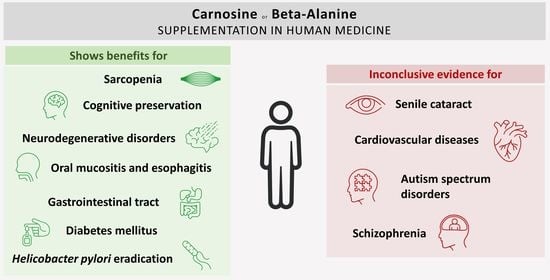
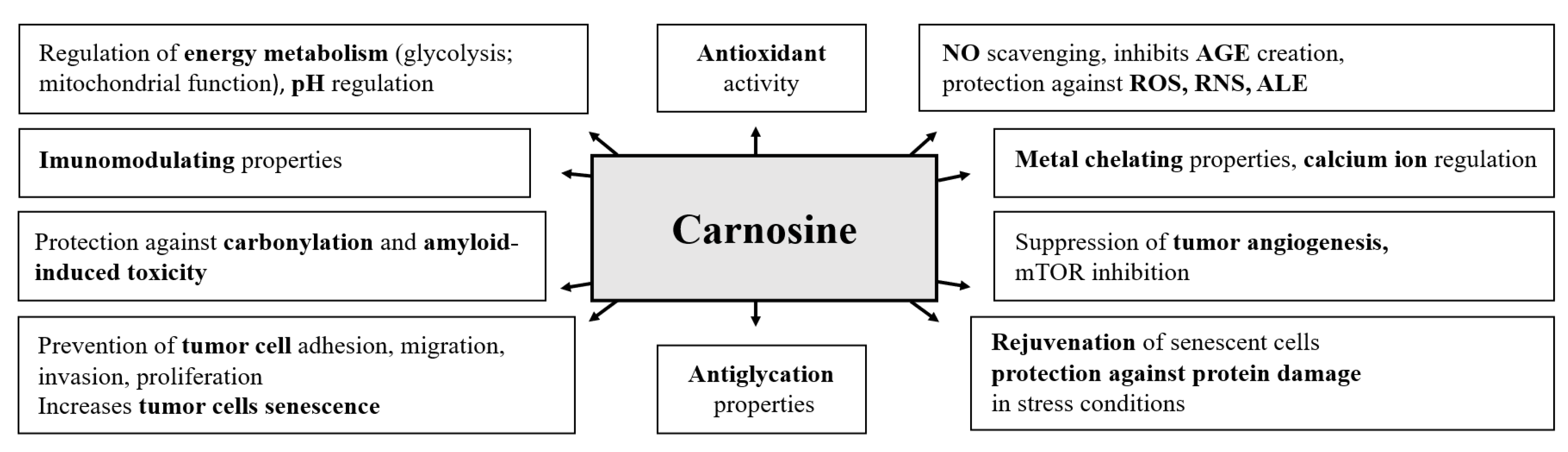
Carnosine and Beta-Alanine Supplementation in Human Medicine: Narrative Review and Critical Assessment
Abstract
1. Introduction
2. Search Strategy and Methodology
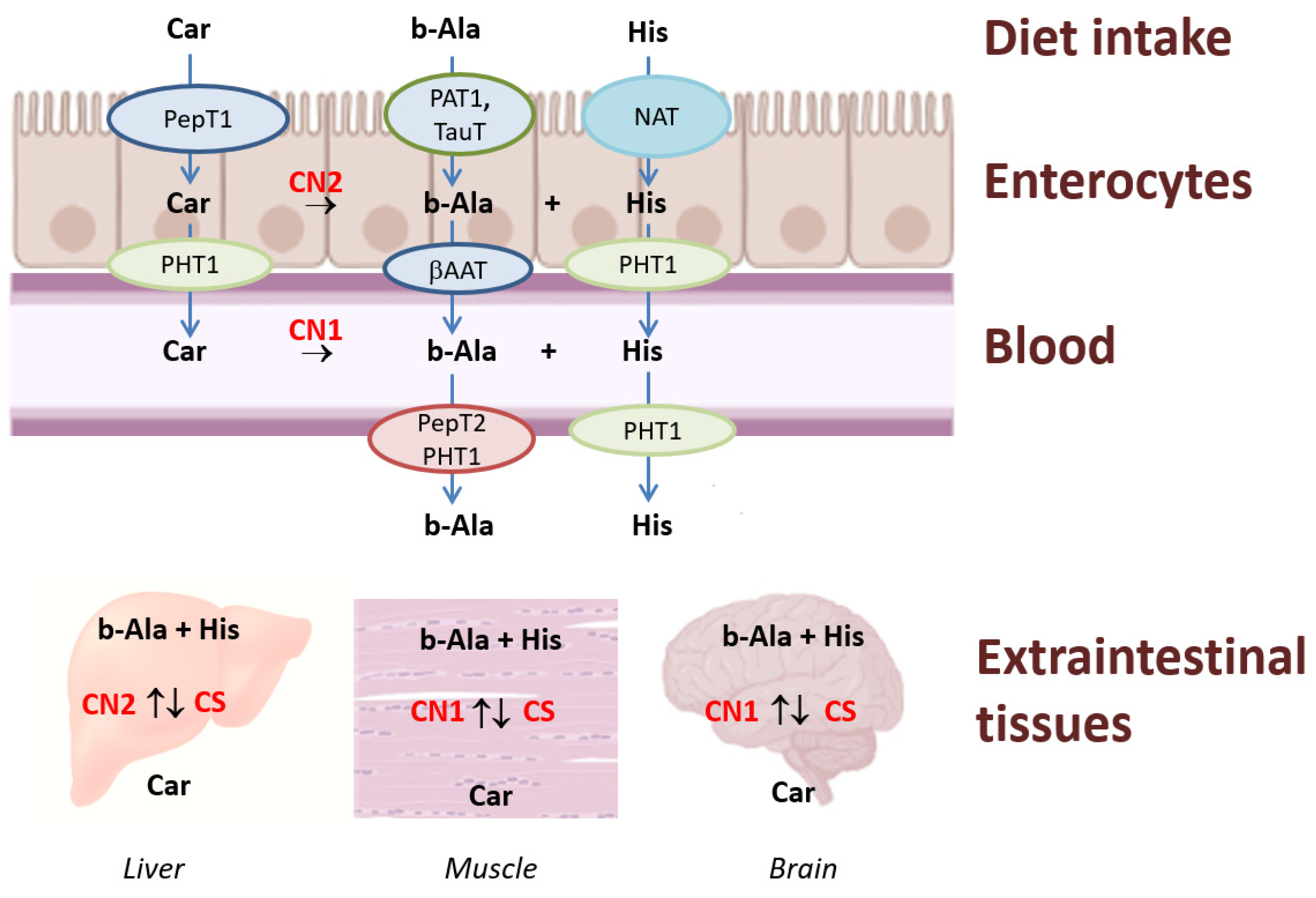
3. Synthesis and Degradation
4. Supplementation and Food Sources
5. Carnosine, Beta-Alanine and Diabetes Mellitus
6. Carnosine, Beta-Alanine, and Neurological Diseases
Neurodegenerative Diseases
7. Carnosine, Beta-Alanine, and Psychiatric Diseases
Autism Spectrum Disorders in Children
8. Carnosine, Beta-Alanine and Cataract
9. Carnosine, Beta-Alanine and Sarcopenia
10. Carnosine, Beta-Alanine and Diseases of the Cardiovascular System
11. Zinc-Carnosine and Oral Mucositis, Loss of Taste and Gastrointestinal Tract
12. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drozak, J.; Veiga-da-Cunha, M.; Vertommen, D.; Stroobant, V.; Van Schaftingen, E. Molecular Identification of Carnosine Synthase as ATP-grasp Domain-containing Protein 1 (ATPGD1). J. Biol. Chem. 2010, 285, 9346–9356. [Google Scholar] [CrossRef]
- Wu, G. Important roles of dietary taurine, creatine, carnosine, anserine and 4-hydroxyproline in human nutrition and health. Amino Acids 2020, 52, 329–360. [Google Scholar] [CrossRef]
- Xing, L.; Chee, M.E.; Zhang, H.; Zhang, W.; Mine, Y. Carnosine—A natural bioactive dipeptide: Bioaccessibility, bioavailability and health benefits. J. Food. Bioact. 2019, 5, 8–17. [Google Scholar] [CrossRef]
- Haus, J.M.; Thyfault, J.P. Therapeutic potential of carbonyl-scavenging carnosine derivative in metabolic disorders. J. Clin. Investig. 2018, 128, 5198–5200. [Google Scholar] [CrossRef] [PubMed]
- Hipkiss, A.R.; Chana, H. Carnosine Protects Proteins against Methylglyoxal-Mediated Modifications. Biochem. Biophys. Res. Commun. 1998, 248, 28–32. [Google Scholar] [CrossRef]
- Dolan, E.; Saunders, B.; Dantas, W.S.; Murai, I.H.; Roschel, H.; Artioli, G.G.; Harris, R.; Bicudo, J.E.P.W.; Sale, C.; Gualano, B. A Comparative Study of Hummingbirds and Chickens Provides Mechanistic Insight on the Histidine Containing Dipeptide Role in Skeletal Muscle Metabolism. Sci. Rep. 2018, 8, 14788. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, H.; Longman, M.; Alany, R.G.; Pierscionek, B. On the Anticataractogenic Effects of L-Carnosine: Is It Best Described as an Antioxidant, Metal-Chelating Agent or Glycation Inhibitor? Oxid Med. Cell. Longev. 2016, 2016, 3240261. [Google Scholar] [CrossRef]
- Nicoletti, V.G.; Santoro, A.M.; Grasso, G.; Vagliasindi, L.I.; Giuffrida, M.L.; Cuppari, C.; Purrello, V.S.; Stella, A.M.G.; Rizzarelli, E. Carnosine interaction with nitric oxide and astroglial cell protection. J. Neurosci. Res. 2007, 85, 2239–2245. [Google Scholar] [CrossRef] [PubMed]
- Corona, C.; Frazzini, V.; Silvestri, E.; Lattanzio, R.; La Sorda, R.; Piantelli, M.; Canzoniero, L.M.T.; Ciavardelli, D.; Rizzarelli, E.; Sensi, S.L. Effects of Dietary Supplementation of Carnosine on Mitochondrial Dysfunction, Amyloid Pathology, and Cognitive Deficits in 3xTg-AD Mice. PLoS ONE 2011, 6, e17971. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and Pathophysiology of Carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef] [PubMed]
- Aldini, G.; de Courten, B.; Regazzoni, L.; Gilardoni, E.; Ferrario, G.; Baron, G.; Altomare, A.; D’Amato, A.; Vistoli, G.; Carini, M. Understanding the antioxidant and carbonyl sequestering activity of carnosine: Direct and indirect mechanisms. Free Radic. Res. 2021, 55, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Holliday, R.; McFarland, G.A. A role for carnosine in cellular maintenance. Biochemistry (Mosc.) 2000, 65, 843–848. [Google Scholar] [PubMed]
- Villari, V.; Attanasio, F.; Micali, N. Control of the Structural Stability of α-Crystallin under Thermal and Chemical Stress: The Role of Carnosine. J. Phys. Chem. B 2014, 118, 13770–13776. [Google Scholar] [CrossRef]
- Nagai, K.; Suda, T. Immunoregulative effects of carnosine and beta-alanine. Nihon Seirigaku Zasshi 1986, 48, 564–571. [Google Scholar]
- Hipkiss, A.R.; Baye, E.; de Courten, B. Carnosine and the processes of ageing. Maturitas 2016, 93, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.D.; Sale, C.; Garner, A.C.; Hipkiss, A.R. Anti-cancer actions of carnosine and the restoration of normal cellular homeostasis. Biochim. Biophys. Acta—Mol. Cell Res. 2021, 1868, 119117. [Google Scholar] [CrossRef]
- Li, X.; Yang, K.; Gao, S.; Zhao, J.; Liu, G.; Chen, Y.; Lin, H.; Zhao, W.; Hu, Z.; Xu, N. Carnosine Stimulates Macrophage-Mediated Clearance of Senescent Skin Cells Through Activation of the AKT2 Signaling Pathway by CD36 and RAGE. Front. Pharmacol. 2020, 11, 593832. [Google Scholar] [CrossRef]
- Mikuła-Pietrasik, J.; Książek, K. L-Carnosine Prevents the Pro-cancerogenic Activity of Senescent Peritoneal Mesothelium Towards Ovarian Cancer Cells. Anticancer Res. 2016, 36, 665–671. [Google Scholar]
- Wu, C.C.; Lai, P.Y.; Hsieh, S.; Cheng, C.C.; Hsieh, S.L. Suppression of Carnosine on Adhesion and Extravasation of Human Colorectal Cancer Cells. Anticancer Res. 2019, 39, 6135–6144. [Google Scholar] [CrossRef]
- Prakash, M.D.; Fraser, S.; Boer, J.C.; Plebanski, M.; de Courten, B.; Apostolopoulos, V. Anti-Cancer Effects of Carnosine—A Dipeptide Molecule. Molecules 2021, 26, 1644. [Google Scholar] [CrossRef]
