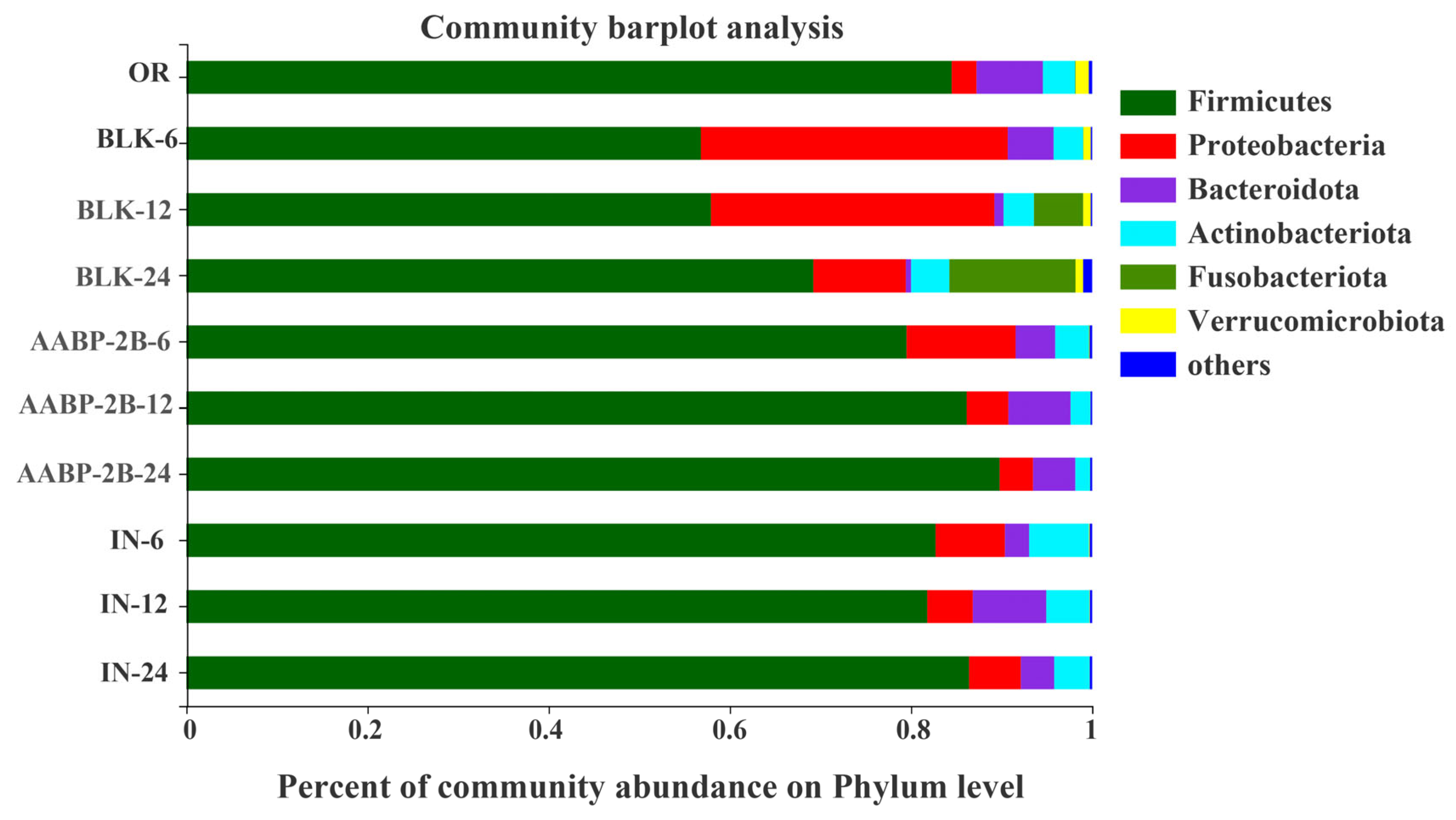
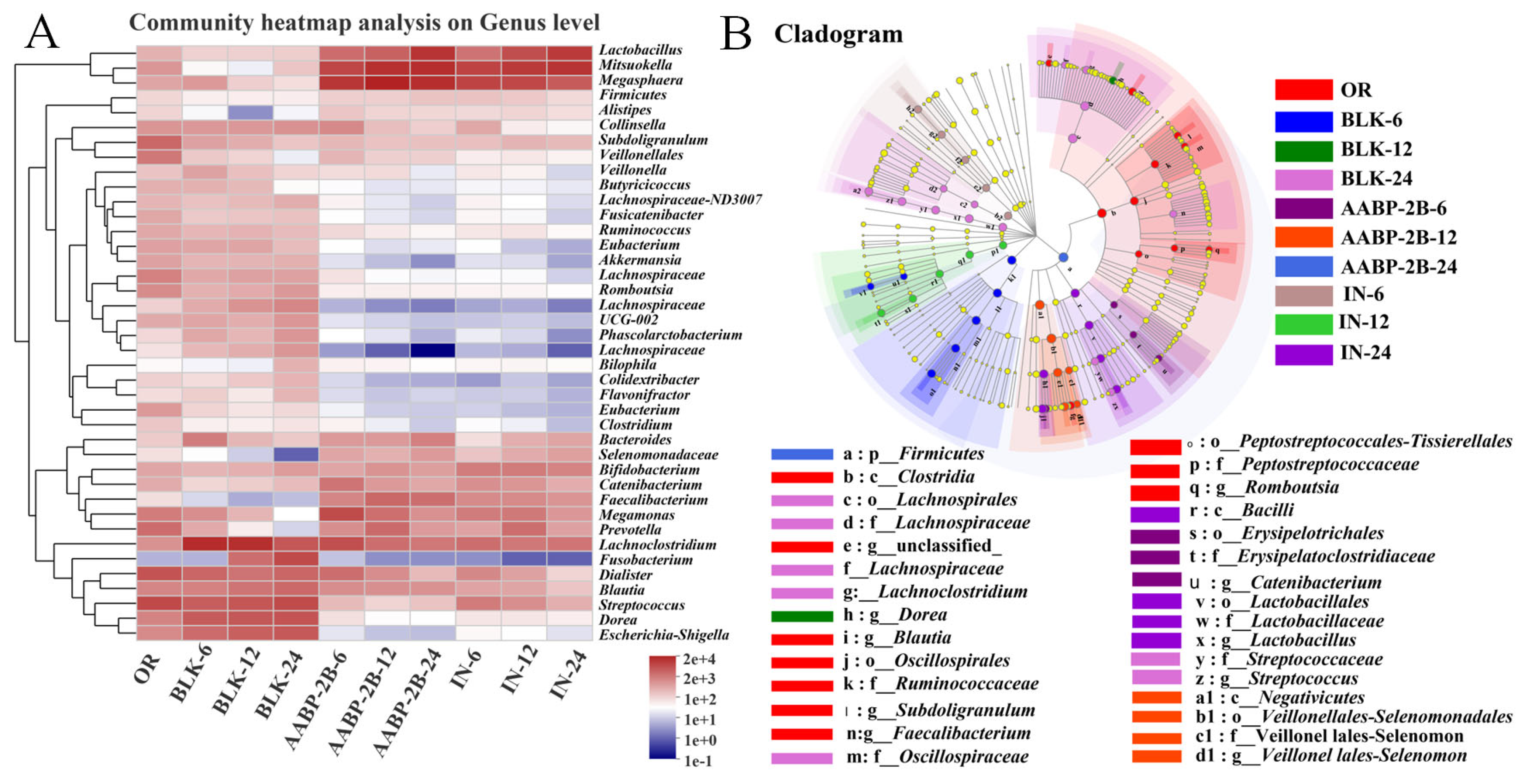
3.5. In Vitro Fermentation of AABP-2B by the Intestinal Flora
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, Y.; Jiang, S.; Zeng, M. In vitro simulated digestion and fermentation characteristics of polysaccharide from oyster (Crassostrea gigas), and its effects on the gut microbiota. Food Res. Int. 2021, 149, 110646. [Google Scholar] [CrossRef] [PubMed]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Brooks, L.; Viardot, A.; Tsakmaki, A.; Stolarczyk, E.; Bewick, G.A. Fermentable carbohydrate stimulates FFAR2-dependent colonic PYY cell expansiontoincrease satiety. Mol. Metab. 2017, 6, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Kza, E.; Hf, B.; Sza, D.; Sn, C.; Yz, A. Polysaccharide from Artocarpus heterophyllus Lam. (jackfruit) pulp modulates gut microbiota composition and improves short-chain fatty acids production. Food Chem. 2021, 364, 130434. [Google Scholar]
- Wei, B.; Qza, C.; Lyab, C.; Ftab, C.; Wei, C.; Qzab, L. Effects of vegetarian diet-associated nutrients on gut microbiota and intestinal physiology. Food Sci. Hum. Wellness 2022, 11, 208–217. [Google Scholar]
- Wu, N.; Gan, R.Y.; Guo, H.; Qin, W. In vitro digestion and fecal fermentation behaviors of a pectic polysaccharide from okra (Abelmoschus esculentus) and its impacts on human gut microbiota. Food Hydrocoll. 2020, 114, 106577. [Google Scholar] [CrossRef]
- Wu, D.; Fu, Y.; Guo, H.; Yuan, Q. In vitro simulated digestion and fecal fermentation of polysaccharides from loquat leaves: Dynamic changes in physicochemical properties and impacts on human gut microbiota. Int. J. Biol. Macromol. 2021, 168, 733–742. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Q.; An, L.; Zhang, J.; Li, Z.; Zhang, J.; Li, Y.; Tuerhong, M.; Ohizumi, Y.; Jin, J.; et al. A fructan from Anemarrhena asphodeloides Bunge showing neuroprotective and immunoregulatory effects. Carbohydr. Polym. 2020, 229, 115477. [Google Scholar] [CrossRef]
