Nutrition-Related N-of-1 Studies Warrant Further Research to Provide Evidence for Dietitians to Practice Personalized (Precision) Medical Nutrition Therapy: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Selection Process
2.4. Data Collection Process and Data Items
2.5. Assessment of Quality
2.6. Data Synthesis
3. Results
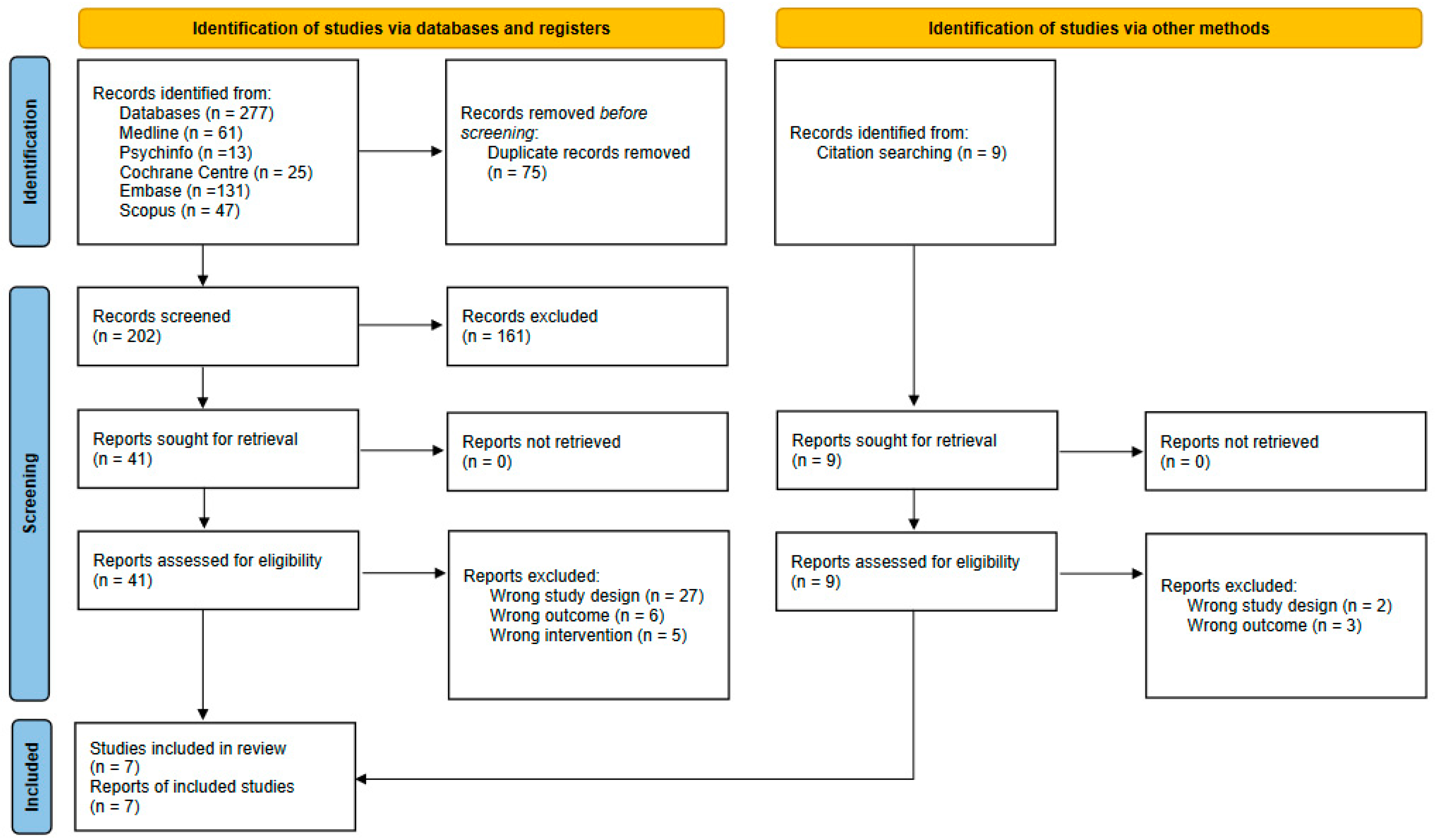
3.1. Study Selection
3.2. Characteristics of Selected Studies
3.3. Quality Assessment of Reporting and Dietary Assessment Methods of the Selected N-of-1 Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sackett, D.L.; Rosenberg, W.M.; Gray, J.A.; Haynes, R.B.; Richardson, W.S. Evidence based medicine: What it is and what it isn’t. BMJ 1996, 312, 71–72. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, I. The Cochrane collaboration: Preparing, maintaining, and disseminating systematic reviews of the effects of health care. Ann. N. Y. Acad. Sci. 1993, 703, 156–165. [Google Scholar] [CrossRef] [PubMed]
- NHMRC. A Guide to the Development, Implementation and Evaluation of Clinical Practice Guidelines; National Health & Medical Research Council: Canberra, Australia, 1998.
