Pomegranate Extract Affects Gut Biofilm Forming Bacteria and Promotes Intestinal Mucosal Healing Regulating the Crosstalk between Epithelial Cells and Intestinal Fibroblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Tissue Collection
2.2. Isolation of Primary Intestinal Fibroblasts
2.3. Cell Culture
2.4. Cell Treatments
2.5. Animal Experiments
2.6. Intestinal Permeability
2.7. Microfluidic Assessment of Biofilm Formation
2.8. Cytotoxicity Assay
2.9. Trans-Epithelial Electric Resistance
2.10. Real-Time PCR
2.11. Collagen Contraction Assay
2.12. Enzyme-Linked Immunosorbent Assay (ELISA)
2.13. Bio-Plex Multiplex Immunoassay
2.14. Histological Preparation and Sirius Red Staining
2.15. Immunofluorescence Staining on Frozen Tissue Sections
2.16. Fluorescence In Situ Hybridization on Frozen Tissues
2.17. Immunofluorescence Staining on HT29CI.16E Cell Line
2.18. Western Blotting
2.19. Statistical Analysis
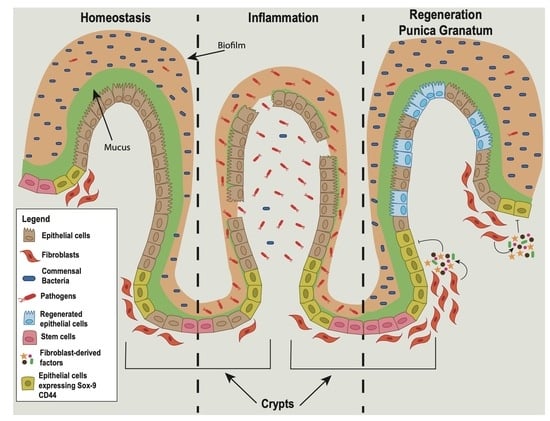
3. Results
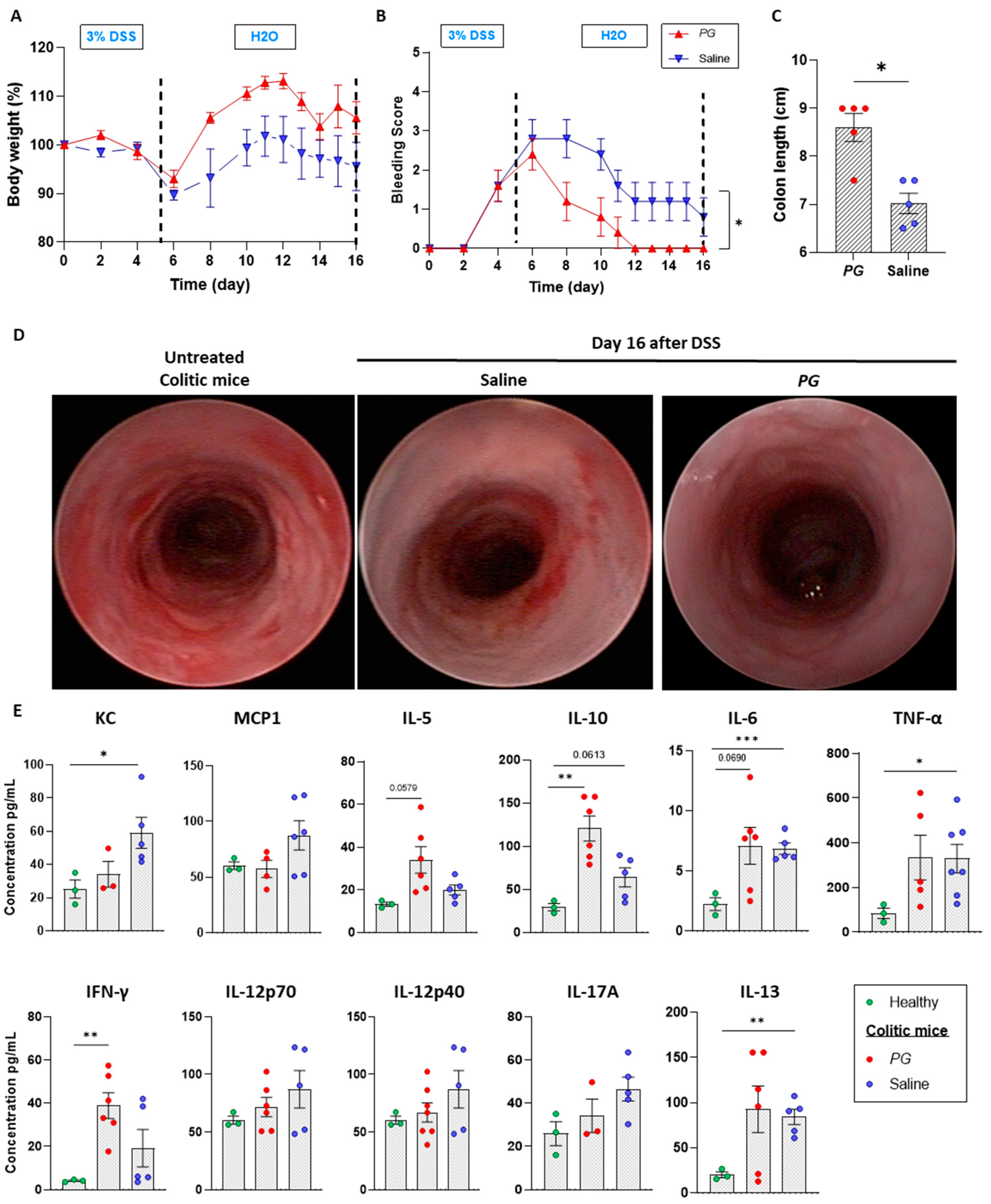
3.1. Pomegranate Extract Accelerates the Recovery Phase of Acute Colitis
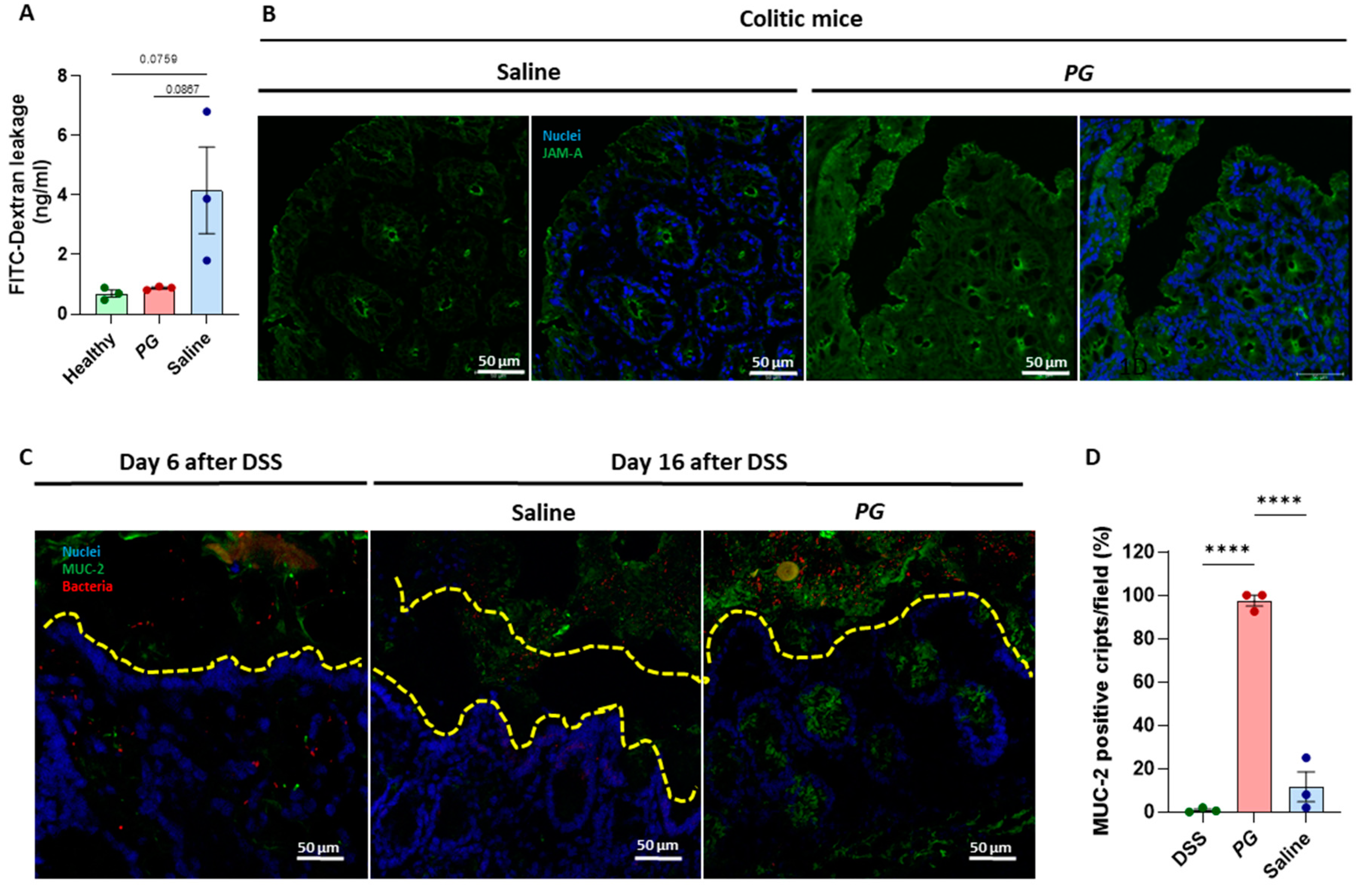
3.2. PG Improves Restoration of Intestinal Barrier In Vivo
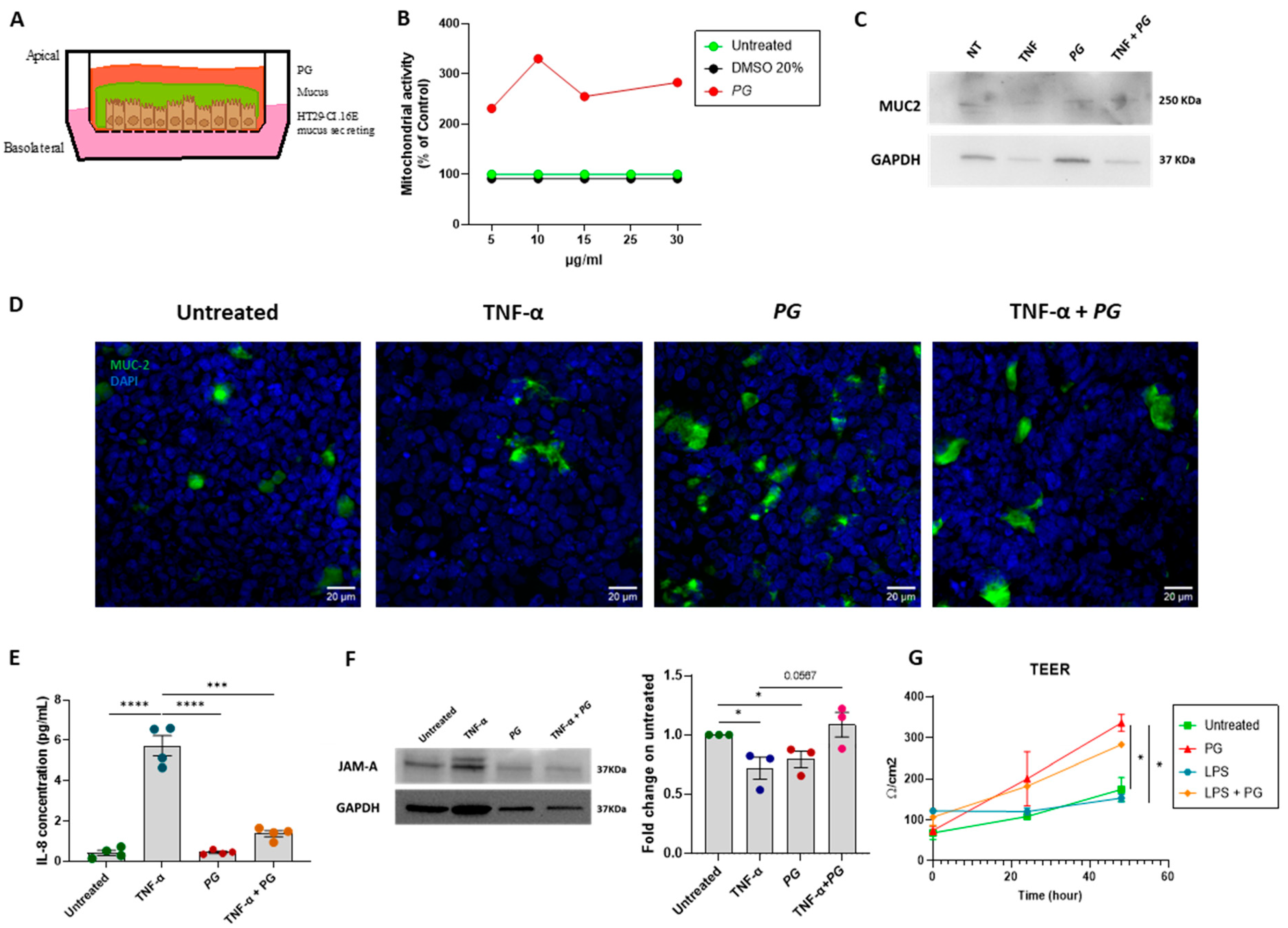
3.3. PG Enhances Epithelial Barrier Integrity In Vitro
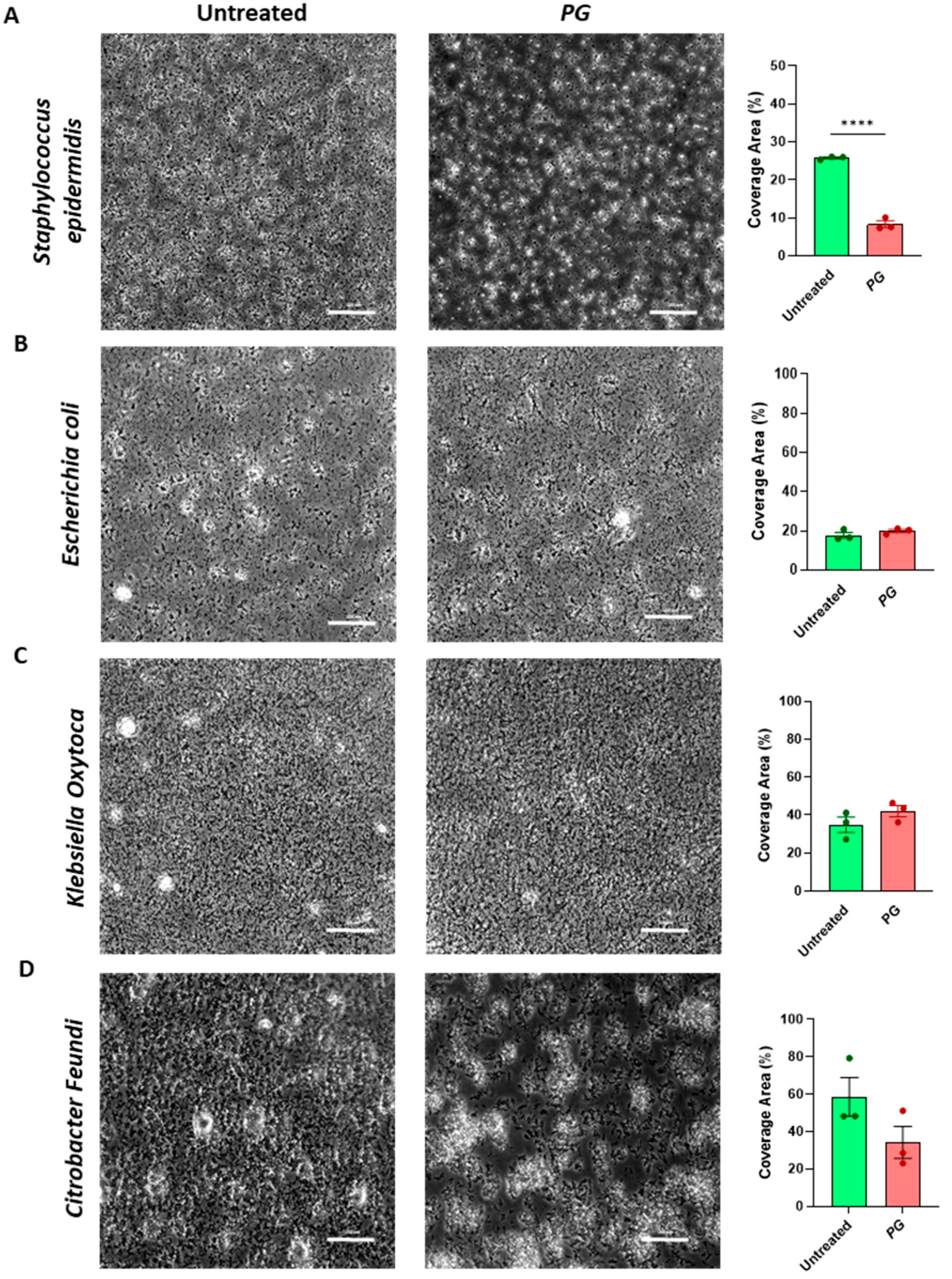
3.4. PG Modulates Gut Microbial Biofilm
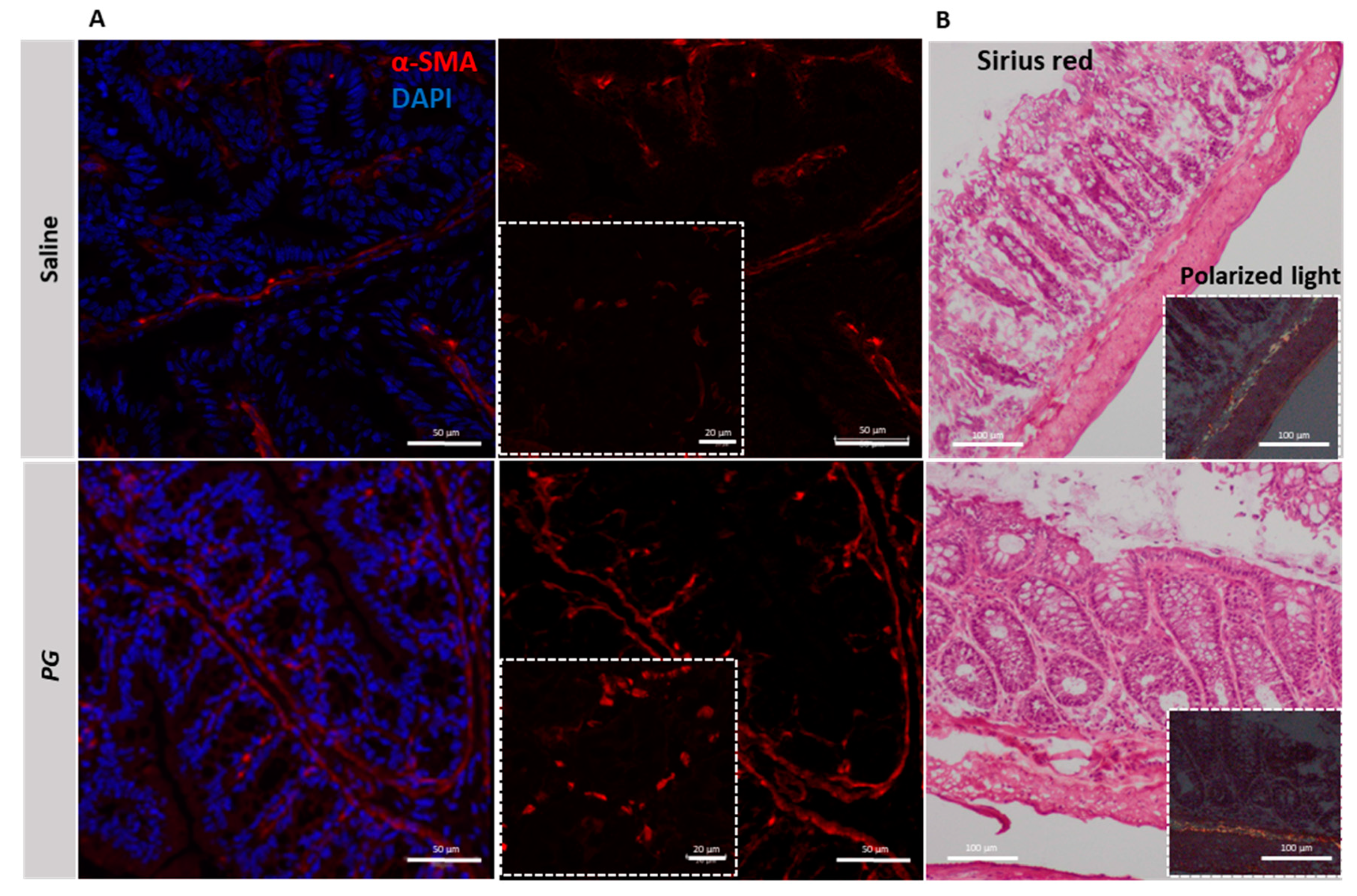
3.5. PG Promotes Fibroblast Accumulation during the Recovery Phase of Acute Colitis
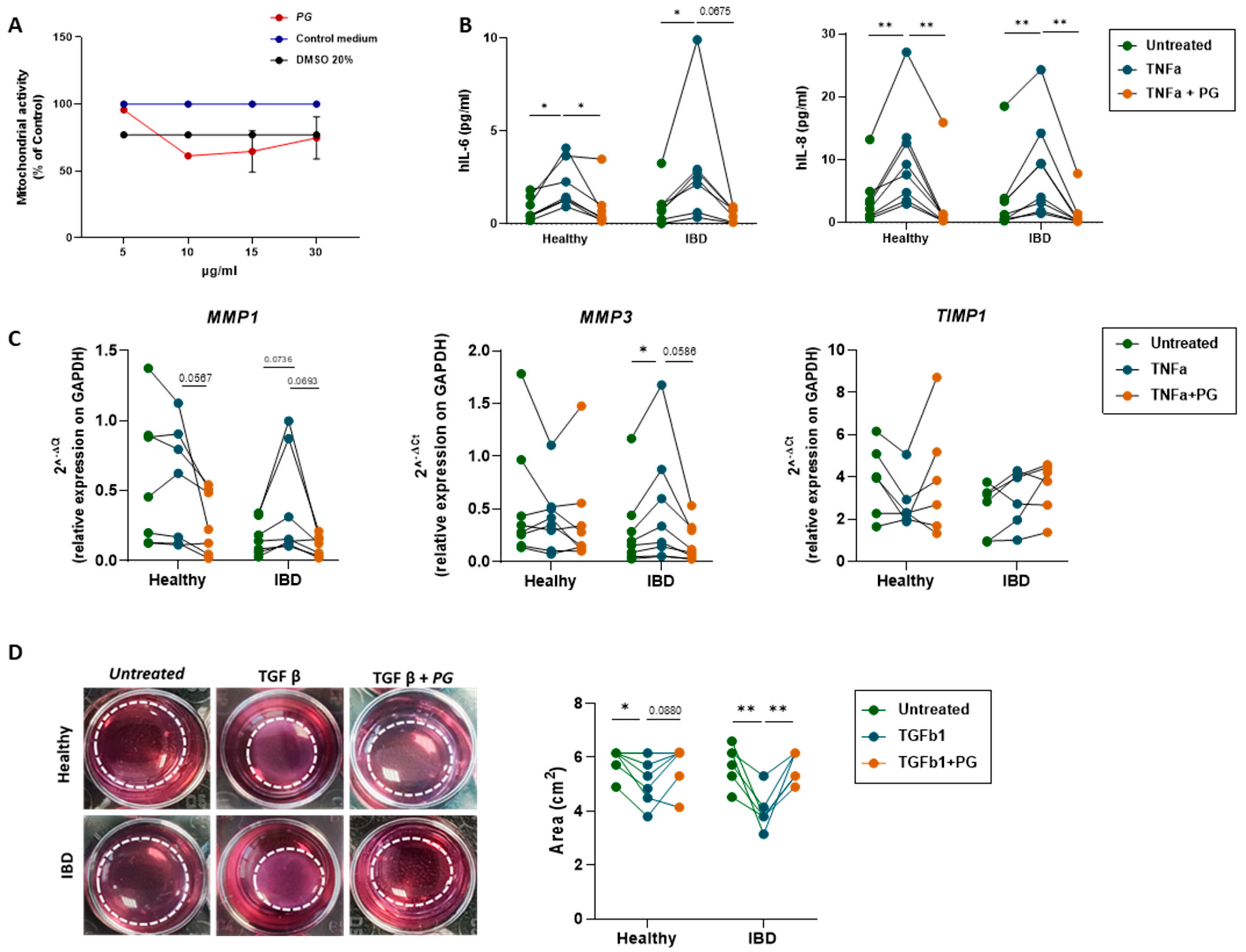
3.6. PG Reverts Fibroblast Activation
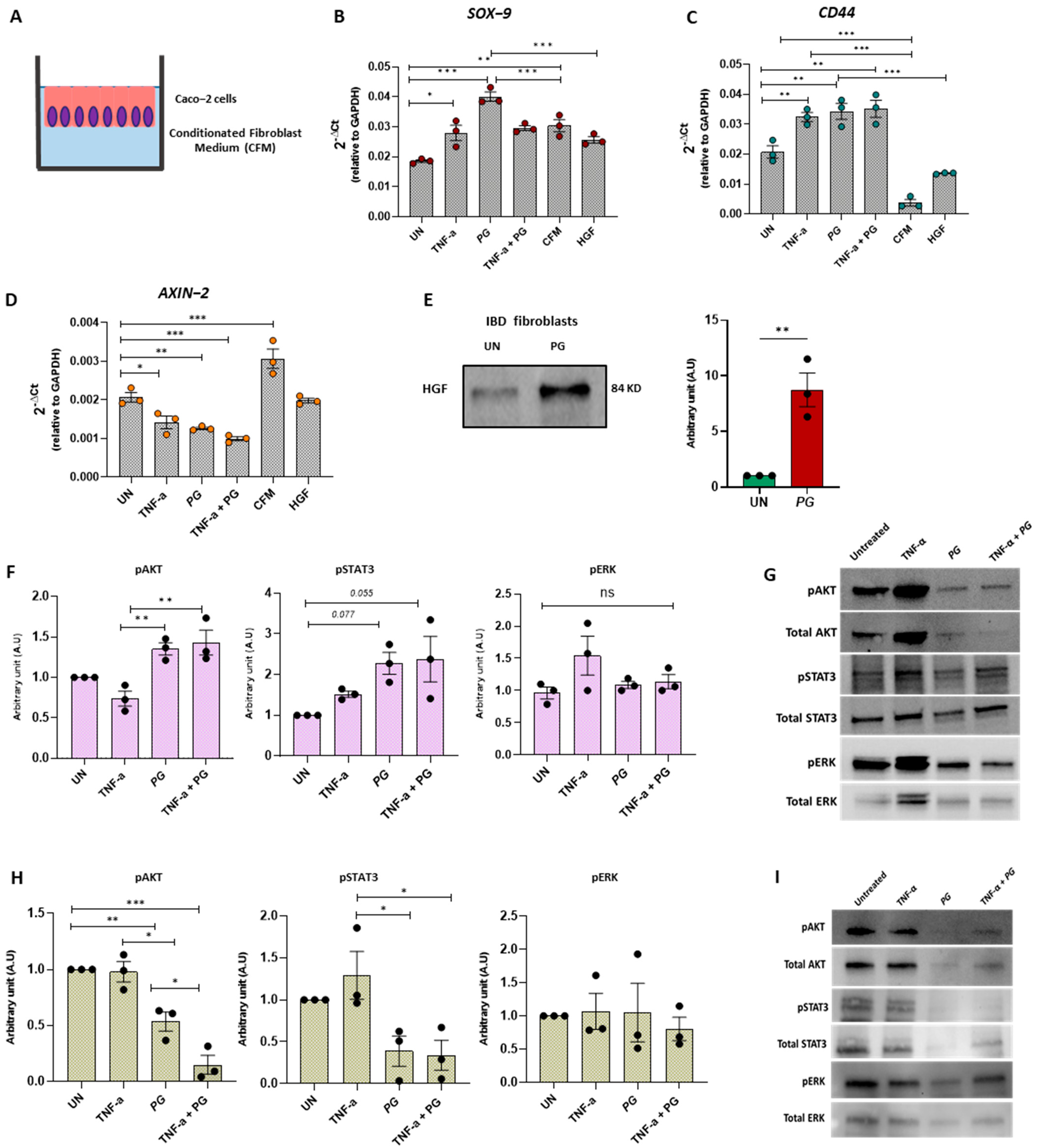
3.7. PG Controls Intestinal Epithelium Repair by Regulating Fibroblast Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Alkhatib, M.; Fayad, C.; Badran, A.; Hamade, K.; Daou, A.; Baydoun, E.; Hijazi, A. Preventive and Therapeutic Effects of Punica granatum (Pomegranate) in Respiratory and Digestive Diseases: A Review. Appl. Sci. 2022, 12, 12326. [Google Scholar] [CrossRef]