- Takahashi, M.; Konno, C.; Hikino, H. Isolation and hypoglycemic activity of anemarans A, B, C and D, glycans of Anemarrhena asphodeloides rhizomes. Planta Med. 1985, 51, 100–102. [Google Scholar] [CrossRef]
- Chen, J.; Wan, L.; Zheng, Q.; Lan, M.; Zhang, X.; Li, Y.; Li, B.; Li, L. Structural characterization and in vitro hypoglycaemic activity of glucomannan from Anemarrhena asphodeloides Bunge. Food Funct. 2022, 13, 1797–1807. [Google Scholar] [CrossRef]
- Floris, A.L.; Eloy, H.L.; Peter, L.L.; Guy, E.R.; Chris, L.W. Alpha-glucosidase inhibitors for patients with type 2 diabetes: Results from a Cochrane systematic review and meta-analysis. Diabetes Care 2005, 28, 154–163. [Google Scholar]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120–126. [Google Scholar] [CrossRef]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; Leleiko, N.; Snapper, S.B. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Huang, Q.; Fu, X.; Liu, R.H. In vitro digestibility and prebiotic potential of a novel polysaccharide from Rosa roxburghii Tratt fruit. J. Funct. Foods 2018, 52, 408–417. [Google Scholar] [CrossRef]
- Zhou, W.; Yan, Y.; Mi, J.; Zhang, H.; Lu, L.; Luo, Q.; Li, X.; Zeng, X.; Cao, Y. Simulated Digestion and Fermentation in Vitro by Human Gut Microbiota of Polysaccharides from Bee Collected Pollen of Chinese Wolfberry. J. Agric. Food Chem. 2018, 66, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, L.; Zhang, X.; Wan, L.; Zheng, Q.; Xu, D.; Li, Y.; Liang, Y.; Chen, M.; Li, B.; et al. Structural characterization of polysaccharide from Centipeda minima and its hypoglycemic activity through alleviating insulin resistance of hepatic HepG2 cells. J. Funct. Foods 2021, 82, 104478. [Google Scholar] [CrossRef]
- Li, X.; Guo, R.; Wu, X.; Liu, X.; Ai, L.; Sheng, Y.; Song, Z.; Wu, Y. Dynamic digestion of tamarind seed polysaccharide: Indigestibility in gastrointestinal simulations and gut microbiota changes in vitro. Carbohydr. Polym. 2020, 239, 116194. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xie, M.; Wan, P.; Chen, D.; Ye, H.; Chen, L.; Zeng, X.; Liu, Z. Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro by human intestinal microbiota of polysaccharides from Fuzhuan brick tea. Food Chem. 2018, 244, 331–339. [Google Scholar] [CrossRef]