- Fisher, A.J.; Medaglia, J.D.; Jeronimus, B.F. Lack of group-to-individual generalizability is a threat to human subjects research. Proc. Natl. Acad. Sci. USA 2018, 115, E6106–E6115. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.D.; Trepanowski, J.F.; Del Gobbo, L.C.; Hauser, M.E.; Rigdon, J.; Ioannidis, J.P.A.; Desai, M.; King, A.C. Effect of Low-Fat vs Low-Carbohydrate Diet on 12-Month Weight Loss in Overweight Adults and the Association with Genotype Pattern or Insulin Secretion: The DIETFITS Randomized Clinical Trial. JAMA 2018, 319, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Zeilstra, D.; Younes, J.A.; Brummer, R.J.; Kleerebezem, M. Perspective: Fundamental Limitations of the Randomized Controlled Trial Method in Nutritional Research: The Example of Probiotics. Adv. Nutr. 2018, 9, 561–571. [Google Scholar] [CrossRef]
- Freedland, K.E. Progress in health-related behavioral intervention research: Making it, measuring it, and meaning it. Health Psychol. 2022, 41, 1–12. [Google Scholar] [CrossRef]
- Hekler, E.; Tiro, J.A.; Hunter, C.M.; Nebeker, C. Precision Health: The Role of the Social and Behavioral Sciences in Advancing the Vision. Ann. Behav. Med. 2020, 54, 805–826. [Google Scholar] [CrossRef]
- Guyatt, G.; Sackett, D.; Taylor, D.W.; Chong, J.; Roberts, R.; Pugsley, S. Determining optimal therapy--randomized trials in individual patients. N. Engl. J. Med. 1986, 314, 889–892. [Google Scholar] [CrossRef]
- Potter, T.; Vieira, R.; de Roos, B. Perspective: Application of N-of-1 Methods in Personalized Nutrition Research. Adv. Nutr. 2021, 12, 579–589. [Google Scholar] [CrossRef]
- Grammatikopoulou, M.G.; Gkouskou, K.K.; Gkiouras, K.; Bogdanos, D.P.; Eliopoulos, A.G.; Goulis, D.G. The Niche of n-of-1 Trials in Precision Medicine for Weight Loss and Obesity Treatment: Back to the Future. Curr. Nutr. Rep. 2022, 11, 133–145. [Google Scholar] [CrossRef]
- Schork, N.J.; Goetz, L.H. Single-Subject Studies in Translational Nutrition Research. Annu. Rev. Nutr. 2017, 37, 395–422. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Haynes, R.B.; Jaeschke, R.Z.; Cook, D.J.; Green, L.; Naylor, C.D.; Wilson, M.C.; Richardson, W.S. Users’ Guides to the Medical Literature: XXV. Evidence-based medicine: Principles for applying the Users’ Guides to patient care. Evidence-Based Medicine Working Group. JAMA 2000, 284, 1290–1296. [Google Scholar] [CrossRef]
- Lillie, E.O.; Patay, B.; Diamant, J.; Issell, B.; Topol, E.J.; Schork, N.J. The n-of-1 clinical trial: The ultimate strategy for individualizing medicine? Per. Med. 2011, 8, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Nikles, C.J.; Glasziou, P.P.; Del Mar, C.B.; Duggan, C.M.; Clavarino, A.; Yelland, M.J. Preliminary experiences with a single-patient trials service in general practice. Med. J. Aust. 2000, 173, 100–103. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.; Quinn, F.; Vieira, R.; O’Brien, N.; White, M.; Johnston, D.W.; Sniehotta, F.F. The state of the art and future opportunities for using longitudinal n-of-1 methods in health behaviour research: A systematic literature overview. Health Psychol. Rev. 2017, 11, 307–323. [Google Scholar] [CrossRef]
- Smith, G.; Williams, L.; O’Donnell, C.; McKechnie, J. A series of n-of-1 studies examining the interrelationships between social cognitive theory constructs and physical activity behaviour within individuals. Psychol. Health 2019, 34, 255–270. [Google Scholar] [CrossRef]
