Food Insecurity and Nutritional Challenges in Adolescent and Young Adult Cancer Survivors in the U.S.A.: A Narrative Review and Call to Action
Abstract
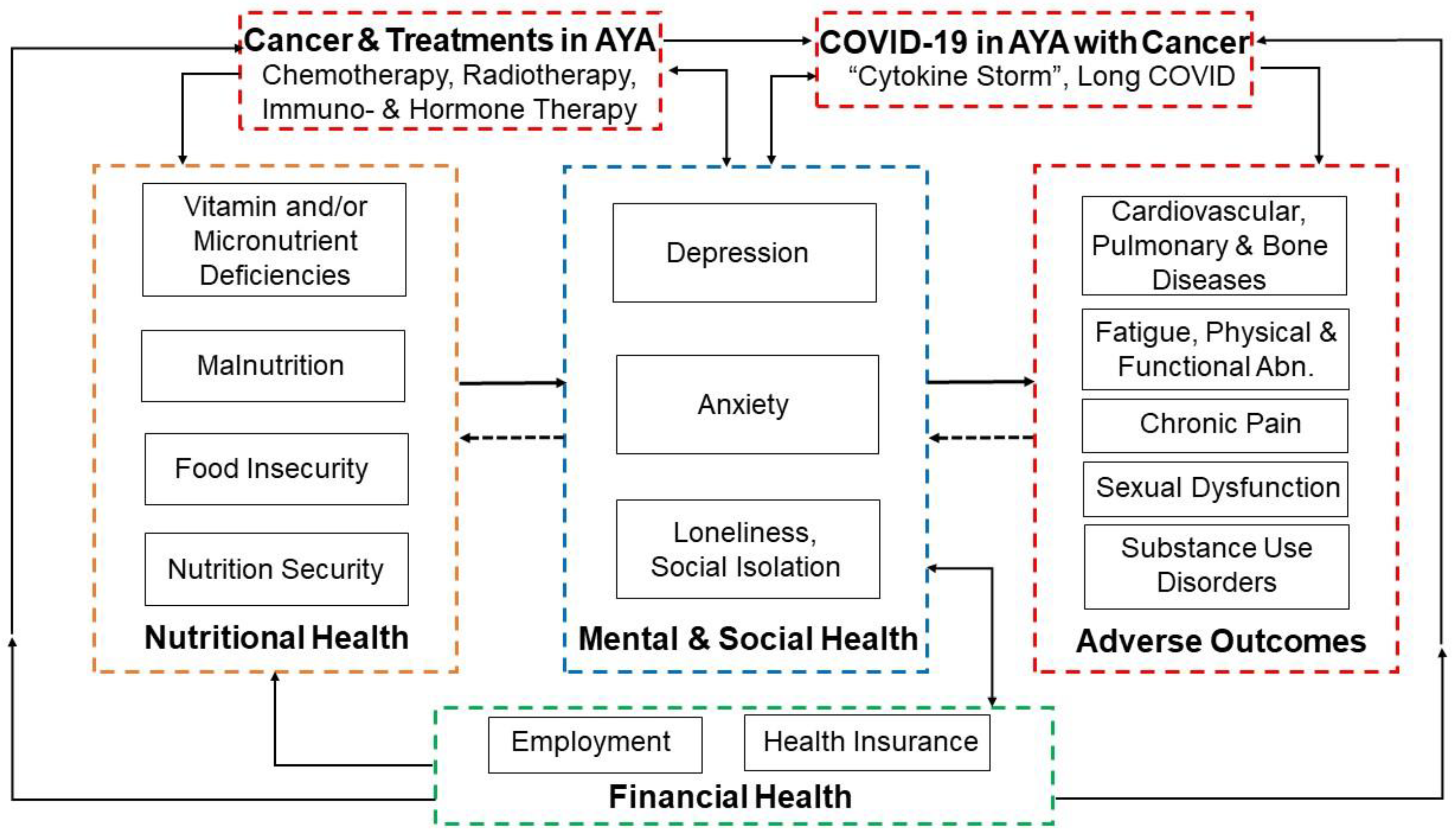
1. Adolescent and Young Adult (AYA) Cancer Survivors “Late Effects”
2. Nutritional Challenges in AYA Cancer Survivors
3. Food Insecurity in AYA Cancer Survivors
4. Financial Insecurity and Mental Health Concerns in AYA Cancer Survivors
5. Potential Exacerbation of Food Insecurity by COVID-19 in AYA Cancer Survivors
6. Differences between Adolescent and Young Adult Cancer Survivors
7. Recommendations for Future Research and Policy to Address Food and Nutrition Security in AYA Cancer Survivors
- Conduct studies to estimate food and nutrition security in AYA cancer survivors to better understand risks in adolescents and young adults as distinct subgroups.
