Bile Salt Hydrolase-Competent Probiotics in the Management of IBD: Unlocking the “Bile Acid Code”
Abstract
:1. Introduction
2. Bile Acids: Between Digestion and Metabolic Signaling
3. Bile Acid Deconjugationand BSH-Competent Microorganisms
4. Bile Acid-Dependent Shaping of Intestinal Microbial Community
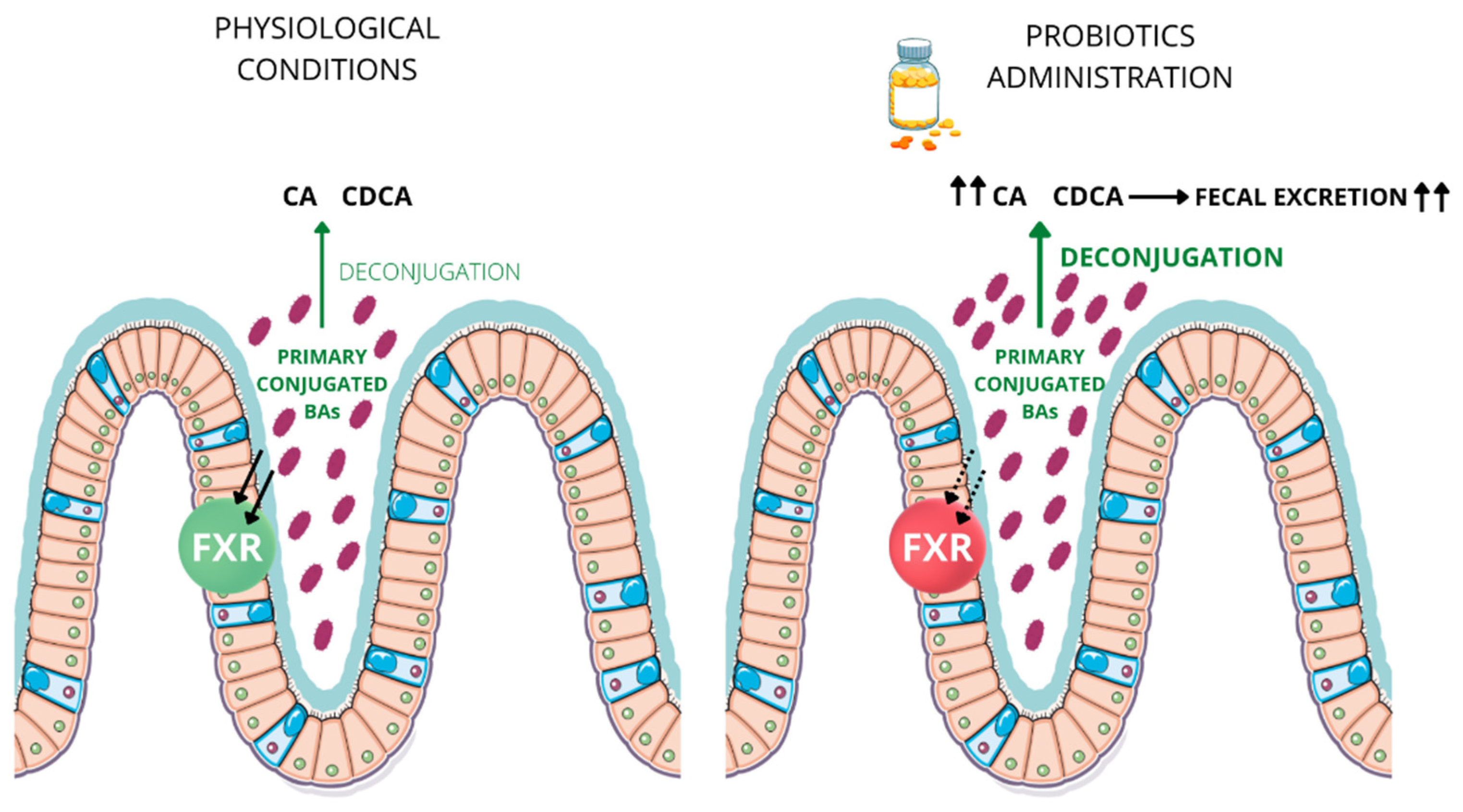
5. Bile Salt Hydrolase Activity and Probiotics
6. BSH-Competent Bacteriaand Probiotics Administrationin Inflammatory Bowel Disease
7. Conclusions and Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pflughoeft, K.J.; Versalovic, J. Human Microbiome in Health and Disease. Annu. Rev. Pathol. 2012, 7, 99–122. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, N.; Kawada, N. Role of the Gut-Liver Axis in Liver Inflammation, Fibrosis, and Cancer: A Special Focus on the Gut Microbiota Relationship. Hepatol. Commun. 2019, 3, 456–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macia, L.; Thorburn, A.N.; Binge, L.C.; Mariño, E.; Rogers, K.E.; Maslowski, K.; Vieira, A.; Kranich, J.; Mackay, C.R. Microbial influences on epithelial integrity and immune function as a basis for inflammatory diseases. Immunol. Rev. 2012, 245, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Wu, L.; Huntington, N.D.; Zhang, X. Crosstalk between Gut Microbiota and Innate Immunity and Its Implication in Autoimmune Diseases. Front. Immunol. 2020, 11, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerdó, T.; Diéguez, E.; Campoy, C. Impact of gut microbiota on neurogenesis and neurological diseases during infancy. Curr. Opin. Pharmacol. 2020, 50, 33–37. [Google Scholar] [CrossRef]
- Ghaisas, S.; Maher, J.; Kanthasamy, A. Gut microbiome in health and disease: Linking the microbiome–gut–brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. Pharmacol. Ther. 2016, 158, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Xing, C.; Long, W.; Wang, H.Y.; Liu, Q.; Wang, R.-F. Impact of microbiota on central nervous system and neurological diseases: The gut-brain axis. J. Neuroinflamm. 2019, 16, 53. [Google Scholar] [CrossRef] [Green Version]
- Berry, S.E.; Valdes, A.M.; Drew, D.A.; Asnicar, F.; Mazidi, M.; Wolf, J. Human postprandial responses to food and potential for precision nutrition. Nat. Med. 2020, 26, 964–973. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Albert, M.J.; Mathan, V.I.; Baker, S.J. Vitamin B12 synthesis by human small intestinal bacteria. Nature 1980, 283, 781–782. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Milani, C.; de Giori, G.S.; Sesma, F.; van Sinderen, D.; Ventura, M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Pascale, A.; Marchesi, N.; Marelli, C.; Coppola, A.; Luzi, L.; Govoni, S. Microbiota and metabolic diseases. Endocrine 2018, 61, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; Van Der Veeken, J.; DeRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [Green Version]
- Dalile, B.; Van, O.L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Stilling, R.M.; van de Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef]
- Wahlström, A.; Sayin, S.I.; Marschall, H.-U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Falany, C.N.; Johnson, M.R.; Barnes, S.; Diasio, R.B. Glycine and taurine conjugation of bile acids by a single enzyme. Molecular cloning and expression of human liver bile acid CoA:amino acid N-acyltransferase. J. Biol. Chem. 1994, 269, 19375–19379. [Google Scholar] [CrossRef]
- Chiang, J.Y. Bile acid metabolism and signaling. Compr. Physiol. 2013, 3, 1191–1212. [Google Scholar] [PubMed] [Green Version]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006, 47, 241–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridlon, J.M.; Harris, S.C.; Bhowmik, S.; Kang, D.-J.; Hylemon, P.B. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 2016, 7, 22–39. [Google Scholar] [CrossRef] [Green Version]
- Alrehaili, B.D.; Lee, M.; Takahashi, S.; Novak, R.; Rimal, B.; Boehme, S.; Trammell, S.A.J.; Grevengoed, T.J.; Kumar, D.; Alnouti, Y.; et al. Bile acid conjugation deficiency causes hypercholanemia, hyperphagia, islet dysfunction, and gut dysbiosis in mice. Hepatol. Commun. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B.; Bajaj, J.S. Bile acids and the gut microbiome. Curr. Opin. Gastroenterol. 2014, 30, 332–338. [Google Scholar] [CrossRef] [Green Version]
- Makishima, M.; Okamoto, A.Y.; Repa, J.J.; Tu, H.; Learned, R.M.; Luk, A.; Hull, M.V.; Lustig, K.D.; Mangelsdorf, D.J.; Shan, B. Identification of a Nuclear Receptor for Bile Acids. Science 1999, 284, 1362–1365. [Google Scholar] [CrossRef]
- Parks, D.J.; Blanchard, S.G.; Bledsoe, R.K.; Chandra, G.; Consler, T.G.; Kliewer, S.A.; Stimmel, J.B.; Willson, T.M.; Zavacki, A.M.; Moore, D.D.; et al. Bile Acids: Natural Ligands for an Orphan Nuclear Receptor. Science 1999, 284, 1365–1368. [Google Scholar] [CrossRef]
- Wang, H.; Chen, J.; Hollister, K.; Sowers, L.C.; Forman, B.M. Endogenous Bile Acids Are Ligands for the Nuclear Receptor FXR/BAR. Mol. Cell 1999, 3, 543–553. [Google Scholar] [CrossRef]
- Gadaleta, R.M.; Cariello, M.; Sabbà, C.; Moschetta, A. Tissue-specific actions of FXR in metabolism and cancer. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2015, 1851, 30–39. [Google Scholar] [CrossRef]
- Inagaki, T.; Choi, M.; Moschetta, A.; Peng, L.; Cummins, C.L.; McDonald, J.G.; Luo, G.; Jones, S.A.; Goodwin, B.; Richardson, J.A.; et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005, 2, 217–225. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.; Ahn, S.-H.; Inagaki, T.; Choi, M.; Ito, S.; Guo, G.L.; Kliewer, S.A.; Gonzalez, F.J. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J. Lipid Res. 2007, 48, 2664–2672. [Google Scholar] [CrossRef] [Green Version]
- Kong, B.; Wang, L.; Chiang, J.Y.; Zhang, Y.; Klaassen, C.D.; Guo, G.L. Mechanism of tissue-specific farnesoid X receptor in suppressing the expression of genes in bile-acid synthesis in mice. Hepatology 2012, 56, 1034–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Repa, J.J.; Mangelsdorf, D.J. The Role of Orphan Nuclear Receptors in the Regulation of Cholesterol Homeostasis. Annu. Rev. Cell Dev. Biol. 2000, 16, 459–481. [Google Scholar] [CrossRef] [PubMed]