- Ding, Q.; Nie, S.; Hu, J.; Zong, X.; Li, Q.; Xie, M. In vitro and in vivo gastrointestinal digestion and fermentation of the polysaccharide from Ganoderma atrum. Food Hydrocoll. 2017, 63, 646–655. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.; Liu, Y.; Li, W.; Niu, A.; Ren, P.; Liu, Y.; Jiang, C.; Inam, M.; Guan, L. Effects of in vitro digestion and fermentation of Nostoc commune Vauch. polysaccharides on properties and gut microbiota. Carbohydr. Polym. 2022, 281, 119055. [Google Scholar] [CrossRef]
- Di, T.; Chen, G.; Sun, Y.; Ou, S.; Zeng, X.; Ye, H. In vitro digestion by saliva, simulated gastric and small intestinal juices and fermentation by human fecal microbiota of sulfated polysaccharides from Gracilaria rubra. J. Funct. Foods 2018, 40, 18–27. [Google Scholar] [CrossRef]
- Zhu, K.; Yao, S.; Zhang, Y.; Liu, Q.; Xu, F.; Wu, G.; Dong, W.; Tan, L. Effects of in vitro saliva, gastric and intestinal digestion on the chemical properties, antioxidant activity of polysaccharide from Artocarpus heterophyllus Lam. (Jackfruit) Pulp. Food Hydrocoll. 2019, 87, 952–959. [Google Scholar] [CrossRef]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.S.; Wang, K.; Ma, K.; Bao, L.; Liu, H.-W. An insoluble polysaccharide from the sclerotium of Poria cocos improves hyperglycemia, hyperlipidemia and hepatic steatosis in ob/ob mice via modulation of gut microbiota. Chin. J. Nat. Med. 2019, 17, 3–14. [Google Scholar] [CrossRef]
- Ding, Y.; Yan, Y.; Peng, Y.; Chen, D.; Mi, J.; Lu, L.; Luo, Q.; Li, X.; Zeng, X.; Cao, Y. In vitro digestion under simulated saliva, gastric and small intestinal conditions and fermentation by human gut microbiota of polysaccharides from the fruits of Lycium barbarum. Int. J. Biol. Macromol. 