- Yisimayili, Z.; Chao, Z. A Review on Phytochemicals, Metabolic Profiles and Pharmacokinetics Studies of the Different Parts (Juice, Seeds, Peel, Flowers, Leaves and Bark) of Pomegranate (Punica granatum L.). Food Chem. 2022, 395, 133600. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of Polyphenols on Gut Microbiota and Implications in Human Health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Câmara, A.B.F.; de Oliveira, K.G.; Santos, M.C.D.; de Lima, R.R.S.; de Lima, K.M.G.; de Carvalho, S.L. Multivariate Assessment for Predicting Antioxidant Activity from Clove and Pomegranate Extracts by MCR-ALS and PLS Models Combined to IR Spectroscopy. Food Chem. 2022, 384, 132321. [Google Scholar] [CrossRef]
- Kupnik, K.; Primožič, M.; Vasić, K.; Knez, Ž.; Leitgeb, M. A Comprehensive Study of the Antibacterial Activity of Bioactive Juice and Extracts from Pomegranate (Punica granatum L.) Peels and Seeds. Plants 2021, 10, 1554. [Google Scholar] [CrossRef] [PubMed]
- Baradaran Rahimi, V.; Ghadiri, M.; Ramezani, M.; Askari, V.R. Antiinflammatory and Anti-cancer Activities of Pomegranate and Its Constituent, Ellagic Acid: Evidence from Cellular, Animal, and Clinical Studies. Phytother. Res. 2020, 34, 685–720. [Google Scholar] [CrossRef]
- Kamali, M.; Tavakoli, H.; Khodadoost, M.; Daghaghzadeh, H.; Kamalinejad, M.; Gachkar, L.; Mansourian, M.; Adibi, P. Efficacy of the Punica Granatum Peels Aqueous Extract for Symptom Management in Ulcerative Colitis Patients. A Randomized, Placebo-Controlled, Clinical Trial. Complement. Ther. Clin. Pract. 2015, 21, 141–146. [Google Scholar] [CrossRef]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 11th ed.; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases with Time, Based on Systematic Review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef]
- Vetrano, S.; Rescigno, M.; Rosaria Cera, M.; Correale, C.; Rumio, C.; Doni, A.; Fantini, M.; Sturm, A.; Borroni, E.; Repici, A.; et al. Unique Role of Junctional Adhesion Molecule-A in Maintaining Mucosal Homeostasis in Inflammatory Bowel Disease. Gastroenterology 2008, 135, 173–184. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Bioactivity of Polyphenols: Preventive and Adjuvant Strategies toward Reducing Inflammatory Bowel Diseases—Promises, Perspectives, and Pitfalls. Oxid. Med. Cell Longev. 2016, 2016, 9346470. [Google Scholar] [CrossRef]
