Effectiveness of Nutritional Supplements for Attenuating the Side Effects of SARS-CoV-2 Vaccines
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
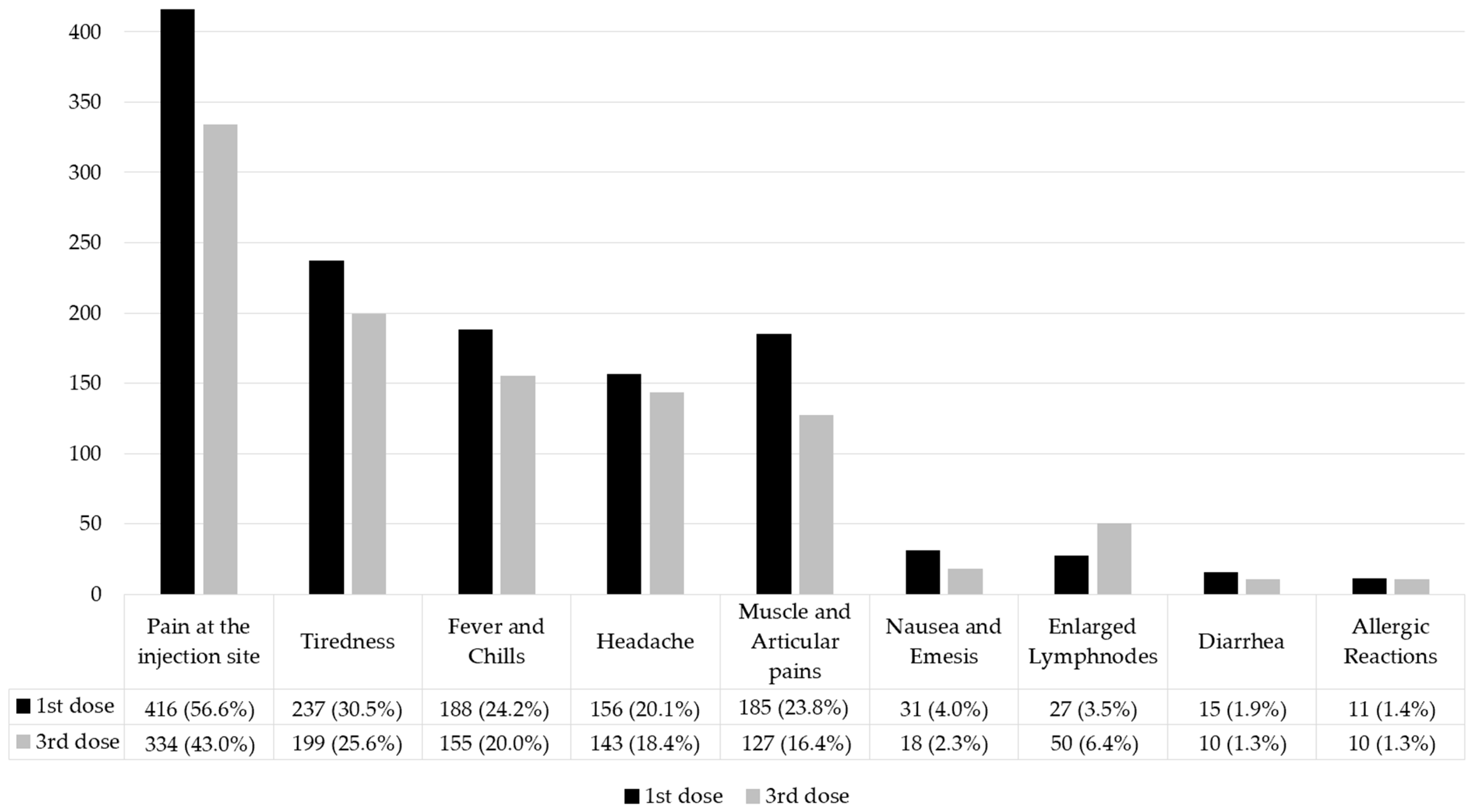
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Questions | Answers |
|---|---|
| Personal Data | |
| Sex at birth | Female/Male. |
| Age | Age in Numbers. |
| Region of residence | Valle d’Aosta (North-West Italy) Piedmont (North-West Italy) Lombardy (North-West Italy) Ligurian (North-West Italy) Trentino-South Tyrol (North-East Italy) Friuli-Venezia Giulia (North-East Italy) Veneto (North-East Italy) Emilia-Romagna (North-East Italy) Tuscany (Central Italy) Marche (Central Italy) Umbria (Central Italy) Lazio (Central Italy) Abruzzo (Southern Italy) Molise (Southern Italy) Campania (Southern Italy) Apulian (Southern Italy) Basilicata (Southern Italy) Calabria (Southern Italy) Sicily (Insular Italy) Sardinia (Insular Italy) |
| County of residence | Aosta (AO) Alexandria (AL) Asti (AT) Biella (BI) Cuneo (CN) Novara (NO) Turin (TO) Verbano-Cusio-Ossola (VB) Vercelli (VC) Bergamo (BG) Brescia (BS) Como (CO) Cremona (CR) Lecco (LC) Lodi (LO) Mantova (MN) Milan (MI) Monza e della Brianza (MB) Pavia (PV) Sondrio (SO) Varese (VA) Genoa (GE) Imperia (IM) La Spezia (SP) Savona (SV) Bolzano (BZ) Trento (TN) Gorizia (GO) Pordenone (PN) Trieste (TS) Udine (UD) Belluno (BL) Padova (PD) Rovigo (RO) Treviso (TV) Venice (VE) Verona (VR) Vicenza (VI) Bologna (BO) Ferrara (FE) Forlì-Cesena (FC) Modena (MO) Parma (PR) Piacenza (PC) Ravenna (RA) Reggio Emilia (RE) Rimini (RN) Arezzo (AR) Florence (FI) Grosseto (GR) Livorno (LI) Lucca (LU) Massa-Carrara (MS) Pisa (PI) Pistoia (PT) Prato (PO) Siena (SI) Ancona (AN) Ascoli Piceno (AP) Fermo (FM) Macerata (MC) Pesaro e Urbino (PU) Perugia (PG) Terni (TR) Frosinone (FR) Latina (LT) Rieti (RI) Rome (RM) Viterbo (VT) Chieti (CH) L’Aquila (AQ) Pescara (PE) Teramo (TE) Campobasso (CB) Isernia (IS) Avellino (AV) Benevento (BN) Caserta (CE) Napoles (NA) Salerno (SA) Bari (BA) Barletta-Andria-Trani (BT) Brindisi (BR) Foggia (FG) Lecce (LE) Taranto (TA) Matera (MT) Potenza (PZ) Catanzaro (CZ) Cosenza (CS) Crotone (KR) Reggio Calabria (RC) Vibo Valenzia (VV) Agrigento (AG) Caltanissetta (CL) Catania (CT) Enna (EN) Messina (ME) Palermo (PA) Ragusa (RG) Siracusa (SR) Trapani (TP) Cagliari (CA) Nuoro (NU) Oristano (OR) Sassari (SS) Sud Sardegna (SU). |
| Current employment | Study Job Unemployed Retiree |
| Degree | Primary School Lower Secondary School Upper Secondary School Graduation Master’s or Doctorate |
| How is your lifestyle? | Sedentary Not very active Moderately active Very active |
| Do you practice any sport(s)? | No Yes, aerobic sports Yes, anaerobic sports Yes, both aerobic and anaerobic sports |
| Do you currently smoke? | No Yes, <5 cigarettes/day Yes, >5 cigarettes/ day |