- Initiate campaigns and educational support programs that specifically target AYA cancer survivors to enhance awareness of existing food support programs (e.g., SNAP and WIC).
- Conduct routine screening for food insecurity, nutrition security and malnutrition in AYA cancer survivors as part of survivorship plans or, preferably, starting at cancer diagnosis and continuing throughout survivorship during clinical follow-up visits.
- Provide professional nutritional support services to AYA cancer survivors, with a particular focus on those who are identified to be at increased risk during screening.
- Provide special provisions in SNAP tailored to AYA cancer survivors with a focus on the needs of young adult cancer survivors to maximize benefits (e.g., similar to the provisions for the elderly).
- Initiate campaigns to reduce the stigma associated with food insecurity screening and the use of food support services.
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, K.D.; Fidler-Benaoudia, M.; Keegan, T.H.; Hipp, H.S.; Jemal, A.; Siegel, R.L. Cancer Statistics for Adolescents and Young Adults, 2020. CA Cancer J. Clin. 2020, 70, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Ness, K.K.; Armenian, S.H.; Kadan-Lottick, N.; Gurney, J.G. Adverse Effects of Treatment in Childhood Acute Lymphoblastic Leukemia: General Overview and Implications for Long-Term Cardiac Health. Expert Rev. Hematol. 2011, 4, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.T.; Stovall, M.; Robison, L.L. Long-Term Effects of Radiation Exposure among Adult Survivors of Childhood Cancer: Results from the Childhood Cancer Survivor Study. Radiat. Res. 2010, 174, 840–850. [Google Scholar] [CrossRef]
- Tukenova, M.; Diallo, I.; Hawkins, M.; Guibout, C.; Quiniou, E.; Pacquement, H.; Dhermain, F.; Shamsaldin, A.; Oberlin, O.; de Vathaire, F. Long-Term Mortality from Second Malignant Neoplasms in 5-Year Survivors of Solid Childhood Tumors: Temporal Pattern of Risk According to Type of Treatment. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2010, 19, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Tukenova, M.; Guibout, C.; Oberlin, O.; Doyon, F.; Mousannif, A.; Haddy, N.; Guérin, S.; Pacquement, H.; Aouba, A.; Hawkins, M.; et al. Role of Cancer Treatment in Long-Term Overall and Cardiovascular Mortality after Childhood Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.; Bhatia, S.; Xu, L.; Cannavale, K.L.; Wong, F.L.; Huang, P.-Y.S.; Cooper, R.; Armenian, S.H. Chronic Comorbidities Among Survivors of Adolescent and Young Adult Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 3161–3174. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Wakefield, C.E.; Laing, D.G. Smell and Taste Disorders Resulting from Cancer and Chemotherapy. Curr. Pharm. Des. 2016, 22, 2253–2263. [Google Scholar] [CrossRef]
- Drareni, K.; Dougkas, A.; Giboreau, A.; Laville, M.; Souquet, P.-J.; Bensafi, M. Relationship between Food Behavior and Taste and Smell Alterations in Cancer Patients Undergoing Chemotherapy: A Structured Review. Semin. Oncol. 2019, 46, 160–172. [Google Scholar] [CrossRef]
