Effects of Ingesting Food Containing Heat-Killed Lactococcus lactis Strain Plasma on Fatigue and Immune-Related Indices after High Training Load: A Randomized, Double-Blind, Placebo-Controlled, and Parallel-Group Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Ethics and Participants
2.2. Test Food
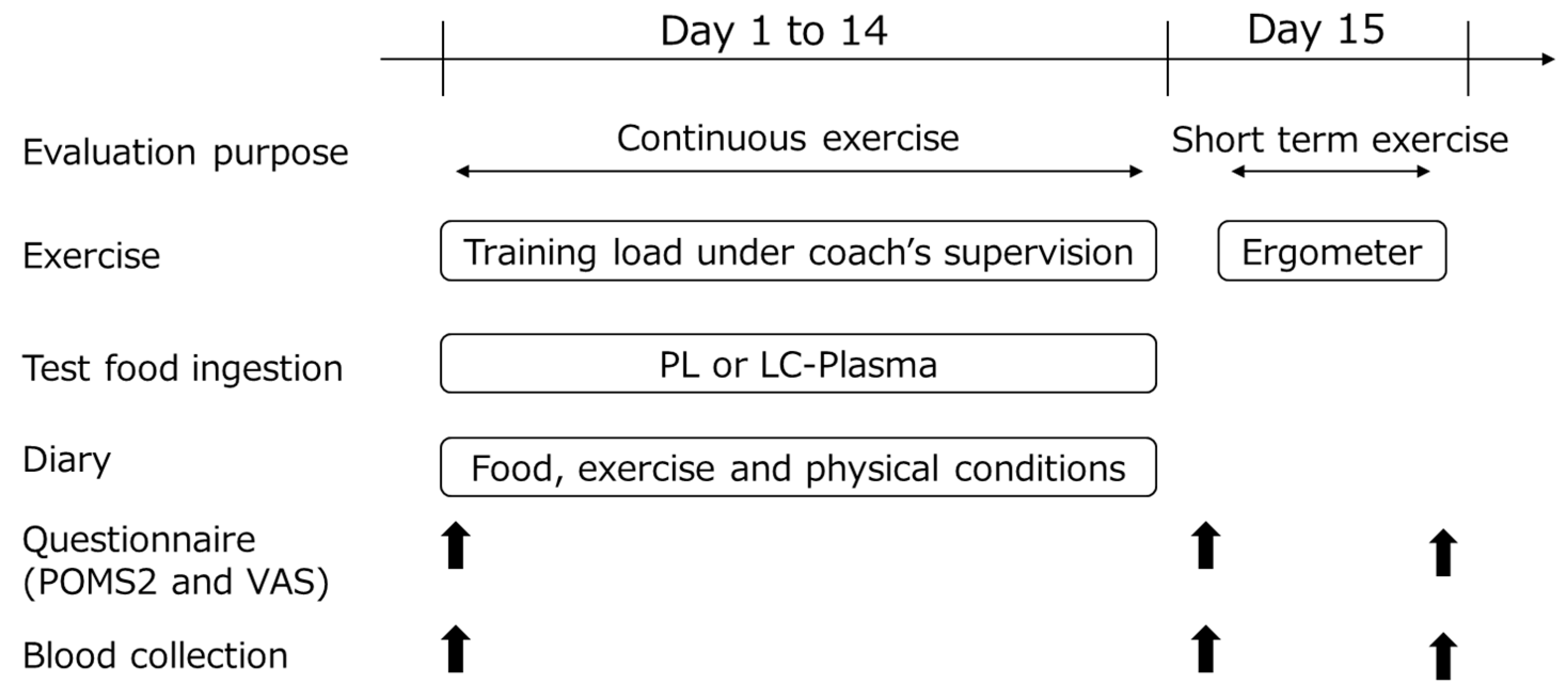
2.3. Study Design
2.4. Evaluation of pDC and mDC Activity
2.5. Evaluation of Hematological Indices
2.6. Evaluation of Physiological Indices
2.7. Participant Diary and Questionnaire
2.8. Statistical Analysis
3. Results
3.1. Participants’ Characteristics
3.2. Assessment for Continuous Exercise
3.3. Assessment for Strenuous Single Exercise
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moreira, A.; Delgado, L.; Moreira, P.; Haahtela, T. Does exercise increase the risk of upper respiratory tract infections? Br. Med. Bull. 2009, 90, 111–131. [Google Scholar] [CrossRef]
- Walsh, N.P.; Oliver, S.J. Exercise, immune function and respiratory infection: An update on the influence of training and environmental stress. Immunol. Cell Biol. 2016, 94, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Reardon, C.L.; Hainline, B.; Aron, C.M.; Baron, D.; Baum, A.L.; Bindra, A.; Budgett, R.; Campriani, N.; Castaldelli-Maia, J.M.; Currie, A.; et al. Mental health in elite athletes: International Olympic Committee consensus statement (2019). Br. J. Sports Med. 2019, 53, 667–699. [Google Scholar] [CrossRef]
- Gleeson, M.; Pyne, D.B. Respiratory inflammation and infections in high performance athletes. Immunol. Cell Biol. 2016, 94, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Pyne, D.B. Special feature for the Olympics: Effects of exercise on the immune system: Exercise effects on mucosal immunity. Immunol. Cell Biol. 2000, 78, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, T.B.; Trudeau, F.B.; Czerwinski, D.; Erredge, S. Immune parameters in athletes before and after strenuous exercise. J. Clin. Immunol. 1982, 2, 173–178. [Google Scholar] [CrossRef]
- Gleeson, M.; Pyne, D.B.; Austin, J.P.; Lynn Francis, J.; Clancy, R.L.; McDonald, W.A.; Fricker, P.A. Epstein-Barr virus reactivation and upper-respiratory illness in elite swimmers. Med. Sci. Sports Exerc. 2002, 34, 411–417. [Google Scholar] [CrossRef]