| Do you have any pathologies? | No Yes, I have respiratory pathology (pulmonary emphysema, chronic obstructive pulmonary disease (COPD), asthma, bronchiectasis, pulmonary fibrosis, OSAS (obstructive sleep apnea syndrome), and respiratory failure). Yes, I have cardiovascular diseases (angina pectoris, myocardial infarction, heart failure, ictus, and peripheral vascular disease). Yes, I have metabolic pathologies (dyslipidemia, diabetes, hyperglycemia, insulin resistance, and gout). Yes, I have neurological pathology (Alzheimer‘s disease, Parkinson’s disease, migraine, epilepsy, headaches, multiple sclerosis (SM), amyotrophic lateral sclerosis (SLA), and other autoimmune diseases). Yes, I have renal pathology. Yes, I have a tumor pathology. Yes, I have three or more pathologies among those listed. Yes, I have fewer pathologies than those listed. |
| Anthropometrics information | |
| Weight | Weight in kilograms (Kg) |
| Height | Height in centimeters (cm) |
| COVID-19 infection and immune response | |
| Did you contract COVID-19? | NoYes, I contracted COVID-19 before having the vaccination. Yes, I contracted COVID-19 after having the first vaccination. Yes, I contracted COVID-19 after having the second vaccination. Yes, I contracted COVID-19 after having the third vaccination. |
| Did you have any symptoms during COVID-19? | Asymptomatic Mild symptoms (cold, diarrhea) Flu symptoms (fever, cough, respiratory distress) Pneumonia. |
| Do you have any differences from your previous state of health after recovering from COVID-19? | Yes/No |
| COVID-19 vaccination and supplementation | |
| Did you get vaccinated for COVID-19? | Yes/No |
| How many doses? | 1 2 3 |
| Which vaccine did you receive at the first dose? | Pfizer Moderna AstraZeneca Jonson & Jonson |
| Did you have any side effects on the first dose? | No Pain at the injection site Tiredness Headache Muscle aches Chills Articular pains Diarrhea Fever Nausea Enlarged lymph nodes Allergic reactions with skin rashes or itching Other |
| How many days did the side effects last? | 0 1 2 3 >4 |
| Did you take the supplements? | No Probiotics Prebiotics (inulin, FOS) Vitamin D Vitamin C Vitamin E B-complex vitamins Zinc Selenium Copper Omega-3 L-arginine L-glutamine Folic acid Lactoferrin |
| For how many days? | I did not hire them. Yes, I only took them a week before the vaccination. Yes, I only got them the week after the vaccination. Yes, I took them both the week before and the week after the vaccination. |
| Who prescribed it for you? | No, I did not hire them. Self-prescription Nutritionist Doctor of general medicine Other. |
| Did you obtain the second dose of the vaccine? | Yes/No |
| Which vaccine did you receive at the second dose? | Pfizer Moderna AstraZeneca Jonson & Jonson. |