- Ruiz, P.A.; Braune, A.; Hölzlwimmer, G.; Quintanilla-Fend, L.; Haller, D. Quercetin Inhibits TNF-Induced NF-ΚB Transcription Factor Recruitment to Proinflammatory Gene Promoters in Murine Intestinal Epithelial Cells. J. Nutr. 2007, 137, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; González-Sarrías, A.; Yáñez-Gascón, M.J.; Selma, M.V.; Azorín-Ortuño, M.; Toti, S.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Anti-Inflammatory Properties of a Pomegranate Extract and Its Metabolite Urolithin-A in a Colitis Rat Model and the Effect of Colon Inflammation on Phenolic Metabolism. J. Nutr. Biochem. 2010, 21, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Hollebeeck, S.; Winand, J.; Hérent, M.-F.; During, A.; Leclercq, J.; Larondelle, Y.; Schneider, Y.-J. Anti-Inflammatory Effects of Pomegranate (Punica Granatum L.) Husk Ellagitannins in Caco-2 Cells, an in Vitro Model of Human Intestine. Food Funct. 2012, 3, 875. [Google Scholar] [CrossRef] [PubMed]
- Hering, N.A.; Luettig, J.; Jebautzke, B.; Schulzke, J.D.; Rosenthal, R. The Punicalagin Metabolites Ellagic Acid and Urolithin A Exert Different Strengthening and Anti-Inflammatory Effects on Tight Junction-Mediated Intestinal Barrier Function In Vitro. Front. Pharmacol. 2021, 12, 610164. [Google Scholar] [CrossRef] [PubMed]
- Oz, H.S.; Chen, T.S.; McClain, C.J.; de Villiers, W.J.S. Antioxidants as Novel Therapy in a Murine Model of Colitis. J. Nutr. Biochem. 2005, 16, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Galvez, J.; de Souza Gracioso, J.; Camuesco, D.; Galvez, J.; Vilegas, W.; Monteiro Souza Brito, A.R.; Zarzuelo, A. Intestinal Antiinflammatory Activity of a Lyophilized Infusion of Turnera Ulmifolia in TNBS Rat Colitis. Fitoterapia 2006, 77, 515–520. [Google Scholar] [CrossRef]