- Hwang, B.; Shin, S.S.; Song, J.H.; Choi, Y.H.; Kim, W.J.; Moon, S.K. Carnosine exerts antitumor activity against bladder cancers in vitro and in vivo via suppression of angiogenesis. J. Nutr. Biochem. 2019, 74, 108230. [Google Scholar] [CrossRef]
- Hipkiss, A.R.; Gaunitz, F. Inhibition of tumour cell growth by carnosine: Some possible mechanisms. Amino Acids 2014, 46, 327–337. [Google Scholar] [CrossRef]
- Dolan, E.; Swinton, P.A.; de Salles Painelli, V.; Hemingway, B.S.; Mazzolani, B.; Smaira, F.I.; Saunders, B.; Artioli, G.G.; Gualano, B. A Systematic Risk Assessment and Meta-Analysis on the Use of Oral β-Alanine Supplementation. Adv. Nutr. 2019, 10, 452–463. [Google Scholar] [CrossRef]
- Saunders, B.; Elliott-Sale, K.; Artioli, G.G.; Swinton, P.A.; Dolan, E.; Roschel, H.; Sale, C.; Gualano, B. β-alanine supplementation to improve exercise capacity and performance: A systematic review and meta-analysis. Br. J. Sports Med. 2017, 51, 658–669. [Google Scholar] [CrossRef]
- Trexler, E.T.; Smith-Ryan, A.E.; Stout, J.R.; Hoffman, J.R.; Wilborn, C.D.; Sale, C.; Kreider, R.B.; Jäger, R.; Earnest, C.P.; Bannock, L.; et al. International society of sports nutrition position stand: Beta-Alanine. J. Int. Soc. Sports Nutr. 2015, 12, 30. [Google Scholar] [CrossRef]
- Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of zinc l-carnosine as a Novel food pursuant to Regulation (EU) 2015/2283 and the bioavailability of zinc from this source in the context of Directive 2002/46/EC on food supplements. EFSA J. 2022, 20, 7332. [Google Scholar] [CrossRef]
- Wu, G. Amino Acids; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar] [CrossRef]
- Artioli, G.G.; Sale, C.; Jones, R.L. Carnosine in health and disease. Eur. J. Sport Sci. 2019, 19, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Spelnikov, D.; Harris, R.C. A kinetic model of carnosine synthesis in human skeletal muscle. Amino Acids 2019, 51, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Asatoor, A.M.; Bandoh, J.K.; Lant, A.F.; Milne, M.D.; Navab, F. Intestinal absorption of carnosine and its constituent amino acids in man. Gut 1970, 11, 250–254. [Google Scholar] [CrossRef]
- Chmielewska, K.; Dzierzbicka, K.; Inkielewicz-Stępniak, I.; Przybyłowska, M. Therapeutic Potential of Carnosine and Its Derivatives in the Treatment of Human Diseases. Chem. Res. Toxicol. 2020, 33, 1561–1578. [Google Scholar] [CrossRef]
- Gaunitz, F.; Hipkiss, A.R. Carnosine and cancer: A perspective. Amino Acids 2012, 43, 135–142. [Google Scholar] [CrossRef]
- Jin, C.-L.; Yang, L.-X.; Wu, X.-H.; Li, Q.; Ding, M.-P.; Fan, Y.-Y.; Zhang, W.-P.; Luo, J.-H.; Chen, Z. Effects of carnosine on amygdaloid-kindled seizures in Sprague–Dawley rats. Neuroscience 2005, 135, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.M.; Pires, J.; Esteves, M.; Graça, B.; Bernardino, L. Histamine: A new immunomodulatory player in the neuron-glia crosstalk. Front. Cell. Neurosci. 2014, 8, 120. [Google Scholar] [CrossRef] [PubMed]
- Charoensin, S.; Laopaiboon, B.; Boonkum, W.; Phetcharaburanin, J.; Villareal, M.O.; Isoda, H.; Duangjinda, M. Thai Native Chicken as a Potential Functional Meat Source Rich in Anserine, Anserine/Carnosine, and Antioxidant Substances. Animals 2021, 11, 902. [Google Scholar] [CrossRef]
- Jones, G.; Smith, M.; Harris, R. Imidazole dipeptide content of dietary sources commonly consumed within the British diet. Proc Nutr. Soc. 2011, 70, E363. [Google Scholar] [CrossRef]
- Sarkar, P.; Kar, K.; Mondal, M.C.; Chakraborty, I.; Kar, M. Elevated level of carbonyl compounds correlates with insulin resistance in type 2 diabetes. Ann. Acad. Med. Singapore 2010, 39, 909–912. [Google Scholar] [CrossRef] [PubMed]
- Freund, M.A.; Chen, B.; Decker, E.A. The Inhibition of Advanced Glycation End Products by Carnosine and Other Natural Dipeptides to Reduce Diabetic and Age-Related Complications. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1367–1378. [Google Scholar] [CrossRef] [PubMed]
- de Courten, B.; Jakubova, M.; de Courten, M.P.; Kukurova, I.J.; Vallova, S.; Krumpolec, P.; Valkovic, L.; Kurdiova, T.; Garzon, D.; Barbaresi, S.; et al. Effects of carnosine supplementation on glucose metabolism: Pilot clinical trial. Obesity 2016, 24, 1027–1034. [Google Scholar] [CrossRef]
- Siriwattanasit, N.; Satirapoj, B.; Supasyndh, O. Effect of Oral carnosine supplementation on urinary TGF-β in diabetic nephropathy: A randomized controlled trial. BMC Nephrol. 2021, 22, 236. [Google Scholar] [CrossRef]
- Karkabounas, S.; Papadopoulos, N.; Anastasiadou, C.; Gubili, C.; Peschos, D.; Daskalou, T.; Fikioris, N.; Simos, Y.V.; Kontargiris, E.; Gianakopoulos, X.; et al. Effects of α -Lipoic Acid, Carnosine, and Thiamine Supplementation in Obese Patients with Type 2 Diabetes Mellitus: A Randomized, Double-Blind Study. J. Med. Food 2018, 21, 1197–1203. [Google Scholar] [CrossRef]
- Nealon, R.S.; Sukala, W.R. The Effect of 28 Days of Beta-alanine Supplementation on Exercise Capacity and Insulin Sensitivity in Individuals with Type 2 Diabetes Mellitus: A Randomised, Double-blind and Placebo-controlled Pilot Trial. Sport Nutr. Ther. 2016, 1, 1–7. [Google Scholar] [CrossRef]
