Quercetin as a Dietary Supplementary Flavonoid Alleviates the Oxidative Stress Induced by Lead Toxicity in Male Wistar Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Lead and Quercetin
2.3. Experimental Design
2.4. Hematological and Biochemical Assays
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magar, R.T.; Sohng, J.K. A review on structure, modifications and structure-activity relation of quercetin and its derivatives. J. Microbiol. Biotech. 2020, 30, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, H.G.; Sanlier, N. A minireview of quercetin: From its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food Sci. Nutr. 2020, 60, 3290–3303. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scolbert, A.; Remesy, C. Bioavailability and bioefficiency of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 2305–2425. [Google Scholar] [CrossRef] [PubMed]
- Wiczkowski, W.; Romaszko, J.; Bucinski, A.; Szawara-Nowak, D.; Honke, J.; Zielinski, H.; Piskula, M.K. Quercetin from shallots (Allium cepa L. var aggregatum) is more bioavailable its glucosoides. J. Nutr. 2008, 138, 885–888. [Google Scholar] [CrossRef]
- Annand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar]
- Abd-Elsalam, H.H.; Gamal, M.; Naguib, I.; Al-Ghobashy, M.A.; Zaazaa, H.E.; Abdelkawy, M. Development of green and efficient extraction methods of quercetin from red onion scales wastes using factorial design for method optimization: A complementary study. Separations 2021, 8, 137. [Google Scholar] [CrossRef]
- Chen, B.L.; Wang, L.T.; Huang, K.H.; Wang, C.C.; Chiang, C.K.; Liu, S.H. Quercetin attenuates renal ischemia/reperfusion injury via an activation of AMP-activated protein kinase-regulated autophagy pathway. J. Nutr. Bioch. 2014, 25, 1226–1234. [Google Scholar] [CrossRef]
- Reyes-Farias, M.; Carrasco-Pozo, C. The anti-cancer effect of quercetin: Molecular implications in cancer metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef]
- Tang, J.; Diao, P.; Shu, X.; Li, L.; Xiong, L. Quercetin and quercitrin attenuates the inflammatory response and oxidative stress in LPS-induced RAW264.7 cells: In vitro assessment and a theoretical model. BioMed Res. IntER. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Yuan, K.; Zhu, Q.; Lu, Q.; Jiang, H.; Zhu, M.; Li, X.; Hunag, G.; Xu, A. Quercetin alleviates rheumatoid arthritis by inhibiting neutrophil inflammatory activities. J. Nutr. Bioch. 2020, 84, 108454. [Google Scholar] [CrossRef]
- Lv, S.; Wang, X.; Jin, S.; Shen, S.; Wang, R.; Tong, P. Quercetin mediates TSC2-RHEB-mTOR pathway to regulate chondrocytes autophagy in knee osteoarthritis. Gene 2022, 820, 146209. [Google Scholar] [CrossRef] [PubMed]
- Neamtu, A.-A.; Maghiar, T.-A.; Alaya, A.; Olah, N.-K.; Turcus, V.; Pelea, D.; Totolici, B.D.; Neamtu, C.; Maghiar, A.M.; Mathe, E. A comprehensive view on the quercetin impact on colorectal cancer. Molecules 2022, 27, 1873. [Google Scholar] [CrossRef]