- Marín, M.; María Giner, R.; Ríos, J.-L.; Carmen Recio, M. Intestinal Anti-Inflammatory Activity of Ellagic Acid in the Acute and Chronic Dextrane Sulfate Sodium Models of Mice Colitis. J. Ethnopharmacol. 2013, 150, 925–934. [Google Scholar] [CrossRef]
- Petti, L. In Vitro Anti-Inflammatory and Protective Effects of Ibidì® on Intestinal Epithelial Cells. Eur. J. Med. Plants 2014, 4, 1022–1035. [Google Scholar] [CrossRef]
- Gracz, A.D.; Fuller, M.K.; Wang, F.; Li, L.; Stelzner, M.; Dunn, J.C.Y.; Martin, M.G.; Magness, S.T. Brief Report: CD24 and CD44 Mark Human Intestinal Epithelial Cell Populations with Characteristics of Active and Facultative Stem Cells. Stem. Cells 2013, 31, 2024–2030. [Google Scholar] [CrossRef]
- Ramalingam, S.; Daughtridge, G.W.; Johnston, M.J.; Gracz, A.D.; Magness, S.T. Distinct Levels of Sox9 Expression Mark Colon Epithelial Stem Cells That Form Colonoids in Culture. Am. J. Physiol.-Gastrointest. Liver. Physiol. 2012, 302, G10–G20. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; De Salvo, C.; Pastorelli, L.; Rana, N.; Senkfor, H.N.; Petito, V.; Di Martino, L.; Scaldaferri, F.; Gasbarrini, A.; Cominelli, F.; et al. IL-33 Promotes Recovery from Acute Colitis by Inducing MiR-320 to Stimulate Epithelial Restitution and Repair. Proc. Natl. Acad. Sci. USA 2018, 115, E9362–E9370. [Google Scholar] [CrossRef]
- Dally, J.; Khan, J.; Voisey, A.; Charalambous, C.; John, H.; Woods, E.; Steadman, R.; Moseley, R.; Midgley, A. Hepatocyte Growth Factor Mediates Enhanced Wound Healing Responses and Resistance to Transforming Growth Factor-Β1-Driven Myofibroblast Differentiation in Oral Mucosal Fibroblasts. Int. J. Mol. Sci. 2017, 18, 1843. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zamora-Ros, R.; Chan, S.; Cross, A.J.; Ward, H.; Jakszyn, P.; Luben, R.; Opstelten, J.L.; Oldenburg, B.; Hallmans, G.; et al. Dietary Polyphenols in the Aetiology of Crohnʼs Disease and Ulcerative Colitis—A Multicenter European Prospective Cohort Study (EPIC). Inflamm. Bowel Dis. 2017, 23, 2072–2082. [Google Scholar] [CrossRef]