- Suzui, M.; Kawai, T.; Kimura, H.; Takeda, K.; Yagita, H.; Okumura, K.; Shek, P.N.; Shephard, R.J. Natural killer cell lytic activity and CD56(dim) and CD56(bright) cell distributions during and after intensive training. J. Appl. Physiol. 2004, 96, 2167–2173. [Google Scholar] [CrossRef]
- Malm, C.; Ekblom, O.; Ekblom, B. Immune system alteration in response to two consecutive soccer games. Acta Physiol. Scand. 2004, 180, 143–155. [Google Scholar] [CrossRef]
- Nayebifar, S.; Afzalpour, M.E.; Kazemi, T.; Eivary, S.H.; Mogharnasi, M. The effect of a 10-week high-intensity interval training and ginger consumption on inflammatory indices contributing to atherosclerosis in overweight women. J. Res. Med. Sci. 2016, 21, 116. [Google Scholar] [CrossRef]
- Hsiao, C.Y.; Hsu, Y.J.; Tung, Y.T.; Lee, M.C.; Huang, C.C.; Hsieh, C.C. Effects of Antrodia camphorata and Panax ginseng supplementation on anti-fatigue properties in mice. J. Vet. Med. Sci. 2018, 80, 284–291. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Behan, P.O. Fatigue in neurological disorders. Lancet 2004, 363, 978–988. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Oliveira, M.; McCauley, T.; Tauler, P.; Lawrence, C. Effects of a Lactobacillus salivarius probiotic intervention on infection, cold symptom duration and severity, and mucosal immunity in endurance athletes. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 235–242. [Google Scholar] [CrossRef]
- Lee, M.C.; Hsu, Y.J.; Ho, H.H.; Hsieh, S.H.; Kuo, Y.W.; Sung, H.C.; Huang, C.C. Lactobacillus salivarius subspecies salicinius SA-03 is a new probiotic capable of enhancing exercise performance and decreasing fatigue. Microorganisms 2020, 8, 545. [Google Scholar] [CrossRef] [PubMed]
- Jounai, K.; Ikado, K.; Sugimura, T.; Ano, Y.; Braun, J.; Fujiwara, D. Spherical lactic acid bacteria activate plasmacytoid dendritic cells immunomodulatory function via TLR9-dependent crosstalk with myeloid dendritic cells. PLoS ONE 2012, 7, e32588. [Google Scholar] [CrossRef]
- Wynn, T.A. Basophils trump dendritic cells as APCs for T(H)2 responses. Nat. Immunol. 2009, 10, 679–681. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, T.; Jounai, K.; Ohshio, K.; Tanaka, T.; Suwa, M.; Fujiwara, D. Immunomodulatory effect of Lactococcus lactis JCM5805 on human plasmacytoid dendritic cells. Clin. Immunol. 2013, 149, 509–518. [Google Scholar] [CrossRef]