| Did you have any side effects on the second dose? | No Pain at the injection site Tiredness Headache Muscle aches Chills Articular pains Diarrhea Fever Nausea Enlarged lymph nodes Allergic reactions with skin rashes or itching Other |
| How many days did the side effects last? | 0 1 2 3 >4 |
| Did you take the supplements? | No Probiotics Prebiotics (inulin, FOS) Vitamin D Vitamin C Vitamin E B-complex vitamins Zinc Selenium Copper Omega-3 L-arginine L-glutamine Folic acid Lactoferrin |
| For how many days? | I did not hire them. Yes, I only took them in the week before the vaccination. Yes, I only got them a week after the vaccination. Yes, I took them both the week before and the week after the vaccination. |
| Who prescribed it for you? | No, I did not hire them. Self-prescription Nutritionist Doctor of general medicine Other |
| Did you obtain the third dose of the vaccine? | Yes/No |
| Which vaccine did you receive at the third dose? | Pfizer Moderna AstraZeneca Jonson & Jonson |
| Did you have any side effects on the third dose? | No Pain at the injection site Tiredness Headache Muscle aches Chills Articular pains Diarrhea Fever Nausea Enlarged lymph nodes Allergic reactions with skin rashes or itching Other |
| How many days did the side effects last? | 0 1 2 3 >4 |
| Did you take the supplements? | No Probiotics Prebiotics (inulin, FOS) Vitamin D Vitamin C Vitamin E B-complex vitamins Zinc Selenium Copper Omega-3 L-arginine L-glutamine Folic acid Lactoferrin |
| For how many days? | I did not hire them. Yes, I only took them in the week before the vaccination. Yes, I only got them a week after the vaccination. Yes, I took them both the week before and the week after the vaccination. |
| Who prescribed it for you? | No, I did not hire them. Self-prescription Nutritionist Doctor of general medicine Other. |
| Did you receive the antibody dosage at least 21 days after one of the vaccine doses? | No, I did not. Yes, I did, but the antibodies were absent. Yes, I did, and the antibodies were present. |
References
- Toor, S.M.; Saleh, R.; Sasidharan Nair, V.; Taha, R.Z.; Elkord, E. T-cell Responses and Therapies against SARS-CoV-2 Infection. Immunology 2021, 162, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Olszak, T.; Neves, J.F.; Dowds, C.M.; Baker, K.; Glickman, J.; Davidson, N.O.; Lin, C.-S.; Jobin, C.; Brand, S.; Sotlar, K.; et al. Protective Mucosal Immunity Mediated by Epithelial CD1d and IL-10. Nature 2014, 509, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Perlot, T.; Penninger, J.M. ACE2—From the Renin–Angiotensin System to Gut Microbiota and Malnutrition. Microbes Infect. 2013, 15, 866–873. [Google Scholar] [CrossRef]
- Epicentro ISS. Vaccini COVID-19. 2023. Available online: https://www.epicentro.iss.it/vaccini/covid-19 (accessed on 12 March 2023).