- Yu, C.; Wang, D.; Yang, Z.; Wang, T. Pharmacological Effects of Polyphenol Phytochemicals on the Intestinal Inflammation via Targeting TLR4/NF-ΚB Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 6939. [Google Scholar] [CrossRef]
- Catanzaro, D.; Rancan, S.; Orso, G.; Dall’Acqua, S.; Brun, P.; Giron, M.C.; Carrara, M.; Castagliuolo, I.; Ragazzi, E.; Caparrotta, L.; et al. Boswellia Serrata Preserves Intestinal Epithelial Barrier from Oxidative and Inflammatory Damage. PLoS ONE 2015, 10, e0125375. [Google Scholar] [CrossRef]
- Cho, J.A.; Park, E. Curcumin Utilizes the Anti-Inflammatory Response Pathway to Protect the Intestine against Bacterial Invasion. Nutr. Res. Pract. 2015, 9, 117. [Google Scholar] [CrossRef]
- Quiros, M.; Nishio, H.; Neumann, P.A.; Siuda, D.; Brazil, J.C.; Azcutia, V.; Hilgarth, R.; O’Leary, M.N.; Garcia-Hernandez, V.; Leoni, G.; et al. Macrophage-Derived IL-10 Mediates Mucosal Repair by Epithelial WISP-1 Signaling. J. Clin. Investig. 2017, 127, 3510–3520. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, S.Z.; Tauro, S.; Das, I.; Tong, H.; Chen, A.C.-H.; Jeffery, P.L.; McDonald, V.; Florin, T.H.; McGuckin, M.A. IL-10 Promotes Production of Intestinal Mucus by Suppressing Protein Misfolding and Endoplasmic Reticulum Stress in Goblet Cells. Gastroenterology 2013, 144, 357–368.e9. [Google Scholar] [CrossRef]
- Gersemann, M.; Becker, S.; Kübler, I.; Koslowski, M.; Wang, G.; Herrlinger, K.R.; Griger, J.; Fritz, P.; Fellermann, K.; Schwab, M.; et al. Differences in Goblet Cell Differentiation between Crohn’s Disease and Ulcerative Colitis. Differentiation 2009, 77, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Schwerbrock, N.M.J.; Makkink, M.K.; van der Sluis, M.; Büller, H.A.; Einerhand, A.W.C.; Sartor, R.B.; Dekker, J. Interleukin 10-Deficient Mice Exhibit Defective Colonic Muc2 Synthesis Before and After Induction of Colitis by Commensal Bacteria: Inflamm. Bowel Dis. 2004, 10, 811–823. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, X.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. The Role of MUC2 Mucin in Intestinal Homeostasis and the Impact of Dietary Components on MUC2 Expression. Int. J. Biol. Macromol. 2020, 164, 884–891. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Blijlevens, N.M.A.; van der Velden, W.J.F.M. Infections in the Immunocompromised Host. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3384–3394.e2. ISBN 978-1-4557-4801-3. [Google Scholar]