- Sevryugin, O.; Kasvis, P.; Vigano, M.; Vigano, A. Taste and Smell Disturbances in Cancer Patients: A Scoping Review of Available Treatments. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2021, 29, 49–66. [Google Scholar] [CrossRef]
- van der Haak, N.; Edwards, S.; Perem, M.; Landorf, E.; Osborn, M. Nutritional Status at Diagnosis, During, and After Treatment in Adolescents and Young Adults with Cancer. J. Adolesc. Young Adult Oncol. 2021, 10, 668–674. [Google Scholar] [CrossRef]
- Arroyave, W.D.; Clipp, E.C.; Miller, P.E.; Jones, L.W.; Ward, D.S.; Bonner, M.J.; Rosoff, P.M.; Snyder, D.C.; Demark-Wahnefried, W. Childhood Cancer Survivors’ Perceived Barriers to Improving Exercise and Dietary Behaviors. Oncol. Nurs. Forum 2008, 35, 121–130. [Google Scholar] [CrossRef]
- Cohen, J.; Wakefield, C.E.; Fleming, C.A.K.; Gawthorne, R.; Tapsell, L.C.; Cohn, R.J. Dietary Intake after Treatment in Child Cancer Survivors. Pediatr. Blood Cancer 2012, 58, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Robien, K.; Ness, K.K.; Klesges, L.M.; Baker, K.S.; Gurney, J.G. Poor Adherence to Dietary Guidelines among Adult Survivors of Childhood Acute Lymphoblastic Leukemia. J. Pediatr. Hematol. Oncol. 2008, 30, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Gianinazzi, M.E.; Kiserud, C.E.; Ruud, E.; Lie, H.C. Who Knows? Information Received, and Knowledge about, Cancer, Treatment and Late Effects in a National Cohort of Long-Term Childhood, Adolescent and Young Adult Cancer Survivors. Cancers 2022, 14, 1534. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.; Bhatia, S.; Xu, L.; Cannavale, K.L.; Wong, F.L.; Huang, P.-Y.S.; Cooper, R.; Armenian, S.H. Incidence, Risk Factors, and Mortality Associated with Second Malignant Neoplasms Among Survivors of Adolescent and Young Adult Cancer. JAMA Netw. Open 2019, 2, e195536. [Google Scholar] [CrossRef]
- Suh, E.; Stratton, K.L.; Leisenring, W.M.; Nathan, P.C.; Ford, J.S.; Freyer, D.R.; McNeer, J.L.; Stock, W.; Stovall, M.; Krull, K.R.; et al. Late Mortality and Chronic Health Conditions in Long-Term Survivors of Early-Adolescent and Young Adult Cancers: A Retrospective Cohort Analysis from the Childhood Cancer Survivor Study. Lancet Oncol. 2020, 21, 421–435. [Google Scholar] [CrossRef]
- Barr, R.D.; Ferrari, A.; Ries, L.; Whelan, J.; Bleyer, W.A. Cancer in Adolescents and Young Adults: A Narrative Review of the Current Status and a View of the Future. JAMA Pediatr. 2016, 170, 495–501. [Google Scholar] [CrossRef]
- Smith, E.C.; Ziogas, A.; Anton-Culver, H. Delay in Surgical Treatment and Survival after Breast Cancer Diagnosis in Young Women by Race/Ethnicity. JAMA Surg. 2013, 148, 516–523. [Google Scholar] [CrossRef]
- Tai, E.; Buchanan, N.; Townsend, J.; Fairley, T.; Moore, A.; Richardson, L.C. Health Status of Adolescent and Young Adult Cancer Survivors. Cancer 2012, 118, 4884–4891. [Google Scholar] [CrossRef]