- Elbarbary, N.S.; Ismail, E.A.R.; El-Naggar, A.R.; Hamouda, M.H.; El-Hamamsy, M. The effect of 12 weeks carnosine supplementation on renal functional integrity and oxidative stress in pediatric patients with diabetic nephropathy: A randomized placebo-controlled trial. Pediatr. Diabetes 2018, 19, 470–477. [Google Scholar] [CrossRef]
- Houjeghani, S.; Kheirouri, S.; Faraji, E.; Jafarabadi, M.A. l -Carnosine supplementation attenuated fasting glucose, triglycerides, advanced glycation end products, and tumor necrosis factor—α levels in patients with type 2 diabetes: A double-blind placebo-controlled randomized clinical trial. Nutr. Res. 2018, 49, 96–106. [Google Scholar] [CrossRef]
- Matthews, J.J.; Dolan, E.; Swinton, P.A.; Santos, L.; Artioli, G.G.; Turner, M.D.; Elliott-Sale, K.J.; Sale, C. Effect of Carnosine or β -Alanine Supplementation on Markers of Glycemic Control and Insulin Resistance in Humans and Animals: A Systematic Review and Meta-analysis. Adv. Nutr. 2021, 12, 2216–2231. [Google Scholar] [CrossRef]
- Sureshkumar, K.; Durairaj, M.; Srinivasan, K.; Goh, K.W.; Undela, K.; Mahalingam, V.T.; Ardianto, C.; Ming, L.C.; Ganesan, R.M. Effect of L-Carnosine in Patients with Age-Related Diseases: A Systematic Review and Meta-Analysis. Front. Biosci. 2023, 28, 18. [Google Scholar] [CrossRef]
- Menon, K.; Marquina, C.; Liew, D.; Mousa, A.; Courten, B. Histidine-containing dipeptides reduce central obesity and improve glycaemic outcomes: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2020, 21, e12975. [Google Scholar] [CrossRef] [PubMed]
- Spaas, J.; Van Noten, P.; Keytsman, C.; Nieste, I.; Blancquaert, L.; Derave, W.; Eijnde, B.O. Carnosine and skeletal muscle dysfunction in a rodent multiple sclerosis model. Amino Acids 2021, 53, 1749–1761. [Google Scholar] [CrossRef]
- Takeuchi, K.; Toyohara, H.; Sakaguchi, M. A hyperosmotic stress-induced mRNA of carp cell encodes Na+- and Cl−-dependent high affinity taurine transporter1The sequence reported in this paper has been deposited in the DDBJ/EMBL/GenBank database with accession no. AB006986.1. Biochim. Biophys. Acta—Biomembr. 2000, 1464, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Lenney, J.F.; George, R.P.; Weiss, A.M.; Kucera, C.M.; Chan, P.W.H.; Rinzler, G.S. Human serum carnosinase: Characterization, distinction from cellular carnosinase, and activation by cadmium. Clin. Chim. Acta 1982, 123, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The Role of Reactive Oxygen Species in the Pathogenesis of Alzheimer’s Disease, Parkinson’s Disease, and Huntington’s Disease: A Mini Review. Oxid. Med. Cell. Longev. 2016, 2016, 8590578. [Google Scholar] [CrossRef]
- Schön, M.; Mousa, A.; Berk, M.; Chia, W.L.; Ukropec, J.; Majid, A.; Ukropcová, B.; de Courten, B. The Potential of Carnosine in Brain-Related Disorders: A Comprehensive Review of Current Evidence. Nutrients 2019, 11, 1196. [Google Scholar] [CrossRef] [PubMed]
- Rokicki, J.; Li, L.; Imabayashi, E.; Kaneko, J.; Hisatsune, T.; Matsuda, H. Daily Carnosine and Anserine Supplementation Alters Verbal Episodic Memory and Resting State Network Connectivity in Healthy Elderly Adults. Front. Aging Neurosci. 2015, 7, 219. [Google Scholar] [CrossRef]
- Szcześniak, D.; Budzeń, S.; Kopeć, W.; Rymaszewska, J. Anserine and carnosine supplementation in the elderly: Effects on cognitive functioning and physical capacity. Arch Gerontol. Geriatr. 2014, 59, 485–490. [Google Scholar] [CrossRef]
- Katakura, Y.; Totsuka, M.; Imabayashi, E.; Matsuda, H.; Hisatsune, T. Anserine/Carnosine Supplementation Suppresses the Expression of the Inflammatory Chemokine CCL24 in Peripheral Blood Mononuclear Cells from Elderly People. Nutrients 2017, 9, 1199. [Google Scholar] [CrossRef]
- Hisatsune, T.; Kaneko, J.; Kurashige, H.; Cao, Y.; Satsu, H.; Totsuka, M.; Katakura, Y.; Imabayashi, E.; Matsuda, H. Effect of Anserine/Carnosine Supplementation on Verbal Episodic Memory in Elderly People. J. Alzheimer’s Dis. 2015, 50, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Baraniuk, J.N.; El-Amin, S.; Corey, R.; Rayhan, R.; Timbol, C. Carnosine Treatment for Gulf War Illness: A Randomized Controlled Trial. Glob. J. Health Sci. 2013, 5, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Solis, M.Y.; Cooper, S.; Hobson, R.M.; Artioli, G.G.; Otaduy, M.C.; Roschel, H.; Robertson, J.; Martin, D.; Painelli, V.S.; Harris, R.C.; et al. Effects of Beta-Alanine Supplementation on Brain Homocarnosine/Carnosine Signal and Cognitive Function: An Exploratory Study. PLoS ONE 2015, 10, e0123857. [Google Scholar] [CrossRef] [PubMed]
- Masuoka, N.; Yoshimine, C.; Hori, M.; Tanaka, M.; Asada, T.; Abe, K.; Hisatsune, T. Effects of Anserine/Carnosine Supplementation on Mild Cognitive Impairment with APOE4. Nutrients 2019, 11, 1626. [Google Scholar] [CrossRef]
- Boldyrev, A.; Fedorova, T.; Stepanova, M.; Dobrotvorskaya, I.; Kozlova, E.; Boldanova, N.; Bagyeva, G.; Ivanova-Smolenskaya, I.; Illarioshkin, S. Carnisone Increases Efficiency of DOPA Therapy of Parkinson’s Disease: A Pilot Study. Rejuvenation Res. 2008, 11, 821–827. [Google Scholar] [CrossRef]
- Cornelli, U. Treatment of Alzheimer’s Disease with a Cholinesterase Inhibitor Combined with Antioxidants. Neurodegener. Dis. 2010, 7, 193–202. [Google Scholar] [CrossRef]
- Checkoway, H.; Lundin, J.I.; Kelada, S.N. Neurodegenerative diseases. IARC Sci. Publ. 2011, 163, 407–419. [Google Scholar]