- Gadaleta, R.M.; Van Erpecum, K.J.; Oldenburg, B.; Willemsen, E.C.L.; Renooij, W.; Murzilli, S.; Klomp, L.W.J.; Siersema, P.D.; Schipper, M.E.; Danese, S.; et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 2011, 60, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Gadaleta, R.M.; Oldenburg, B.; Willemsen, E.C.; Spit, M.; Murzilli, S.; Salvatore, L. Activation of bile salt nuclear receptor FXR is repressed by pro-inflammatory cytokines activating NF-kappaB signaling in the intestine. Biochim. Biophys. Acta 2011, 1812, 851–858. [Google Scholar] [CrossRef]
- Vavassori, P.; Mencarelli, A.; Renga, B.; Distrutti, E.; Fiorucci, S. The Bile Acid Receptor FXR Is a Modulator of Intestinal Innate Immunity. J. Immunol. 2009, 183, 6251–6261. [Google Scholar] [CrossRef] [Green Version]
- Cariello, M.; Zerlotin, R.; Pasculli, E.; Piccinin, E.; Peres, C.; Porru, E.; Roda, A.; Gadaleta, R.M.; Moschetta, A. Intestinal FXR Activation via Transgenic Chimera or Chemical Agonism Prevents Colitis-Associated and Genetically-Induced Colon Cancer. Cancers 2022, 14, 3081. [Google Scholar] [CrossRef]
- Maran, R.R.; Thomas, A.; Roth, M.; Sheng, Z.; Esterly, N.; Pinson, D.; Gao, X.; Zhang, Y.; Ganapathy, V.; Gonzalez, F.J.; et al. Farnesoid X Receptor Deficiency in Mice Leads to Increased Intestinal Epithelial Cell Proliferation and Tumor Development. J. Pharmacol. Exp. Ther. 2009, 328, 469–477. [Google Scholar] [CrossRef] [Green Version]
- Modica, S.; Murzilli, S.; Salvatore, L.; Schmidt, D.R.; Moschetta, A. Nuclear Bile Acid Receptor FXR Protects against Intestinal Tumorigenesis. Cancer Res. 2008, 68, 9589–9594. [Google Scholar] [CrossRef] [Green Version]
- Inagaki, T.; Moschetta, A.; Lee, Y.-K.; Peng, L.; Zhao, G.; Downes, M.; Yu, R.T.; Shelton, J.M.; Richardson, J.A.; Repa, J.J.; et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 3920–3925. [Google Scholar] [CrossRef] [Green Version]
- Gadaleta, R.M.; Garcia-Irigoyen, O.; Cariello, M.; Scialpi, N.; Peres, C.; Vetrano, S. Fibroblast Growth Factor 19 modulates intestinal microbiota and inflammation in presence of Farnesoid X Receptor. eBioMedicine 2020, 54, 102719. [Google Scholar] [CrossRef] [PubMed]
- Turroni, F.; Ribbera, A.; Foroni, E.; Van Sinderen, D.; Ventura, M. Human gut microbiota and bifidobacteria: From composition to functionality. Antonie Leeuwenhoek 2008, 94, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Massafra, V.; van Mil, S.W.C. Farnesoid X receptor: A “homeostat” for hepatic nutrient metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Begley, M.; Hill, C.; Gahan, C.G. Bile salt hydrolase activity in probiotics. Appl. Environ. Microbiol. 2006, 72, 1729–1738. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.K.; Singhania, R.R.; Pandey, A.; Chincholkar, S.B. Probiotic Bile Salt Hydrolase: Current Developments and Perspectives. Appl. Biochem. Biotechnol. 2009, 162, 166–180. [Google Scholar] [CrossRef]
- Batta, A.K.; Salen, G.; Arora, R.; Shefer, S.; Batta, M.; Person, A. Side chain conjugation prevents bacterial 7-dehydroxylation of bile acids. J. Biol. Chem. 1990, 265, 10925–10928. [Google Scholar] [CrossRef]
- Geng, W.; Lin, J. Bacterial bile salt hydrolase: An intestinal microbiome target for enhanced animal health. Anim. Health Res. Rev. 2016, 17, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Jones, B.V.; Begley, M.; Hill, C.; Gahan, C.G.M.; Marchesi, J.R. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc. Natl. Acad. Sci. USA 2008, 105, 13580–13585. [Google Scholar] [CrossRef] [Green Version]
- Bateup, J.M.; McConnell, M.A.; Jenkinson, H.F.; Tannock, G.W. Comparison of Lactobacillus strains with respect to bile salt hydrolase activity, colonization of the gastrointestinal tract, and growth rate of the murine host. Appl. Environ. Microbiol. 1995, 61, 1147–1149. [Google Scholar] [CrossRef] [Green Version]
- Corzo, G.; Gilliland, S.E. Bile Salt Hydrolase Activity of Three Strains of Lactobacillus acidophilus. J. Dairy Sci. 1999, 82, 472–480. [Google Scholar] [CrossRef]
- Grill, J.; Schneider, F.; Crociani, J.; Ballongue, J. Purification and Characterization of Conjugated Bile Salt Hydrolase from Bifidobacterium longum BB536. Appl. Environ. Microbiol. 1995, 61, 2577–2582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.-B.; Yi, S.-H.; Lee, B.H. Purification and Characterization of Three Different Types of Bile Salt Hydrolases from Bifidobacterium Strains. J. Dairy Sci. 2004, 87, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, H.; Hashiba, H.; Kok, J.; Mierau, I. Bile Salt Hydrolase of Bifidobacterium longum—Biochemical and Genetic Characterization. Appl. Environ. Microbiol. 2000, 66, 2502–2512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishinaka, M.; Umeda, A.; Kuroki, S. High concentrations of conjugated bile acids inhibit bacterial growth of Clostridium perfringens and induce its extracellular cholylglycine hydrolase. Steroids 1994, 59, 485–489. [Google Scholar] [CrossRef]
- Masuda, N. Deconjugation of bile salts by Bacteroids and Clostridium. Microbiol. Immunol. 1981, 25, 1–11. [Google Scholar] [CrossRef]
- Franz, C.M.; Specht, I.; Haberer, P.; Holzapfel, W.H. Bile Salt Hydrolase Activity of Enterococci Isolated from Food: Screening and Quantitative Determination. J. Food Prot. 2001, 64, 725–729. [Google Scholar] [CrossRef]
- Yao, L.; Seaton, S.C.; Ndousse-Fetter, S.; Adhikari, A.A.; DiBenedetto, N.; Mina, A.; Banks, A.S.; Bry, L.; Devlin, A.S. A selective gut bacterial bile salt hydrolase alters host metabolism. eLife 2018, 7, e37182. [Google Scholar] [CrossRef]
- Song, Z.; Cai, Y.; Lao, X.; Wang, X.; Lin, X.; Cui, Y.; Kalavagunta, P.K.; Liao, J.; Jin, L.; Shang, J.; et al. Taxonomic profiling and populational patterns of bacterial bile salt hydrolase (BSH) genes based on worldwide human gut microbiome. Microbiome 2019, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Begley, M.; Gahan, C.G.; Hill, C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 2005, 29, 625–651. [Google Scholar] [CrossRef] [Green Version]