- Barnett, M.; McDonnell, G.; DeRosa, A.; Schuler, T.; Philip, E.; Peterson, L.; Touza, K.; Jhanwar, S.; Atkinson, T.M.; Ford, J.S. Psychosocial Outcomes and Interventions among Cancer Survivors Diagnosed during Adolescence and Young Adulthood (AYA): A Systematic Review. J. Cancer Surviv. Res. Pract. 2016, 10, 814–831. [Google Scholar] [CrossRef]
- Bradford, N.K.; McDonald, F.E.J.; Bibby, H.; Kok, C.; Patterson, P. Psychological, Functional and Social Outcomes in Adolescent and Young Adult Cancer Survivors over Time: A Systematic Review of Longitudinal Studies. Psycho-Oncology 2022, 31, 1448–1458. [Google Scholar] [CrossRef]
- Poudel, P.G.; Bauer, H.E.; Srivastava, D.K.; Krull, K.R.; Hudson, M.M.; Robison, L.L.; Wang, Z.; Huang, I.-C. Online Platform to Assess Complex Social Relationships and Patient-Reported Outcomes Among Adolescent and Young Adult Cancer Survivors. JCO Clin. Cancer Inform. 2021, 5, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Rabin, C.; Simpson, N.; Morrow, K.; Pinto, B. Behavioral and Psychosocial Program Needs of Young Adult Cancer Survivors. Qual. Health Res. 2011, 21, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Toriola, A.T.; Nguyen, N.; Scheitler-Ring, K.; Colditz, G.A. Circulating 25-Hydroxyvitamin D Levels and Prognosis among Cancer Patients: A Systematic Review. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2014, 23, 917–933. [Google Scholar] [CrossRef] [PubMed]
- Schepisi, G.; Gianni, C.; Bleve, S.; De Padova, S.; Menna, C.; Lolli, C.; Filograna, A.; Conteduca, V.; Urbini, M.; Gallà, V.; et al. Vitamin D Deficiency in Testicular Cancer Survivors: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 5145. [Google Scholar] [CrossRef]
- Simmons, J.H.; Chow, E.J.; Koehler, E.; Esbenshade, A.; Smith, L.-A.; Sanders, J.; Friedman, D. Significant 25-Hydroxyvitamin D Deficiency in Child and Adolescent Survivors of Acute Lymphoblastic Leukemia: Treatment with Chemotherapy Compared with Allogeneic Stem Cell Transplant. Pediatr. Blood Cancer 2011, 56, 1114–1119. [Google Scholar] [CrossRef]
- Bhandari, R.; Teh, J.B.; Herrera, C.; Echevarria, M.; Lindenfeld, L.; Wong, F.L.; Wilson, K.; Armenian, S.H. Prevalence and Risk Factors for Vitamin D Deficiency in Long-Term Childhood Cancer Survivors. Pediatr. Blood Cancer 2021, 68, e29048. [Google Scholar] [CrossRef]
- Matyjaszek-Matuszek, B.; Lenart-Lipińska, M.; Woźniakowska, E. Clinical Implications of Vitamin D Deficiency. Prz. Menopauzalny Menopause Rev. 2015, 14, 75–81. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Tuo, L.; Zhai, Q.; Cui, J.; Chen, D.; Xu, D. Relationship between Maternal Vitamin D Levels and Adverse Outcomes. Nutrients 2022, 14, 4230. [Google Scholar] [CrossRef]
- Cormick, G.; Betran, A.P.; Romero, I.B.; Cormick, M.S.; Belizán, J.M.; Bardach, A.; Ciapponi, A. Effect of Calcium Fortified Foods on Health Outcomes: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 316. [Google Scholar] [CrossRef]
- Stoltzfus, R.J. Iron Deficiency: Global Prevalence and Consequences. Food Nutr. Bull. 2003, 24, S99–S103. [Google Scholar] [CrossRef]
- Greenberg, J.A.; Bell, S.J.; Guan, Y.; Yu, Y.-H. Folic Acid Supplementation and Pregnancy: More than Just Neural Tube Defect Prevention. Rev. Obstet. Gynecol. 2011, 4, 52–59. [Google Scholar] [PubMed]