- Hébert, J.R.; Frongillo, E.A.; Adams, S.A.; Turner-McGrievy, G.M.; Hurley, T.G.; Miller, D.R.; Ockene, I.S. Perspective: Randomized Controlled Trials Are Not a Panacea for Diet-Related Research. Adv. Nutr. 2016, 7, 423–432. [Google Scholar] [CrossRef]
- Academy of Nutrition and Dietetics. Revised 2017 Standards of Practice in Nutrition Care and Standards of Professional Performance for Registered Dietitian Nutritionists. J. Acad. Nutr. Diet. 2018, 118, 132–140.e115. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Shamseer, L.; Sampson, M.; Bukutu, C.; Schmid, C.H.; Nikles, J.; Tate, R.; Johnston, B.C.; Zucker, D.; Shadish, W.R.; Kravitz, R.; et al. CONSORT extension for reporting N-of-1 trials (CENT) 2015: Explanation and elaboration. BMJ 2015, 350, h1793. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Burrows, T.; Golley, R.K.; Khambalia, A.; McNaughton, S.A.; Magarey, A.; Rosenkranz, R.R.; Allman-Farinelli, M.; Rangan, A.M.; Truby, H.; Collins, C. The quality of dietary intake methodology and reporting in child and adolescent obesity intervention trials: A systematic review. Obes. Rev. 2012, 13, 1125–1138. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Siopis, G.; Wong, H.Y.; Allman-Farinelli, M. Poor quality of dietary assessment in randomized controlled trials of nutritional interventions for type 2 diabetes may affect outcome conclusions: A systematic review and meta-analysis. Nutrition 2022, 94, 111498. [Google Scholar] [CrossRef]
- Feltham, S.; Westman, E.C. A case study of overfeeding 3 different diets. Curr. Opin. Endocrinol. Diabetes Obes. 2021, 28, 446–452. [Google Scholar] [CrossRef]
- Karkar, R.; Schroeder, J.; Epstein, D.A.; Pina, L.R.; Scofield, J.; Fogarty, J.; Kientz, J.A.; Munson, S.A.; Vilardaga, R.; Zia, J. TummyTrials: A Feasibility Study of Using Self-Experimentation to Detect Individualized Food Triggers. In Proceedings of the 2017 CHI Conference on Human Factors in Computing Systems, Denver, CO, USA, 6–11 May 2017; pp. 6850–6863. [Google Scholar] [CrossRef]
- Miller, C.K.; Weinhold, K.R.; Mitchell, D.C. Using Ecological Momentary Assessment to Track Goal Progress Toward the Adoption of a Low Glycemic Index Diet Among Adults With Type 2 Diabetes: A Pilot Study. Top. Clin. Nutr. 2016, 31, 323–334. [Google Scholar] [CrossRef]
- Gkouskou, K.K.; Grammatikopoulou, M.G.; Lazou, E.; Sanoudou, D.; Goulis, D.G.; Eliopoulos, A.G. Genetically-Guided Medical Nutrition Therapy in Type 2 Diabetes Mellitus and Pre-diabetes: A Series of n-of-1 Superiority Trials. Front. Nutr. 2022, 9, 772243. [Google Scholar] [CrossRef]
- Ma, Y.; Fu, Y.; Tian, Y.; Gou, W.; Miao, Z.; Yang, M.; Ordovás, J.M.; Zheng, J.S. Individual Postprandial Glycemic Responses to Diet in n-of-1 Trials: Westlake N-of-1 Trials for Macronutrient Intake (WE-MACNUTR). J. Nutr. 2021, 151, 3158–3167. [Google Scholar] [CrossRef]
- Tian, Y.; Ma, Y.; Fu, Y.; Zheng, J.S. Application of n-of-1 Clinical Trials in Personalized Nutrition Research: A Trial Protocol for Westlake N-of-1 Trials for Macronutrient Intake (WE-MACNUTR). Curr. Dev. Nutr. 2020, 4, nzaa143. [Google Scholar] [CrossRef]
- Hendriks, M.A.L.; van Wanroij, J.W.M.; Laros-van Gorkom, B.A.P.; Nijhuis-van der Sanden, M.W.G.; Hoogeboom, T.J. The SLIM study-Shared medical appointments to change lifestyles of overweight people with haemophilia: A randomized multiple baseline (n-of-1) design. Haemophilia 2021, 27, 606–617. [Google Scholar] [CrossRef]