- Kokubo, T.; Wakai, S.; Fujiwara, D.; Kanauchi, O.; Jounai, K.; Ichikawa, H.; Takuma, M.; Kanaya, Y.; Shiraoka, R. Lactococcus lactis strain plasma improves subjective physical state and presenteeism: A randomized, open-label crossover study among healthy office workers. Prev. Nutr. Food Sci. 2020, 25, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Komano, Y.; Tsuji, R.; Fujiwara, D.; Fujii, T.; Kanauchi, O. The effects of plasmacytoid dendritic cell-stimulative lactic acid bacteria, Lactococcus lactis strain plasma, on exercise-induced fatigue and recovery via immunomodulatory action. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 354–358. [Google Scholar] [CrossRef]
- Komano, Y.; Shimada, K.; Naito, H.; Fukao, K.; Ishihara, Y.; Fujii, T.; Kokubo, T.; Daida, H. Efficacy of heat-killed Lactococcus lactis JCM 5805 on immunity and fatigue during consecutive high intensity exercise in male athletes: A randomized, placebo-controlled, double-blinded trial. J. Int. Soc. Sports Nutr. 2018, 15, 39. [Google Scholar] [CrossRef] [PubMed]
- Holt, A.C.; Plews, D.J.; Oberlin-Brown, K.T.; Merien, F.; Kilding, A.E. Cardiac Parasympathetic and Anaerobic Performance Recovery After High-Intensity Exercise in Rowers. Int. J. Sports Physiol. Perform. 2019, 14, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, R.; Katayose, M.; Yamada, Y.; Neki, T.; Kamoda, T.; Tamai, K.; Yamazaki, K.; Iwamoto, E. High-but not moderate-intensity exercise acutely attenuates hypercapnia-induced vasodilation of the internal carotid artery in young men. Eur. J. Appl. Physiol. 2021, 121, 2471–2485. [Google Scholar] [CrossRef]
- Parimbelli, M.; Pezzotti, E.; Negro, M.; Calanni, L.; Allemano, S.; Bernardi, M.; Berardinelli, A.; D’Antona, G. Nutrition and Exercise in a Case of Carnitine Palmitoyl-Transferase II Deficiency. Front. Physiol. 2021, 12, 637406. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.J.; Ringenbach, S.D.R. The effect of acute exercise on the performance of verbal fluency in adolescents and young adults with Down syndrome: A pilot study. J. Intellect. Disabil. Res. 2019, 63, 614–623. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Physical Activity Guidelines for Health Promotion; Ministry of Health, Labour and Welfare: Tokyo, Japan, 2013. Available online: https://www.mhlw.go.jp/stf/houdou/2r9852000002xple-att/2r9852000002xpqt.pdf (accessed on 1 September 2017).
