Combination of Lactobacillus plantarum HAC03 and Garcinia cambogia Has a Significant Anti-Obesity Effect in Diet-Induced Obesity Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Culture Conditions and Natural Product
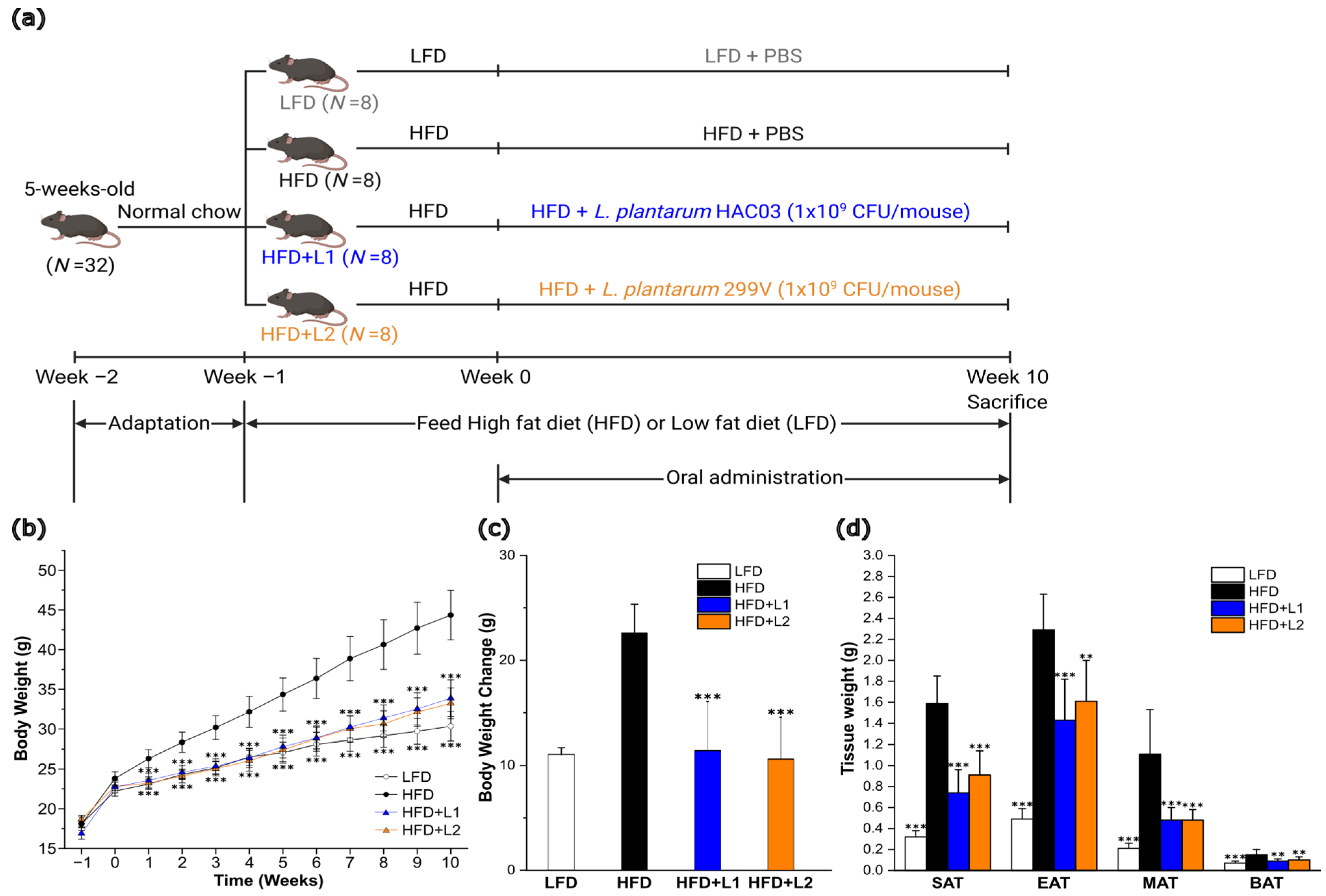
2.2. Animal Experiments
2.3. Serum Analysis
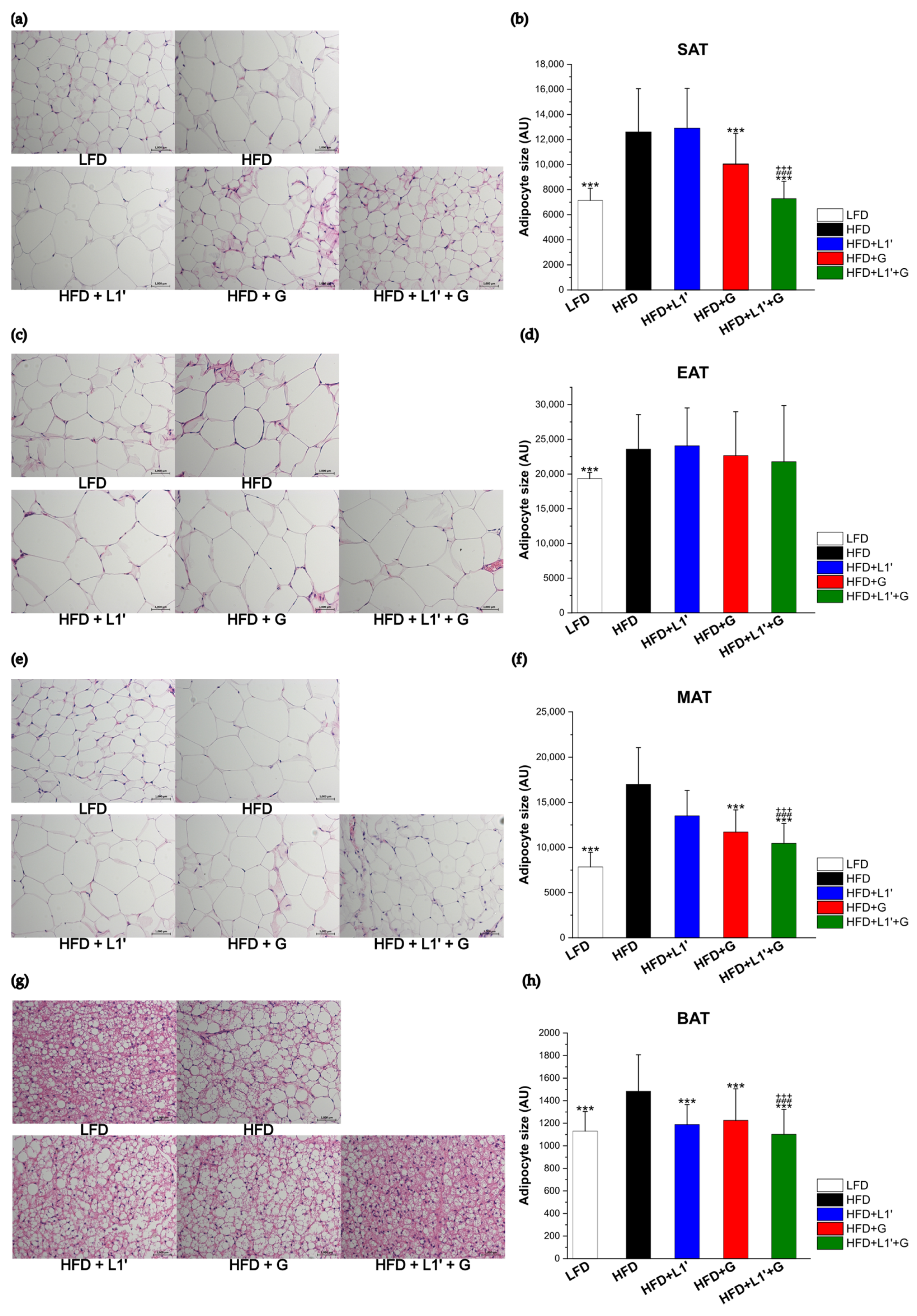
2.4. Histological Analysis
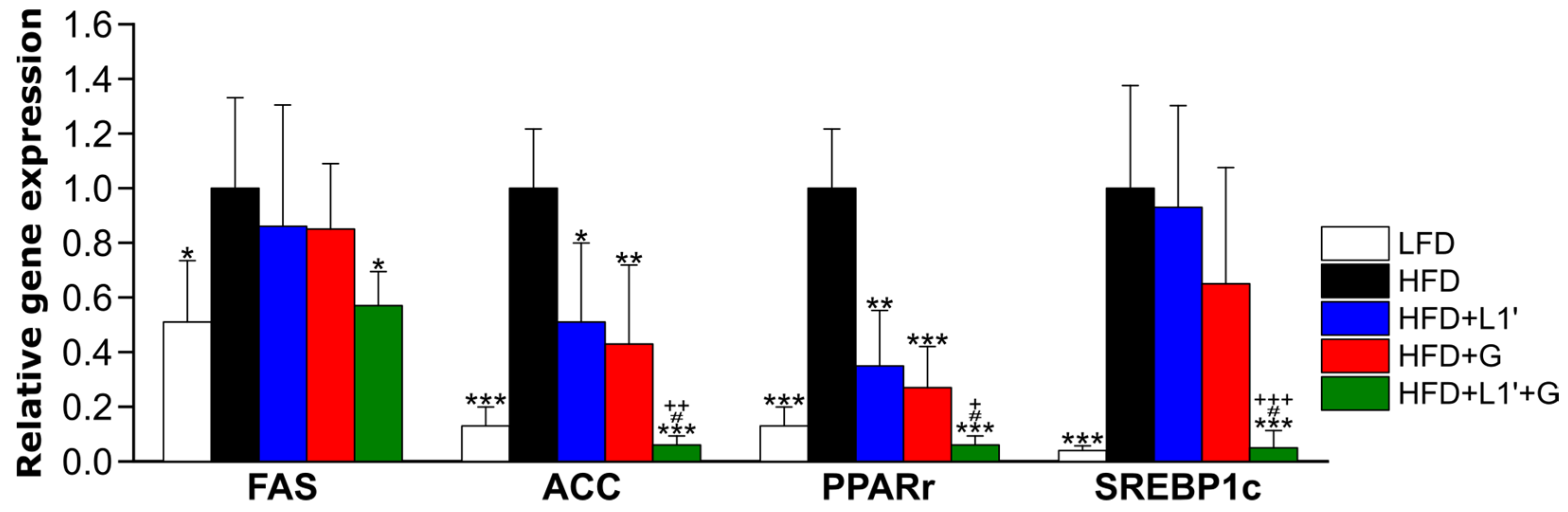
2.5. Real-Time RT PCR
2.6. Gut Microbiota Analysis
2.7. Statistical Analyses
3. Results
3.1. L. plantarum HAC03 Has an Anti-Obesity Effect on Dietary-Induced Obesity Mice
3.2. Combining L. plantarum HAC03 with G. cambogia Extract Has a Synergic Effect on Losing Weight
3.3. Combining L. plantarum HAC03 and G. cambogia Has a Synergic Effect on Decreasing the Biochemical Markers of Obesity
3.4. Combining L. plantarum HAC03 and G. cambogia Has a Synergic Effect on Decreasing the Adipocyte Size
3.5. Combining L. plantarum HAC03 and G. cambogia Has a Synergic Effect on Decreasing mRNA Expression of Fatty Acid Synthesis
3.6. Combining L. plantarum HAC03 and G. cambogia Affect Gut Microbiota Composition
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OECD; World Health Organization (WHO). Overweight and Obesity; OECD: Paris, France, 2020.
- James, W.P.T. WHO Recognition of the Global Obesity Epidemic. Int. J. Obes. 2008, 32, S120–S126. [Google Scholar] [CrossRef] [PubMed]
- Abarca-Gómez, L.; Abdeen, Z.A.; Hamid, Z.A.; Abu-Rmeileh, N.M.; Acosta-Cazares, B.; Acuin, C.; Adams, R.J.; Aekplakorn, W.; Afsana, K.; Aguilar-Salinas, C.A.; et al. Worldwide Trends in Body-Mass Index, Underweight, Overweight, and Obesity from 1975 to 2016: A Pooled Analysis of 2416 Population-Based Measurement Studies in 128·9 Million Children, Adolescents, and Adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Rufino, A.T.; Costa, V.M.; Carvalho, F.; Fernandes, E. Flavonoids as Antiobesity Agents: A Review. Med. Res. Rev. 2021, 41, 556–585. [Google Scholar] [CrossRef]