- Kwasnicka, D.; Boroujerdi, M.; O’Gorman, A.; Anderson, M.; Craig, P.; Bowman, L.; McCann, M. An N-of-1 study of daily alcohol consumption following minimum unit pricing implementation in Scotland. Addiction 2021, 116, 1725–1733. [Google Scholar] [CrossRef]
- Celis-Morales, C.; Livingstone, K.M.; Marsaux, C.F.; Macready, A.L.; Fallaize, R.; O’Donovan, C.B.; Woolhead, C.; Forster, H.; Walsh, M.C.; Navas-Carretero, S.; et al. Effect of personalized nutrition on health-related behaviour change: Evidence from the Food4Me European randomized controlled trial. Int. J. Epidemiol. 2017, 46, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Mathers, J.C. Paving the way to better population health through personalised nutrition. ESFA J. 2019, 17, e170713. [Google Scholar] [CrossRef] [PubMed]
- Braakhuis, A.; Monnard, C.R.; Ellis, A.; Rozga, M. Consensus Report of the Academy of Nutrition and Dietetics: Incorporating Genetic Testing into Nutrition Care. J. Acad. Nutr. Diet. 2021, 121, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.C.; Allman-Farinelli, M.; Chen, J.; Gauglitz, J.M.; Hamideh, D.; Jankowska, M.M.; Johnson, A.J.; Rangan, A.; Spruijt-Metz, D.; Yang, J.A.; et al. Perspective: A Framework for Addressing Dynamic Food Consumption Processes. Adv. Nutr. 2022, 13, 992–1008. [Google Scholar] [CrossRef] [PubMed]
- Deroover, K.; Bucher, T.; Vandelanotte, C.; de Vries, H.; Duncan, M.J. Practical Nutrition Knowledge Mediates the Relationship Between Sociodemographic Characteristics and Diet Quality in Adults: A Cross-Sectional Analysis. Am. J. Health Promot. 2020, 34, 59–62. [Google Scholar] [CrossRef]
- Sebastian, A.T.; Rajkumar, E.; Tejaswini, P.; Lakshmi, R.; Romate, J. Applying social cognitive theory to predict physical activity and dietary behavior among patients with type-2 diabetes. Health Psychol. Res. 2021, 9, 24510. [Google Scholar] [CrossRef]
- Goldstein, S.P.; Zhang, F.; Klasnja, P.; Hoover, A.; Wing, R.R.; Thomas, J.G. Optimizing a Just-in-Time Adaptive Intervention to Improve Dietary Adherence in Behavioral Obesity Treatment: Protocol for a Microrandomized Trial. JMIR Res. Protoc. 2021, 10, e33568. [Google Scholar] [CrossRef]
- Chevance, G.; Perski, O.; Hekler, E.B. Innovative methods for observing and changing complex health behaviors: Four propositions. Transl. Behav. Med. 2021, 11, 676–685. [Google Scholar] [CrossRef]
- Kravitz, R.L.; Aguilera, A.; Chen, E.J.; Choi, Y.K.; Hekler, E.; Karr, C.; Kim, K.K.; Phatak, S.; Sarkar, S.; Schueller, S.M.; et al. Feasibility, Acceptability, and Influence of mHealth-Supported N-of-1 Trials for Enhanced Cognitive and Emotional Well-Being in US Volunteers. Front. Public Health 2020, 8, 260. [Google Scholar] [CrossRef]
- Allman-Farinelli, M. Using digital media to measure diet. CAB Rev. 2018, 13, 1–7. [Google Scholar] [CrossRef]
- Allman-Farinelli, M.; Gemming, L. Technology Interventions to Manage Food Intake: Where Are We Now? Curr. Diab. Rep. 2017, 17, 103. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Allman-Farinelli, M.; Yang, J.A.; Taylor, J.C.; Gemming, L.; Hekler, E.; Rangan, A. Enhancing Nutrition Care Through Real-Time, Sensor-Based Capture of Eating Occasions: A Scoping Review. Front. Nutr. 2022, 9, 852984. [Google Scholar] [CrossRef] [PubMed]