- Smitherman, J.; Harber, D.A. A case of mistaken identity, a case of lead toxicity. Am. J. Vet. Med. 1991, 20, 795–798. [Google Scholar]
- Waalkes, M.P. Metal Characteristics. In Metal Toxicology; Gogerin, A., Klasassen, A., Waalkes, M.P., Eds.; Academic Press: San Diego, CA, USA, 1995. [Google Scholar]
- Deng, Q.; Li, X.X.; Fang, Y.; Chen, X.; Xue, J. Therapeutic potential of quercetin as an antiatherosclerotic agent in atherosclerotic cardiovascular disease: A review. Evid. Based Complement. Altern. Med. 2020, 20, 5926381. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Kim, J.E.; Song, Y.J. Antiviral activities of quercetin and isoquercitrin against human herpesviruses. Molecules 2020, 25, 2379. [Google Scholar] [CrossRef] [PubMed]
- Akyuz, E.; Paudel, Y.N.; Polat, A.K.; Dundar, H.E.; Angelopoulou, E. Angelopoulou. Enlightening the neuroprotective effect of quercetin in epilepsy: From mechanism to therapeutic opportunities. Epilepsy Behav. 2021, 115, 107701. [Google Scholar] [CrossRef]
- Zou, H.; Ye, H.; Kamaraj, R.; Zhang, T.; Zhang, J.; Pavek, P. A review on pharmacological activities and synergistic effect of quercetin with small molecule agents. Phytomedicine 2021, 92, 153736. [Google Scholar] [CrossRef]
- Deepika; Maurya, P.K. Health benefits of quercetin in age-related diseases. Molecules 2022, 27, 2498. [Google Scholar] [CrossRef]
- Nguyen, T.L.A.; Bhattacharya, D. Antimicrobial activity of quercetin: An approach to its mechanistic principle. Molecules 2022, 27, 2494. [Google Scholar] [CrossRef]
- Gasmi, A.; Mujawdiya, P.K.; Lysiuk, R.; Shanaida, M.; Peana, M.; Gasmi Benahmed, A.; Beley, N.; Kovalska, N.; Bjørklund, G. Quercetin in the prevention and treatment of coronavirus infections. A focus on SARS-Co-2. Pharmaceuticals 2022, 15, 1049. [Google Scholar] [CrossRef]
- Remigante, A.; Spinelli, S.; Straface, E.; Gambardella, L.; Caruso, D.; Falliti, G.; Dossena, S.; Marino, A.; Morabito, R. Antioxidant activity of quercetin in a H2O2-induced oxidative stress model in red blood cells: Functional role of Band 3 protein. Int. J. Mol. Sci. 2022, 23, 10991. [Google Scholar] [CrossRef] [PubMed]
- Al-Balushi, H.; Hannemann, A.; Rees, D.; Brewin, J.; Gibson, J.S. The effect of antioxidant on the properties of red blood cells from patients with sickle cell anemia. Front. Physiol. 2019, 10, 976. [Google Scholar] [CrossRef]
- Begum, A.N.; Terao, J. Protective effect of quercetin against cigarette tar extract-induced impairment of erythrocyte deformability. Nutr. Biochem. 2002, 13, 265–272. [Google Scholar] [CrossRef]
- Muthukumaran, S.; Sudheer, A.R.; Nalini, N.; Menon, V.P. Effect of quercetin on nicotine-induced biochemical changes and DNA damage in rat peripheral blood lymphocytes. Redox Rep. 2008, 13, 217–224. [Google Scholar] [CrossRef]