- Patel, D.K.; Stanford, F.C. Safety and Tolerability of New-Generation Anti-Obesity Medications: A Narrative Review. Postgrad. Med. 2018, 130, 173–182. [Google Scholar] [CrossRef]
- Iepsen, E.W.; Torekov, S.S.; Holst, J.J. Liraglutide for Type 2 Diabetes and Obesity: A 2015 Update. Expert. Rev. Cardiovasc. Ther. 2015, 13, 753–767. [Google Scholar] [CrossRef]
- Donnelly, D. The Structure and Function of the Glucagon-like Peptide-1 Receptor and Its Ligands. Br. J. Pharmacol. 2012, 166, 27–41. [Google Scholar] [CrossRef]
- Barrea, L.; Pugliese, G.; Muscogiuri, G.; Laudisio, D.; Colao, A.; Savastano, S. New-Generation Anti-Obesity Drugs: Naltrexone/Bupropion and Liraglutide. An Update for Endocrinologists and Nutritionists. Minerva Endocrinol. 2020, 45, 127–137. [Google Scholar] [CrossRef]
- Kern, E.; VanWagner, L.B.; Yang, G.-Y.; Rinella, M.E. Liraglutide-Induced Autoimmune Hepatitis. JAMA Intern. Med. 2014, 174, 984–987. [Google Scholar] [CrossRef]
- Singh, S.; Chang, H.-Y.; Richards, T.M.; Weiner, J.P.; Clark, J.M.; Segal, J.B. Glucagonlike Peptide 1-Based Therapies and Risk of Hospitalization for Acute Pancreatitis in Type 2 Diabetes Mellitus: A Population-Based Matched Case-Control Study. JAMA Intern. Med. 2013, 173, 534–539. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, H.; Dong, P. Liraglutide Reduces Systolic Blood Pressure in Patients with Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Trials. Clin. Exp. Hypertens. 2020, 42, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Marso, S.P.; Neeland, I.J. Liraglutide for Weight Management: A Critical Review of the Evidence. Obes. Sci. Pract. 2017, 3, 3–14. [Google Scholar] [CrossRef]
- Hicks, B.M.; Yin, H.; Yu, O.H.Y.; Pollak, M.N.; Platt, R.W.; Azoulay, L. Glucagon-like Peptide-1 Analogues and Risk of Breast Cancer in Women with Type 2 Diabetes: Population Based Cohort Study Using the UK Clinical Practice Research Datalink. BMJ 2016, 355, i5340. [Google Scholar] [CrossRef]