- Yiru, S.; Salley, J.; Muth, E.; Hoover, A. Assessing the Accuracy of a Wrist Motion Tracking Method for Counting Bites Across Demographic and Food Variables. IEEE J. Biomed. Health Inform. 2017, 21, 599–606. [Google Scholar] [CrossRef]
- Battaglia, B.; Lee, L.; Jia, S.; Partridge, S.; Allman-Farinelli, M. The use of mobile-based ecological momentary assessment (mEMA) methodology to assess dietary intake, food consumption behaviours and context in young people: A systematic review. Healthcare 2022, 10, 1329. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.J.; Raubenheimer, D. Obesity: The protein leverage hypothesis. Obes. Rev. 2005, 6, 133–142. [Google Scholar] [CrossRef]
- Liao, Y.; Skelton, K.; Dunton, G.; Bruening, M. A Systematic Review of Methods and Procedures Used in Ecological Momentary Assessments of Diet and Physical Activity Research in Youth: An Adapted STROBE Checklist for Reporting EMA Studies (CREMAS). J. Med. Internet Res. 2016, 18, e151. [Google Scholar] [CrossRef]
- Krone, T.; Boessen, R.; Bijlsma, S.; van Stokkum, R.; Clabbers, N.D.S.; Pasman, W.J. The possibilities of the use of N-of-1 and do-it-yourself trials in nutritional research. PLoS ONE 2020, 15, e0232680. [Google Scholar] [CrossRef]
- Vohra, S.; Moher, D. Complementary and alternative medicine in Canadian children: A call for action. Paediatr. Child Health 2005, 10, 154–156. [Google Scholar] [CrossRef]
- Hekler, E.B.; Klasnja, P.; Chevance, G.; Golaszewski, N.M.; Lewis, D.; Sim, I. Why we need a small data paradigm. BMC Med. 2019, 17, 133. [Google Scholar] [CrossRef]
- Nikles, J.; Onghena, P.; Vlaeyen, J.W.S.; Wicksell, R.K.; Simons, L.E.; McGree, J.M.; McDonald, S. Establishment of an International Collaborative Network for N-of-1 Trials and Single-Case Designs. Contemp. Clin. Trials. Commun. 2021, 23, 100826. [Google Scholar] [CrossRef]
| Authors, Year, Country | N | Participant Characteristics | Aim/s | Study Design | Intervention/s | Comparator/s | Outcome/s |
|---|---|---|---|---|---|---|---|
| Interventional Studies | |||||||
| Feltham and Westman, 2021, USA [26] | N = 1 M | Age: 29 y | To determine any differential effects of hypercaloric feeding of 3 diets on body composition | N-of-1 3 × 21 d periods (ABC) separated by 3 mo wash-out Analysis Differences in repeated measures of weight and waist before and after diet | 5800 kcal diets Low-carb: 6% CHO 72% fat 22% protein Low-fat: 64%CHO 23% fat 13% protein Very-low-fat vegan: 68% CHO 15.5% fat 16.5% protein | Baseline measurement | Caloric intake (Δ per 21 d): 121,676 kcal (low-carb) 121,653 kcal (low-fat) 121,674 kcal (very-low-fat vegan) Weight Δ kg: 1.3 (low-carb) 7.1 (low-fat) 4.7 (v low-fat vegan) Waist Δ cm: −3 (low-carb) 9.25 (low-fat) 7.75 (v low-fat vegan) |
| Gkouskou et al., 2022, Greece [29] | N = 3 M | Age: 45, 54, 67 y BMI: 23.5, 26.2, 31.5 kg/m2 Pre-diabetes (n = 2) Type 2 diabetes (n = 1) | To assess the effectiveness of precision genetically guided MNT interventions vs. conventional MNT | N-of-1 quasi-experimental, AB cross-over trial 8 weeks conventional MNT (A) 1 week wash-out 8 weeks precision MNT (B) Analysis Differences in percentage change in measures from baseline to end of each MNT period | Precision diet based on nutrigenetic testing: 1800–2200 kcal/day 25–32 g fiber 45–49%E CHO 26–37%E fat 16–25%E protein 7–9%E SFA | Conventional MNT: 1750–2200 kcal/day 33 g fiber 45–50%E CHO 20–32%E fat 18–30%E protein 7%E SFA | %Δ body weight: I = −1.4 to −7.4, C = 0 to −4.9 %Δ SBP/DBP: I = 0 to −8.6/−1.4 to −5.9, C = 0 to −3.4/0 to −1.2 %Δ HbA1c: I = −3.6 to −8.5%, C = 0% %Δ FPG: I = −6.7 to −25.5, C = 0 to −6.5 |