- Jarocki, P.; Targoński, Z. Genetic Diversity of Bile Salt Hydrolases among Human Intestinal Bifidobacteria. Curr. Microbiol. 2013, 67, 286–292. [Google Scholar] [CrossRef] [Green Version]
- Liang, L.; Yi, Y.; Lv, Y.; Qian, J.; Lei, X.; Zhang, G. A Comprehensive Genome Survey Provides Novel Insights into Bile Salt Hydrolase (BSH) in Lactobacillaceae. Molecules 2018, 23, 1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coleman, J.P.; Hudson, L.L. Cloning and characterization of a conjugated bile acid hydrolase gene from Clostridium perfringens. Appl. Environ. Microbiol. 1995, 61, 2514–2520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dussurget, O.; Cabanes, D.; Dehoux, P.; Lecuit, M.; Buchrieser, C.; Glaser, P. Listeria monocytogenes bile salt hydrolase is a PrfA-regulated virulence factor involved in the intestinal and hepatic phases of listeriosis. Mol. Microbiol. 2002, 45, 1095–1106. [Google Scholar] [CrossRef]
- Delpino, M.V.; Marchesini, M.I.; Estein, S.M.; Comerci, D.J.; Cassataro, J.; Fossati, C.A.; Baldi, P.C. A Bile Salt Hydrolase of Brucella abortus Contributes to the Establishment of a Successful Infection through the Oral Route in Mice. Infect. Immun. 2007, 75, 299–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shankar, N.; Baghdayan, A.S.; Gilmore, M.S. Modulation of virulence within a pathogenicity island in vancomycin-resistant Enterococcus faecalis. Nature 2002, 417, 746–750. [Google Scholar] [CrossRef]
- Dean, M.; Cervellati, C.; Casanova, E.; Squerzanti, M.; Lanzara, V.; Medici, A.; De Laureto, P.P.; Bergamini, C.M. Characterization of Cholylglycine Hydrolase from a Bile-Adapted Strain of Xanthomonas maltophilia and Its Application for Quantitative Hydrolysis of Conjugated Bile Salts. Appl. Environ. Microbiol. 2002, 68, 3126–3128. [Google Scholar] [CrossRef] [Green Version]
- Margolles, A.; Gueimonde, M.; Sánchez, B. Genome Sequence of the Antarctic Psychrophile Bacterium Planococcusantarcticus DSM 14505. J. Bacteriol. 2012, 194, 4465. [Google Scholar] [CrossRef] [Green Version]
- Funabashi, M.; Grove, T.L.; Wang, M.; Varma, Y.; McFadden, M.E.; Brown, L.C.; Guo, C.; Higginbottom, S.; Almo, S.C.; Fischbach, M.A. A metabolic pathway for bile acid dehydroxylation by the gut microbiome. Nature 2020, 582, 566–570. [Google Scholar] [CrossRef]
- Jin, W.-B.; Li, T.-T.; Huo, D.; Qu, S.; Li, X.V.; Arifuzzaman, M.; Lima, S.F.; Shi, H.-Q.; Wang, A.; Putzel, G.G.; et al. Genetic manipulation of gut microbes enables single-gene interrogation in a complex microbiome. Cell 2022, 185, 547–562.e22. [Google Scholar] [CrossRef]
- Devlin, A.S.; Fischbach, M.A. A biosynthetic pathway for a prominent class of microbiota-derived bile acids. Nat. Chem. Biol. 2015, 11, 685–690. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.J.; Plichta, D.; Hogstrom, L.; Borren, N.Z.; Lau, H.; Gregory, S.M.; Tan, W.; Khalili, H.; Clish, C.; Vlamakis, H.; et al. Multi-omics reveal microbial determinants impacting responses to biologic therapies in inflammatory bowel disease. Cell Host Microbe 2021, 29, 1294–1304.e4. [Google Scholar] [CrossRef] [PubMed]
- Doden, H.L.; Ridlon, J.M. Microbial Hydroxysteroid Dehydrogenases: From Alpha to Omega. Microorganisms 2021, 9, 469. [Google Scholar] [CrossRef] [PubMed]
- Quinn, R.A.; Melnik, A.V.; Vrbanac, A.; Fu, T.; Patras, K.A.; Christy, M.P.; Bodai, Z.; Belda-Ferre, P.; Tripathi, A.; Chung, L.K.; et al. Global chemical effects of the microbiome include new bile-acid conjugations. Nature 2020, 579, 123–129. [Google Scholar] [CrossRef]
- de Aguiar Vallim, T.Q.; Tarling, E.J.; Edwards, P.A. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013, 17, 657–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayin, S.I.; Wahlström, A.; Felin, J.; Jäntti, S.; Marschall, H.-U.; Bamberg, K.; Angelin, B.; Hyötyläinen, T.; Orešič, M.; Bäckhed, F. Gut Microbiota Regulates Bile Acid Metabolism by Reducing the Levels of Tauro-beta-muricholic Acid, a Naturally Occurring FXR Antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef] [Green Version]
- Islam, K.S.; Fukiya, S.; Hagio, M.; Fujii, N.; Ishizuka, S.; Ooka, T.; Ogura, Y.; Hayashi, T.; Yokota, A. Bile Acid Is a Host Factor That Regulates the Composition of the Cecal Microbiota in Rats. Gastroenterology 2011, 141, 1773–1781. [Google Scholar] [CrossRef]
- Jones, M.L.; Martoni, C.J.; Ganopolsky, J.G.; Labbé, A.; Prakash, S. The human microbiome and bile acid metabolism: Dysbiosis, dysmetabolism, disease and intervention. Expert Opin. Biol. Ther. 2014, 14, 467–482. [Google Scholar] [CrossRef]
- Sagar, N.M.; Cree, I.A.; Covington, J.A.; Arasaradnam, R.P. The interplay of the gut microbiome, bile acids, and volatile organic compounds. Gastroenterol. Res. Pract. 2015, 2015, 398585. [Google Scholar] [CrossRef] [Green Version]
- Degirolamo, C.; Rainaldi, S.; Bovenga, F.; Murzilli, S.; Moschetta, A. Microbiota Modification with Probiotics Induces Hepatic Bile Acid Synthesis via Downregulation of the Fxr-Fgf15 Axis in Mice. Cell Rep. 2014, 7, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Herrema, H.; Meissner, M.; van Dijk, T.H.; Brufau, G.; Boverhof, R.; Oosterveer, M.H. Bile salt sequestration induces hepatic de novo lipogenesis through farnesoid X receptor- and liver X receptor alpha-controlled metabolic pathways in mice. Hepatology 2010, 51, 806–816. [Google Scholar] [CrossRef]
- Coleman, R.; Lowe, P.J.; Billington, D. Membrane lipid composition and susceptibility to bile salt damage. Biochim. Biophys. Acta BBA Biomembr. 1980, 599, 294–300. [Google Scholar] [CrossRef]
- Taranto, M.P.; Perez-Martinez, G.; Font d, V. Effect of bile acid on the cell membrane functionality of lactic acid bacteria for oral administration. Res. Microbiol. 2006, 157, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Fukiya, S.; Yokota, A. Comprehensive evaluation of the bactericidal activities of free bile acids in the large intestine of humans and rodents. J. Lipid Res. 2017, 58, 1143–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clements, W.D.; Parks, R.; Erwin, P.; Halliday, M.I.; Barr, J.; Rowlands, B.J. Role of the gut in the pathophysiology of extrahepatic biliary obstruction. Gut 1996, 39, 587–593. [Google Scholar] [CrossRef] [Green Version]