- França, A.; Gaio, V.; Lopes, N.; Melo, L.D.R. Virulence Factors in Coagulase-Negative Staphylococci. Pathogens 2021, 10, 170. [Google Scholar] [CrossRef] [PubMed]
- Ballén, V.; Cepas, V.; Ratia, C.; Gabasa, Y.; Soto, S.M. Clinical Escherichia coli: From Biofilm Formation to New Antibiofilm Strategies. Microorganisms 2022, 10, 1103. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial Biofilms: From the Natural Environment to Infectious Diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Barranquero, J.A.; Reen, F.J.; McCarthy, R.R.; O’Gara, F. Deciphering the Role of Coumarin as a Novel Quorum Sensing Inhibitor Suppressing Virulence Phenotypes in Bacterial Pathogens. Appl. Microbiol. Biotechnol. 2015, 99, 3303–3316. [Google Scholar] [CrossRef] [PubMed]
- Onsare, J.G.; Arora, D.S. Antibiofilm Potential of Flavonoids Extracted from Moringa Oleifera Seed Coat against Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans. J. Appl. Microbiol. 2015, 118, 313–325. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, X.; Zhang, F.; Feng, L.; Li, J. Inhibition of Quorum Sensing, Biofilm, and Spoilage Potential in Shewanella Baltica by Green Tea Polyphenols. J. Microbiol. 2015, 53, 829–836. [Google Scholar] [CrossRef]
- Tsou, L.K.; Lara-Tejero, M.; RoseFigura, J.; Zhang, Z.J.; Wang, Y.-C.; Yount, J.S.; Lefebre, M.; Dossa, P.D.; Kato, J.; Guan, F.; et al. Antibacterial Flavonoids from Medicinal Plants Covalently Inactivate Type III Protein Secretion Substrates. J. Am. Chem. Soc. 2016, 138, 2209–2218. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Spit, M.; Koo, B.-K.; Maurice, M.M. Tales from the Crypt: Intestinal Niche Signals in Tissue Renewal, Plasticity and Cancer. Open Biol. 2018, 8, 180120. [Google Scholar] [CrossRef]