- Rivera, R.L.; Maulding, M.K.; Eicher-Miller, H.A. Effect of Supplemental Nutrition Assistance Program-Education (SNAP-Ed) on Food Security and Dietary Outcomes. Nutr. Rev. 2019, 77, 903–921. [Google Scholar] [CrossRef] [PubMed]
- Dreizen, S.; McCredie, K.B.; Keating, M.J.; Andersson, B.S. Nutritional Deficiencies in Patients Receiving Cancer Chemotherapy. Postgrad. Med. 1990, 87, 163–167, 170. [Google Scholar] [CrossRef]
- Barazzoni, R.; Gortan Cappellari, G. Double Burden of Malnutrition in Persons with Obesity. Rev. Endocr. Metab. Disord. 2020, 21, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Badr, H.; Paxton, R.J.; Ater, J.L.; Urbauer, D.; Demark-Wahnefried, W. Health Behaviors and Weight Status of Childhood Cancer Survivors and Their Parents: Similarities and Opportunities for Joint Interventions. J. Am. Diet. Assoc. 2011, 111, 1917–1923. [Google Scholar] [CrossRef]
- Zhang, F.F.; Kelly, M.J.; Saltzman, E.; Must, A.; Roberts, S.B.; Parsons, S.K. Obesity in Pediatric ALL Survivors: A Meta-Analysis. Pediatrics 2014, 133, e704–e715. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.J.; Pihoker, C.; Hunt, K.; Wilkinson, K.; Friedman, D.L. Obesity and Hypertension among Children after Treatment for Acute Lymphoblastic Leukemia. Cancer 2007, 110, 2313–2320. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.A.; Stancel, H.H.; Klesges, L.M.; Tyc, V.L.; Hinds, P.S.; Wu, S.; Hudson, M.M.; Kahalley, L.S. Eating Behavior and BMI in Adolescent Survivors of Brain Tumor and Acute Lymphoblastic Leukemia. J. Pediatr. Oncol. Nurs. Off. J. Assoc. Pediatr. Oncol. Nurses 2014, 31, 41–50. [Google Scholar] [CrossRef]
- Galaniha, L.T.; Nolden, A.A. The Role of Saliva in Taste Dysfunction among Cancer Patients: Mechanisms and Potential Treatment. Oral Oncol. 2022, 133, 106030. [Google Scholar] [CrossRef]
- Nock, N.L.; Jiang, H.; Borato, L.; Alberts, J.; Dimitropoulos, A. Insights to the Neural Response to Food Cues in Class III Compared with Class I and II Obese Adults Using a Sample of Endometrial Cancer Survivors Seeking Weight Loss. Nutr. Diabetes 2020, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. The State of Food Security and Nutrition in the World 2022; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [Google Scholar]
- Shankar, P.; Chung, R.; Frank, D.A. Association of Food Insecurity with Children’s Behavioral, Emotional, and Academic Outcomes: A Systematic Review. J. Dev. Behav. Pediatr. JDBP 2017, 38, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Robien, K.; Clausen, M.; Sullo, E.; Ford, Y.; Griffith, K.; Le, D.; Wickersham, K.; Wallington, S. Prevalence of Food Insecurity Among Cancer Survivors in the United States: A Scoping Review. J. Acad. Nutr. Diet. 2022, 123, 330–346. [Google Scholar] [CrossRef] [PubMed]
- Jella, T.; Cwalina, T.B.; Hamadani, M. Epidemiology of Food Insecurity in a Nationally Representative Sample of Lymphoma Patients. Clin. Lymphoma Myeloma Leuk. 2022, 22, e128–e134. [Google Scholar] [CrossRef]