- Fonteh, A.N.; Harrington, R.J.; Tsai, A.; Liao, P.; Harrington, M.G. Free amino acid and dipeptide changes in the body fluids from Alzheimer’s disease subjects. Amino Acids 2007, 32, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.A.; Jiménez-Jiménez, F.J.; Gomez, P.; Vargas, C.; Navarro, J.A.; Ortí-Pareja, M.; Gasalla, T.; Benito-León, J.; Bermejo, F.; Arenas, J. Decreased cerebrospinal fluid levels of neutral and basic amino acids in patients with Parkinson’s disease. J. Neurol. Sci. 1997, 150, 123–127. [Google Scholar] [CrossRef]
- Millan, M.J.; Fone, K.; Steckler, T.; Horan, W.P. Negative symptoms of schizophrenia: Clinical characteristics, pathophysiological substrates, experimental models and prospects for improved treatment. Eur. Neuropsychopharmacol. 2014, 24, 645–692. [Google Scholar] [CrossRef]
- Beck, K.; Javitt, D.C.; Howes, O.D. Targeting glutamate to treat schizophrenia: Lessons from recent clinical studies. Psychopharmacology (Berl.) 2016, 233, 2425–2428. [Google Scholar] [CrossRef] [PubMed]
- Ghajar, A.; Khoaie-Ardakani, M.-R.; Shahmoradi, Z.; Alavi, A.-R.; Afarideh, M.; Shalbafan, M.-R.; Ghazizadeh-Hashemi, M.; Akhondzadeh, S. L-carnosine as an add-on to risperidone for treatment of negative symptoms in patients with stable schizophrenia: A double-blind, randomized placebo-controlled trial. Psychiatry Res. 2018, 262, 94–101. [Google Scholar] [CrossRef]
- Chengappa, K.N.R.; Turkin, S.R.; DeSanti, S.; Bowie, C.R.; Brar, J.S.; Schlicht, P.J.; Murphy, S.L.; Hetrick, M.L.; Bilder, R.; Fleet, D. A preliminary, randomized, double-blind, placebo-controlled trial of l-carnosine to improve cognition in schizophrenia. Schizophr. Res. 2012, 142, 145–152. [Google Scholar] [CrossRef]
- Woo, H.-I.; Chun, M.-R.; Yang, J.-S.; Lim, S.-W.; Kim, M.-J.; Kim, S.-W.; Myung, W.-J.; Kim, D.-K.; Lee, S.-Y. Plasma Amino Acid Profiling in Major Depressive Disorder Treated With Selective Serotonin Reuptake Inhibitors. CNS Neurosci. Ther. 2015, 21, 417–424. [Google Scholar] [CrossRef]
- Ali-Sisto, T.; Tolmunen, T.; Kraav, S.-L.; Mäntyselkä, P.; Valkonen-Korhonen, M.; Honkalampi, K.; Ruusunen, A.; Velagapudi, V.; Lehto, S.M. Serum levels of carnosine may be associated with the duration of MDD episodes. J. Affect Disord. 2023, 320, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Sauer, A.K.; Stanton, J.E.; Hans, S.; Grabrucker, A.M. Autism Spectrum Disorders: Etiology and Pathology. In Autism Spectrum Disorders; Exon Publications: Brisbane, Australia, 2021. [Google Scholar] [CrossRef]
- Anyachor, C.P.; Dooka, D.B.; Orish, C.N.; Amadi, C.N.; Bocca, B.; Ruggieri, F.; Senofonte, M.; Frazzoli, C.; Orisakwe, O.E. Mechanistic considerations and biomarkers level in nickel-induced neurodegenerative diseases: An updated systematic review. IBRO Neurosci. Reports 2022, 13, 136–146. [Google Scholar] [CrossRef]
- Błażewicz, A.; Grabrucker, A.M. Metal Profiles in Autism Spectrum Disorders: A Crosstalk between Toxic and Essential Metals. Int. J. Mol. Sci. 2022, 24, 308. [Google Scholar] [CrossRef] [PubMed]
- Abraham, D.A.; Undela, K.; Narasimhan, U.; Rajanandh, M.G. Effect of L-Carnosine in children with autism spectrum disorders: A systematic review and meta-analysis of randomised controlled trials. Amino Acids 2021, 53, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Bala, K.A.; Doğan, M.; Mutluer, T.; Kaba, S.; Aslan, O.; Balahoroğlu, R.; Çokluk, E.; Üstyol, L.; Kocaman, S. Plasma amino acid profile in autism spectrum disorder (ASD). Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 923–929. [Google Scholar]
- Ming, X.; Stein, T.P.; Barnes, V.; Rhodes, N.; Guo, L. Metabolic Perturbance in Autism Spectrum Disorders: A Metabolomics Study. J. Proteome Res. 2012, 11, 5856–5862. [Google Scholar] [CrossRef] [PubMed]
- Chez, M.G.; Buchanan, C.P.; Aimonovitch, M.C.; Becker, M.; Schaefer, K.; Black, C.; Komen, J. Double-Blind, Placebo-Controlled Study of L-Carnosine Supplementation in Children with Autistic Spectrum Disorders. J. Child Neurol. 2002, 17, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Mehrazad-Saber, Z.; Kheirouri, S.; Noorazar, S.G. Effects of l-Carnosine Supplementation on Sleep Disorders and Disease Severity in Autistic Children: A Randomized, Controlled Clinical Trial. Basic Clin. Pharmacol. Toxicol. 2018, 123, 72–77. [Google Scholar] [CrossRef]
- Hajizadeh-Zaker, R.; Ghajar, A.; Mesgarpour, B.; Afarideh, M.; Mohammadi, M.R.; Akhondzadeh, S. l-Carnosine As an Adjunctive Therapy to Risperidone in Children with Autistic Disorder: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Child Adolesc. Psychopharmacol. 2018, 28, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Ghajar, A.; Aghajan-Nashtaei, F.; Afarideh, M.; Mohammadi, M.R.; Akhondzadeh, S. l-Carnosine as Adjunctive Therapy in Children and Adolescents with Attention-Deficit/Hyperactivity Disorder: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Child Adolesc. Psychopharmacol. 2018, 28, 331–338. [Google Scholar] [CrossRef]
- Esposito, D.; Belli, A.; Ferri, R.; Bruni, O. Sleeping without Prescription: Management of Sleep Disorders in Children with Autism with Non-Pharmacological Interventions and over-the-Counter Treatments. Brain Sci. 2020, 10, 441. [Google Scholar] [CrossRef]