- National Center for Immunization and Respiratory Diseases (U.S.); Division of Viral Diseases. Understanding MRNA COVID-19 Vaccines. 2021. Available online: https://stacks.cdc.gov/view/cdc/111218 (accessed on 3 November 2021).
- Goyal, K.; Goel, H.; Baranwal, P.; Tewary, A.; Dixit, A.; Pandey, A.K.; Benjamin, M.; Tanwar, P.; Dey, A.; Khan, F.; et al. Immunological Mechanisms of Vaccine-Induced Protection against SARS-CoV-2 in Humans. Immuno 2021, 1, 442–456. [Google Scholar] [CrossRef]
- Di Pasquale, A.; Bonanni, P.; Garçon, N.; Stanberry, L.R.; El-Hodhod, M.; Tavares Da Silva, F. Vaccine Safety Evaluation: Practical Aspects in Assessing Benefits and Risks. Vaccine 2016, 34, 6672–6680. [Google Scholar] [CrossRef] [PubMed]
- Al Khames Aga, Q.A.; Alkhaffaf, W.H.; Hatem, T.H.; Nassir, K.F.; Batineh, Y.; Dahham, A.T.; Shaban, D.; Al Khames Aga, L.A.; Agha, M.Y.R.; Traqchi, M. Safety of COVID-19 Vaccines. J. Med. Virol. 2021, 93, 6588–6594. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Kaki, M.; Potluri, V.S.; Kahar, P.; Khanna, D. A Comprehensive Review of SARS-CoV-2 Vaccines: Pfizer, Moderna & Johnson & Johnson. Hum. Vaccines Immunother. 2022, 18, 2002083. [Google Scholar] [CrossRef]
- Suardi, C.; Cazzaniga, E.; Graci, S.; Dongo, D.; Palestini, P. Link between Viral Infections, Immune System, Inflammation and Diet. Int. J. Environ. Res. Public Health 2021, 18, 2455. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P.; Pivari, F.; Soldati, L.; Attinà, A.; Leggeri, C.; Cinelli, G.; Tarsitano, M.G.; Caparello, G.; Carrano, E.; et al. COVID-19: Is There a Role for Immunonutrition in Obese Patient? J. Transl. Med. 2020, 18, 415. [Google Scholar] [CrossRef]
- Zhao, L.; Wirth, M.D.; Petermann-Rocha, F.; Parra-Soto, S.; Mathers, J.C.; Pell, J.P.; Ho, F.K.; Celis-Morales, C.A.; Hébert, J.R. Diet-Related Inflammation Is Associated with Worse COVID-19 Outcomes in the UK Biobank Cohort. Nutrients 2023, 15, 884. [Google Scholar] [CrossRef]
- Nursyifa Fadiyah, N.; Megawati, G.; Luftimas, D.E. Potential of Omega 3 Supplementation for Coronavirus Disease 2019 (COVID-19): A Scoping Review. Int. J. Gen. Med. 2022, 15, 3915–3922. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System–Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef]
- Calder, P.C. Nutrition, Immunity and COVID-19. BMJ Nutr. Prev. Health 2020, 3, 74–92. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.; Carr, A.; Gombart, A.; Eggersdorfer, M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Foods to Deliver Immune-Supporting Nutrients. Curr. Opin. Food Sci. 2022, 43, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Berger, M.M.; Gombart, A.F.; McComsey, G.A.; Martineau, A.R.; Eggersdorfer, M. Micronutrients to Support Vaccine Immunogenicity and Efficacy. Vaccines 2022, 10, 568. [Google Scholar] [CrossRef]
- Ferro, Y.; Pujia, R.; Maurotti, S.; Boragina, G.; Mirarchi, A.; Gnagnarella, P.; Mazza, E. Mediterranean Diet a Potential Strategy against SARS-CoV-2 Infection: A Narrative Review. Medicina 2021, 57, 1389. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P.; Pivari, F.; Soldati, L.; Attinà, A.; Cinelli, G.; Leggeri, C.; Caparello, G.; Barrea, L.; Scerbo, F.; et al. Eating Habits and Lifestyle Changes during COVID-19 Lockdown: An Italian Survey. J. Transl. Med. 2020, 18, 229. [Google Scholar] [CrossRef]
- Agenzia Italiana del Farmaco. Quattordicesimo Rapporto Sulla Sorveglianza Dei Vaccini Anti-COVID-19. Available online: https://www.aifa.gov.it/documents/20142/1315190/Rapporto_sorveglianza_vaccini_COVID-19_14.pdf (accessed on 9 December 2022).