- Ilan, Y. Leaky gut and the liver: A role for bacterial translocation in nonalcoholic steatohepatitis. World J. Gastroenterol. 2012, 18, 2609–2618. [Google Scholar] [CrossRef] [PubMed]
- Wigg, A.J.; Roberts-Thomson, I.C.; Dymock, R.B.; McCarthy, P.J.; Grose, R.H.; Cummins, A.G. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut 2001, 48, 206–211. [Google Scholar] [CrossRef] [Green Version]
- Fouts, D.E.; Torralba, M.; Nelson, K.E.; Brenner, D.A.; Schnabl, B. Bacterial translocation and changes in the intestinal microbiome in mouse models of liver disease. J. Hepatol. 2012, 56, 1283–1292. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo-Zúñiga, V.; Bartolí, R.; Planas, R.; Hofmann, A.F.; Viñado, B.; Hagey, L.R.; Hernández, J.M.; Mañé, J.; Alvarez-Gonzalez, M.A.; Ausina, V.; et al. Oral bile acids reduce bacterial overgrowth, bacterial translocation, and endotoxemia in cirrhotic rats. Hepatology 2003, 37, 551–557. [Google Scholar] [CrossRef]
- Berg, R.D. Bacterial translocation from the gastrointestinal tract. Adv. Exp. Med. Biol. 1999, 473, 11–30. [Google Scholar] [CrossRef]
- Cahill, C.J.; Pain, J.A.; Bailey, M.E. Bile salts, endotoxin and renal function in obstructive jaundice. Surg. Gynecol. Obstet. 1987, 165, 519–522. [Google Scholar]
- Ding, J.W.; Andersson, R.; Soltesz, V.; Willén, R.; Bengmark, S. The Role of Bile and Bile Acids in Bacterial Translocation in Obstructive Jaundice in Rats. Eur. Surg. Res. 1993, 25, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.J.; Torrealba, V.; Hudd, C.; Knight, M. The effect of preoperative bile salt administration on postoperative renal function in patients with obstructive jaundice. Br. J. Surg. 1982, 69, 706–708. [Google Scholar] [CrossRef] [PubMed]
- Marzano, M.; Fosso, B.; Colliva, C.; Notario, E.; Passeri, D.; Intranuovo, M. Farnesoid X receptor activation by the novel agonist TC-100 (3a, 7a, 11ß-Trihydroxy-6a-ethyl-5ß-cholan-24-oic Acid) preserves the intestinal barrier integrity and promotes intestinal microbial reshaping in a mouse model of obstructed bile acid flow. Biomed. Pharmacother. 2022, 153, 1–9. [Google Scholar] [CrossRef]
- Claus, S.P.; Tsang, T.M.; Wang, Y.; Cloarec, O.; Skordi, E.; Martin, F.-P.; Rezzi, S.; Ross, A.; Kochhar, S.; Holmes, E.; et al. Systemic multicompartmental effects of the gut microbiome on mouse metabolic phenotypes. Mol. Syst. Biol. 2008, 4, 219. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, P.; Hochrath, K.; Horvath, A.; Chen, P.; Seebauer, C.T.; Llorente, C.; Wang, L.; Alnouti, Y.; Fouts, D.E.; Stärkel, P.; et al. Modulation of the intestinal bile acid/farnesoid X receptor/fibroblast growth factor 15 axis improves alcoholic liver disease in mice. Hepatology 2018, 67, 2150–2166. [Google Scholar] [CrossRef]
- Martin, F.-P.; Dumas, M.-E.; Wang, Y.; Legido-Quigley, C.; Yap, I.K.S.; Tang, H.; Zirah, S.; Murphy, G.M.; Cloarec, O.; Lindon, J.C.; et al. A top-down systems biology view of microbiome-mammalian metabolic interactions in a mouse model. Mol. Syst. Biol. 2007, 3, 112. [Google Scholar] [CrossRef] [Green Version]
- Swann, J.R.; Want, E.J.; Geier, F.M.; Spagou, K.; Wilson, I.D.; Sidaway, J.E. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4523–4530. [Google Scholar] [CrossRef] [Green Version]
- Wostmann, B.S. Intestinal Bile Acids and Cholesterol Absorption in the Germfree Rat. J. Nutr. 1973, 103, 982–990. [Google Scholar] [CrossRef]
- Friedman, E.S.; Li, Y.; Shen, T.-C.D.; Jiang, J.; Chau, L.; Adorini, L.; Babakhani, F.; Edwards, J.; Shapiro, D.; Zhao, C.; et al. FXR-Dependent Modulation of the Human Small Intestinal Microbiome by the Bile Acid Derivative Obeticholic Acid. Gastroenterology 2018, 155, 1741–1752.e5. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Dong, W.; Liu, L.; Xu, M.; Wang, Y.; Liu, T.; Zhang, Y.; Wang, B.; Cao, H. Interplay between bile acids and the gut microbiota promotes intestinal carcinogenesis. Mol. Carcinog. 2019, 58, 1155–1167. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Cen, M.; Shen, Y.; Zhu, Y.; Cheng, F.; Tang, L.; Hu, W.; Dai, N. Deoxycholic Acid-Induced Gut Dysbiosis Disrupts Bile Acid Enterohepatic Circulation and Promotes Intestinal Inflammation. Dig. Dis. Sci. 2021, 66, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.L.; Tomaro-Duchesneau, C.; Martoni, C.J.; Prakash, S. Cholesterol lowering with bile salt hydrolase-active probiotic bacteria, mechanism of action, clinical evidence, and future direction for heart health applications. Expert Opin. Biol. Ther. 2013, 13, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Joyce, S.A.; MacSharry, J.; Casey, P.G.; Kinsella, M.; Murphy, E.F.; Shanahan, F.; Hill, C.; Gahan, C.G.M. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc. Natl. Acad. Sci. USA 2014, 111, 7421–7426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherji, A.; Kobiita, A.; Ye, T.; Chambon, P. Homeostasis in Intestinal Epithelium Is Orchestrated by the Circadian Clock and Microbiota Cues Transduced by TLRs. Cell 2013, 153, 812–827. [Google Scholar] [CrossRef] [Green Version]
- Angelakis, E.; Merhej, V.; Raoult, D. Related actions of probiotics and antibiotics on gut microbiota and weight modification. Lancet Infect. Dis. 2013, 13, 889–899. [Google Scholar] [CrossRef]
- Hernandez-Gomez, J.G.; Lopez-Bonilla, A.; Trejo-Tapia, G.; Avila-Reyes, S.V.; Jimenez-Aparicio, A.R.; Hernandez-Sanchez, H. In Vitro Bile Salt Hydrolase (BSH) Activity Screening of Different Probiotic Microorganisms. Foods 2021, 10, 674. [Google Scholar] [CrossRef]
- Briand, F.; Sulpice, T.; Giammarinaro, P.; Roux, X. Saccharomyces boulardii CNCM I-745 changes lipidemic profile and gut microbiota in a hamster hypercholesterolemic model. Benef. Microbes 2019, 10, 555–567. [Google Scholar] [CrossRef]
- Ryan, J.J.; Hanes, D.A.; Schafer, M.B.; Mikolai, J.; Zwickey, H. Effect of the Probiotic Saccharomyces boulardii on Cholesterol and Lipoprotein Particles in Hypercholesterolemic Adults: A Single-Arm, Open-Label Pilot Study. J. Altern. Complement. Med. 2015, 21, 288–293. [Google Scholar] [CrossRef] [Green Version]