- Michalickova, D.; Minic, R.; Dikic, N.; Andjelkovic, M.; Kostic-Vucicevic, M.; Stojmenovic, T.; Nikolic, I.; Djordjevic, B. Lactobacillus helveticus Lafti L10 supplementation reduces respiratory infection duration in a cohort of elite athletes: A randomized, double-blind, placebo-controlled trial. Appl. Physiol. Nutr. Metab. 2016, 41, 782–789. [Google Scholar] [CrossRef]
- Kume, S.; Nishimura, Y.; Mizuno, K.; Sakimoto, N.; Hori, H.; Tamura, Y.; Yamato, M.; Mitsuhashi, R.; Akiba, K.; Koizumi, J.I.; et al. Music improves subjective feelings leading to cardiac autonomic nervous modulation: A pilot study. Front. Neurosci. 2017, 11, 108. [Google Scholar] [CrossRef]
- Shibata, T.; Kanayama, M.; Haida, M.; Fujimoto, S.; Oroguchi, T.; Sata, K.; Mita, N.; Kutsuzawa, T.; Ikeuchi, M.; Kondo, M.; et al. Lactococcus lactis JCM5805 activates anti-viral immunity and reduces symptoms of common cold and influenza in healthy adults in a randomized controlled trial. J. Funct. Foods 2016, 24, 492–500. [Google Scholar] [CrossRef]
- Oshima, S.; Takehata, C.; Sasahara, I.; Lee, E.; Akama, T.; Taguchi, M. Changes in stress and appetite responses in male power-trained athletes during intensive training camp. Nutrients 2017, 9, 912. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Johanssen, L.M.; Lee, J.W.; Arabatzis, K. Infectious episodes in runners before and after the Los Angeles Marathon. J. Sports Med. Phys. Fit. 1990, 30, 316–328. [Google Scholar]
- Kindermann, W. Creatine Kinase Levels After Exercise. Dtsch. Arztebl. Int. 2016, 113, 344. [Google Scholar] [CrossRef] [PubMed]
- McCusker, R.H.; Kelley, K.W. Immune-neural connections: How the immune system’s response to infectious agents influences behavior. J. Exp. Biol. 2013, 216, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Louati, K.; Berenbaum, F. Fatigue in chronic inflammation—A link to pain pathways. Arthritis Res. Ther. 2015, 17, 254. [Google Scholar] [CrossRef] [PubMed]
- Stoffels, B.; Hupa, K.J.; Snoek, S.A.; van Bree, S.; Stein, K.; Schwandt, T.; Vilz, T.O.; Lysson, M.; Veer, C.V.; Kummer, M.P.; et al. Postoperative ileus involves interleukin-1 receptor signaling in enteric glia. Gastroenterology 2014, 146, 176–187.e171. [Google Scholar] [CrossRef] [PubMed]
- Mensah, F.K.F.; Bansal, A.S.; Ford, B.; Cambridge, G. Chronic fatigue syndrome and the immune system: Where are we now? Neurophysiol. Clin. 2017, 47, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Esquius, L.; Javierre, C.; Llaudo, I.; Rama, I.; Oviedo, G.R.; Massip-Salcedo, M.; Aguilar-Martinez, A.; Nino, O.; Lloberas, N. Impact of Olive Oil Supplement Intake on Dendritic Cell Maturation after Strenuous Physical Exercise: A Preliminary Study. Int. J. Environ. Res. Public Health 2021, 18, 4128. [Google Scholar] [CrossRef]