- Pandey, A.K.; Patnaik, R.; Muresanu, D.F.; Sharma, A.; Sharma, H.S. Quercetin in hypoxia-induced oxidative stress: Novel target for neuroprotection. Int. Rev. Neurobiol. 2012, 102, 107–146. [Google Scholar] [PubMed]
- He, D.; Guo, X.; Zhang, E.; Zi, F.; Chen, J.; Chen, Q.; Lin, X.; Yang, L.; Li, Y.; Wu, W.; et al. Quercetin induces cell apoptosis of myeloma and displays a synergistic effect with dexamethasone in vitro and in vivo xenograft models. Oncotarget 2016, 7, 45489–45499. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, J.; Zhao, D.; Wang, C.; Zhang, Y.; Wang, Y. Therapeutic effect and mechanism of action of quercetin in a rat model of osteoarthritis. J. Int. Med. Res. 2020, 48. [Google Scholar] [CrossRef] [PubMed]
- Poisonero-Vaquero, S.; Garcia-Mediavilla, M.V.; Jorquera, F.; Majano, P.L.; Benet, M.; Jover, R.; González-Gallego, J.; Sánchez-Campos, S. Modulation of PI3k-LxRx-dependent lipogenesis mediated by oxidative/nitrosative stress contributes to inhibition of HCV replication by quercetin. Lab. Investig. 2014, 94, 262–274. [Google Scholar] [CrossRef]
- Wu, W.; Li, R.; Li, X.; He, J.; Jiang, S.; Liu, S.; Yang, J. Quercetin as an antiviral agent inhibits influenza A virus (IAV) entry. Viruses 2016, 8, 6. [Google Scholar] [CrossRef]
- Agrawal, P.K.; Agrawal, C.; Blunden, G. Quercetin: Antiviral significance and possible COVID-19 integrative considerations. Nat. Prod. Commun. 2020, 15, 1934578X2097629. [Google Scholar] [CrossRef]
- Biancatelli, R.M.L.C.; Berrill, M.; Catravas, J.D.; Marik, P.E. Quercetin and vitamin C: An experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19). Front. Immunol. 2020, 11, 1451. [Google Scholar] [CrossRef]
- Boretti, A. Quercetin supplementation and COVID-19. Nat. Prod. Commun. 2021, 16, 1934578X2110427. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P.; D’Angelo, A.; Di Pierro, F. A role for quercetin in coronavirus disease 2019 (COVID-19). Phytother. Res. 2021, 35, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Pavek, P.; Kamaraj, R.; Ren, L.; Zhang, T. Dietary phytochemicals as modulators of human pregnane X receptor. Crit. Rev. Food Sci. Nutr. 2021, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Egan, K.B.; Tsai, R.J.; Chuke, S.O. Integrating Childhood and Adult Blood Lead Surveillance to Improve Identification and Intervention Efforts. J. Public Health Manag. Pract. JPHMP 2019, 25 (Suppl. S1), S98–S104. [Google Scholar] [CrossRef]
- Whitehead, L.S.; Buchanan, S.D. Childhood Lead Poisoning: A Perpetual Environmental Justice Issue? J. Public Health Manag. Sapractice JPHMP 2019, 25 (Suppl. S1), S115–S120. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Shirazi, F.M.; Saeedi, F.; Roshanravan, B.; Pourbagher-Shahri, A.M.; Khorasani, E.Y.; Farkhondeh, T.; Aaseth, J.O.; Abdollahi, M.; Mehrpour, O. A systematic review of clinical and laboratory findings of lead poisoning: Lessons from case reports. Toxicol. App. Pharmacol. 2021, 429, 115681. [Google Scholar] [CrossRef]