- Duboc, H.; Rajca, S.; Rainteau, D.; Benarous, D.; Maubert, M.-A.; Quervain, E.; Thomas, G.; Barbu, V.; Humbert, L.; Despras, G.; et al. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut 2013, 62, 531–539. [Google Scholar] [CrossRef]
- Ogilvie, L.A.; Jones, B.V. Dysbiosis modulates capacity for bile acid modification in the gut microbiomes of patients with inflammatory bowel disease: A mechanism and marker of disease? Gut 2012, 61, 1642–1643. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Dong, W.; Wang, S.; Zhang, Y.; Liu, T.; Xie, R.; Wang, B.; Cao, H. Deoxycholic acid disrupts the intestinal mucosal barrier and promotes intestinal tumorigenesis. Food Funct. 2018, 9, 5588–5597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.; Shen, Y.; Cen, M.; Zhu, Y.; Cheng, F.; Tang, L.; Zheng, X.; Kim, J.J.; Dai, N.; Hu, W. Modulation of the Gut Microbiota-farnesoid X Receptor Axis Improves Deoxycholic Acid-induced Intestinal Inflammation in Mice. J. Crohn’s Colitis 2021, 15, 1197–1210. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.R.; Haileselassie, Y.; Nguyen, L.P.; Tropini, C.; Wang, M.; Becker, L.S. Dysbiosis-Induced Secondary Bile Acid Deficiency Promotes Intestinal Inflammation. Cell Host Microb. 2020, 27, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef]
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 2019, 4, 293–305. [Google Scholar] [CrossRef]
- Jakubczyk, D.; Leszczyńska, K.; Górska, S. The Effectiveness of Probiotics in the Treatment of Inflammatory Bowel Disease (IBD)—A Critical Review. Nutrients 2020, 12, 1973. [Google Scholar] [CrossRef]
- Huttenhower, C.; Kostic, A.D.; Xavier, R.J. Inflammatory Bowel Disease as a Model for Translating the Microbiome. Immunity 2014, 40, 843–854. [Google Scholar] [CrossRef] [Green Version]
- Jia, B.; Park, D.; Hahn, Y.; Jeon, C.O. Metagenomic analysis of the human microbiome reveals the association between the abundance of gut bile salt hydrolases and host health. Gut Microbes 2020, 11, 1300–1313. [Google Scholar] [CrossRef]
- Carlsson, A.H.; Yakymenko, O.; Olivier, I.; Hakansson, F.; Postma, E.; Keita, A.V. Faecalibacteriumprausnitzii supernatant improves intestinal barrier function in mice DSS colitis. Scand. J. Gastroenterol. 2013, 48, 1136–1144. [Google Scholar] [CrossRef]
- Laval, L.; Martin, R.; Natividad, J.; Chain, F.; Miquel, S.; de Maredsous, C.D.; Capronnier, S.; Sokol, H.; Verdu, E.; van HylckamaVlieg, J.E.; et al. Lactobacillus rhamnosusCNCM I-3690 and the commensal bacterium Faecalibacterium prausnitzii A2-165 exhibit similar protective effects to induced barrier hyper-permeability in mice. Gut Microbes 2015, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Traina, G.; Menchetti, L.; Rappa, F.; Casagrande-Proietti, P.; Barbato, O.; Leonardi, L.; Carini, F.; Piro, F.; Brecchia, G. Probiotic mixture supplementation in the preventive management of trinitrobenzenesulfonic acid-induced inflammation in a murine model. J. Biol. Regul. Homeost. Agents 2016, 30, 895–901. [Google Scholar] [PubMed]
- Chae, J.M.; Heo, W.; Cho, H.T.; Lee, D.H.; Kim, J.H.; Rhee, M.S.; Park, T.S.; Kim, Y.K.; Lee, J.H.; Kim, Y.J. Effects of Orally-Administered Bifidobacterium animalis subsp. lactis Strain BB12 on Dextran Sodium Sulfate-Induced Colitis in Mice. J. Microbiol. Biotechnol. 2018, 28, 1800–1805. [Google Scholar] [CrossRef] [PubMed]
- Duranti, S.; Gaiani, F.; Mancabelli, L.; Milani, C.; Grandi, A.; Bolchi, A.; Santoni, A.; Lugli, G.A.; Ferrario, C.; Mangifesta, M.; et al. Elucidating the gut microbiome of ulcerative colitis: Bifidobacteria as novel microbial biomarkers. FEMS Microbiol. Ecol. 2016, 92, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elian, S.D.; Souza, E.L.; Vieira, A.T.; Teixeira, M.M.; Arantes, R.M.; Nicoli, J.R. Bifidobacterium longum subsp. infantis BB-02 attenuates acute murine experimental model of inflammatory bowel disease. Benef. Microb. 2015, 6, 277–286. [Google Scholar] [CrossRef]
- Javed, N.H.; Alsahly, M.B.; Khubchandani, J. Oral Feeding of Probiotic Bifidobacteriuminfantis: Colonic Morphological Changes in Rat Model of TNBS-Induced Colitis. Scientifica 2016, 2016, 9572596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satish Kumar, C.S.; Kondal, R.K.; Reddy, A.G.; Vinoth, A.; Ch, S.R.; Boobalan, G. Protective effect of Lactobacillus plantarum 21, a probiotic on trinitrobenzenesulfonic acid-induced ulcerative colitis in rats. Int. Immunopharmacol. 2015, 25, 504–510. [Google Scholar] [CrossRef]
- Hrdý, J.; Alard, J.; Couturier-Maillard, A.; Boulard, O.; Boutillier, D.; Delacre, M.; Lapadatescu, C.; Cesaro, A.; Blanc, P.; Pot, B.; et al. Lactobacillus reuteri 5454 and Bifidobacterium animalis ssp. lactis 5764 improve colitis while differentially impacting dendritic cells maturation and antimicrobial responses. Sci. Rep. 2020, 10, 5345. [Google Scholar] [CrossRef] [Green Version]
- Biagioli, M.; Laghi, L.; Carino, A.; Cipriani, S.; Distrutti, E.; Marchianò, S.; Parolin, C.; Scarpelli, P.; Vitali, B.; Fiorucci, S. Metabolic Variability of a Multispecies Probiotic Preparation Impacts on the Anti-inflammatory Activity. Front. Pharmacol. 2017, 8, 505. [Google Scholar] [CrossRef] [Green Version]
- Hegazy, S.K.; El-Bedewy, M.M. Effect of probiotics on pro-inflammatory cytokines and NF-kappaB activation in ulcerative colitis. World J. Gastroenterol. 2010, 16, 4145–4151. [Google Scholar] [CrossRef]
- Kato, K.; Mizuno, S.; Umesaki, Y.; Ishii, Y.; Sugitani, M.; Imaoka, A.; Otsuka, M.; Hasunuma, O.; Kurihara, R.; Iwasaki, A.; et al. Randomized placebo-controlled trial assessing the effect of bifidobacteria-fermented milk on active ulcerative colitis. Aliment. Pharmacol. Ther. 2004, 20, 1133–1141. [Google Scholar] [CrossRef]