- McArdle, W.D.; Katch, F.I.; Katch, V.L. Essentials of Exercise Physiology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2015. [Google Scholar]
- Kindermann, W.; Schnabel, A.; Schmitt, W.M.; Biro, G.; Cassens, J.; Weber, F. Catecholamines, growth hormone, cortisol, insulin, and sex hormones in anaerobic and aerobic exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1982, 49, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; Nabekura, Y.; Gwon, O.; Yamazaki, F.; Homma, S.; Ikegami, H. Heart rate and plasma catecholamines responses to exercise at various intensities. J. Phys. Fit Sport Med. 1992, 41, 313–321. [Google Scholar] [CrossRef]
- Wyller, V.B.; Saul, J.P.; Amlie, J.P.; Thaulow, E. Sympathetic predominance of cardiovascular regulation during mild orthostatic stress in adolescents with chronic fatigue. Clin. Physiol. Funct. Imaging 2007, 27, 231–238. [Google Scholar] [CrossRef]
- Zhong, H.; Eungpinichpong, W.; Wang, X.; Chatchawan, U.; Wanpen, S.; Buranruk, O. Effects of mechanical-bed massage on exercise-induced back fatigue in athletes. J. Phys. Ther. Sci. 2018, 30, 365–372. [Google Scholar] [CrossRef]
- Parrado, E.; Cervantes, J.; Pintanel, M.; Rodas, G.; Capdevila, L. Perceived tiredness and heart rate variability in relation to overload during a field hockey World Cup. Percept. Mot. Skills 2010, 110, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Perini, R.; Veicsteinas, A. Heart rate variability and autonomic activity at rest and during exercise in various physiological conditions. Eur. J. Appl. Physiol. 2003, 90, 317–325. [Google Scholar] [CrossRef]
- Arai, Y.; Saul, J.P.; Albrecht, P.; Hartley, L.H.; Lilly, L.S.; Cohen, R.J.; Colucci, W.S. Modulation of cardiac autonomic activity during and immediately after exercise. Am. J. Physiol. 1989, 256, H132–H141. [Google Scholar] [CrossRef] [PubMed]
- Karayigit, R.; Naderi, A.; Akca, F.; Cruz, C.; Sarshin, A.; Yasli, B.C.; Ersoz, G.; Kaviani, M. Effects of Different Doses of Caffeinated Coffee on Muscular Endurance, Cognitive Performance, and Cardiac Autonomic Modulation in Caffeine Naive Female Athletes. Nutrients 2020, 13, 2. [Google Scholar] [CrossRef]
- Kageta, T.; Tsuchiya, Y.; Morishima, T.; Hasegawa, Y.; Sasaki, H.; Goto, K. Influences of increased training volume on exercise performance, physiological and psychological parameters. J. Sports Med. Phys. Fitness 2016, 56, 913–921. [Google Scholar] [PubMed]
- Rodrigues, F.L.; Silva, L.E.; Hott, S.C.; Bomfim, G.F.; da Silva, C.A.; Fazan, R., Jr.; Resstel, L.B.; Tostes, R.C.; Carneiro, F.S. Toll-like receptor 9 plays a key role in the autonomic cardiac and baroreflex control of arterial pressure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R714–R723. [Google Scholar] [CrossRef]