- Elashoff, M.; Matveyenko, A.V.; Gier, B.; Elashoff, R.; Butler, P.C. Pancreatitis, Pancreatic, and Thyroid Cancer With Glucagon-Like Peptide-1–Based Therapies. Gastroenterology 2011, 141, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Sorbara, M.T.; Pamer, E.G. Microbiome-Based Therapeutics. Nat. Rev. Microbiol. 2022, 20, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Belizário, J.E.; Faintuch, J. Microbiome and Gut Dysbiosis. Exp. Suppl. 2018, 109, 459–476. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Prifti, E.; Belda, E.; Ichou, F.; Kayser, B.D.; Dao, M.C.; Verger, E.O.; Hedjazi, L.; Bouillot, J.-L.; Chevallier, J.-M.; et al. Major Microbiota Dysbiosis in Severe Obesity: Fate after Bariatric Surgery. Gut 2019, 68, 70–82. [Google Scholar] [CrossRef]
- Amabebe, E.; Robert, F.O.; Agbalalah, T.; Orubu, E.S.F. Microbial Dysbiosis-Induced Obesity: Role of Gut Microbiota in Homoeostasis of Energy Metabolism. Br. J. Nutr. 2020, 123, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.A.; Shapiro, J.A.; Church, T.R.; Miller, G.; Trinh-Shevrin, C.; Yuen, E.; Friedlander, C.; Hayes, R.B.; Ahn, J. A Taxonomic Signature of Obesity in a Large Study of American Adults. Sci. Rep. 2018, 8, 9749. [Google Scholar] [CrossRef]
- Duan, M.; Wang, Y.; Zhang, Q.; Zou, R.; Guo, M.; Zheng, H. Characteristics of Gut Microbiota in People with Obesity. PLoS ONE 2021, 16, e0255446. [Google Scholar] [CrossRef]
- Jin, J.; Cheng, R.; Ren, Y.; Shen, X.; Wang, J.; Xue, Y.; Zhang, H.; Jia, X.; Li, T.; He, F.; et al. Distinctive Gut Microbiota in Patients with Overweight and Obesity with Dyslipidemia and Its Responses to Long-Term Orlistat and Ezetimibe Intervention: A Randomized Controlled Open-Label Trial. Front. Pharmacol. 2021, 12, 732541. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, C.; Zhang, H.; Qu, G.; Li, C.; Liu, L. Biotransformation of Polyphenols in Apple Pomace Fermented by β-Glucosidase-Producing Lactobacillus Rhamnosus L08. Foods 2021, 10, 1343. [Google Scholar] [CrossRef] [PubMed]