- Kruis, W.; Fric, P.; Pokrotnieks, J.; Lukas, M.; Fixa, B.; Kaščák, M.; Kamm, M.A.; Weismueller, J.; Beglinger, C.; Stolte, M.; et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut 2004, 53, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Uemura, Y.; Kanai, T.; Kunisaki, R.; Suzuki, Y.; Yokoyama, K.; Yoshimura, N.; Hibi, T. Efficacy of Bifidobacterium breve Fermented Milk in Maintaining Remission of Ulcerative Colitis. Dig. Dis. Sci. 2018, 63, 1910–1919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamaki, H.; Nakase, H.; Inoue, S.; Kawanami, C.; Itani, T.; Ohana, M. Efficacy of probiotic treatment with Bifidobacterium longum 536 for induction of remission in active ulcerative colitis: A randomized, double-blinded, placebo-controlled multicenter trial. Dig. Endosc. 2016, 28, 67–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia Vilela, E.; De Lourdes De Abreu Ferrari, M.; Oswaldo Da Gama Torres, H.; Guerra Pinto, A. Carolina Carneiro Aguirre, A.; Paiva Martins, F.; Sales Da Cunha, A. Influence of Saccharomyces boulardii on the intestinal permeability of patients with Crohn’s disease in remission. Scand. J. Gastroenterol. 2008, 43, 842–848. [Google Scholar] [CrossRef]
- Wildt, S.; Nordgaard, I.; Hansen, U.; Brockmann, E.; Rumessen, J.J. A randomised double-blind placebo-controlled trial with Lactobacillus acidophilus La-5 and Bifidobacterium animalis subsp. lactis BB-12 for maintenance of remission in ulcerative colitis. J. Crohns Colitis 2011, 5, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Sood, A.; Midha, V.; Makharia, G.K.; Ahuja, V.; Singal, D.; Goswami, P.; Tandon, R.K. The Probiotic Preparation, VSL#3 Induces Remission in Patients with Mild-to-Moderately Active Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2009, 7, 1202–1209.e1. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; Brandimarte, G.; Papa, A.; Giglio, A.; Elisei, W.; Giorgetti, G.M.; Forti, G.; Morini, S.; Hassan, C.; Pistoia, M.A.; et al. Treatment of Relapsing Mild-to-Moderate Ulcerative Colitis with the Probiotic VSL#3 as Adjunctive to a Standard Pharmaceutical Treatment: A Double-Blind, Randomized, Placebo-Controlled Study. Am. J. Gastroenterol. 2010, 105, 2218–2227. [Google Scholar] [CrossRef] [Green Version]
- Huynh, H.Q.; Debruyn, J.; Guan, L.; Diaz, H.; Li, M.; Girgis, S.; Turner, J.; Fedorak, R.; Madsen, K. Probiotic preparation VSL#3 induces remission in children with mild to moderate acute ulcerative colitis: A pilot study. Inflamm. Bowel Dis. 2009, 15, 760–768. [Google Scholar] [CrossRef]
- Fedorak, R.N.; Feagan, B.G.; Hotte, N.; Leddin, D.; Dieleman, L.A.; Petrunia, D.M. The probiotic VSL#3 has anti-inflammatory effects and could reduce endoscopic recurrence after surgery for Crohn’s disease. Clin. Gastroenterol. Hepatol. 2015, 13, 928–935. [Google Scholar]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humaran, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [Green Version]
- Pappa, H.M.; Mitchell, P.D.; Jiang, H.; Kassiff, S.; Filip-Dhima, R.; DiFabio, D.; Quinn, N.; Lawton, R.C.; Varvaris, M.; Van Straaten, S.; et al. Treatment of Vitamin D Insufficiency in Children and Adolescents with Inflammatory Bowel Disease: A Randomized Clinical Trial Comparing Three Regimens. J. Clin. Endocrinol. Metab. 2012, 97, 2134–2142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pappa, H.M.; Mitchell, P.D.; Jiang, H.; Kassiff, S.; Filip-Dhima, R.; DiFabio, D.; Quinn, N.; Lawton, R.C.; Bronzwaer, M.E.S.; Koenen, K.; et al. Maintenance of Optimal Vitamin D Status in Children and Adolescents with Inflammatory Bowel Disease: A Randomized Clinical Trial Comparing Two Regimens. J. Clin. Endocrinol. Metab. 2014, 99, 3408–3417. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Khazai, N.B.; Ziegler, T.R.; Nanes, M.S.; Abrams, S.A.; Tangpricha, V. Vitamin D-mediated calcium absorption in patients with clinically stable Crohn’s disease: A pilot study. Mol. Nutr. Food Res. 2010, 54, 1085–1091. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Khalili, H.; Higuchi, L.M.; Bao, Y.; Korzenik, J.R.; Giovannucci, E.L. Higher predicted vitamin D status is associated with reduced risk of Crohn’s disease. Gastroenterology 2012, 142, 482–489. [Google Scholar] [CrossRef] [Green Version]
- Cantorna, M.T.; Munsick, C.; Bemiss, C.; Mahon, B.D. 1,25-Dihydroxycholecalciferol Prevents and Ameliorates Symptoms of Experimental Murine Inflammatory Bowel Disease. J. Nutr. 2000, 130, 2648–2652. [Google Scholar] [CrossRef] [Green Version]
- Jørgensen, S.P.G.; Agnholt, J.; Glerup, H.; Lyhne, S.; Villadsen, G.E.; Hvas, C.L.; Bartels, L.E.; Kelsen, J.; Christensen, L.A.; Dahlerup, J.F. Clinical trial: Vitamin D3 treatment in Crohn’s disease—A randomized double-blind placebo-controlled study. Aliment. Pharmacol. Ther. 2010, 32, 377–383. [Google Scholar] [CrossRef]
- Khalili, H.; Huang, E.S.; Ananthakrishnan, A.N.; Higuchi, L.; Richter, J.M.; Fuchs, C.S. Geographical variation and incidence of inflammatory bowel disease among US women. Gut 2012, 61, 1686–1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dryden, G.W.; Lam, A.; Beatty, K.; Qazzaz, H.H.; McClain, C.J. A Pilot Study to Evaluate the Safety and Efficacy of an Oral Dose of (−)-Epigallocatechin-3-Gallate–Rich Polyphenon E in Patients with Mild to Moderate Ulcerative Colitis. Inflamm. Bowel Dis. 2013, 19, 1904–1912. [Google Scholar] [CrossRef]
- Ajouz, H.; Mukherji, D.; Shamseddine, A. Secondary bile acids: An underrecognized cause of colon cancer. World J. Surg. Oncol. 2014, 12, 164. [Google Scholar] [CrossRef] [Green Version]
| Trial Identifier | Trail Phase (Status) | Title | Conditions | Interventions |
|---|---|---|---|---|
| NCT03266484 | Active, not recruiting | Effect of a Probiotic Mixture on the Gut Microbiome and Fatigue in Patients With Quiescent Inflammatory Bowel Disease | Crohn’s Disease Ulcerative Colitis |
|
| NCT01765998 | Unknown | The Effect of Probiotics on Exacerbation of Inflammatory Bowel Disease Exacerbation (Crohn’s Disease) | Crohn’s Disease |
|
| NCT01765439 | Active, not recruiting | The Effect of VSL#3 Probiotic Preparation on the Bile Acid Metabolism in Patients With Inflammatory Bowel Disease | Crohn’s Disease Ulcerative Colitis | Dietary Supplement: VSL#3 |
| NCT00175292 | Completed | A Randomized Controlled Trial of VSL#3 for the Prevention of Endoscopic Recurrence Following Surgery for Crohn’s Disease | Crohn’s Disease Inflammatory Bowel Disease | Drug: Probiotic—VSL#3 |