| Hendriks et al., 2021, Netherlands [32] | N = 5 M | Age: 30–64 y BMI = 29.61–38.88 kg/m2 Hemophilia A (n = 3), Hemophilia B (n = 1) Factor VII Deficiency (n = 1) | To evaluate feasibility and efficacy of shared medical appointment in people with hemophilia to improve PA and eating habits (diet) | Randomized A B A’ N-of-1 trial staggered phase Baseline: 2–4 weeks A Intervention: 7 weeks B post-intervention: 2–4 weeks A’ Analysis Visually using 2-SD band method or Randomisation test | Weekly 2-h shared medical appointments using multiple behavioral change techniques | Baseline measurements | Primary: PA increased Diet (1/5 patients) Secondary: pain in rest NS pain during PA NS, patient-specific complaints NS, kinesiophobia NS motivation for PA and diet NS Decrease BMI 2/5 Decrease BP 2/5 |
| Karkar et al., 2017, USA [27] | 10 F 5 M | Age: 20–69 y Food intolerances Rome IV IBS criteria | To evaluate the feasibility of TummyTrial app to conduct self-experiments for diagnostic self-tracking | Feasibility study on TummyTrials, an app that enables N-of-1 study design. Alternating Treatment Design: AB at random for 12 d Analysis Visualization of symptoms with timeline plots and trend plots | Breakfast with potential IBS food trigger (B) | Breakfast without potential IBS food trigger (A) | Participant compliance: 12/15 100% compliance for the 12 d Change in Pre- and post-IBS Symptom severity score NS Feasible and acceptable to patients |
| Ma et al., 2021, China [30] Tian et al. [31] | 19 F 9 M | Age: 22–34 y BMI: 17.2–31.9 kg/m2 Healthy | To investigate individual variability in postprandial glycemic response to diets with different proportions of Fat:CHO | N-of-1 randomized trial 6 d washout Then, three cycles of 3 d treatment 6 d washout 3 d treatment AB BA AB BA AB BA Analysis Difference in means between diets for individuals. Determined clinical meaningful difference. Further analysis of group effects using a hierarchical Bayesian model | Isocaloric diets HF-LC: Fat 60–70%E CHO 15–25%E Protein 15%E LF-HC: Fat 10–20%E CHO 65–75% Protein 15%E | Baseline measurements of individuals | Primary: MPG 10/28 participants showed clinically meaningful difference (3 HF and 7 HC responders) MAGE 9/28 clinical difference (4 HF and 5 HC responders) AUC24 NS Secondary: collected microbiome and urine metabolomic profiles (NR) |
| Miller et al., 2016, USA [28] | 4 F 2 M | Age: 58–62 y BMI: 25–44 kg/m2 T2DM ≥ 1 y no insulin therapy | To assess the feasibility of using mobile EMA to monitor low GI food intake and goal attainment after group education and individual counselling | N-of-1 pilot study AB 6-week group education A 6-week goal setting and individual counselling with EMA for data collection and monitoring B Analysis Changes in servings of low GI foods | Group-based education followed by individual counseling for goal attainment to increase low GI foods | Serves of low GI foods at baseline | Compliance: 79.3% EMA prompts completed; 16.4% ignored; 4.2% refused Servings of low GI foods: Mean Increase (SD) 1.2 (0.1) GI goal attainment 3/6 participants Acceptable and feasible program |