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- D’Errico, S.; Zanon, M.; Montanaro, M.; Radaelli, D.; Sessa, F.; Di Mizio, G.; Montana, A.; Corrao, S.; Salerno, M.; Pomara, C. More than Pneumonia: Distinctive Features of SARS-CoV-2 Infection. From Autopsy Findings to Clinical Implications: A Systematic Review. Microorganisms 2020, 8, 1642. [Google Scholar] [CrossRef]
- Wagner, A.; Garner-Spitzer, E.; Jasinska, J.; Kollaritsch, H.; Stiasny, K.; Kundi, M.; Wiedermann, U. Age-Related Differences in Humoral and Cellular Immune Responses after Primary Immunisation: Indications for Stratified Vaccination Schedules. Sci. Rep. 2018, 8, 9825. [Google Scholar] [CrossRef] [PubMed]
- Sharma, E.; Revinipati, S.; Bhandari, S.; Thakur, S.; Goyal, S.; Ghose, A.; Bajpai, S.; Muhammad, W.; Boussios, S. Efficacy and Safety of COVID-19 Vaccines—An Update. Diseases 2022, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Klugar, M.; Riad, A.; Mekhemar, M.; Conrad, J.; Buchbender, M.; Howaldt, H.-P.; Attia, S. Side Effects of MRNA-Based and Viral Vector-Based COVID-19 Vaccines among German Healthcare Workers. Biology 2021, 10, 752. [Google Scholar] [CrossRef] [PubMed]
- Almufty, H.B.; Mohammed, S.A.; Abdullah, A.M.; Merza, M.A. Potential Adverse Effects of COVID19 Vaccines among Iraqi Population; a Comparison between the Three Available Vaccines in Iraq; a Retrospective Cross-Sectional Study. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102207. [Google Scholar] [CrossRef] [PubMed]
- Nassar, R.I.; Alnatour, D.; Thiab, S.; Nassar, A.; El-Hajji, F.; Basheti, I.A. Short-Term Side Effects of COVID-19 Vaccines: A Cross-Sectional Study in Jordan. Hum. Vaccines Immunother. 2022, 18, 2082792. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Franza, L.; Monsignore, D.; Esposito, E.; Rio, P.; Gasbarrini, A.; Gambassi, G.; Cianci, R.; De Lorenzo, A. Vaccines, Microbiota and Immunonutrition: Food for Thought. Vaccines 2022, 10, 294. [Google Scholar] [CrossRef]
- Saresella, M.; Piancone, F.; Marventano, I.; Hernis, A.; Trabattoni, D.; Invernizzi, M.; La Rosa, F.; Clerici, M. Innate Immune Responses to Three Doses of the BNT162b2 MRNA SARS-CoV-2 Vaccine. Front. Immunol. 2022, 13, 947320. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex Differences in Immune Responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Sumaily, K.M. The Roles and Pathogenesis Mechanisms of a Number of Micronutrients in the Prevention and/or Treatment of Chronic Hepatitis, COVID-19 and Type-2 Diabetes Mellitus. Nutrients 2022, 14, 2632. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-J.; Chang, H.-S.; Yang, Y.-P.; Lin, T.-W.; Lai, W.-Y.; Lin, Y.-Y.; Chang, C.-C. The Role of Micronutrient and Immunomodulation Effect in the Vaccine Era of COVID-19. J. Chin. Med. Assoc. 2021, 84, 821–826. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.; Lely, A.; Navis, G.; van Goor, H. Tissue Distribution of ACE2 Protein, the Functional Receptor for SARS Coronavirus. A First Step in Understanding SARS Pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Shatizadeh Malekshahi, S.; Yavarian, J.; Shafiei-Jandaghi, N. Usage of Peptidases by SARS-CoV-2 and Several Human Coronaviruses as Receptors: A Mysterious Story. Biotechnol. Appl. Biochem. 2022, 69, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Di Lisa, F.; Oelze, M.; Kröller-Schön, S.; Steven, S.; Schulz, E.; Münzel, T. Crosstalk of Mitochondria with NADPH Oxidase via Reactive Oxygen and Nitrogen Species Signalling and Its Role for Vascular Function: Redox Crosstalk of Mitochondria with NADPH Oxidase and ENOS Uncoupling. Br. J. Pharmacol. 2017, 174, 1670–1689. [Google Scholar] [CrossRef] [PubMed]