2018, 125, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Dong, J.; Wang, S.; Shao, W.; Ahmed, A.F.; Zhang, Y.; Kang, W. Immunomodulatory effects of Nigella sativa seed polysaccharides by gut microbial and proteomic technologies. Int. J. Biol. Macromol. 2021, 184, 483–496. [Google Scholar] [CrossRef]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 2019, 26, 222–235. [Google Scholar] [CrossRef]
- Xu, S.-Y.; Jude, J.A.; Na, L.; Rui-Yan, D.; Wei-Yi, C.; Kit-Leong, C. Microbial catabolism of Porphyra haitanensis polysaccharides by human gut microbiota. Food Chem. 2019, 289, 177–186. [Google Scholar] [CrossRef]
- Shin, N.R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Zhang, X.; Aweya, J.J.; Huang, Z.-X.; Kang, Z.-Y.; Bai, Z.-H.; Li, K.-H.; He, X.-T.; Liu, Y.; Chen, X.-Q.; Cheong, K.-L. In vitro fermentation of Gracilaria lemaneiformis sulfated polysaccharides and its agaro-oligosaccharides by human fecal inocula and its impact on microbiota. Carbohydr. Polym. 2020, 234, 115894. [Google Scholar] [CrossRef]
- Martínez, N.; Hidalgo-Cantabrana, C.; Delgado, S.; Margolles, A.; Sánchez, B. Filling the gap between collection, transport and storage of the human gut microbiota. Sci. Rep. 2019, 9, 8327. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zheng, J.; Mao, G.; Hu, W.; Ye, X.; Linhardt, R.J.; Chen, S. Rethinking the impact of RG-I mainly from fruits and vegetables on dietary health. Crit. Rev. Food Sci. Nutr. 2020, 60, 2938–2960. [Google Scholar] [CrossRef] [PubMed]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee, Y.S.; De Vadder, F.; Arora, T.; Hallen, A.; Martens, E.; Björck, I.; Bäckhed, F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015, 22, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Martín, R.; Miquel, S.; Chain, F.; Natividad, J.M.; Jury, J.; Lu, J.; Sokol, H.; Theodorou, V.; Bercik, P.; Verdu, E.F.; et al. Faecalibacterium prausnitzii prevents physiological damages in a chronic low-grade inflammation murine model. BMC Microbiol. 2015, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2010, 27, 104–119. [Google Scholar] [CrossRef]