| PL Group | LC-Plasma Group | p Value | |
|---|---|---|---|
| Age (years) | 19.8 ± 1.3 | 19.9 ± 1.2 | 0.800 |
| Body height (cm) | 171.6 ± 3.7 | 171.5 ± 5.4 | 0.935 |
| Weight (kg) | 56.3 ± 1.8 | 56.4 ± 4.1 | 0.989 |
| BMI a (kg/m2) | 19.1 ± 0.9 | 19.2 ± 1.0 | 0.972 |
| Group | Day 1 | Day 15 | p Value (Effect Size) | ||
|---|---|---|---|---|---|
| Intragroup Comparison | Intergroup Comparison | ||||
| pDC CD86 | PL | 312 ± 132 | 196 ± 55 | 0.000 ** (1.185) | 0.015 * (0.963) |
| LC-Plasma | 347 ± 144 | 271 ± 101 | 0.047 * (0.633) | ||
| pDC HLA-DR | PL | 4918 ± 1265 | 4246 ± 1328 | 0.009 ** (0.535) | 0.510 (0.246) |
| LC-Plasma | 4764 ± 732 | 4519 ± 920 | 0.189 (0.305) | ||
| Indices | Group | Cumulative Days | p Value | |
|---|---|---|---|---|
| 1, 2, 3 | 4, 5 | |||
| Physical conditions a | PL | 141 | 83 | 0.798 |
| LC-Plasma | 135 | 73 | ||
| Nasal congestion/nasal discharge b | PL | 2 | 222 | 0.624 |
| LC-Plasma | 4 | 206 | ||
| Pharyngeal pain b | PL | 1 | 223 | 1.000 |
| LC-Plasma | 1 | 209 | ||
| Cough b | PL | 1 | 223 | 1.000 |
| LC-Plasma | 0 | 210 | ||
| Joint pain b | PL | 1 | 223 | 0.034 * |
| LC-Plasma | 8 | 202 | ||
| Chill b | PL | 1 | 223 | 0.187 |
| LC-Plasma | 5 | 204 | ||
| Fatigue b | PL | 151 | 72 | 0.035 * |
| LC-Plasma | 120 | 89 | ||
| Malaise b | PL | 71 | 153 | 0.933 |
| LC-Plasma | 68 | 141 | ||
| Muscle pain b | PL | 113 | 111 | 0.657 |
| LC-Plasma | 100 | 109 | ||
| Group | Day 1 | Day 15 | p Value (Effect Size) | ||
|---|---|---|---|---|---|
| Intragroup Comparison | Intergroup Comparison | ||||
| Anger–Hostility | PL | 45.3 ± 4.9 | 43.2 ± 6.3 | 0.158 (0.250) | 0.708 (0.067) |
| LC-Plasma | 41.9 ± 3.5 | 42.6 ± 7.1 | 0.919 (0.019) | ||
| Confusion–Bewilderment | PL | 48.6 ± 5.6 | 47.2 ± 8.7 | 0.505 (0.118) | 0.519 (0.116) |
| LC-Plasma | 48.1 ± 9.9 | 46.5 ± 10.8 | 0.328 (0.179) | ||
| Depression–Dejection | PL | 49.8 ± 4.9 | 48.3 ± 5.7 | 0.347 (0.166) | 0.826 (0.040) |
| LC-Plasma | 48.7 ± 3.4 | 47.9 ± 6.5 | 0.363 (0.166) | ||
| Fatigue–Inertia | PL | 46.8 ± 5.7 | 50.8 ± 9.5 | 0.094 (0.296) | 0.266 (0.200) |
| LC-Plasma | 47.2 ± 7.4 | 47.1 ± 8.6 | 0.551 (0.109) | ||
| Tension–Anxiety | PL | 45.3 ± 8.3 | 46.7 ± 10.2 | 0.650 (0.080) | 0.193 (0.234) |
| LC-Plasma | 43.6 ± 8.1 | 42.3 ± 9.4 | 0.477 (0.130) | ||
| Vigor–Activity | PL | 54.3 ± 9.2 | 54.6 ± 11.3 | 0.842 (0.035) | 0.751 (0.057) |
| LC-Plasma | 58.0 ± 9.9 | 56.4 ± 10.4 | 0.363 (0.166) | ||
| Friendliness | PL | 54.8 ± 10.5 | 52.6 ± 12.3 | 0.529 (0.111) | 0.842 (0.036) |
| LC-Plasma | 60.3 ± 6.7 | 53.9 ± 6.1 | 0.002 ** (0.559) | ||