- Alarcon, W.A. Elevated Blood Lead Levels Among Employed Adults—United States, 1994–2013. MMWR Morb. Mortal. Wkly. Rep. 2016, 63, 59–65. [Google Scholar] [CrossRef]
- Raymond, J.; Brown, M.J. Childhood Blood Lead Levels in Children Aged <5 Years—United States, 2009–2014. MMWR Surveill. Summ 2017, 66, 1–10. [Google Scholar]
- Wang, H.; Shi, H.; Chang, L.; Zhang, X.; Li, J.; Yang, Y.; Jiang, Y. Association of blood lead with calcium, iron, zinc and hemoglobin in children aged 0-7 years: A large population-based study. Biol. Trace Elem. Res. 2012, 149, 143–147. [Google Scholar] [CrossRef]
- Reddy, Y.S.; Aparna, Y.; Ramalaksmi, B.A.; Kumar, B.D. Lead and trace element levels in placenta, maternal and cord blood: A cross-sectional pilot study. J. Obstet. Gynaecol. Res. 2014, 40, 2184–2190. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Jang, T.W.; Chae, H.J.; Choi, W.J.; Ha, M.N.; Ye, B.J.; Kim, B.G.; Jeon, M.J.; Kim, S.Y.; Hong, Y.S. Evaluation and management of lead exposure. Ann. Occup. Environ. Medicine. 2015, 15, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Bergeson, L.L. The proposed lead NAAQS: Is consideration of cost in the clean air act future. Environ. Qual. Mang. 2008, 18, 79–84. [Google Scholar] [CrossRef]
- Rubin, R.; Strayer, D.S. Environmental and Nutritional Pathology. Rubin Pathology, Clinicopathologic Foundations of Medicine, 5th ed.; Lippincot Williams & Wilkins: Baltimore, MD, USA, 1986. [Google Scholar]
- Mitra, P.; Sharma, S.; Purohit, P.; Sharma, P. Clinical and molecular aspects of lead toxicity: An update. Crit. Rev. Clin. Lab. Sci. 2017, 54, 506–528. [Google Scholar] [CrossRef]
- Piasek, M.; Kostial, K.; Bunare, L. The effect of lead exposure on pathological changes in the liver and kidney in relation to age in rats. Arch. Hg. Rada. Toksikol. 1989, 40, 15–21. [Google Scholar]
- Sokol, R.Z.; Berman, N. The effect of age of exposure on lead-induced testicular toxicity. Toxicology 1991, 69, 269–278. [Google Scholar] [CrossRef]
- Tulas, I.; Reddy, N.M.; Rallenaino, J.V. Accumulation of lead and effects on total lipid derivatives in fresh water fish. Ecotoxicol. Environ. Saf. 1992, 23, 33–38. [Google Scholar] [CrossRef]
- Busselberg, D.; Evans, M.L.; Haas, H.L.; Carpenter, D.O. Blockade of mammalian and invertebrate calcium channels by lead. Neurotoxicology 1993, 14, 249–258. [Google Scholar]
- Nehru, B.; Kaushal, S. Alterations in the hepatic enzymes following experimental lead poisoning. Biol. Trace Elem. Res. 1993, 38, 27–34. [Google Scholar] [CrossRef]
- Sherlock, S.; Dodey, J. Diseases of the Liver and Biliary System; Blackwell Scientific Publication: London, UK, 1993. [Google Scholar]
- Benjamin, M.M. Outline of Veterinary Clinical Pathology, 3rd ed.; The Iowa State University Press: Ames, Iowa, 1974. [Google Scholar]
- Coles, E.H. Veterinary Clinical Pathology, 3rd ed.; W.B. Saunders Co.: Philadelphia, PA, USA, 1986. [Google Scholar]