- Tamboli, C.P.; Neut, C.; Desreumaux, P.; Colombel, J.F. Dysbiosis in Inflammatory Bowel Disease. Gut 2004, 53, 1–4. [Google Scholar] [CrossRef]
- Felis, G.E.; Dellaglio, F. Taxonomy of Lactobacilli and Bifidobacteria. Curr. Issues Intest. Microbiol. 2007, 8, 44–61. [Google Scholar]
- Bull, M.; Plummer, S.; Marchesi, J.; Mahenthiralingam, E. The Life History of Lactobacillus Acidophilus as a Probiotic: A Tale of Revisionary Taxonomy, Misidentification and Commercial Success. FEMS Microbiol. Lett. 2013, 349, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Oh, S. Lactobacillus acidophilus as a Probiotics. J. Dairy. Sci. Biotechnol. 2019, 37, 155–166. [Google Scholar] [CrossRef]
- Arasu, M.V.; Al-Dhabi, N.A.; Ilavenil, S.; Choi, K.C.; Srigopalram, S. In Vitro Importance of Probiotic Lactobacillus Plantarum Related to Medical Field. Saudi J. Biol. Sci. 2016, 23, S6–S10. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-L.; Zhang, L.-Q.; Yang, Y.; Yin, B.-C.; Ye, B.-C.; Zhou, Y. Advances in the Role and Mechanism of Lactic Acid Bacteria in Treating Obesity. Food Bioeng. 2022, 1, 101–115. [Google Scholar] [CrossRef]
- Aggarwal, J.; Swami, G.; Kumar, M. Probiotics and Their Effects on Metabolic Diseases: An Update. J. Clin. Diagn. Res. 2013, 7, 173–177. [Google Scholar] [CrossRef]
- Park, S.; Ji, Y.; Park, H.; Lee, K.; Park, H.; Beck, B.R.; Shin, H.; Holzapfel, W.H. Evaluation of Functional Properties of Lactobacilli Isolated from Korean White Kimchi. Food Control 2016, 69, 5–12. [Google Scholar] [CrossRef]
- Ivanova, S.; Delattre, C.; Karcheva-Bahchevanska, D.; Benbasat, N.; Nalbantova, V.; Ivanov, K. Plant-Based Diet as a Strategy for Weight Control. Foods 2021, 10, 3052. [Google Scholar] [CrossRef]
- Golzarand, M.; Omidian, M.; Toolabi, K. Effect of Garcinia Cambogia Supplement on Obesity Indices: A Systematic Review and Dose-Response Meta-Analysis. Complement. Ther. Med. 2020, 52, 102451. [Google Scholar] [CrossRef]