| NCT01078935 | Unknown | The Effect of Probiotics on the Rate of Recovery of Inflammatory Bowel Disease Exacerbation, Endothelial Function, and Markers of Inflammation | Crohn’s Disease Ulcerative Colitis |
|
| NCT01173588 | Completed | Effect of Yogurt Added With Bifidobacteria and Soluble Fiber on Bowel Function | Inflammatory Bowel Disease | Other: yogurt added with bifidobacteria and soluble fiber (YBF) |
| NCT01479660 | Unknown | Role of Healthy Bacteria in Ulcerative Colitis | Ulcerative Colitis |
|
| NCT00510978 | Unknown | Probiotics in GastroIntestinal Disorders (PROGID) | Crohn’s Disease Ulcerative Colitis |
|
| NCT01632462 | Unknown | A Prospective, Placebo Controlled, Double-Blind, Cross-over Study on the Effects of a Probiotic Preparation (VSL#3) on Metabolic Profile, Intestinal Permeability, Microbiota, Cytokines and Chemokines Expression and Other Inflammatory Markers in Pediatric Patients With Crohn’s Disease | Crohn’s Disease | Drug: VSL#3 |
| NCT01548014 | Unknown | The Effect of a Probiotic Preparation (VSL#3) Plus Infliximab in Children With Crohn’s Disease | Crohn’s Disease |
|
| NCT00374374 | Completed | Treatment With Lactobacillus Rhamnosus and Lactobacillus Acidophilus for Patients With Active Colonic Crohn’s Disease | Crohn’s Disease |
|
| NCT03565939 | Completed | Probiotic Treatment of Ulcerative Colitis With Trichuris Suis Ova (TSO) (PROCTO) | Ulcerative Colitis Chronic Moderate |
|
| NCT00114465 | Completed | VSL#3 Versus Placebo in Maintenance of Remission in Crohn’s Disease | Crohn’s Disease |
|
| NCT00944736 | Completed | Effect of VSL#3 on Intestinal Permeability in Pediatric Crohn’s Disease | Crohn’s Disease |
|
| NCT04842149 | Recruiting | The Effects of Bifidobacterium Breve Bif195 for Small Intestinal Crohn’s Disease | Crohn’s Disease |
|
| NCT04223479 | Completed | Effect of Probiotic Supplementation on the Immune System in Patients With Ulcerative Colitis in Amman, Jordan | Ulcerative Colitis |
|
| NCT01698970 | Completed | Effect of the Consumption of a Probiotic Strain on the Prevention of Post-operative Recurrence in Crohn’s Disease | Crohn’s Disease |
|
| NCT01772615 | Completed | Treatment of Ulcerative Colitis With Ciprofloxacin and E. Coli Nissle | Ulcerative Colitis |
|
| NCT02488954 | Terminated | Interest of Propionibacterium Freudenreichii for the Treatment of Mild to Moderate Ulcerative Colitis (EMMENTAL) | Ulcerative Colitis | Other: Probiotics in the form of cheese portion |
| NCT04305535 | Unknown | Impact of an Oligomeric Diet in Intestinal Absorption and Inflammatory Markers in Patients With Crohn Disease | Crohn Disease Absorption; Disorder: Protein Absorption; Disorder Absorption; Disorder: Fat Absorption; Disorder: Carbohydrate Malnutrition |
|
| NCT00305409 | Completed | Synbiotic Treatment in Crohn’s Disease Patients | Crohn’s Disease | Drug: Synbiotic (Synergy I/B. longum) |
| NCT04804046 | Recruiting | Synbiotics and Post-op Crohn’s Disease | Crohn’s Disease |
|
| NCT00803829 | Completed | Synbiotic Treatment of Ulcerative Colitis Patients | Ulcerative Colitis |
|
| NCT00951548 | Completed | Food Supplementation With VSL#3 as a Support to Standard Pharmaceutical Therapy in Ulcerative Colitis | Ulcerative Colitis |
|
| NCT00374725 | Completed | Treatment of Ulcerative Colitis With a Combination of Lactobacillus Rhamnosus and Lactobacillus Acidophilus. | Ulcerative Colitis | Behavioral: Administration of probiotic (L. rhamnosus and L. acidophilus) |
| NCT03415711 | Terminated | PRObiotic VSL#3® for Maintenance of Clinical and Endoscopic REMission in Ulcerative Colitis | Ulcerative Colitis |
|
| NCT04102852 | Recruiting | Lactobacillus Rhamnosus GG (ATCC 53103) in Mild-moderately Active UC Patients | Ulcerative Colitis Chronic Mild Ulcerative Colitis Chronic Moderate | Dietary Supplement: Lactobacillus rhamnosus GG ATCC 53103 |
| NCT00367705 | Unknown | VSL#3 Treatment in Children With Crohn’s Disease | Crohn’s Disease |
|
| NCT04969679 | Completed | Additive Effect of Probiotics (Mutaflor®) in Patients With Ulcerative Colitis on 5-ASA Treatment | Ulcerative Colitis |
|
| NCT00268164 | Terminated | Lactobacillus Acidophilus and Bifidobacterium Animalis Subsp. Lactis, Maintenance Treatment in Ulcerative Colitis | Ulcerative Colitis | Drug: lactobacilus acidophilus & bifidobacterium animalis/lactis |
| Trial Identifier | Trail Phase (Status) | Title | Conditions | Interventions |
|---|---|---|---|---|
| Short Chain Fatty Acids | ||||
| NCT05456763 | Completed | Butyrate in Pediatric Inflammatory Bowel Disease | IBD |
|
| Tryptophan Metabolites | ||||
| NCT04089501 | Completed | The Role of the Pregnane X Receptor (PXR) in Indole Signaling and Intestinal Permeability in Inflammatory Bowel Disease | IBD |
|
| Bile Acid Metabolites | ||||
| NCT03724175 | Recruiting | The Role of Secondary Bile Acids in Intestinal Inflammation | Ulcerative Colitis Pouchitis |
|
| Vitamines | ||||
| NCT03145896 | Unknown | The Correlation Between Anemia of Chronic Diseases, Hepcidin and Vitamin D in IBD Patients | IBD | Dietary Supplement: vitamin D |
| NCT03162432 | Completed | High Dose Interval Vitamin D Supplementation in Patients With IBD Receiving Remicade | IBD Ulcerative Colitis Crohn Disease | Drug: Vitamin D3 |
| NCT02076750 | Completed | Weekly Vitamin D in Pediatric IBD | IBD Skin Pigmentation | Dietary Supplement: Vitamin D3 (cholecalciferol) |
| NCT00621257 | Terminated Has Results [141,142] | Vitamin D Levels in Children With IBD | IBD Crohn’s Disease Ulcerative Colitis |
|
| NCT02256605 | Completed | Vitamin D3 Supplementation in Pediatric IBD: Weely vs Daily Dosing Regimens | IBD | Dietary Supplement: Vitamin D-3 |
| NCT04225819 | Recruiting | Adjunctive Treatment With Vitamin D3 in Patients With Active IBD | IBD Crohn Disease Ulcerative Colitis Vitamin D3 Deficiency |
|
| NCT03496246 | Unknown | Vitamin D Status in Inflammatory Bowel Disease (vdsinibd) | Vitamin D Deficiency IBD |
|
| NCT04991324 | Not yet recruiting | Cholecalciferol Comedication in IBD—the 5C-study (5C) | IBD | Drug: Vitamin D3 |