- Doughan, A.K.; Harrison, D.G.; Dikalov, S.I. Molecular Mechanisms of Angiotensin II–Mediated Mitochondrial Dysfunction: Linking Mitochondrial Oxidative Damage and Vascular Endothelial Dysfunction. Circ. Res. 2008, 102, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Wosniak, J.; Santos, C.X.C.; Kowaltowski, A.J.; Laurindo, F.R.M. Cross-Talk Between Mitochondria and NADPH Oxidase: Effects of Mild Mitochondrial Dysfunction on Angiotensin II-Mediated Increase in Nox Isoform Expression and Activity in Vascular Smooth Muscle Cells. Antioxid. Redox Signal. 2009, 11, 1265–1278. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Wauquier, F.; Eid, A.A.; Roman, L.J.; Ghosh-Choudhury, G.; Khazim, K.; Block, K.; Gorin, Y. Nox4 NADPH Oxidase Mediates Peroxynitrite-Dependent Uncoupling of Endothelial Nitric-Oxide Synthase and Fibronectin Expression in Response to Angiotensin II. J. Biol. Chem. 2013, 288, 28668–28686. [Google Scholar] [CrossRef]
- Sharif, N.; Opu, R.R.; Khan, A.; Alzahrani, K.J.; Banjer, H.J.; Alzahrani, F.M.; Haque, N.; Khan, S.; Soumik, S.T.; Zhang, M.; et al. Impact of Zinc, Vitamins C and D on Disease Prognosis among Patients with COVID-19 in Bangladesh: A Cross-Sectional Study. Nutrients 2022, 14, 5029. [Google Scholar] [CrossRef]
- Gao, D.; Xu, M.; Wang, G.; Lv, J.; Ma, X.; Guo, Y.; Zhang, D.; Yang, H.; Jiang, W.; Deng, F.; et al. The Efficiency and Safety of High-Dose Vitamin C in Patients with COVID-19: A Retrospective Cohort Study. Aging 2021, 13, 7020–7034. [Google Scholar] [CrossRef]
- Thomas, S.; Patel, D.; Bittel, B.; Wolski, K.; Wang, Q.; Kumar, A.; Il’Giovine, Z.J.; Mehra, R.; McWilliams, C.; Nissen, S.E.; et al. Effect of High-Dose Zinc and Ascorbic Acid Supplementation vs Usual Care on Symptom Length and Reduction Among Ambulatory Patients With SARS-CoV-2 Infection: The COVID A to Z Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e210369. [Google Scholar] [CrossRef]
- Olczak-Pruc, M.; Swieczkowski, D.; Ladny, J.R.; Pruc, M.; Juarez-Vela, R.; Rafique, Z.; Peacock, F.W.; Szarpak, L. Vitamin C Supplementation for the Treatment of COVID-19: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4217. [Google Scholar] [CrossRef]
- Shakoor, H.; Feehan, J.; Al Dhaheri, A.S.; Ali, H.I.; Platat, C.; Ismail, L.C.; Apostolopoulos, V.; Stojanovska, L. Immune-Boosting Role of Vitamins D, C, E, Zinc, Selenium and Omega-3 Fatty Acids: Could They Help against COVID-19? Maturitas 2021, 143, 1–9. [Google Scholar] [CrossRef]
- Newton, S.; Owusu-Agyei, S.; Filteau, S.; Gyan, T.; Kirkwood, B.R. Vitamin A Supplements Are Well Tolerated with the Pentavalent Vaccine. Vaccine 2008, 26, 6608–6613. [Google Scholar] [CrossRef]
- Heller, R.A.; Sun, Q.; Hackler, J.; Seelig, J.; Seibert, L.; Cherkezov, A.; Minich, W.B.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Prediction of Survival Odds in COVID-19 by Zinc, Age and Selenoprotein P as Composite Biomarker. Redox Biol. 2021, 38, 101764. [Google Scholar] [CrossRef]