- Hashizume, K.; Tsukahara, T.; Yamada, K.; Koyama, H.; Ushida, K. Megasphaera elsdenii JCM1772T Normalizes Hyperlactate Production in the Large Intestine of Fructooligosaccharide-Fed Rats by Stimulating Butyrate Production. J. Nutr. 2003, 133, 3187–3190. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, E.J.; Iljazovic, A.; Amend, L.; Lesker, T.R.; Renault, T.; Thiemann, S.; Hao, L.; Roy, U.; Gronow, A.; Charpentier, E.; et al. Distinct Polysaccharide Utilization Determines Interspecies Competition between Intestinal Prevotella spp. Cell Host Microbe 2020, 28, 838–852. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Lan, M.; Zhang, X.; Jiao, W.; Chen, Z.; Li, L.; Li, B. Effects of Simulated In Vitro Digestion on the Structural Characteristics, Inhibitory Activity on α-Glucosidase, and Fermentation Behaviours of a Polysaccharide from Anemarrhena asphodeloides Bunge. Nutrients 2023, 15, 1965. https://doi.org/10.3390/nu15081965
Chen J, Lan M, Zhang X, Jiao W, Chen Z, Li L, Li B. Effects of Simulated In Vitro Digestion on the Structural Characteristics, Inhibitory Activity on α-Glucosidase, and Fermentation Behaviours of a Polysaccharide from Anemarrhena asphodeloides Bunge. Nutrients. 2023; 15(8):1965. https://doi.org/10.3390/nu15081965
Chicago/Turabian StyleChen, Juncheng, Meijuan Lan, Xia Zhang, Wenjuan Jiao, Zhiyi Chen, Lin Li, and Bing Li. 2023. "Effects of Simulated In Vitro Digestion on the Structural Characteristics, Inhibitory Activity on α-Glucosidase, and Fermentation Behaviours of a Polysaccharide from Anemarrhena asphodeloides Bunge" Nutrients 15, no. 8: 1965. https://doi.org/10.3390/nu15081965
APA StyleChen, J., Lan, M., Zhang, X., Jiao, W., Chen, Z., Li, L., & Li, B. (2023). Effects of Simulated In Vitro Digestion on the Structural Characteristics, Inhibitory Activity on α-Glucosidase, and Fermentation Behaviours of a Polysaccharide from Anemarrhena asphodeloides Bunge. Nutrients, 15(8), 1965. https://doi.org/10.3390/nu15081965