| Observational Study | |||||||
| Kwasnicka et al., 2020, Scotland [33] | 16 M 8 F 1 NI | Age: 16–65 y Current or recent history of alcohol dependence | To assess interpersonal differences in psychological and social factors associated with daily alcohol intake and effects of maximum unit price (MUP) and contextual factors | N-of-1 observational study 3 × 12-week waves of data collection pre-MUP (n = 11) pre- and post-MUP (n = 11) post-MUP (n = 3) used mixed methods as interviews after n-of-1 observation 15/25 analyzed Analysis Visualisation of changes over time by individuals. Regression and multilevel models for combined data | NA observational | NA observational | Amount and type of alcohol consumed: small decrease after MUP implementation amongst heaviest drinkers Less difference amongst less frequent or mainly abstinent drinkers Variation among participants as to internal and external factors associated with alcohol intake |
| Author, Year Reference | Title and Abstract | Introduction | Methods | Results | Discussion | Other Information |
|---|---|---|---|---|---|---|
| (a) N-of-1 trials (including feasibility and pilot studies) | ||||||
| Feltham and Westman 2021 [26] | No | Yes | Few a | Few a | Yes | Few a |
| Gkouskou et al., 2022 [29] | Yes | Yes | Most b | Most b | Yes | Yes |
| Hendriks et al., 2021 [32] | Yes | Yes | Yes | Most b | Yes | Most b |
| Karkar et al., 2017 [27] | Yes | Yes | Most b | Most b | Yes | Most b |
| Ma et al., 2021 [30] Tian et al., [31] | Yes | Yes | Most b | Most b | Yes | Yes |
| Miller et al., 2016 [28] | Most b | Yes | Most b | Most b | Yes | Few a |
| (b) N-of-1 observational study | ||||||
| Kwasnicka et al., 2020 [33] | Yes | Yes | Most b | Yes | Yes | Yes |
| Author, Year | Dietary Assessment Method | Validation | Data Collection | Data Analysis | Score | Overall Rating |
|---|---|---|---|---|---|---|
| Feltham and Westman, 2021 [26] | NR | NR | Participant used nutrition information on packaging and a supermarket website to determine calorie and macronutrient compositions | NR | 0 | Poor |
| Gkouskou et al., 2022 [29] | 24-h Recall | NR | Administered by a trained researcher via phone interview, but did not specify whether subjects were trained for data collection. The 24 h recall was reviewed/checked by a trained person. Did not specify days of recall, nutrient database(s) used nor comment on aids or multiple passes | Data coded and analyzed by a trained individual | 1 | Poor |
| Hendriks et al., 2021 [32] | Single Diet question on a visual analogue scale | NR | Self-reported eating habits via visual analogue scale were completed digitally in a secured data entry platform. Did not specify whether subjects were trained for data collection. Dietary Questions provided. | Unclear, but appears principal researcher carried out the analysis | 1.25 | Poor |
| Karkar et al., 2017 [27] | EMA questions | NR | Self-reported compliance with experimental menu condition via TummyTrials app), subjects were trained for data collection. Prompts to complete and compliance assessed by app. Weekend and weekdays were considered at six days’ recording. Dietary questionnaire (app EMA) supplied within text | Analysis built into app system and documented by researchers (consultation with dietitians) | 3.0 | Acceptable |