- Siafis, S.; Çıray, O.; Wu, H.; Schneider-Thoma, J.; Bighelli, I.; Krause, M.; Rodolico, A.; Ceraso, A.; Deste, G.; Huhn, M.; et al. Pharmacological and dietary-supplement treatments for autism spectrum disorder: A systematic review and network meta-analysis. Mol. Autism. 2022, 13, 10. [Google Scholar] [CrossRef]
- Ann Abraham, D.; Narasimhan, U.; Christy, S.; Muhasaparur Ganesan, R. Effect of l-Carnosine as adjunctive therapy in the management of children with autism spectrum disorder: A randomized controlled study. Amino Acids 2020, 52, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Lindfield, R.; Vishwanath, K.; Ngounou, F.; Khanna, R. The challenges in improving outcome of cataract surgery in low and middle income countries. Indian J. Ophthalmol. 2012, 60, 464. [Google Scholar] [CrossRef]
- Wang, A.M.; Ma, C.; Xie, Z.H.; Shen, F. Use of carnosine as a natural anti-senescence drug for human beings. Biochemistry (Mosc). 2000, 65, 869–871. [Google Scholar]
- Babizhayev, M.A.; Deyev, A.I.; Yermakova, V.N.; Semiletov, Y.A.; Davydova, N.G.; Kurysheva, N.I.; Zhukotskii, A.V.; Goldman, I.M. N-Acetylcarnosine, a natural histidine-containing dipeptide, as a potent ophthalmic drug in treatment of human cataracts. Peptides 2001, 22, 979–994. [Google Scholar] [CrossRef]
- Babizhayev, M.; Kasus-Jacobi, A. State of the Art Clinical Efficacy and Safety Evaluation of N-acetylcarnosine Dipeptide Ophthalmic Prodrug. Principles for the Delivery, Self-Bioactivation, Molecular Targets and Interaction with a Highly Evolved Histidyl-Hydrazide Structure in the Treatm. Curr. Clin. Pharmacol. 2009, 4, 4–37. [Google Scholar] [CrossRef] [PubMed]
- Babizhayev, M.A.; Burke, L.; Micans, P.; Richer, S.P. N-acetylcarnosine sustained drug delivery eye drops to control the signs of ageless vision: Glare sensitivity, cataract amelioration and quality of vision currently available treatment for the challenging 50,000-patient population. Clin. Interv. Aging 2009, 4, 31–50. [Google Scholar] [CrossRef] [PubMed]
- Dubois, V.D.P.; Bastawrous, A. N-acetylcarnosine (NAC) drops for age-related cataract. Cochrane Database Syst. Rev. 2017, 2, CD009493. [Google Scholar] [CrossRef]
- Yang, Q.-J.; Zhao, J.-R.; Hao, J.; Li, B.; Huo, Y.; Han, Y.-L.; Wan, L.-L.; Li, J.; Huang, J.; Lu, J.; et al. Serum and urine metabolomics study reveals a distinct diagnostic model for cancer cachexia. J. Cachexia Sarcopenia Muscle. 2018, 9, 71–85. [Google Scholar] [CrossRef]
- Stuerenburg, H.J. The roles of carnosine in aging of skeletal muscle and in neuromuscular diseases. Biochemistry (Mosc.) 2000, 65, 862–865. [Google Scholar]
- Hipkiss, A.R. Would Carnosine or a Carnivorous Diet Help Suppress Aging and Associated Pathologies? Ann. N. Y. Acad. Sci. 2006, 1067, 369–374. [Google Scholar] [CrossRef]
- Hipkiss, A.R. Glycation, ageing and carnosine: Are carnivorous diets beneficial? Mech. Ageing Dev. 2005, 126, 1034–1039. [Google Scholar] [CrossRef]
- del Favero, S.; Roschel, H.; Solis, M.Y.; Hayashi, A.P.; Artioli, G.G.; Otaduy, M.C.; Benatti, F.B.; Harris, R.C.; Wise, J.A.; Leite, C.C.; et al. Beta-alanine (CarnosynTM) supplementation in elderly subjects (60–80 years): Effects on muscle carnosine content and physical capacity. Amino Acids 2012, 43, 49–56. [Google Scholar] [CrossRef] [PubMed]
- McCormack, W.P.; Stout, J.R.; Emerson, N.S.; Scanlon, T.C.; Warren, A.M.; Wells, A.J.; Gonzalez, A.M.; Mangine, G.T.; Robinson, E.H., 4th; Fragala, M.S.; et al. Oral nutritional supplement fortified with beta-alanine improves physical working capacity in older adults: A randomized, placebo-controlled study. Exp. Gerontol. 2013, 48, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Furst, T.; Massaro, A.; Miller, C.; Williams, B.T.; LaMacchia, Z.M.; Horvath, P.J. β-Alanine supplementation increased physical performance and improved executive function following endurance exercise in middle aged individuals. J. Int. Soc. Sports Nutr. 2018, 15, 32. [Google Scholar] [CrossRef]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants. 2020, 9, 864. [Google Scholar] [CrossRef]
- Roberts, P.R.; Zaloga, G.P. Cardiovascular effects of carnosine. Biochemistry (Mosc.) 2000, 65, 856–861. [Google Scholar]
- Stvolinsky, S.L.; Dobrota, D. Anti-ischemic activity of carnosine. Biochemistry (Mosc.) 2000, 65, 849–855. [Google Scholar] [PubMed]
- Lombardi, C.; Carubelli, V.; Lazzarini, V.; Vizzardi, E.; Bordonali, T.; Ciccarese, C.; Castrini, A.I.; Cas, A.D.; Nodari, S.; Metra, M. Effects of oral administration of orodispersible levo-carnosine on quality of life and exercise performance in patients with chronic heart failure. Nutrition 2015, 31, 72–78. [Google Scholar] [CrossRef]
- Yoshikawa, F.; Nakajima, T.; Hanada, M.; Hirata, K.; Masuyama, T.; Aikawa, R. Beneficial effect of polaprezinc on cardiac function post-myocardial infarction: A prospective and randomized clinical trial. Medicine 2019, 98, e14637. [Google Scholar] [CrossRef]
- Hall, A.G.; King, J.C. Zinc Fortification: Current Trends and Strategies. Nutrients 2022, 14, 3895. [Google Scholar] [CrossRef]