- Parasuraman, S.; Maithli, M. Antioxidant and drug metabolism. Free. Radic. Antioxid. 2014, 4, 1–2. [Google Scholar]
- Urso, M.L.; Clarkson, P.M. Oxidative stress, exercise, and antioxidant supplementation. Toxicology 2003, 189, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.; Wang, N.; Zhang, Z.; Lao, L.; Wong, C. The role of oxidative stress and antioxidants in liver diseases. Int. J. Med. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, V.; Trujillo, M.; Diederichs, T.; Dick, T.P.; Radi, R. Redox-sensitive GFP fusions for monitoring the catalytic mechanism and inactivation of peroxiredoxins in living cells. Redox Biol. 2018, 14, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Salvamani, S.; Gunasekaran, B.; Shaharuddn, N.A.; Ahmed, S.A.; Shukar, M.Y. Antiatherosclerotic effects of plant flavonoids. Biomed. Res. Int. 2014, 480258. [Google Scholar]
- Augus, S.T.; Eka, M.; Oh, L.K.; Keizo, H. Isolation and characterization of antioxidant protein functions from melinjo (Gnetum gnemon) seeds. J. Agric. Food Chem. 2011, 59, 5648–5656. [Google Scholar]
- Yigit, A.A.; Panda, A.K.; Cherian, G. The avian embryo and its antioxidant defense system. World Poult. Sci. 2014, 70, 563–574. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Negulescu, G.P. Methods for total antioxidant activity determination: A review. Bioch. Analyt. Bioch. 2011, 1, 106. [Google Scholar] [CrossRef]
- Case, A.J. On the origin of superoxide dismutase: An evolutionary perspective of superoxide-mediated redox signaling. Antioxidants 2017, 6, 82. [Google Scholar] [CrossRef]
- Pham-Huy, Z.A.; He, H.; Pham-Huy, C. Free radicals and antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar]
- Feng, P.; Ding, H.; Lin, H.; Chen, W. AOD: The antioxidant protein database. Sci. Rep. 2017, 7, 7449. [Google Scholar] [CrossRef]
- Hounkpatin, A.S.; Edorth, P.A.; Guedenan, P.A.; Limba, C.G.; Ogunkanmi, A. Hematological evaluation of Wister rats exposed to chronic doses of cadmium, mercury and combined cadmium and mercury. Afr. J. Biotechnol. 2003, 12, 3731–3737. [Google Scholar]
- Lodia, S.; Kansala, Z. Antioxidant activity of Rubia cordfolia against lead toxicity. Int. J. Phamacol. Sci. Res. 2012, 3, 2224–2232. [Google Scholar]
- Johar, D.; Roth, J.C.; Bay, G.; Walker, J.N.; Kroczak, T.J.; Los, M. Inflammatory response, reactive oxygen species, programmed (necrotic-like and apoptotic) cell death and cancer. Rocz. Akad. Med. Bialymst. 2004, 49, 31–39. [Google Scholar] [PubMed]
- Dhu, P.; Gorg, M.L.; Dhawn, D.K. Protective role of zinc in nickel induced hepatotoxicity in rats. Chem. Biol. Interaction. 2004, 150, 199–209. [Google Scholar]
- Firoozichahak, A.; Rahimnejad, S.; Rahmani, A.; Parvizimehr, A.; Aghaei, A.; Rahimpoor, R. Effect of occupational exposure to lead on serum levels of lipid profile and liver enzymes: An occupational cohort study. Toxicol. Rep. 2022, 9, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Packer, L. Thiol homeostasis and supplements in physical exercise. Am. J. Clin. Nutr. 2000, 72, 653–669. [Google Scholar] [CrossRef]