- Chuah, L.O.; Ho, W.Y.; Beh, B.K.; Yeap, S.K. Updates on Antiobesity Effect of Garcinia Origin (−)-HCA. Evid. Based Complement. Altern. Med. 2013, 2013, 751658. [Google Scholar] [CrossRef]
- Khwairakpam, A.D.; Banik, K.; Girisa, S.; Shabnam, B.; Shakibaei, M.; Fan, L.; Arfuso, F.; Monisha, J.; Wang, H.; Mao, X.; et al. The Vital Role of ATP Citrate Lyase in Chronic Diseases. J. Mol. Med. 2020, 98, 71–95. [Google Scholar] [CrossRef]
- Ohia, S.E.; Awe, S.O.; LeDay, A.M.; Opere, C.A.; Bagchi, D. Effect of Hydroxycitric Acid on Serotonin Release from Isolated Rat Brain Cortex. Res. Commun. Mol. Pathol. Pharmacol. 2001, 109, 210–216. [Google Scholar]
- Baky, M.H.; Fahmy, H.; Farag, M.A. Recent Advances in Garcinia Cambogia Nutraceuticals in Relation to Its Hydroxy Citric Acid Level. A Comprehensive Review of Its Bioactive Production, Formulation, and Analysis with Future Perspectives. ACS Omega 2022, 7, 25948–25957. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-Y.; Kim, B.; Hyun, C.-K. Lactobacillus Rhamnosus GG Reverses Insulin Resistance but Does Not Block Its Onset in Diet-Induced Obese Mice. J. Microbiol. Biotechnol. 2015, 25, 753–757. [Google Scholar] [CrossRef] [PubMed]
- King, W.L.; Siboni, N.; Williams, N.L.R.; Kahlke, T.; Nguyen, K.V.; Jenkins, C.; Dove, M.; O’Connor, W.; Seymour, J.R.; Labbate, M. Variability in the Composition of Pacific Oyster Microbiomes Across Oyster Families Exhibiting Different Levels of Susceptibility to OsHV-1 Μvar Disease. Front. Microbiol. 2019, 10, 473. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Glöckner, F.O.; Yilmaz, P.; Quast, C.; Gerken, J.; Beccati, A.; Ciuprina, A.; Bruns, G.; Yarza, P.; Peplies, J.; Westram, R.; et al. 25 Years of Serving the Community with Ribosomal RNA Gene Reference Databases and Tools. J. Biotechnol. 2017, 261, 169–176. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Lahti, L.; Shetty, S. Microbiome R Package. Bioconductor. 2017. Available online: https://doi.org/10.18129/B9.bioc.microbiome (accessed on 27 December 2022).