- Li, M.; Sun, Z.; Zhang, H.; Liu, Z. Recent advances on polaprezinc for medical use (Review). Exp. Ther. Med. 2021, 22, 1445. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Liu, H.; Ooi, T.C.; Rajab, N.F.; Cao, H.; Sharif, R. Zinc carnosine: Frontiers advances of supplement for cancer therapy. Biomed Pharmacother. 2022, 151, 113157. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Medeiros, D.M. Nutrition: Real People, Real Choices; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2008; ISBN ISBN-13 978-0757579035, ISBN-10 0757579035. [Google Scholar]
- Sakagami, M.; Ikeda, M.; Tomita, H.; Ikui, A.; Aiba, T.; Takeda, N.; Inokuchi, A.; Kurono, Y.; Nakashima, M.; Shibasaki, Y.; et al. A zinc-containing compound, Polaprezinc, is effective for patients with taste disorders: Randomized, double-blind, placebo-controlled, multi-center study. Acta Otolaryngol. 2009, 129, 1115–1120. [Google Scholar] [CrossRef]
- Fujii, H.; Hirose, C.; Ishihara, M.; Iihara, H.; Imai, H.; Tanaka, Y.; Matsuhashi, N.; Takahashi, T.; Yamaguchi, K.; Yoshida, K.; et al. Improvement of Dysgeusia by Polaprezinc, a Zinc-L-carnosine, in Outpatients Receiving Cancer Chemotherapy. Anticancer. Res. 2018, 38, 6367–6373. [Google Scholar] [CrossRef]
- Doi, H.; Fujiwara, M.; Suzuki, H.; Niwa, Y.; Nakayama, M.; Shikata, T.; Odawara, S.; Takada, Y.; Kimura, T.; Kamikonya, N.; et al. Polaprezinc reduces the severity of radiation-induced mucositis in head and neck cancer patients. Mol. Clin. Oncol. 2015, 3, 381–386. [Google Scholar] [CrossRef]
- Hayashi, H.; Kobayashi, R.; Suzuki, A.; Ishihara, M.; Nakamura, N.; Kitagawa, J.; Kanemura, N.; Kasahara, S.; Kitaichi, K.; Hara, T.; et al. Polaprezinc prevents oral mucositis in patients treated with high-dose chemotherapy followed by hematopoietic stem cell transplantation. Anticancer Res. 2014, 34, 7271–7277. [Google Scholar]
- Hayashi, H.; Kobayashi, R.; Suzuki, A.; Yamada, Y.; Ishida, M.; Shakui, T.; Kitagawa, J.; Hayashi, H.; Sugiyama, T.; Takeuchi, H.; et al. Preparation and clinical evaluation of a novel lozenge containing polaprezinc, a zinc-L-carnosine, for prevention of oral mucositis in patients with hematological cancer who received high-dose chemotherapy. Med. Oncol. 2016, 33, 91. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Kobayashi, R.; Shakui, T.; Kubota, Y.; Fukita, M.; Kuze, B.; Aoki, M.; Sugiyama, T.; Mizuta, K.; Itoh, Y. Effect of polaprezinc on oral mucositis, irradiation period, and time to discharge in patients with head and neck cancer. Head Neck 2016, 38, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; FitzGerald, A.J.; Marchbank, T.; Ntatsaki, E.; Murray, D.; Ghosh, S.; Playford, R.J. Zinc carnosine, a health food supplement that stabilises small bowel integrity and stimulates gut repair processes. Gut 2007, 56, 168–175. [Google Scholar] [CrossRef]
- Watanabe, T.; Ishihara, M.; Matsuura, K.; Mizuta, K.; Itoh, Y. Polaprezinc prevents oral mucositis associated with radiochemotherapy in patients with head and neck cancer. Int. J. Cancer 2010, 127, 1984–1990. [Google Scholar] [CrossRef]
- Yanase, K.; Funaguchi, N.; Iihara, H.; Yamada, M.; Kaito, D.; Endo, J.; Ito, F.; Ohno, Y.; Tanaka, H.; Itoh, Y.; et al. Prevention of radiation esophagitis by polaprezinc (zinc L-carnosine) in patients with non-small cell lung cancer who received chemoradiotherapy. Int. J. Clin. Exp. Med. 2015, 8, 16215–16222. [Google Scholar] [PubMed]
- Tan, B.; Luo, H.-Q.; Xu, H.; Lv, N.-H.; Shi, R.-H.; Luo, H.-S.; Li, J.-S.; Ren, J.-L.; Zou, Y.-Y.; Li, Y.-Q.; et al. Polaprezinc combined with clarithromycin-based triple therapy for Helicobacter pylori-associated gastritis: A prospective, multicenter, randomized clinical trial. PLoS ONE 2017, 12, e0175625. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, T.; Sarukura, N.; Ueda, C.; Kitamura, Y.; Kalubi, B.; Toda, N.; Abe, K.; Yamamoto, S.; Takeda, N. Effects of zinc supplementation on serum zinc concentration and ratio of apo/holo-activities of angiotensin converting enzyme in patients with taste impairment. Auris Nasus Larynx 2010, 37, 190–194. [Google Scholar] [CrossRef]
- Masayuki, F.; Norihiko, K.; Keita, T.; Miwa, I.; Masayuki, I.; Toshihiko, I.; Hiromi, F.; Chikaaki, M.; Norio, N. Efficacy and safety of polaprezinc as a preventive drug for radiation-induced stomatitis. Nihon Igaku Hoshasen Gakkai Zasshi 2002, 62, 144–150. [Google Scholar]
- Kitagawa, J.; Kobayashi, R.; Nagata, Y.; Kasahara, S.; Ono, T.; Sawada, M.; Ohata, K.; Kato-Hayashi, H.; Hayashi, H.; Shimizu, M.; et al. Polaprezinc for prevention of oral mucositis in patients receiving chemotherapy followed by hematopoietic stem cell transplantation: A multi-institutional randomized controlled trial. Int. J. Cancer 2021, 148, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.H.; Park, J.C.; Lee, Y.C.; Lee, S.K.; Shin, S.K.; Chung, H.; Park, J.J.; Kim, J.-H.; Youn, Y.H.; Park, H. Comparison of the Efficacy of Polaprezinc Plus Proton Pump Inhibitor and Rebamipide Plus Proton Pump Inhibitor Treatments for Endoscopic Submucosal Dissection-induced Ulcers. J. Clin. Gastroenterol. 2021, 55, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.; Kalman, D. A Review of Zinc-L-Carnosine and Its Positive Effects on Oral Mucositis, Taste Disorders, and Gastrointestinal Disorders. Nutrients 2020, 12, 665. [Google Scholar] [CrossRef]
- FDA (Food and Drug Administration). NDI 134—Zinc Carnosine—Original NDI Notification. Published Online 2007. Available online: https://www.regulations.gov/document/FDA-2003-S-0732-0044 (accessed on 1 February 2023).

