Do Poor Diet and Lifestyle Behaviors Modify the Genetic Susceptibility to Impulsivity in the General Population?
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Outcome: Impulsivity
2.3. Predictor: ADHD PRS
2.4. Moderator: Diet
2.5. Secondary Moderators: Other Lifestyle Variables
2.6. Covariates
2.7. Statistical Analyses
2.8. Sensitivity Analyses
3. Results
3.1. Participants’ Characteristics
3.2. G: ADHD PRS and Impulsivity
3.3. GxE: Moderation by Diet Indicators
3.4. Exploration: Other Lifestyles
3.5. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Lifelines Cohort Study-UGLI Group Authors
- Department of Genetics, University Medical Center Groningen, University of Groningen, 9700 Groningen, The Netherlands
- Department of Pediatrics, University Medical Center Groningen, University of Groningen, 9700 Groningen, The Netherlands
- Department of Epidemiology, University Medical Center Groningen, University of Groningen, 9700 Groningen, The Netherlands
- Institute for Molecular Bioscience, The University of Queensland, Brisbane, QLD 4072, Australia
References
- Moeller, F.G.; Barratt, E.S.; Dougherty, D.M.; Schmitz, J.M.; Swann, A.C. Psychiatric aspects of impulsivity. Am. J. Psychiatry 2001, 158, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Potenza, M.N. To do or not to do? The complexities of addiction, motivation, self-control, and impulsivity. Am. J. Psychiatry 2007, 164, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, J.; Bernardi, S.; Potenza, M.N.; Grant, J.E.; Marsh, R.; Wang, S.; Blanco, C. Impulsivity in the general population: A national study. J. Psychiatr. Res. 2012, 46, 994–1001. [Google Scholar] [CrossRef]
- Faraone, S.V.; Asherson, P.; Banaschewski, T.; Biederman, J.; Buitelaar, J.K.; Ramos-Quiroga, J.A.; Rohde, L.A.; Sonuga-Barke, E.J.S.; Tannock, R.; Franke, B. Attention-deficit/hyperactivity disorder. Nat. Rev. Dis. Prim. 2015, 1, 15020. [Google Scholar] [CrossRef]
- Sciberras, E.; Streatfeild, J.; Ceccato, T.; Pezzullo, L.; Scott, J.G.; Middeldorp, C.M.; Hutchins, P.; Paterson, R.; Bellgrove, M.A.; Coghill, D. Social and Economic Costs of Attention-Deficit/Hyperactivity Disorder Across the Lifespan. J. Atten. Disord. 2022, 26, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Bezdjian, S.; Baker, L.A.; Tuvblad, C. Genetic and environmental influences on impulsivity: A meta-analysis of twin, family and adoption studies. Clin. Psychol. Rev. 2011, 31, 1209. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Roige, S.; Fontanillas, P.; Elson, S.L.; Gray, J.C.; de Wit, H.; MacKillop, J.; Palmer, A.A. Genome-Wide Association Studies of Impulsive Personality Traits (BIS-11 and UPPS-P) and Drug Experimentation in up to 22,861 Adult Research Participants Identify Loci in the CACNA1I and CADM2 genes. J. Neurosci. 2019, 39, 2562–2572. [Google Scholar] [CrossRef]
- Demontis, D.; Walters, R.K.; Martin, J.; Mattheisen, M.; Als, T.D.; Agerbo, E.; Baldursson, G.; Belliveau, R.; Bybjerg-Grauholm, J.; Bækvad-Hansen, M.; et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat. Genet. 2019, 51, 63–75. [Google Scholar] [CrossRef]
- Demontis, D.; Walters, G.B.; Athanasiadis, G.; Walters, R.; Therrien, K.; Nielsen, T.T.; Farajzadeh, L.; Voloudakis, G.; Bendl, J.; Zeng, B.; et al. Genome-wide analyses of ADHD identify 27 risk loci, refine the genetic architecture and implicate several cognitive domains. Nat. Genet. 2023, 55, 198–208. [Google Scholar] [CrossRef]