| Kwasnicka et al., 2020 [33] | EMA questions | NR | Self-reported alcohol consumption via smartphone survey; subjects were trained for data collection and the data reviewed by researchers as appropriate for this type of survey. Daily surveys sent at 7 p.m. to the mobile phones of participants or a study phone for 12 weeks, weekend and weekdays considered, and authors reported compliance with EMA prompts. Questions on amounts and types of alcohol in the previous 24 h. Questionnaire (EMA) supplied. | The researchers conducted the analysis | 3.0 | Acceptable |
| Ma et al., 2021 [30] Tian et al., 2020 [31] | Weighed food (known composition) provided and food checklist to track | Weighed food provided for which nutrient composition calculated and standard recipes used | All food provided via the University canteen. Diets designed by dietitian and participants sign in to show they consume the meal provided. Food checklist not provided and did not discuss method of checking; however, compliance with meals reported as 98% | Dietitian prepared diet of known composition. Did not specify whether checklist coded and analyzed by a trained individual | 5.5 | Good |
| Miller et al. 2016 [28] | 24-Hour Recall | NR Unclear if validated by University of Minnesota service | Administered by trained staff via an interactive phone interview and collected unannounced. Nutrient database reported. Multiple days of recall (1 weekend day and 2 weekdays selected at random), multiple pass approach used, and a food amounts booklet was provided to help participant estimate portion sizes. | Analysis and coded by the University of Minnesota 24 h recall service | 3.0 | Acceptable |
| EMA questions | Checklist used to prompt completion. Statistics on agreement with 24 h recall days not reported | Self-reported servings of low GI foods via PRO-Diary EMA; subjects were trained for data collection. Checklist of low-GI foods used to assist entry and memory. 6 weeks of EMA recording, weekend and weekdays considered, and authors reported compliance with EMA prompts | Did not specify whether coded and analyzed by a trained individual | 3.0 | Acceptable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allman-Farinelli, M.; Boljevac, B.; Vuong, T.; Hekler, E. Nutrition-Related N-of-1 Studies Warrant Further Research to Provide Evidence for Dietitians to Practice Personalized (Precision) Medical Nutrition Therapy: A Systematic Review. Nutrients 2023, 15, 1756. https://doi.org/10.3390/nu15071756
Allman-Farinelli M, Boljevac B, Vuong T, Hekler E. Nutrition-Related N-of-1 Studies Warrant Further Research to Provide Evidence for Dietitians to Practice Personalized (Precision) Medical Nutrition Therapy: A Systematic Review. Nutrients. 2023; 15(7):1756. https://doi.org/10.3390/nu15071756
Chicago/Turabian StyleAllman-Farinelli, Margaret, Brianna Boljevac, Tiffany Vuong, and Eric Hekler. 2023. "Nutrition-Related N-of-1 Studies Warrant Further Research to Provide Evidence for Dietitians to Practice Personalized (Precision) Medical Nutrition Therapy: A Systematic Review" Nutrients 15, no. 7: 1756. https://doi.org/10.3390/nu15071756
APA StyleAllman-Farinelli, M., Boljevac, B., Vuong, T., & Hekler, E. (2023). Nutrition-Related N-of-1 Studies Warrant Further Research to Provide Evidence for Dietitians to Practice Personalized (Precision) Medical Nutrition Therapy: A Systematic Review. Nutrients, 15(7), 1756. https://doi.org/10.3390/nu15071756