- Baron, E.S.G. Thiol groups of biological importance. In Advances in Enzymology and Related Areas of Molecular Biology; Wiley Online Library: Hoboken, NJ, USA, 2006; Volume 11, pp. 201–266. [Google Scholar]
- Von Ossowski, I.; Hausner, G.; Loewen, P.C. Molecular evolutionary analysis based on the amino acid sequence of catalase. J. Mol. Evol. 1993, 37, 71–76. [Google Scholar] [CrossRef]
- Lardinosis, O.M. Reactions of bovine liver catalase with superoxide with superoxide radicals and hydrogen peroxide. Free Radic. Res. 1995, 22, 251–274. [Google Scholar] [CrossRef]
- Habib, L.K.; Lee, M.T.C.; Yang, J. Inhibitors of catalase-amyloid interactions protect cells from β-amyloid induced oxidative stress and toxicity. J. Biol. Chem. 2010, 285, 38933–38943. [Google Scholar] [CrossRef]
- Deleve, L.D.; Kaplowitz, N. Glutathione metabolism and its role in hepatoxicity. Pharmacol. Ther. 1991, 52, 287–305. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Alfonso-Prieto, M.; Biarnes, X.; Vidosschi, P.; Roviva, C. The molecular mechanism of the catalase reaction. J. Am. Chem. Soc. 2009, 131, 11751–11761. [Google Scholar] [CrossRef] [PubMed]
- De Cavanagh, E.M.; Inserria, F.; Ferder, L.; Fraga, C.G. Enalapril and captopril enhance glutathione-dependent antioxidant defenses in mouse tissues. Am. J. Physiol. 2000, 278, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Berrnner, A. Oxygen free radicals and metallothionein. Free Radic. Biol. Med. 1993, 14, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Torun, A.N.; Kulaksizoglu, S.; Kulaksizoglu, M.; Pamuk, B.O.; Isbilen, E.; Tutuncu, N.B. Serum total antioxidant status and lipid peroxidation marker malondialdehyde levels in overt and subclinical hypothyroidism. Clin. Endocrinol. 2009, 70, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Coline, R.; Clement, V.; Anne, L.; Frederic, B. Use of H2O2 to cause oxidative stress, the catalase issue. Int. J. Mol. Sci. 2020, 21, 9149. [Google Scholar]
- Hanasaki, Y.; Ogawa, S.; Fukui, S. The correlation between active oxygen scavenging and antioxidative effects of flavonoids. Free Radic. Bio. Med. 1994, 16, 845–850. [Google Scholar] [CrossRef]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its main pharmacological activity and potential application in clinical medicine. Oxidative Med. Cell. Longev. 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Tsai, C.F.; Chen, G.W.; Chen, Y.C.; Shen, C.K.; Lu, D.Y.; Yang, L.Y.; Chen, J.H.; Yeh, W.L. Regulatory effects of quercetin on M1/M2 macrophage polarization and oxidative/antioxidative balance. Nutrients 2021, 14, 67. [Google Scholar] [CrossRef]
- Zhu, X.; Li, N.; Wang, Y.; Ding, L.; Chen, H.; Yu, Y.; Shi, X. Protective effects of quercetin on UVB irradiation-induced cytotoxicity through ROS clearance in keratinocyte cells. Oncol. Rep. 2017, 37, 209–218. [Google Scholar] [CrossRef]