| Author (Year) | Study Design | Intervention | Number of Patients | Effect |
|---|---|---|---|---|
| de Courten et al. (2016) [39] | double-blind RCT | Carnosine orally (2 g in 2 doses/day) or placebo; 12 weeks | 30 | Preserved insulin sensitivity and insulin secretion, normalized glucose intolerance and reduced 2-h insulin levels after o-GTT in a subgroup of individuals with impaired glucose tolerance |
| Siriwattanasit et al. (2021) [40] | RCT | Carnosine (2 g/day) or placebo; 12 weeks | 40 | Nephroprotective effect of oral supplementation to decrease urinary TGF-β. |
| Karkabounas et al. (2018) [41] | double-blind RCT | Alpha-lipoic acid (7 mg/kg bodyweight), carnosine (6 mg/kg bodyweight), thiamine (1 mg/kg bodyweight) or placebo; 8 weeks | 82 | Supplementation effectively reduced glucose concentration in patients with T2DM. |
| Nealon et al. (2016) [42] | double-blind RCT | Beta-alanine (4 g split into 3 doses/day) or placebo; 28 days | 12 | Beta-alanine supplementation can increase exercise capacity in individuals with T2DM. |
| Elbarbary et al. (2018) [43] | double-blind RCT | Patients with diabetic nephropathy received supplemented carnosine (1 g/day) or placebo; 12 weeks | 90 | Oral supplementation with L-carnosine for 12 weeks resulted in a significant improvement of oxidative stress, glycemic control and renal function. |
| Houjeghani et al. (2018) [44] | double-blind RCT | Patients with T2DM received carnosine (500 mg, 2×/day, capsules) or placebo | 54 | Carnosine supplementation lowered fasting glucose, serum levels of triglycerides, AGEs and tumor necrosis factor-α without changing sRAGE, IL-6 or IL-1β levels in T2DM patients. |
| Author (Year) | Study Design | Intervention | Number of Patients | Effect |
|---|---|---|---|---|
| Rokicki et al. (2015) [53] | double-blind RCT | Carnosine/anserine (1:3 ratio; 500 mg/day) or placebo; 3 months | 31 | Intervention group had better verbal episodic memory performance and decreased connectivity in the default mode network, the posterior cingulate cortex and the right frontal parietal network. A correlation between the extent of cognitive and neuroimaging changes was observed. |
| Szcześniak et al. (2014) [54] | RCT | Chicken meat extract containing anserine and carnosine (2:1 ratio; 1 g/day) or placebo; 13 weeks | 51 | Mean values of Short Test of Mental Status (STMS) scores increased in the intervention group (in the subscores of construction/copying, abstraction and recall), and promising effects on physical capacity. |
| Katakura et al. (2017) [55] | double-blind RCT | Anserine/carnosine (3:1 ratio; 1 g/day) or placebo; 3 months | 60 | Supplementation may preserve verbal episodic memory, probably owing to inflammatory chemokine CCL24 suppression in the blood. |
| Hisatsune et al. (2015) [56] | double-blind RCT | Anserine/carnosine (3:1 ratio; 1 g/day) or placebo; 3 months | 39 | MRI analysis showed a suppression in the age-related decline in brain blood flow in the posterior cingulate cortex area. Delayed recall verbal memory showed significant preservation in the intervention group. |
| Baraniuk et al. (2013) [57] | double-blind RCT | Carnosine (500, 1000 and 1500 mg/day, increasing at 4-week intervals) or placebo; 12 weeks | 25 | Supplementation may have beneficial cognitive effects. Fatigue, pain, hyperalgesia, activity and other outcomes were resistant to treatment. |
| Solis et al. (2015) [58] | double-blind RCT | Beta-alanine (6.4 g/day) supplementation or placebo; 28 days | 19 | Supplementation did not influence cognitive function before or after exercise in trained cyclists. |
| Masuoka et al. (2019) [59] | double-blind RCT | Anserine (750 mg/day) and carnosine (250 mg/day) or placebo; 12 weeks | 54 | Protective effects against cognitive decline in APOE4 (+) MCI subjects exist. |
| Boldyrev et al. (2008) [60] | two-arm, prospective | In addition to basic PD therapy, carnosine (1.5 g/day); 30 days | 36 | The combination of carnosine with basic therapy may be a useful way to increase efficiency of PD treatment. |
| Cornelli et al. (2010) [61] | two-arm, RCT | Carnosine (100 mg/day) with a mixture of antioxidants (beta-carotene, selenium, cysteine, ginko biloba and coenzyme Q10) and vitamins (B1, B2, B3, B6, B9, B12, C, D and E) on Alzheimer’s disease (AD) patients treated with donepezil; 6 months | 52 | A reduction in oxidative stress parameters and an improvement in mini-mental state examination, version 2 (MMSE-2) scores were observed. |
| Author (Year) | Study Design | Intervention | Number of Patients | Effect |
|---|---|---|---|---|
| Ghajar et al. (2018) [80] | double-blind RCT | Carnosine (2 g/day in two doses) or placebo; 8 weeks | 60 | Administration of carnosine along with therapy resulted in a reduction in negative symptoms of schizophrenia without an increase in side effects. |
| Chengappa et al. (2012) [68] | double-blind RCT | Carnosine (2 g/day) or placebo; 3 months | 75 | Intervention group performed significantly faster on non-reversal condition trials of the set-shifting test. The strategic target detection test displayed improved strategic efficiency and fewer perseverative errors. |
| Chez et al. (2002) [77] | double-blind RCT | Carnosine supplementation (800 mg/day) or placebo; 8 weeks | 31 | Improved communication skills and behavior in children with ASD. The authors also reported improvements in receptive speech and social attention, a reduction in apraxia and an overall improvement in brain function. |
| Mehrazad-Saber et al. (2018) [78] | double-blind RCT | Carnosine (500 mg/day) or placebo; 2 months | 43 | Carnosine supplementation did not change anthropometric indices and showed no effect on autism severity, whereas it significantly reduced sleep duration, parasomnias and total sleep disorders scores. |
| Hajizadeh-Zaker et al. (2018) [79] | double-blind RCT | Carnosine (800 mg/day in 2 doses) or placebo in addition to risperidone; 10 weeks | 70 | Carnosine supplementation resulted in a reduction in hyperactivity and non-compliance in children with ASD. |
| Ghajar et al. (2018) [67] | double-blind RCT | Carnosine (2 g/day in two divided doses) or placebo; 8 weeks | 63 | Carnosine add-on therapy reduced the primary negative symptoms of patients with schizophrenia. |
| Ann Abraham et al. (2020) [83] | double-blind RCT | Carnosine (10–15 mg/kg in 2 divided doses/day) or placebo; 2 months | 63 | No statistically significant difference was observed for any of the outcome measures assessed. |