- Hackler, J.; Heller, R.A.; Sun, Q.; Schwarzer, M.; Diegmann, J.; Bachmann, M.; Moghaddam, A.; Schomburg, L. Relation of Serum Copper Status to Survival in COVID-19. Nutrients 2021, 13, 1898. [Google Scholar] [CrossRef] [PubMed]
- Doaei, S.; Gholami, S.; Rastgoo, S.; Gholamalizadeh, M.; Bourbour, F.; Bagheri, S.E.; Samipoor, F.; Akbari, M.E.; Shadnoush, M.; Ghorat, F.; et al. The Effect of Omega-3 Fatty Acid Supplementation on Clinical and Biochemical Parameters of Critically Ill Patients with COVID-19: A Randomized Clinical Trial. J. Transl. Med. 2021, 19, 128. [Google Scholar] [CrossRef] [PubMed]
- Sedighiyan, M.; Abdollahi, H.; Karimi, E.; Badeli, M.; Erfanian, R.; Raeesi, S.; Hashemi, R.; Vahabi, Z.; Asanjarani, B.; Mansouri, F.; et al. Omega-3 Polyunsaturated Fatty Acids Supplementation Improve Clinical Symptoms in Patients with COVID-19: A Randomised Clinical Trial. Int. J. Clin. Pract. 2021, 75, e14854. [Google Scholar] [CrossRef] [PubMed]
- Lynn, D.J.; Benson, S.C.; Lynn, M.A.; Pulendran, B. Modulation of Immune Responses to Vaccination by the Microbiota: Implications and Potential Mechanisms. Nat. Rev. Immunol. 2022, 22, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Mak, W.Y. Modulation of Gut Microbiota to Enhance Health and Immunity. Available online: https://clinicaltrials.gov/ct2/show/NCT04884776 (accessed on 9 December 2022).
- Bozkurt, H.S.; Quigley, E.M. The Probiotic Bifidobacterium in the Management of Coronavirus: A Theoretical Basis. Int. J. Immunopathol. Pharmacol. 2020, 34, 205873842096130. [Google Scholar] [CrossRef]
- Merra, G.; Capacci, A.; Cenname, G.; Esposito, E.; Dri, M.; Di Renzo, L.; Marchetti, M. The “Microbiome”: A Protagonist in COVID-19 Era. Microorganisms 2022, 10, 296. [Google Scholar] [CrossRef]
- Istituto Nazionale Di Statistica. Uso Di Internet 2019. 2019. Available online: https://www.istat.it/donne-uomini/bloc-3c.html (accessed on 10 July 2022).
- Il Sole 24 Ore. 56° Rapporto CENSIS. 2022. Available online: https://www.sanita24.ilsole24ore.com/art/dal-governo/2022-12-02/-56-rapporto-censis-61percento-italiani-ottimista-futuro-ssn-migliorera-anche-grazie-lezione-pandemia-plebiscito-sanita-e-investimento-il-937percento-scenario-popolo-spaventato-guerra-che-ricerca-110017.php?uuid=AEPYZ0LC (accessed on 2 December 2022).
| Whole Sample (N = 776) | |
|---|---|
| Females | 553 (71.3) |
| Males | 223 (28.7) |
| Weight (kg) | 69 ± 15 |
| Height (cm) | 168 ± 9 |
| BMI (kg/m2) | 24 ± 5 |
| Sedentary lifestyle | 313 (40.3) |
| Active lifestyle | 463 (59.7) |
| Age (years) | 43 ± 18 |
| Groups by age | |
| 18–24 years | 65 (8.4; M 12.1) |
| 25–42 years | 335 (43.2; M 45.6) |
| 43–60 years | 274 (35.3; M 30.9) |
| Over 60 | 102 (13.1; M 19.7) |
| Affected by COVID-19 | 273 (35.2) |
| Vaccinated for COVID-19 | 776 (100) |
| Groups of vaccinated | |
| One dose | 11 (1.4) |
| Two doses | 139 (17.9) |
| Three doses | 626 (80.7) |
| Pre- and Probiotics | Vitamins D, E, C, and B | Zinc, Selenium, and Copper | Omega-3 | |
|---|---|---|---|---|
| 1st dose | 775 (99.9%) | 208 (26.8%) | 36 (4.6%) | 20 (2.6%) |
| 3rd dose | 20 (2.6%) | 194 (25.0%) | 27 (3.5%) | 19 (2.4%) |
| COVID-19 Symptoms | Comorbidities | ||
|---|---|---|---|
| COVID-19 symptoms | Correlation coefficient | 1.000 | −0.08 * |
| Sig. (2-code) | 0.03 | ||
| N | 776 | 770 | |