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for Prediction of Metagenome Functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic Biomarker Discovery and Explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Tallarida, R.J. Quantitative Methods for Assessing Drug Synergism. Genes Cancer 2011, 2, 1003. [Google Scholar] [CrossRef]
- Han, J.-H.; Park, M.-H.; Myung, C.-S. Garcinia Cambogia Ameliorates Non-Alcoholic Fatty Liver Disease by Inhibiting Oxidative Stress-Mediated Steatosis and Apoptosis through NRF2-ARE Activation. Antioxidants 2021, 10, 1226. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial Ecology: Human Gut Microbes Associated with Obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Johansson, M.L.; Molin, G.; Jeppsson, B.; Nobaek, S.; Ahrné, S.; Bengmark, S. Administration of Different Lactobacillus Strains in Fermented Oatmeal Soup: In Vivo Colonization of Human Intestinal Mucosa and Effect on the Indigenous Flora. Appl. Environ. Microbiol. 1993, 59, 15–20. [Google Scholar] [CrossRef]
- Tomar, M.; Rao, R.P.; Dorairaj, P.; Koshta, A.; Suresh, S.; Rafiq, M.; Kumawat, R.; Paramesh, R.; Babu, U.V.; Venkatesh, K.V. A Clinical and Computational Study on Anti-Obesity Effects of Hydroxycitric Acid. RSC Adv. 2019, 9, 18578–18588. [Google Scholar] [CrossRef]
- Heo, J.; Seo, M.; Park, H.; Lee, W.K.; Guan, L.L.; Yoon, J.; Caetano-Anolles, K.; Ahn, H.; Kim, S.-Y.; Kang, Y.-M.; et al. Gut Microbiota Modulated by Probiotics and Garcinia Cambogia Extract Correlate with Weight Gain and Adipocyte Sizes in High Fat-Fed Mice. Sci. Rep. 2016, 6, 33566. [Google Scholar] [CrossRef]
- Kasarala, G.; Tillmann, H.L. Standard Liver Tests. Clin. Liver Dis. 2016, 8, 13–18. [Google Scholar] [CrossRef]
- Jena, B.S.; Jayaprakasha, G.K.; Singh, R.P.; Sakariah, K.K. Chemistry and Biochemistry of (-)-Hydroxycitric Acid from Garcinia. J. Agric. Food Chem. 2002, 50, 10–22. [Google Scholar] [CrossRef]
- Jia, Y.; Wu, C.; Kim, J.; Kim, B.; Lee, S.-J. Astaxanthin Reduces Hepatic Lipid Accumulations in High-Fat-Fed C57BL/6J Mice via Activation of Peroxisome Proliferator-Activated Receptor (PPAR) Alpha and Inhibition of PPAR Gamma and Akt. J. Nutr. Biochem. 2016, 28, 9–18. [Google Scholar] [CrossRef]
- Xu, H.F.; Luo, J.; Zhao, W.S.; Yang, Y.C.; Tian, H.B.; Shi, H.B.; Bionaz, M. Overexpression of SREBP1 (Sterol Regulatory Element Binding Protein 1) Promotes de Novo Fatty Acid Synthesis and Triacylglycerol Accumulation in Goat Mammary Epithelial Cells. J. Dairy. Sci. 2016, 99, 783–795. [Google Scholar] [CrossRef]
- Palmas, V.; Pisanu, S.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Vascellari, S.; Loviselli, A.; Manzin, A.; et al. Gut Microbiota Markers Associated with Obesity and Overweight in Italian Adults. Sci. Rep. 2021, 11, 5532. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut Microbiota from Twins Discordant for Obesity Modulate Metabolism in Mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef]
- Whittaker, R.H. Evolution and Measurement of Species Diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Pinart, M.; Dötsch, A.; Schlicht, K.; Laudes, M.; Bouwman, J.; Forslund, S.K.; Pischon, T.; Nimptsch, K. Gut Microbiome Composition in Obese and Non-Obese Persons: A Systematic Review and Meta-Analysis. Nutrients 2021, 14, 12. [Google Scholar] [CrossRef]
- Hart, C.; Cock, I.E. An Examination of the Antimicrobial and Anticancer Properties of Garcinia Cambogia Fruit Pericarp Extracts. Biol. Eng. Med. Sci. Rep. 2016, 2, 55–63. [Google Scholar] [CrossRef]
- Su, X. Elucidating the Beta-Diversity of the Microbiome: From Global Alignment to Local Alignment. mSystems 2021, 6, e00363-21. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lordan, C.; Ross, R.P.; Cotter, P.D. Gut Microbes from the Phylogenetically Diverse Genus Eubacterium and Their Various Contributions to Gut Health. Gut Microbes 2020, 12, 1802866. [Google Scholar] [CrossRef]