- Trego, M.L.; Baba, Z.M.; DiSantis, K.I.; Longacre, M.L. Food Insecurity among Adult Cancer Survivors in the United States. J. Cancer Surviv. Res. Pract. 2019, 13, 641–652. [Google Scholar] [CrossRef]
- McDougall, J.A.; Anderson, J.; Adler Jaffe, S.; Guest, D.D.; Sussman, A.L.; Meisner, A.L.W.; Wiggins, C.L.; Jimenez, E.Y.; Pankratz, V.S. Food Insecurity and Forgone Medical Care Among Cancer Survivors. JCO Oncol. Pract. 2020, 16, e922–e932. [Google Scholar] [CrossRef]
- Burton-Obanla, A.A.; Sloane, S.; Koester, B.; Gundersen, C.; Fiese, B.H.; Arthur, A.E. Oncology Registered Dietitian Nutritionists’ Knowledge, Attitudes, and Practices Related to Food Insecurity among Cancer Survivors: A Qualitative Study. J. Acad. Nutr. Diet. 2022, 122, 2267–2287. [Google Scholar] [CrossRef]
- Jones, A.D.; Ngure, F.M.; Pelto, G.; Young, S.L. What Are We Assessing When We Measure Food Security? A Compendium and Review of Current Metrics. Adv. Nutr. 2013, 4, 481–505. [Google Scholar] [CrossRef]
- Simelane, K.S.; Worth, S. Food and Nutrition Security Theory. Food Nutr. Bull. 2020, 41, 367–379. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Fleischhacker, S.; Andrés, J.R. Prioritizing Nutrition Security in the US. JAMA 2021, 325, 1605–1606. [Google Scholar] [CrossRef]
- Bellizzi, K.M.; Smith, A.; Schmidt, S.; Keegan, T.H.M.; Zebrack, B.; Lynch, C.F.; Deapen, D.; Shnorhavorian, M.; Tompkins, B.J.; Simon, M.; et al. Positive and Negative Psychosocial Impact of Being Diagnosed with Cancer as an Adolescent or Young Adult. Cancer 2012, 118, 5155–5162. [Google Scholar] [CrossRef]
- Shih, Y.-C.T.; Xu, Y.; Bradley, C.; Giordano, S.H.; Yao, J.; Yabroff, K.R. Costs around the First Year of Diagnosis for 4 Common Cancers among the Privately Insured. JNCI J. Natl. Cancer Inst. 2022, 114, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Ketterl, T.G.; Syrjala, K.L.; Casillas, J.; Jacobs, L.A.; Palmer, S.C.; McCabe, M.S.; Ganz, P.A.; Overholser, L.; Partridge, A.; Rajotte, E.J.; et al. Lasting Effects of Cancer and Its Treatment on Employment and Finances in Adolescent and Young Adult Cancer Survivors. Cancer 2019, 125, 1908–1917. [Google Scholar] [CrossRef] [PubMed]
- Guy, G.P.; Yabroff, K.R.; Ekwueme, D.U.; Smith, A.W.; Dowling, E.C.; Rechis, R.; Nutt, S.; Richardson, L.C. Estimating the Health and Economic Burden of Cancer among Those Diagnosed as Adolescents and Young Adults. Health Aff. Proj. Hope 2014, 33, 1024–1031. [Google Scholar] [CrossRef]
- Salsman, J.M.; Bingen, K.; Barr, R.D.; Freyer, D.R. Understanding, Measuring, and Addressing the Financial Impact of Cancer on Adolescents and Young Adults. Pediatr. Blood Cancer 2019, 66, e27660. [Google Scholar] [CrossRef]
- Kale, H.P.; Carroll, N.V. Self-Reported Financial Burden of Cancer Care and Its Effect on Physical and Mental Health-Related Quality of Life among US Cancer Survivors. Cancer 2016, 122, 283–289. [Google Scholar] [CrossRef]
- Anestin, A.S.; Lippé, S.; Robaey, P.; Bertout, L.; Drouin, S.; Krajinovic, M.; Michon, B.; Rondeau, É.; Samoilenko, M.; Laverdière, C.; et al. Psychological Risk in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia and Its Association with Functional Health Status: A PETALE Cohort Study. Pediatr. Blood Cancer 2018, 65, e27356. [Google Scholar] [CrossRef]