| Woo et al. (2015) [69] | two-arm prospective | SSRI; 6 weeks | 68/22 (90 total) | A potential was shown to measure therapeutic response. Patients with MDD, after 6 weeks of SSRI treatment, had alterations of amino acids, including beta-alanine (and alanine, beta-aminoisobutyric acid, cystathionine, ethanolamine, glutamic acid, homocystine, methionine, O-phospho-L-serine and sarcosine). |
| Ali Sisto et al. (2023) [70] | two-arm, prospective | Antidepressant quetiapine; 40 weeks | 99/253 (352 total) | The use of any antipsychotic medication was associated with lowered carnosine levels. Elevated serum levels of carnosine were also associated with a longer duration of the depressive episode. |
| Author (Year) | Study Design | Intervention | Number of Patients | Effect |
|---|---|---|---|---|
| del Favero et al. (2012) [94] | double-blind RCT | Beta-alanine (3.2 g divided into 4 doses/day) or placebo; 12 weeks | 18 | Supplementation is effective in increasing the muscle carnosine content in healthy elderly subjects, with improvement in exercise capacity. |
| McCormack et al. (2013) [95] | double-blind RCT, three-arm | (1) ONS (containing 8 oz; 230 kcal; 12 g protein, 31 g cholesterol, 6g fat); 12 weeks (2) ONS plus beta-alanine (800 mg, 2×/day); 12 weeks (3) ONS plus beta-alanine (1200 mg, 2×/day); 12 weeks | 60 | ONS fortified with beta-alanine may improve physical working capacity, muscle quality and function in older men and women. |
| Furst et al. (2018) [96] | double-blind RCT | Beta-alanine (2.4 g/day) or placebo; 28 days | 12 | Supplementation increased exercise capacity and eliminated endurance exercise-induced declines in executive function seen after recovery. |
| Author (Year) | Study Design | Intervention | Number of Patients | Effect |
|---|---|---|---|---|
| Doi et al. (2015) [108] | two-arm, prospective | 1 min ZnC mouth rinse (37.5 mg/10 mL, 4×/day) | 32 | Grade 3 mucositis was observed less frequently according to clinical findings and symptomatology. ZnC promoted recovery. |
| Watanabe et al. (2010) [113] | RCT | ZnC oral rinse | 16/15 (31 total) | Use of analgesics was less frequent and the amount of food intake was significantly higher. Tumor response rate was not affected in patients receiving ZnC. |
| Hayashi et al. (2016) [110] | prospective, three-arm | ZnC lozenge (18.75 mg 4×/day), ZnC suspension (75 mg in 4 doses/day) | 19/31/16 (66 total) | ZnC lozenge was highly effective for prevention of moderate to severe oral mucositis in patients receiving high-dose chemotherapy for HSCT. The efficacy of lozenge preparation was comparable suspension. |
| Hayashi et al. (2014) [109] | retrospective | ZnC (500 mg) in 20mL P-AG, mouth rinse | 36 | Reduced the incidence of moderate-to-severe oral mucositis, and pain was significantly relieved. Incidence of xerostomia and taste disturbance tended to be lowered, but not significantly. |
| Yanase et al. (2015) [114] | retrospective | 60 mL P-AG and 150 mg ZnC (3×/day) | 19/19 (38 total) | ZnC highly effective in suppressing the development of radiation esophagitis without affecting the tumor response rate. |
| Sakagami et al. (2009) [106] | double-blind RCT, multi-center | ZnC (17 mg, 34 mg or 68 mg/day; 12 weeks) | 28/27/26/28 (109 total) | An amount of 68 mg of ZnC showed a significant improvement in gustatory sensitivity. |
| Fujii et al. (2018) [107] | retrospective | ZnC (150 mg; 2×/day), until symptom disappearance | 80 | The administration of 150 mg of ZnC to patients (with pancreatic cancer treated with fluoropyrimidines) with grade 2 dysgeusia significantly shortened its duration. |
| Mahmood et al. (2007) [112] | RCT | ZnC (37.5 mg; 2×/day) before and after 5 days of indomethacin treatment (50 mg; 3×/day) | 10 | ZnC, at concentrations likely to be found in the gut lumen, stabilized gut mucosa. |
| Tan et al. (2017) [115] | RCT, multi-center | ZnC (150 mg/day) combined with triple therapy; ZnC (300 mg/day) combined with triple therapy; triple therapy | 113/108/111 (332 total) | Confirmed the effectiveness of the zinc compound in improving HP eradication rate. |
| Takaoka et al. (2010) [116] | two-arm, prospective | 150 mg of ZnC orally | 12/28 (40 total) | No significant correlation between improvement of VAS pain score and the zinc concentration in the serum after zinc supplementation. |
| Masayuki et al. (2002) [117] | two-arm, prospective | ZnC and 2% carmellose sodium | 19 | ZnC was found to have efficacy and safety as a preventive drug for radiation-induced stomatitis. |
| Suzuki et al. (2016) [111] | retrospective | P-AG oral rinse | 104 | P-AG was found to be effective in preventing severe oral mucositis and reducing the irradiation period and median time to discharge after completion of radiotherapy. |
| Baraniuk et al. (2013) [57] | RCT | Carnosine (500 mg, 1000 mg and 1500 mg increasing at 4-week intervals) | 25 | Decrease in diarrhea associated with irritable bowel syndrome. |
| Kitagawa et al. (2021) [118] | RCT, multi-center | ZnC lozenge (18.75 mg) 4×/day, until 35 days after transplantation | 47/41 (88 total) | In patients receiving high-dose chemotherapy followed by hematopoietic stem cell transplantation, grade ≥2 oral mucositis was significantly reduced in the intervention group. |
| Jung et al. (2021) [119] | RCT | ZnC (150 mg/day), pantoprazole or rebamipide (300 mg/day), and pantoprazole | 200 | ZnC plus PPI treatment showed noninferiority to rebamipide, with PPI treatment of the ulcer healing rate at 4 weeks after endoscopic submucosal dissection. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cesak, O.; Vostalova, J.; Vidlar, A.; Bastlova, P.; Student, V., Jr. Carnosine and Beta-Alanine Supplementation in Human Medicine: Narrative Review and Critical Assessment. Nutrients 2023, 15, 1770. https://doi.org/10.3390/nu15071770
Cesak O, Vostalova J, Vidlar A, Bastlova P, Student V Jr. Carnosine and Beta-Alanine Supplementation in Human Medicine: Narrative Review and Critical Assessment. Nutrients. 2023; 15(7):1770. https://doi.org/10.3390/nu15071770
Chicago/Turabian StyleCesak, Ondrej, Jitka Vostalova, Ales Vidlar, Petra Bastlova, and Vladimir Student, Jr. 2023. "Carnosine and Beta-Alanine Supplementation in Human Medicine: Narrative Review and Critical Assessment" Nutrients 15, no. 7: 1770. https://doi.org/10.3390/nu15071770
APA StyleCesak, O., Vostalova, J., Vidlar, A., Bastlova, P., & Student, V., Jr. (2023). Carnosine and Beta-Alanine Supplementation in Human Medicine: Narrative Review and Critical Assessment. Nutrients, 15(7), 1770. https://doi.org/10.3390/nu15071770