- Wong, J.; Piceno, Y.M.; DeSantis, T.Z.; Pahl, M.; Andersen, G.L.; Vaziri, N.D. Expansion of Urease- and Uricase-Containing, Indole- and p-Cresol-Forming and Contraction of Short-Chain Fatty Acid-Producing Intestinal Microbiota in ESRD. AJN 2014, 39, 230–237. [Google Scholar] [CrossRef]
- Biddle, A.; Stewart, L.; Blanchard, J.; Leschine, S. Untangling the Genetic Basis of Fibrolytic Specialization by Lachnospiraceae and Ruminococcaceae in Diverse Gut Communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Li, L.; Batt, S.M.; Wannemuehler, M.; Dispirito, A.; Beitz, D.C. Effect of Feeding of a Cholesterol-Reducing Bacterium, Eubacterium Coprostanoligenes, to Germ-Free Mice. Lab. Anim. Sci. 1998, 48, 253–255. [Google Scholar]
- Sheridan, P.O.; Martin, J.C.; Lawley, T.D.; Browne, H.P.; Harris, H.M.B.; Bernalier-Donadille, A.; Duncan, S.H.; O’Toole, P.W.; Scott, K.P.; Flint, H.J. Polysaccharide Utilization Loci and Nutritional Specialization in a Dominant Group of Butyrate-Producing Human Colonic Firmicutes. Microb. Genom. 2016, 2, e000043. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.M.A.E.; Bast, A.; Vanhoutvin, S.A.L.W.; Fischer, M.A.J.G.; Kodde, A.; Troost, F.J.; Venema, K.; Brummer, R.-J.M. Butyrate Modulates Oxidative Stress in the Colonic Mucosa of Healthy Humans. Clin. Nutr. 2009, 28, 88–93. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.-J. Review Article: The Role of Butyrate on Colonic Function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Yu, P.; Ke, C.; Guo, J.; Zhang, X.; Li, B. Lactobacillus Plantarum L15 Alleviates Colitis by Inhibiting LPS-Mediated NF-ΚB Activation and Ameliorates DSS-Induced Gut Microbiota Dysbiosis. Front. Immunol. 2020, 11, 575173. [Google Scholar] [CrossRef]
- Teng, Y.; Wang, Y.; Guan, W.; Wang, C.; Yu, H.; Li, X.; Wang, Y. Effect of Lactobacillus Plantarum LP104 on Hyperlipidemia in High-Fat Diet Induced C57BL/6N Mice via Alteration of Intestinal Microbiota. J. Funct. Foods 2022, 95, 105176. [Google Scholar] [CrossRef]
- Wu, T.-R.; Lin, C.-S.; Chang, C.-J.; Lin, T.-L.; Martel, J.; Ko, Y.-F.; Ojcius, D.M.; Lu, C.-C.; Young, J.D.; Lai, H.-C. Gut Commensal Parabacteroides Goldsteinii Plays a Predominant Role in the Anti-Obesity Effects of Polysaccharides Isolated from Hirsutella Sinensis. Gut 2019, 68, 248–262. [Google Scholar] [CrossRef] [PubMed]
- John, O.D.; Mouatt, P.; Majzoub, M.E.; Thomas, T.; Panchal, S.K.; Brown, L. Physiological and Metabolic Effects of Yellow Mangosteen (Garcinia Dulcis) Rind in Rats with Diet-Induced Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 272. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hu, G.; Wang, A.; Long, G.; Yang, Y.; Wang, D.; Zhong, N.; Jia, J. Black Tea Reduces Diet-Induced Obesity in Mice via Modulation of Gut Microbiota and Gene Expression in Host Tissues. Nutrients 2022, 14, 1635. [Google Scholar] [CrossRef] [PubMed]
- Okabe, K.; Yaku, K.; Tobe, K.; Nakagawa, T. Implications of Altered NAD Metabolism in Metabolic Disorders. J. Biomed. Sci. 2019, 26, 34. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Yoshino, J. Adipose Tissue NAD+ Biology in Obesity and Insulin Resistance: From Mechanism to Therapy. Bioessays 2017, 39, 227. [Google Scholar] [CrossRef]
- Fernández de Palencia, P.; de la Plaza, M.; Amárita, F.; Requena, T.; Peláez, C. Diversity of Amino Acid Converting Enzymes in Wild Lactic Acid Bacteria. Enzym. Microb. Technol. 2006, 38, 88–93. [Google Scholar] [CrossRef]
- Novin, Z.S.; Ghavamzadeh, S.; Mehdizadeh, A. The Weight Loss Effects of Branched Chain Amino Acids and Vitamin B6: A Randomized Controlled Trial on Obese and Overweight Women. Int. J. Vitam. Nutr. Res. 2019, 8, 1–2. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef]