- Ander, M.; Grönqvist, H.; Cernvall, M.; Engvall, G.; Hedström, M.; Ljungman, G.; Lyhagen, J.; Mattsson, E.; von Essen, L. Development of Health-related Quality of Life and Symptoms of Anxiety and Depression among Persons Diagnosed with Cancer during Adolescence: A 10-year Follow-up Study. Psycho-Oncology 2016, 25, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Bakouny, Z.; Labaki, C.; Grover, P.; Awosika, J.; Gulati, S.; Hsu, C.-Y.; Alimohamed, S.I.; Bashir, B.; Berg, S.; Bilen, M.A.; et al. Interplay of Immunosuppression and Immunotherapy Among Patients with Cancer and COVID-19. JAMA Oncol. 2022, 9, 128–134. [Google Scholar] [CrossRef]
- Jordan, T.; Siuka, D.; Rotovnik, N.K.; Pfeifer, M. COVID-19 and Vitamin D—A Systematic Review. Zdr. Varst. 2022, 61, 124–132. [Google Scholar] [CrossRef]
- Singh, A.; Chidharla, A.; Agarwal, K.; Singh, P.; Jain, N.; Hassen, G.; Abdelwahed, S.; Bhandari, R.; Patel, K.; Gupta, S.; et al. Vitamin D: The Missing Nutrient Behind the Two Deadly Pandemics, COVID-19 and Cardiovascular Diseases. Cureus 2022, 14, e24133. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.A.; Coleman-Jensen, A. Food Insecurity, Chronic Disease, and Health among Working-Age Adults; U.S. Department of Agriculture, Economic Research Service: Washington, DC, USA, 2017.
- Brown, H.; Mills, S.; Albani, V. Socioeconomic Risks of Food Insecurity during the COVID-19 Pandemic in the UK: Findings from the Understanding Society COVID Survey. BMC Public Health 2022, 22, 590. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, F.; Salam, R.A.; Lassi, Z.S.; Das, J.K. The Intertwined Relationship between Malnutrition and Poverty. Front. Public Health 2020, 8, 453. [Google Scholar] [CrossRef] [PubMed]
- Waters, A.R.; Kepka, D.; Ramsay, J.M.; Mann, K.; Vaca Lopez, P.L.; Anderson, J.S.; Ou, J.Y.; Kaddas, H.K.; Palmer, A.; Ray, N.; et al. COVID-19 Vaccine Hesitancy Among Adolescent and Young Adult Cancer Survivors. JNCI Cancer Spectr. 2021, 5, Pkab049. [Google Scholar] [CrossRef]
- Verbruggen, L.C.; Wang, Y.; Armenian, S.H.; Ehrhardt, M.J.; van der Pal, H.J.H.; van Dalen, E.C.; van As, J.W.; Bardi, E.; Baust, K.; Berger, C.; et al. Guidance Regarding COVID-19 for Survivors of Childhood, Adolescent, and Young Adult Cancer: A Statement from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Pediatr. Blood Cancer 2020, 67, e28702. [Google Scholar] [CrossRef]
- Yan, A.; Howden, K.; Mahar, A.L.; Scott, I.; Glidden, C.; Deleemans, J.; Chalifour, K.; Eaton, G.; Gupta, A.; Bolton, J.M.; et al. Experiences of Adolescent and Young Adult Cancer Survivors during the COVID-19 Pandemic. J. Cancer Surviv. 2022, 17, 370–383. [Google Scholar] [CrossRef]