- Walter, R.J.; Sonnentag, S.J.; Munoz-Sagredo, L.; Merkel, M.; Richert, L.; Bunert, F.; Heneka, Y.M.; Loustau, T.; Hodder, M.; Ridgway, R.A.; et al. Wnt Signaling Is Boosted during Intestinal Regeneration by a CD44-Positive Feedback Loop. Cell Death Dis. 2022, 13, 168. [Google Scholar] [CrossRef]
- Bastide, P.; Darido, C.; Pannequin, J.; Kist, R.; Robine, S.; Marty-Double, C.; Bibeau, F.; Scherer, G.; Joubert, D.; Hollande, F.; et al. Sox9 Regulates Cell Proliferation and Is Required for Paneth Cell Differentiation in the Intestinal Epithelium. J. Cell Biol. 2007, 178, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Al-Zahrani, K.N.; Abou-Hamad, J.; Pascoal, J.; Labrèche, C.; Garland, B.; Sabourin, L.A. AKT-Mediated Phosphorylation of Sox9 Induces Sox10 Transcription in a Murine Model of HER2-Positive Breast Cancer. Breast Cancer Res. 2021, 23, 55. [Google Scholar] [CrossRef]
- Liu, S.; Cheng, C. Akt Signaling Is Sustained by a CD44 Splice Isoform–Mediated Positive Feedback Loop. Cancer Res. 2017, 77, 3791–3801. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.; Karin, E.; Terzic, J.; Mucida, D.; Yu, G.-Y.; Vallabhapurapu, S.; Scheller, J.; Rose-John, S.; Cheroutre, H.; Eckmann, L.; et al. IL-6 and Stat3 Are Required for Survival of Intestinal Epithelial Cells and Development of Colitis-Associated Cancer. Cancer Cell 2009, 15, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Allam, A.; Yakou, M.; Pang, L.; Ernst, M.; Huynh, J. Exploiting the STAT3 Nexus in Cancer-Associated Fibroblasts to Improve Cancer Therapy. Front. Immunol. 2021, 12, 767939. [Google Scholar] [CrossRef] [PubMed]
| Score | Weight Loss (%) | Stool Consistency | Rectal Bleeding |
|---|---|---|---|
| 0 | <1 | Normal | Negative |
| 1 | 1–5 | ||
| 2 | 5–10 | Loose | Positive |
| 3 | 10–15 | Diarrhea | Slight bleeding |
| 4 | >15 | Gross bleeding |
| Gene | Forward | Reverse |
|---|---|---|
| GAPDH CD44 | 5′ GGAGCGAGATCCCTCCAAAAT 3′ 5′ CTGCCGCTTTGCAGGTGTA 3′ | 5′ GGCTGTTGTCATACTTCTCATGG 3′ 5′ CATTGTGGGCAAGGTGCTATT 3′ |
| MMP1 | 5′ CTCTGGAGTAATGTCACACCTCT 3′ | 5′ TGTTGGTCCACCTTTCATCTTC 3′ |