- Prochazkova, D.; Bousova, I.; Wilhemova, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 313–523. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.Y.; Ambigaipalan, P.; Shahidi, F. Preparation of quercetin esters and their antioxidant activity. J. Agric. Food Chem. 2019, 67, 10653–10659. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhu, Y.; Liu, N.; Fan, D.; Wang, M.; Zhao, Y. Antioxidative properties and chemical changes of quercetin in fish oil: Quercetin reacts with free fatty acids to form its ester derivatives. J. Agric. Food Chem. 2021, 69, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Babenkova, I.V.; Osipov, A.N.; Teselkin, Y.O. The effects of dihydroquercetin on catalytic activity of iron (II) ions in the Fenton reaction. Bull. Exp. Biol. Med. 2018, 165, 347–350. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Y.; Yu, H.; Gao, C.; Liu, L.; Xing, M.; Liu, L.; Yao, P. Quercetin attenuates chronic ethanol hepatotoxicity: Implication of free iron uptake and release. Food Chem. Toxicol. 2014, 67, 131–138. [Google Scholar] [CrossRef]
- Dong, B.; Shi, Z.; Dong, Y.; Chen, J.; Wu, Z.X.; Wu, W.; Chen, Z.S.; Han, C. Quercetin ameliorates oxidative stress induced cell apoptosis of seminal vesicles via activating Nrf2 in type 1 diabetic rats. Biomed. Pharmacother. 2022, 151, 113108. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, X.; Zhu, G.; Liu, H.; Chen, J.; Wang, Y.; He, X. Quercetin inhibits TNF-α induced HUVECs apoptosis and inflammation via downregulating NF-kB and AP-1 signaling pathway in vitro. Medicine 2020, 99, e22241. [Google Scholar] [CrossRef]
- Li, F.; Liu, J.; Tang, S.; Yan, J.; Chen, H.; Li, D.; Yan, X. Quercetin regulates inflammation, oxidative stress, apoptosis, and mitochondrial structure and function in H9C2 cells by promoting PVT1 expression. Acta Histochem. 2021, 123, 151819. [Google Scholar] [CrossRef]
- Odbayar, T.O.; Kimura, T.; Tsushida, T.; Ide, T. Isoenzyme-specific up-regulation of glutathione transferase and aldo-keto reductase mRNA expression by dietary quercetin in rat liver. Mol. Cell. Biochem. 2009, 325, 121–130. [Google Scholar] [CrossRef]
- Kobori, M.; Takahashi, Y.; Akimoto, Y.; Sakurai, M.; Matsunaga, I.; Nishimuro, H.; Ippoushi, K.; Oike, H.; Ohnishi-Kameyama, M. Chronic high intake of quercetin reduces oxidative stress and induces expression of the antioxidant enzymes in the liver and visceral adipose tissues in mice. J. Funct. Foods. 2015, 15, 551–560. [Google Scholar] [CrossRef]
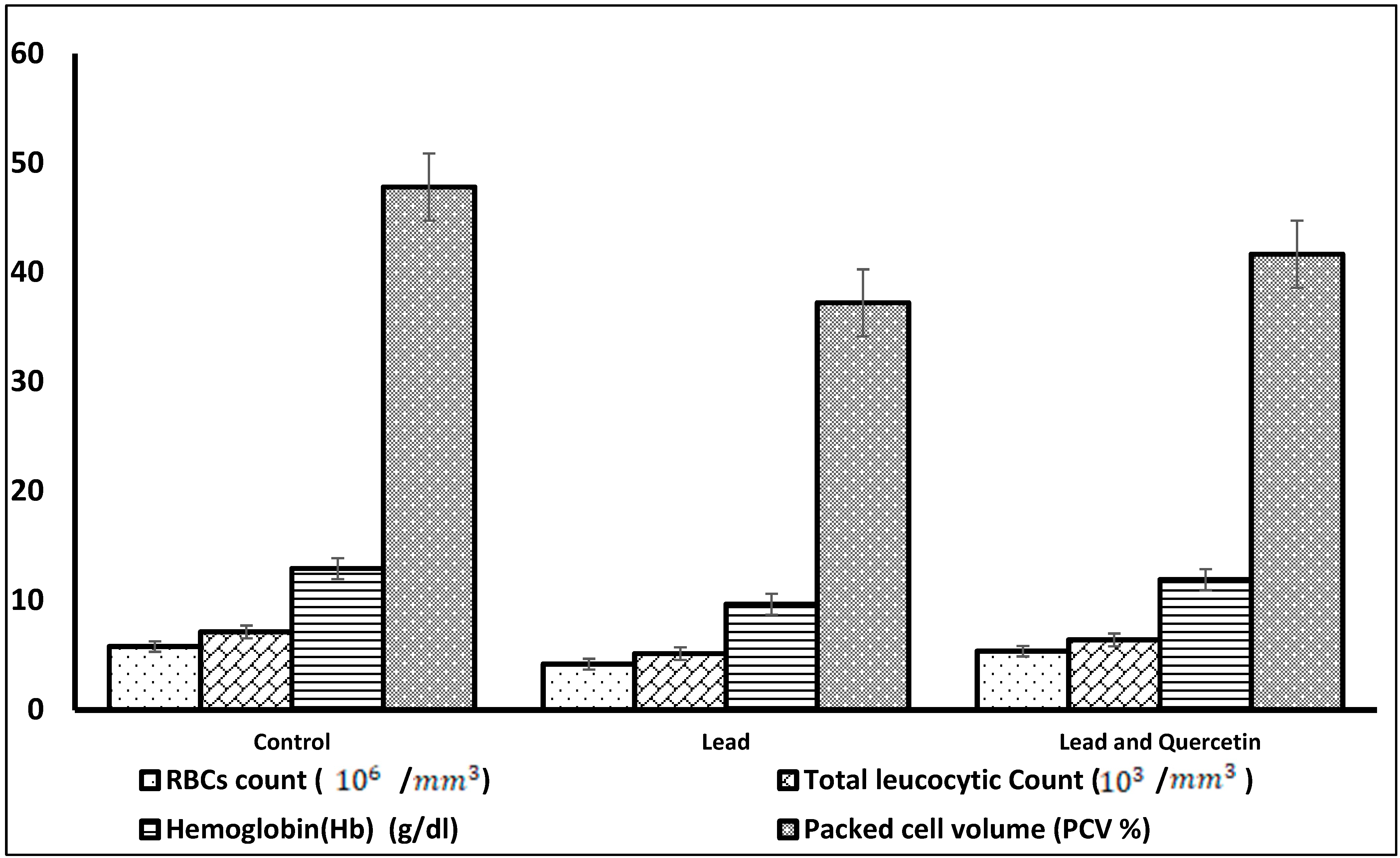
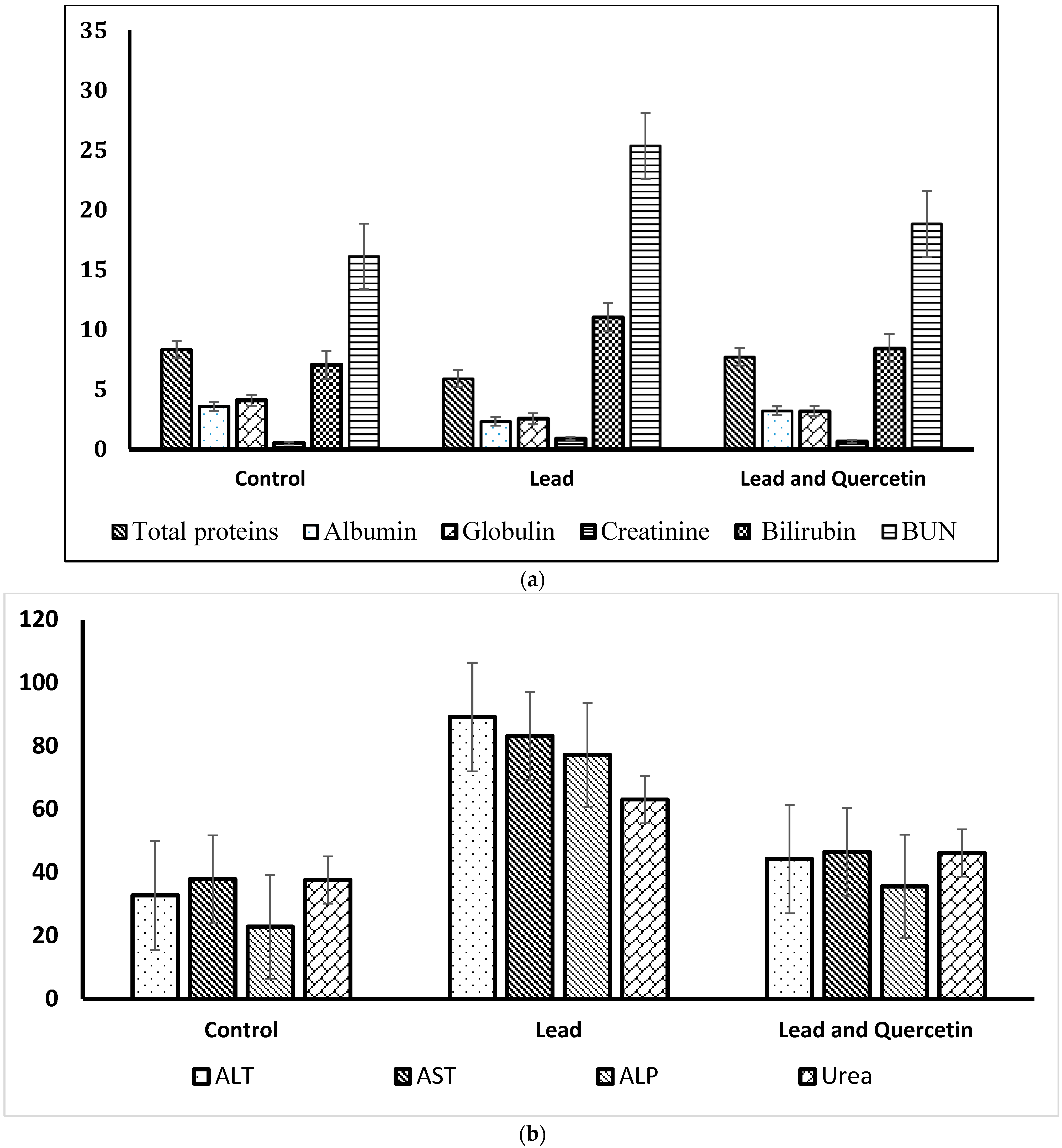
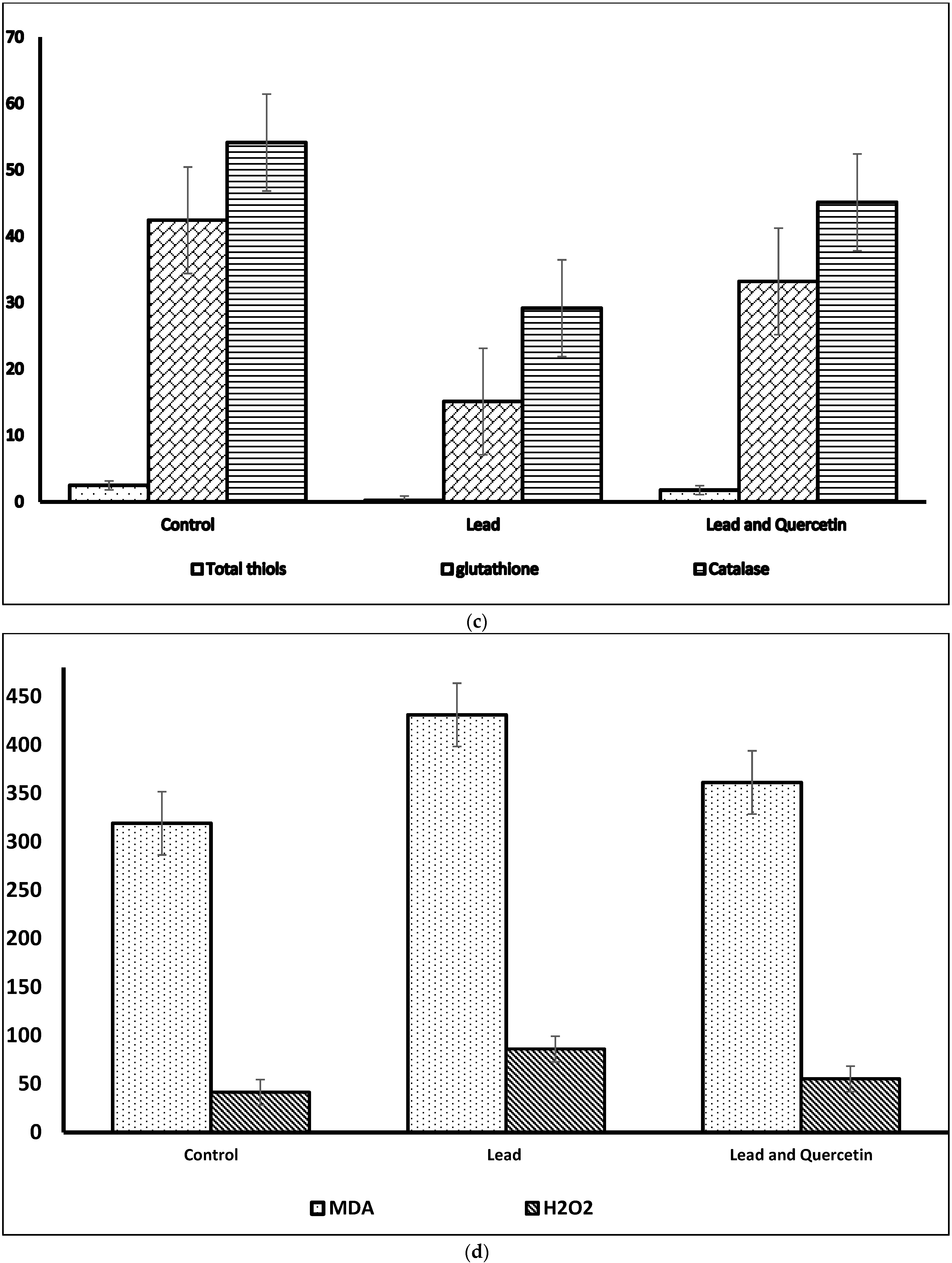
| Parameter | Control | Lead | Lead and Quercetin Phytosome |
|---|---|---|---|
| RBCs count (106/mm3) | 5.77 ± 0.08 | 4.17 * ± 0.19 | 5.34 ** ± 0.07 |