- Maguire, D.; Talwar, D.; Shiels, P.G.; McMillan, D. The Role of Thiamine Dependent Enzymes in Obesity and Obesity Related Chronic Disease States: A Systematic Review. Clin. Nutr. ESPEN 2018, 25, 8–17. [Google Scholar] [CrossRef]
- Rahim, F.; Ullah, H.; Javid, M.T.; Wadood, A.; Taha, M.; Ashraf, M.; Shaukat, A.; Junaid, M.; Hussain, S.; Rehman, W.; et al. Synthesis, in Vitro Evaluation and Molecular Docking Studies of Thiazole Derivatives as New Inhibitors of α-Glucosidase. Bioorg. Chem. 2015, 62, 15–21. [Google Scholar] [CrossRef]
- Chen, K.; Yao, X.; Tang, T.; Chen, L.-M.; Xiao, C.; Wang, J.-Y.; Chen, H.-F.; Jiang, Z.-X.; Liu, Y.; Zheng, X. Thiazole-Based and Thiazolidine-Based Protein Tyrosine Phosphatase 1B Inhibitors as Potential Anti-Diabetes Agents. Med. Chem. Res. 2021, 30, 519–534. [Google Scholar] [CrossRef]
- Chandel, V.; Biswas, D.; Roy, S.; Vaidya, D.; Verma, A.; Gupta, A. Current Advancements in Pectin: Extraction, Properties and Multifunctional Applications. Foods 2022, 11, 2683. [Google Scholar] [CrossRef] [PubMed]
- CHANDA, M.; REMPEL, G.L. Separation of Hydroxycitric Acid Lactone from Fruit Pectins and Polyhydroxyphenols on Poly(4-Vinylpyridine) Weak-Base Resin. Sep. Sci. Technol. 2000, 35, 869–902. [Google Scholar] [CrossRef]


| Groups | Feed Type | Treatment |
|---|---|---|
| LFD | Low fat diet | 200 µL PBS |
| HFD | High fat diet | 200 µL PBS |
| HFD + L1 | High fat diet | L. plantarum HAC03 (1 × 109 CFU/mouse) |
| HFD + L2 | High fat diet | L. plantarum 299V (1 × 109 CFU/mouse) |
| HFD + L1′ | High fat diet | L. plantarum HAC03 (1 × 108 CFU/mouse) |
| HFD + G | High fat diet | G. cambogia (200 mg/kg) |
| HFD + L1′ + G | High fat diet | L. plantarum HAC03 (1 × 108 CFU/mouse) + G. cambogia (200 mg/kg) |
| Gene | Forward Primer 5′-3′ | Reverse Primer 5′-3′ |
|---|---|---|
| FAS | CTGGACTCGCTCATGGGTG | CATTTCCTGAAGTTTCCGCAG |
| ACC | TGACAGACTGATCGCAGAGAAAG | TGGAGAGCCCCACACACA |
| PPAR | AGTGGAGACCGCCCAGG | GCAGCAGGTTGTCTTGGATGT |
| SREBP1c | AGCAGCCCCTAGAACAAACAC | CAGCAGTGAGTCTGCCTTGAT |
| Parameter | Groups | ||||
|---|---|---|---|---|---|
| LFD | HFD | HFD + L1′ | HFD + G | HFD + L1′ + G | |
| ALT (U/L) | 34.5 ± 6.59 *** | 70.8 ± 9.80 | 69.1 ± 12.84 | 60.0 ± 9.00 | 36.1 ± 4.84 *** +++/### |
| AST (U/L) | 78.7 ± 6.83 *** | 110.2 ± 10.06 | 108.5 ± 7.04 | 88.7 ± 7.79 *** | 72,6 ± 11.22 *** +++/# |
| TG (mg/dL) | 65.0 ± 4.08 *** | 122.5 ± 18.87 | 95.0 ± 22.91 * | 100.0 ±23.73 | 78.3 ± 8.50 *** |
| T-chol (mg/dL) | 118.3 ± 8.22 *** | 171.0 ± 17.68 | 147.3 ± 8.65 * | 160.7 ± 4.92 | 138.3 ± 3.09 ** ### |
| HDL (mg/dL) | 60.4 ± 4.64 *** | 73.0 ± 1.13 | 69.5 ± 5.95 | 74.7 ± 0.85 | 74.2 ± 1.00 |
| LDL (mg/dL) | 9.4 ± 0.98 ** | 13.9 ± 2.24 | 9.8 ± 2.14 * | 11.7 ± 0.22 | 9.3 ± 1.13 ** ### |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Y.-G.; Lee, T.; Ro, J.; Oh, S.; Kwak, J.-H.; Kim, A.-R. Combination of Lactobacillus plantarum HAC03 and Garcinia cambogia Has a Significant Anti-Obesity Effect in Diet-Induced Obesity Mice. Nutrients 2023, 15, 1859. https://doi.org/10.3390/nu15081859
Kang Y-G, Lee T, Ro J, Oh S, Kwak J-H, Kim A-R. Combination of Lactobacillus plantarum HAC03 and Garcinia cambogia Has a Significant Anti-Obesity Effect in Diet-Induced Obesity Mice. Nutrients. 2023; 15(8):1859. https://doi.org/10.3390/nu15081859
Chicago/Turabian StyleKang, Youn-Goo, Taeyoung Lee, Jaeyoung Ro, Sanghun Oh, Jin-Hwan Kwak, and Ah-Ram Kim. 2023. "Combination of Lactobacillus plantarum HAC03 and Garcinia cambogia Has a Significant Anti-Obesity Effect in Diet-Induced Obesity Mice" Nutrients 15, no. 8: 1859. https://doi.org/10.3390/nu15081859
APA StyleKang, Y.-G., Lee, T., Ro, J., Oh, S., Kwak, J.-H., & Kim, A.-R. (2023). Combination of Lactobacillus plantarum HAC03 and Garcinia cambogia Has a Significant Anti-Obesity Effect in Diet-Induced Obesity Mice. Nutrients, 15(8), 1859. https://doi.org/10.3390/nu15081859