| MMP3 | 5′ CTGGACTCCGACACTCTGGA 3′ | 5′ CAGGAAAGGTTCTGAAGTGACC 3′ |
| TIMP1 | 5′ ACCACCTTATACCAGCGTTATGA 3′ | 5′ GGTGTAGACGAACCGGATGTC 3′ |
| AXIN2 SOX9 | 5′ CAACACCAGGCGGAACGAA 3′ 5′ AGCGAACGCACATCAAGAC 3′ | 5′ GCCCAATAAGGAGTGTAAGGACT 3′ 5′ CTGTAGGCGATCTGTTGGGG 3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzo, G.; Pineda Chavez, S.E.; Vandenkoornhuyse, E.; Cárdenas Rincón, C.L.; Cento, V.; Garlatti, V.; Wozny, M.; Sammarco, G.; Di Claudio, A.; Meanti, L.; et al. Pomegranate Extract Affects Gut Biofilm Forming Bacteria and Promotes Intestinal Mucosal Healing Regulating the Crosstalk between Epithelial Cells and Intestinal Fibroblasts. Nutrients 2023, 15, 1771. https://doi.org/10.3390/nu15071771
Rizzo G, Pineda Chavez SE, Vandenkoornhuyse E, Cárdenas Rincón CL, Cento V, Garlatti V, Wozny M, Sammarco G, Di Claudio A, Meanti L, et al. Pomegranate Extract Affects Gut Biofilm Forming Bacteria and Promotes Intestinal Mucosal Healing Regulating the Crosstalk between Epithelial Cells and Intestinal Fibroblasts. Nutrients. 2023; 15(7):1771. https://doi.org/10.3390/nu15071771
Chicago/Turabian StyleRizzo, Giulia, Samuel Elias Pineda Chavez, Elisa Vandenkoornhuyse, Cindy Lorena Cárdenas Rincón, Valeria Cento, Valentina Garlatti, Marek Wozny, Giusy Sammarco, Alessia Di Claudio, Lisa Meanti, and et al. 2023. "Pomegranate Extract Affects Gut Biofilm Forming Bacteria and Promotes Intestinal Mucosal Healing Regulating the Crosstalk between Epithelial Cells and Intestinal Fibroblasts" Nutrients 15, no. 7: 1771. https://doi.org/10.3390/nu15071771
APA StyleRizzo, G., Pineda Chavez, S. E., Vandenkoornhuyse, E., Cárdenas Rincón, C. L., Cento, V., Garlatti, V., Wozny, M., Sammarco, G., Di Claudio, A., Meanti, L., Elangovan, S., Romano, A., Roda, G., Loy, L., Dal Buono, A., Gabbiadini, R., Lovisa, S., Rusconi, R., Repici, A., ... Vetrano, S. (2023). Pomegranate Extract Affects Gut Biofilm Forming Bacteria and Promotes Intestinal Mucosal Healing Regulating the Crosstalk between Epithelial Cells and Intestinal Fibroblasts. Nutrients, 15(7), 1771. https://doi.org/10.3390/nu15071771