| NCT04828031 | Recruiting | Vitamin D Regulation of Gut Specific B Cells and Antibodies Targeting Gut Bacteria in Inflammatory Bowel Disease | IBD Ulcerative Colitis Crohn Disease | Drug: Vitamin D |
| NCT04331639 | Recruiting | High Dose Interval Vitamin D Supplementation in Patients With Inflammatory Bowel Disease Receiving Biologic Therapy | IBD Crohn Disease Ulcerative Colitis Vitamin D Deficiency | Dietary Supplement: vitamin D3 |
| NCT01877577 | Completed | Supplementation of Vitamin D3 in Patients With Inflammatory Bowel Diseases and Hypovitaminosis D | Crohn’s Disease Ulcerative Colitis | Dietary Supplement: Vitamin D3 |
| NCT00742781 | Completed Has Results | Vitamin D Supplementation in Crohn’s Patients | IBD | Dietary Supplement: Vitamin D |
| NCT01121796 | Unknown | Influence of Vitamin D on Inflammatory Bowel Disease Remission | IBD |
|
| NCT01792388 | Completed | Vitd and Barrier Function in IBD | Crohn’s Disease |
|
| NCT00152841 | Terminated | Effect of Iron and Vitamin E Supplementation on Disease Activity in Patients With Either Crohn’s Disease or Ulcerative Colitis | Crohn’s Disease Ulcerative Colitis Mild or Moderate Anaemia |
|
| NCT00114803 | Completed | Nasal Calcitonin in the Treatment of Bone Mineral Loss in Children and Adolescents With Inflammatory Bowel Disease | Ulcerative Colitis Crohn’s Disease | Drug: Calcitonin nasal spray (salmon) |
| NCT04913467 | Recruiting | Effect of Ileocolonic Delivered Vitamins and an Anti-Inflammatory Diet on Crohn’s Disease and Healthy Volunteers | Crohn Disease |
|
| NCT03718182 | Unknown | Can Vitamin D Supplementation in People With Crohn’s Disease Improve Symptoms as an Adjunct Therapy? | Crohn Disease Vitamin D Deficiency | Dietary Supplement: Cholecalciferol |
| NCT02615288 | Completed | High Dose Vitamin D3 in Crohn’s Disease | Crohn Disease | Dietary Supplement: Vitamin D3 |
| NCT01692808 | Completed | Bioavailability of Vitamin D in Children and Adolescents With Crohn’s Disease | Crohn’s Disease |
|
| NCT02186275 | Completed | The Vitamin D in Pediatric Crohn’s Disease | Crohn’s Disease |
|
| NCT03999580 | Recruiting | The Vitamin D in Pediatric Crohn’s Disease (ViDiPeC-2) | Crohn Disease | Drug: vitamin D3 |
| NCT01235325 | Completed | The Effect of Vitamin K Supplementation on Bone Health in Adult Crohn’s Disease Patients | Supplementation Bone Health Crohn’s Disease |
|
| NCT00132184 | Unknown | Vitamin D Treatment for Crohn’s Disease | Crohn Disease | Drug: Vitamin D |
| NCT01369667 | Completed | Vitamin D Supplementation in Adult Crohn’s Disease | Crohn Disease |
|
| NCT01864616 | Terminated | The Impact of Vitamin D on Disease Activity in Crohn’s Disease | Crohn Disease | Dietary Supplement: Vitamin D3 |
| NCT00427804 | Completed Has Results [143] | Tumor Necrosis Factor Decreases Vitamin D Dependant Calcium Absorption | Rheumatoid Arthritis Crohn’s Disease | Drug: calcitriol |
| NCT04308850 | Not yet recruiting | Exploring the Effects of Vitamin D Supplementation on the Chronic Course of Patients With Crohn’s Disease With Vitamin D Deficiency | Crohn’s Disease Vitamin D Deficiency Vitamin D Supplement | Drug: Vitamin D drops |
| NCT04134065 | Unknown | The Effect of Vitamin D in Crohn’s Disease | Crohn’s Disease Vitamin D Deficiency |
|
| NCT02704624 | Unknown | Effects of Supplementation of Vitamin D in Patients With Crohn’s Disease | Crohn Disease Vitamin D Deficiency Fatigue Sarcopenia Muscle Weakness Disorder of Bone Density and Structure, Unspecified |
|
| NCT02208310 | Terminated Has Results [144,145,146,147] | Trial of High Dose Vitamin D in Patient’s With Crohn’s Disease | Crohn’s Disease Vitamin D Deficiency |
|
| NCT03615378 | Terminated | Maintenance Dosing of Vitamin D in Crohn’s Disease | Crohns Disease Vitamin D Deficiency |
|
| NCT04309058 | Unknown | Observation of the Effect of Vitamin D Supplementation on Chronic Course of Patients With Ulcerative Colitis Based on Vitamin D Receptor Fok I Gene Polymorphism | Ulcerative Colitis Vitamin D Deficiency Vitamin D Supplement | Drug: Vitamin D drops |
| NCT01046773 | Terminated | Vitamin D Supplementation as Non-toxic Immunomodulation in Children With Crohn’s Disease | Crohn’s Disease Vitamin D Deficiency | Drug: Cholecalciferol |
| NCT04276649 | Completed | A Retrospective Analysis: the Influence of Caltrate Supplement on the Effect of Mesalazine in Ulcerative Colitis | Ulcerative Colitis Vitamin D Deficiency Vitamin D Supplement | Drug: Caltrate |
| NCT04259060 | Not yet recruiting | Hydroxocobalamin Approach for Reducing of Calprotectin With Butyrate for Ulcerative Colitis Remission | Ulcerative Colitis |
|
| Sulfur-Containing Metabolites | ||||
| NCT01282905 | Completed | Hydrogen Sulfide Detoxification and Butyrate Metabolism in Ulcerative Colitis | Ulcerative Colitis | / |
| NCT04474561 | Completed | Reduced Sulfur Diet in Ulcerative Colitis Patients | Ulcerative Colitis Diet Habit | Other: Reduced sulfur diet |
| Polyphenol Metabolites | ||||
| NCT00718094 | Completed Has Results [148] | Pilot Study of Green Tea Extract (Polyphenon E®)in Ulcerative Colitis | Mild to Moderately Active Ulcerative Colitis |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gadaleta, R.M.; Cariello, M.; Crudele, L.; Moschetta, A. Bile Salt Hydrolase-Competent Probiotics in the Management of IBD: Unlocking the “Bile Acid Code”. Nutrients 2022, 14, 3212. https://doi.org/10.3390/nu14153212
Gadaleta RM, Cariello M, Crudele L, Moschetta A. Bile Salt Hydrolase-Competent Probiotics in the Management of IBD: Unlocking the “Bile Acid Code”. Nutrients. 2022; 14(15):3212. https://doi.org/10.3390/nu14153212
Chicago/Turabian StyleGadaleta, Raffaella Maria, Marica Cariello, Lucilla Crudele, and Antonio Moschetta. 2022. "Bile Salt Hydrolase-Competent Probiotics in the Management of IBD: Unlocking the “Bile Acid Code”" Nutrients 14, no. 15: 3212. https://doi.org/10.3390/nu14153212
APA StyleGadaleta, R. M., Cariello, M., Crudele, L., & Moschetta, A. (2022). Bile Salt Hydrolase-Competent Probiotics in the Management of IBD: Unlocking the “Bile Acid Code”. Nutrients, 14(15), 3212. https://doi.org/10.3390/nu14153212