| TMD a | PL | 10.9 ± 11.8 | 11.1 ± 15.3 | 0.605 (0.091) | 0.212 (0.224) |
| LC-Plasma | 6.5 ± 12.5 | 6.1 ± 18.2 | 0.414 (0.149) | ||
| Group | Day 1 | Day 15 | p Value (Effect Size) | ||
|---|---|---|---|---|---|
| Intragroup Comparison | Intergroup Comparison | ||||
| TGF-β (pg/mL) | PL | 502 ± 95 | 403 ± 65 | 0.001 ** (1.256) | 0.461 (0.287) |
| LC-Plasma | 536 ± 127 | 423 ± 79 | 0.002 ** (1.106) | ||
| IL-6 (pg/mL) | PL | 22.4 ± 17.5 | 17.0 ± 23.4 | 0.398 (0.270) | 0.291 (0.406) |
| LC-Plasma | 39.5 ± 34.7 | 29.1 ± 37.2 | 0.296 (0.299) | ||
| CPK (U/L) | PL | 227 ± 136 | 307 ± 132 | 0.012 * (0.617) | 0.094 (0.638) |
| LC-Plasma | 153 ± 74 | 231 ± 113 | 0.029 * (0.845) | ||
| Cathepsin L (pg/mL) | PL | 1464 ± 1142 | 883 ± 1074 | 0.002 ** (0.541) | 0.881 (0.058) |
| LC-Plasma | 1371 ± 1025 | 943 ± 1077 | 0.071 (0.421) | ||
| Adrenaline (ng/mL) | PL | 0.85 ± 0.77 | 1.58 ± 1.25 | 0.021 * (0.726) | 0.891 (0.057) |
| LC-Plasma | 0.82 ± 0.85 | 1.66 ± 1.66 | 0.033 * (0.659) | ||
| 8-OHdG (ng/mL) | PL | 0.46 ± 0.10 | 0.19 ± 0.08 | 0.000 ** (3.079) | 0.546 (0.291) |
| LC-Plasma | 0.42 ± 0.07 | 0.17 ± 0.06 | 0.000 ** (3.969) | ||
| Testosterone (ng/mL) | PL | 23.5 ± 16.0 | 24.3 ± 15.0 | 0.451 (0.053) | 0.740 (0.128) |
| LC-Plasma | 28.3 ± 37.2 | 27.6 ± 35.0 | 0.625 (0.020) | ||
| Leptin (ng/mL) | PL | 50.5 ± 16.2 | 49.5 ± 19.8 | 0.793 (0.057) | 0.303 (0.395) |
| LC-Plasma | 54.0 ± 17.6 | 57.9 ± 24.1 | 0.355 (0.191) | ||
| LF/HF | PL | 1.56 ± 2.83 | 3.09 ± 4.24 | 0.014 * (0.670) | 0.968 (0.014) |
| LC-Plasma | 0.54 ± 0.69 | 3.04 ± 2.79 | 0.005 ** (1.273) | ||
| Group | Before Exercise | After Exercise | p Value (Effect Size) | ||
|---|---|---|---|---|---|
| Intragroup Comparison | Intergroup Comparison | ||||
| Are you tired? | PL | 646 ± 187 | 876 ± 138 | 0.001 ** (0.603) | 0.179 (0.241) |
| LC-Plasma | 546 ± 185 | 838 ± 117 | 0.001 ** (0.623) | ||
| Are you well? | PL | 481 ± 227 | 523 ± 279 | 0.394 (0.151) | 0.406 (0.149) |
| LC-Plasma | 515 ± 203 | 598 ± 263 | 0.140 (0.279) | ||
| Are you feeling physical listlessness? | PL | 483 ± 207 | 696 ± 207 | 0.007 ** (0.475) | 0.874 (0.028) |
| LC-Plasma | 480 ± 198 | 722 ± 194 | 0.009 ** (0.492) | ||
| Are you feeling muscle pain? | PL | 364 ± 293 | 511 ± 226 | 0.039 * (0.366) | 0.737 (0.06) |
| LC-Plasma | 337 ± 244 | 464 ± 251 | 0.064 (0.350) | ||
| Group | Day 15 Pre Exercise | Day 15 Post Exercise | p Value (Effect Size) | ||
|---|---|---|---|---|---|
| Intragroup Comparison | Intergroup Comparison | ||||
| TGF-β (pg/mL) | PL | 403 ± 65 | 401 ± 60 | 0.929 (0.033) | 0.230 (0.475) |
| LC-Plasma | 423 ± 79 | 438 ± 98 | 0.495 (0.174) | ||
| IL-6 (pg/mL) | PL | 17.0 ± 23.4 | 76.7 ± 53.2 | 0.000 ** (1.500) | 0.319 (0.376) |