| Comorbidities | Correlation coefficient | −0.08 * | 1.000 |
| Sig. (2-code) | 0.03 | ||
| N | 770 | 770 |
| Comorbidities | Vaccination Side Effects | Supplement Intake | ||
|---|---|---|---|---|
| Comorbidities | Correlation coefficient | 1 | 0.03 | 0.07 * |
| Sig. (2-code) | 0.38 | 0.047 | ||
| N | 770 | 770 | 770 | |
| Vaccination side effects | Correlation coefficient | −0.03 | 1.000 | −0.001 |
| Sig. (2-code) | 0.38 | 0.98 | ||
| N | 770 | 776 | 776 | |
| Supplement intake | Correlation coefficient | 0.07 * | −0.001 | 1.000 |
| Sig. (2-code) | 0.047 | 0.98 | ||
| N | 770 | 776 | 776 |
| Comorbidities | Vaccination Side Effects | Supplement Intake | ||
|---|---|---|---|---|
| Comorbidities | Correlation coefficient | 1.000 | 0.008 | 0.06 |
| Sig. (2-code) | 0.82 | 0.12 | ||
| N | 770 | 770 | 770 | |
| Vaccination side effects | Correlation coefficient | 0.008 | 1.000 | 0.59 |
| Sig. (2-code) | 0.82 | 0.000 ** | ||
| N | 770 | 776 | 776 | |
| Supplement intake | Correlation coefficient | 0.06 | 0.59 | 1.000 |
| Sig. (2-code) | 0.12 | 0.000 ** | ||
| N | 770 | 776 | 776 |
| Supplement Intake | |||
|---|---|---|---|
| At the Start of the Vaccination Cycle | During the Vaccination Cycle | At the End of the Vaccination Cycle | |
| Side Effects | p-Value | p-Value | p-Value |
| Pain in the injection site | 0.71 | 0.80 | 0.48 |
| Tiredness | 0.53 | 0.58 | 0.76 |
| Headache | 0.30 | 0.78 | 0.40 |
| Articular and muscle pains | 0.14 | 0.30 | 0.24 |
| Fever | 0.73 | 0.25 | 0.10 |
| Nausea | 0.99 | 0.57 | 0.04 * |
| Diarrhea | 0.007 * | 0.10 | 0.001 ** |
| Enlarged lymph nodes | 0.53 | 0.052 | 0.98 |
| Allergic reactions | 0.38 | 0.48 | 0.99 |
| Side Effects | ||
|---|---|---|
| At the Start of the Vaccination Cycle | At the End of the Vaccination Cycle | |
| Supplementation | p-Value | p-Value |
| Omega-3 | 0.001 ** | 0.29 |
| Vitamins (D, E, C and B) | 0.80 | 0.005 * |
| Minerals (zinc, selenium and copper) | 0.02 * | 0.28 |
| Biotics (prebiotics and probiotics) | 0.17 | 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gualtieri, P.; Trombetta, D.; Smeriglio, A.; Frank, G.; Alibrandi, A.; Leggeri, G.; Marchetti, M.; Zingale, I.; Fanelli, S.; Stocchi, A.; et al. Effectiveness of Nutritional Supplements for Attenuating the Side Effects of SARS-CoV-2 Vaccines. Nutrients 2023, 15, 1807. https://doi.org/10.3390/nu15081807
Gualtieri P, Trombetta D, Smeriglio A, Frank G, Alibrandi A, Leggeri G, Marchetti M, Zingale I, Fanelli S, Stocchi A, et al. Effectiveness of Nutritional Supplements for Attenuating the Side Effects of SARS-CoV-2 Vaccines. Nutrients. 2023; 15(8):1807. https://doi.org/10.3390/nu15081807
Chicago/Turabian StyleGualtieri, Paola, Domenico Trombetta, Antonella Smeriglio, Giulia Frank, Angela Alibrandi, Giulia Leggeri, Marco Marchetti, Ilaria Zingale, Silvia Fanelli, Arianna Stocchi, and et al. 2023. "Effectiveness of Nutritional Supplements for Attenuating the Side Effects of SARS-CoV-2 Vaccines" Nutrients 15, no. 8: 1807. https://doi.org/10.3390/nu15081807
APA StyleGualtieri, P., Trombetta, D., Smeriglio, A., Frank, G., Alibrandi, A., Leggeri, G., Marchetti, M., Zingale, I., Fanelli, S., Stocchi, A., & Di Renzo, L. (2023). Effectiveness of Nutritional Supplements for Attenuating the Side Effects of SARS-CoV-2 Vaccines. Nutrients, 15(8), 1807. https://doi.org/10.3390/nu15081807