| Total leucocytic | 7.11 ± 0.04 | 5.12 * ± 0.31 | 6.38 ** ± 0.06 |
| Count | |||
| (103/mm3) | |||
| Hemoglobin (Hb) | 12.90 ± 0.21 | 9.64 * ± 0.44 | 11.89 ** ± 0.53 |
| (g/dL) | |||
| Packed cell volume | 47.81 ± 0.32 | 37.22 * ± 0.79 | 41.67 ** ± 0.60 |
| (PCV%) |
| Parameter | Control | Lead | Lead and Quercetin Phytosome |
|---|---|---|---|
| a. Levels of total proteins (g/dL), albumin (g/dL), globulin (g/dL), creatinine (mg/dL), urea (mg/dL), BUN (mg/dL) and bilirubin (mg/dL). | |||
| Total proteins | 8.33 ± 0.15 | 5.91 * ± 0.19 | 7.71 ** ± 0.13 |
| Albumin | 3.58 ± 0.06 | 2.34 * ± 0.18 | 3.22 ** ± 0.11 |
| Globulin | 4.08 ± 0.07 | 2.57 * ± 0.24 | 3.19 ± 0.16 |
| Creatinine | 0.51 ± 0.14 | 0.91 * ± 0.47 | 0.66 ** ± 0.31 |
| Urea | 37.66 ± 0.71 | 63.07 * ± 0.49 | 46.18 ** ± 0.81 |
| BUN | 16.11 ± 1.04 | 25.35 * ± 1.34 | 18.83 ** ± 1.62 |
| Bilirubin | 7.03 ± 0.31 | 11.05 * ± 0.46 | 8.44 ** ± 0.49 |
| b. Levels of alanine transferase (ALT) (IU/L), aspartate transferase (AST) (IU/L) and alkaline phosphatase (ALP) (IU/L). | |||
| ALT | 32.79 ± 0.33 | 89.17 * ± 0.51 | 44.29 ** ± 0.64 |
| AST | 37.88 ± 0.71 | 83.13 * ± 0.62 | 46.55 ** ± 0.59 |
| ALP | 22.87 ± 0.57 | 77.22 * ± 0.42 | 35.61 ** ± 0.34 |
| c. Levels of total thiols (mmol/L), glutathione (µg/mL), catalase(IU/L), malondialdehyde (MDA) (nmol/mL) and hydrogen peroxide (H2O2) (mmol/L). | |||
| Total thiols | 2.46 ± 0.27 | 0.231 * ± 0.041 | 1.75 ** ± 0.39 |
| Glutathione | 42.41 ± 1.41 | 15.09 * ± 0.37 | 33.19 ** ± 1.12 |
| Catalase | 54.11 ± 1.63 | 29.15 * ± 1.05 | 45.11 **± 1.28 |
| MDA | 319.11 ± 3.23 | 431.17 * ± 3.78 | 361.27 ** ± 2.62 |
| H2O2 | 41.37 ± 1.60 | 86.11 * ± 1.13 | 55. 27 ** ± 1.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Zharani, M.; Mubarak, M.; Rudayni, H.A.; Al-Doaiss, A.A.; Abd-Elwahab, M.M.; Al-Eissa, M.S. Quercetin as a Dietary Supplementary Flavonoid Alleviates the Oxidative Stress Induced by Lead Toxicity in Male Wistar Rats. Nutrients 2023, 15, 1888. https://doi.org/10.3390/nu15081888
Al-Zharani M, Mubarak M, Rudayni HA, Al-Doaiss AA, Abd-Elwahab MM, Al-Eissa MS. Quercetin as a Dietary Supplementary Flavonoid Alleviates the Oxidative Stress Induced by Lead Toxicity in Male Wistar Rats. Nutrients. 2023; 15(8):1888. https://doi.org/10.3390/nu15081888
Chicago/Turabian StyleAl-Zharani, Mohammed, Mohammed Mubarak, Hassan Ahmed Rudayni, Amin A. Al-Doaiss, Mahmoud M. Abd-Elwahab, and Mohammed S. Al-Eissa. 2023. "Quercetin as a Dietary Supplementary Flavonoid Alleviates the Oxidative Stress Induced by Lead Toxicity in Male Wistar Rats" Nutrients 15, no. 8: 1888. https://doi.org/10.3390/nu15081888
APA StyleAl-Zharani, M., Mubarak, M., Rudayni, H. A., Al-Doaiss, A. A., Abd-Elwahab, M. M., & Al-Eissa, M. S. (2023). Quercetin as a Dietary Supplementary Flavonoid Alleviates the Oxidative Stress Induced by Lead Toxicity in Male Wistar Rats. Nutrients, 15(8), 1888. https://doi.org/10.3390/nu15081888