| LC-Plasma | 29.1 ± 37.2 | 95.3 ± 48.8 | 0.000 ** (1.579) | ||
| CPK (U/L) | PL | 307 ± 132 | 311 ± 140 | 0.680 (0.030) | 0.111 (0.605) |
| LC-Plasma | 231 ± 113 | 239 ± 102 | 0.445 (0.077) | ||
| Cathepsin L (pg/mL) | PL | 883 ± 1074 | 575 ± 1205 | 0.334 (0.279) | 0.295 (0.403) |
| LC-Plasma | 943 ± 1077 | 1041 ± 1185 | 0.701 (0.090) | ||
| Adrenaline (ng/mL) | PL | 1.58 ± 1.25 | 4.31 ± 2.68 | 0.001 ** (1.348) | 0.462 (0.274) |
| LC-Plasma | 1.66 ± 1.66 | 3.66 ± 2.19 | 0.000 ** (1.065) | ||
| 8-OHdG (ng/mL) | PL | 0.19 ± 0.08 | 0.18 ± 0.11 | 0.882 (0.107) | 0.714 (0.214) |
| LC-Plasma | 0.17 ± 0.06 | 0.20 ± 0.08 | 0.117 (0.439) | ||
| Testosterone (ng/mL) | PL | 24.3 ± 15.0 | 21.4 ± 18.8 | 0.030 * (0.176) | 0.542 (0.234) |
| LC-Plasma | 27.6 ± 35.0 | 28.2 ± 38.7 | 0.817 (0.017) | ||
| Leptin (ng/mL) | PL | 49.5 ± 19.8 | 58.1 ± 20.5 | 0.011 * (0.441) | 0.560 (0.218) |
| LC-Plasma | 57.9 ± 24.1 | 63.4 ± 29.2 | 0.024 * (0.213) | ||
| LF/HF | PL | 3.09 ± 4.24 | 4.23 ± 3.54 | 0.128 (0.087) | 0.009 ** (1.047) |
| LC-Plasma | 3.04 ± 2.79 | 1.50 ± 1.28 | 0.061 (0.734) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komano, Y.; Fukao, K.; Shimada, K.; Naito, H.; Ishihara, Y.; Fujii, T.; Kokubo, T.; Daida, H. Effects of Ingesting Food Containing Heat-Killed Lactococcus lactis Strain Plasma on Fatigue and Immune-Related Indices after High Training Load: A Randomized, Double-Blind, Placebo-Controlled, and Parallel-Group Study. Nutrients 2023, 15, 1754. https://doi.org/10.3390/nu15071754
Komano Y, Fukao K, Shimada K, Naito H, Ishihara Y, Fujii T, Kokubo T, Daida H. Effects of Ingesting Food Containing Heat-Killed Lactococcus lactis Strain Plasma on Fatigue and Immune-Related Indices after High Training Load: A Randomized, Double-Blind, Placebo-Controlled, and Parallel-Group Study. Nutrients. 2023; 15(7):1754. https://doi.org/10.3390/nu15071754
Chicago/Turabian StyleKomano, Yuta, Kosuke Fukao, Kazunori Shimada, Hisashi Naito, Yoshihiko Ishihara, Toshio Fujii, Takeshi Kokubo, and Hiroyuki Daida. 2023. "Effects of Ingesting Food Containing Heat-Killed Lactococcus lactis Strain Plasma on Fatigue and Immune-Related Indices after High Training Load: A Randomized, Double-Blind, Placebo-Controlled, and Parallel-Group Study" Nutrients 15, no. 7: 1754. https://doi.org/10.3390/nu15071754
APA StyleKomano, Y., Fukao, K., Shimada, K., Naito, H., Ishihara, Y., Fujii, T., Kokubo, T., & Daida, H. (2023). Effects of Ingesting Food Containing Heat-Killed Lactococcus lactis Strain Plasma on Fatigue and Immune-Related Indices after High Training Load: A Randomized, Double-Blind, Placebo-Controlled, and Parallel-Group Study. Nutrients, 15(7), 1754. https://doi.org/10.3390/nu15071754