- Betts, A.C.; Shay, L.A.; Allicock, M.; Preston, S.M.; Grimes, A.; Murphy, C.C. Impacts of the Early COVID-19 Pandemic among a National Sample of Adolescent and Young Adult Cancer Survivors in the United States. J. Adolesc. Young Adult Oncol. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Waters, A.R.; Bybee, S.; Warner, E.L.; Kaddas, H.K.; Kent, E.E.; Kirchhoff, A.C. Financial Burden and Mental Health among LGBTQIA+ Adolescent and Young Adult Cancer Survivors During the COVID-19 Pandemic. Front. Oncol. 2022, 12, 832635. [Google Scholar] [CrossRef]
- Lauer, A.L. Treatment of Anxiety and Depression in Adolescents and Young Adults with Cancer. J. Pediatr. Oncol. Nurs. 2015, 32, 278–283. [Google Scholar] [CrossRef]
- Patel, K.G.; Borno, H.T.; Seligman, H.K. Food Insecurity Screening: A Missing Piece in Cancer Management. Cancer 2019, 125, 3494–3501. [Google Scholar] [CrossRef]
- Gattu, R.K.; Paik, G.; Wang, Y.; Ray, P.; Lichenstein, R.; Black, M.M. The Hunger Vital Sign Identifies Household Food Insecurity among Children in Emergency Departments and Primary Care. Children 2019, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.; Capra, S.; Bauer, J.; Banks, M. Development of a Valid and Reliable Malnutrition Screening Tool for Adult Acute Hospital Patients. Nutr. Burbank Los Angel. Cty. Calif 1999, 15, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Haines, E.R.; Lux, L.; Smitherman, A.B.; Kessler, M.L.; Schonberg, J.; Dopp, A.; Stover, A.M.; Powell, B.J.; Birken, S.A. An Actionable Needs Assessment for Adolescents and Young Adults with Cancer: The AYA Needs Assessment & Service Bridge (NA-SB). Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2021, 29, 4693–4704. [Google Scholar] [CrossRef]
- Supplemental Nutrition Assistance Program (SNAP) | Food and Nutrition Service. Available online: https://www.fns.usda.gov/snap/supplemental-nutrition-assistance-program (accessed on 1 February 2023).
- About WIC-WIC at a Glance | Food and Nutrition Service. Available online: https://www.fns.usda.gov/wic/about-wic-glance (accessed on 1 February 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogland-Hand, C.; Ciesielski, T.H.; Daunov, K.; Bean, M.K.; Nock, N.L. Food Insecurity and Nutritional Challenges in Adolescent and Young Adult Cancer Survivors in the U.S.A.: A Narrative Review and Call to Action. Nutrients 2023, 15, 1731. https://doi.org/10.3390/nu15071731
Ogland-Hand C, Ciesielski TH, Daunov K, Bean MK, Nock NL. Food Insecurity and Nutritional Challenges in Adolescent and Young Adult Cancer Survivors in the U.S.A.: A Narrative Review and Call to Action. Nutrients. 2023; 15(7):1731. https://doi.org/10.3390/nu15071731
Chicago/Turabian StyleOgland-Hand, Callie, Timothy H. Ciesielski, Katherine Daunov, Melanie K. Bean, and Nora L. Nock. 2023. "Food Insecurity and Nutritional Challenges in Adolescent and Young Adult Cancer Survivors in the U.S.A.: A Narrative Review and Call to Action" Nutrients 15, no. 7: 1731. https://doi.org/10.3390/nu15071731
APA StyleOgland-Hand, C., Ciesielski, T. H., Daunov, K., Bean, M. K., & Nock, N. L. (2023). Food Insecurity and Nutritional Challenges in Adolescent and Young Adult Cancer Survivors in the U.S.A.: A Narrative Review and Call to Action. Nutrients, 15(7), 1731. https://doi.org/10.3390/nu15071731