- Larsson, H.; Anckarsater, H.; Råstam, M.; Chang, Z.; Lichtenstein, P. Childhood attention-deficit hyperactivity disorder as an extreme of a continuous trait: A quantitative genetic study of 8,500 twin pairs. J. Child Psychol. Psychiatry 2012, 53, 73–80. [Google Scholar] [CrossRef]
- Martin, J.; Hamshere, M.L.; Stergiakouli, E.; O’Donovan, M.C.; Thapar, A. Genetic risk for attention-deficit/hyperactivity disorder contributes to neurodevelopmental traits in the general population. Biol. Psychiatry 2014, 76, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Agnew-Blais, J.C.; Belsky, D.W.; Caspi, A.; Danese, A.; Moffitt, T.E.; Polanczyk, G.V.; Sugden, K.; Wertz, J.; Williams, B.S.; Lewis, C.M.; et al. Polygenic Risk and the Course of Attention-Deficit/Hyperactivity Disorder from Childhood to Young Adulthood: Findings From a Nationally Representative Cohort. J. Am. Acad. Child Adolesc. Psychiatry 2021, 60, 1147. [Google Scholar] [CrossRef] [PubMed]
- Stergiakouli, E.; Hamshere, M.; Holmans, P.; Langley, K.; Zaharieva, I.; Hawi, Z.; Kent, L.; Gill, M.; Williams, N.; Owen, M.J.; et al. Investigating the contribution of common genetic variants to the risk and pathogenesis of ADHD. Am. J. Psychiatry 2012, 169, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Bénard, M.; Bellisle, F.; Kesse-Guyot, E.; Julia, C.; Andreeva, V.A.; Etilé, F.; Reach, G.; Dechelotte, P.; Tavolacci, M.-P.; Hercberg, S.; et al. Impulsivity is associated with food intake, snacking, and eating disorders in a general population. Am. J. Clin. Nutr. 2019, 109, 117–126. [Google Scholar] [CrossRef]
- Wiles, N.J.; Northstone, K.; Emmett, P.; Lewis, G. “Junk food” diet and childhood behavioural problems: Results from the ALSPAC cohort. Eur. J. Clin. Nutr. 2009, 63, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Loewen, O.K.; Maximova, K.; Ekwaru, J.P.; Ohinmaa, A.; Veugelers, P.J. Adherence to Life-Style Recommendations and Attention-Deficit/Hyperactivity Disorder: A Population-Based Study of Children Aged 10 to 11 Years. Psychosom. Med. 2020, 82, 305–315. [Google Scholar] [CrossRef]
- Assary, E.; Vincent, J.P.; Keers, R.; Pluess, M. Gene-environment interaction and psychiatric disorders: Review and future directions. Semin. Cell Dev. Biol. 2018, 77, 133–143. [Google Scholar] [CrossRef]
- Li, L.; Taylor, M.J.; Bälter, K.; Xie, T.; Solberg, B.S.; Haavik, J.; Vásquez, A.A.; Hartman, C.A.; Larsson, H. Gene-Environment Interactions in Attention-Deficit/Hyperactivity Disorder Symptom Dimensions: The Role of Unhealthy Food Habits. Genes 2021, 13, 47. [Google Scholar] [CrossRef]
- Kahn, R.S.; Khoury, J.; Nichols, W.C.; Lanphear, B.P. Role of dopamine transporter genotype and maternal prenatal smoking in childhood hyperactive-impulsive, inattentive, and oppositional behaviors. J. Pediatr. 2003, 143, 104–110. [Google Scholar] [CrossRef]
- Brookes, K.-J.; Mill, J.; Guindalini, C.; Curran, S.; Xu, X.; Knight, J.; Chen, C.-K.; Huang, Y.-S.; Sethna, V.; Taylor, E.; et al. A common haplotype of the dopamine transporter gene associated with attention-deficit/hyperactivity disorder and interacting with maternal use of alcohol during pregnancy. Arch. Gen. Psychiatry 2006, 63, 74–81. [Google Scholar] [CrossRef]
- Palladino, V.S.; McNeill, R.; Reif, A.; Kittel-Schneider, S. Genetic risk factors and gene-environment interactions in adult and childhood attention-deficit/hyperactivity disorder. Psychiatr. Genet. 2019, 29, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Sijtsma, A.; Rienks, J.; van der Harst, P.; Navis, G.; Rosmalen, J.G.M.; Dotinga, A. Cohort Profile Update: Lifelines, a three-generation cohort study and biobank. Int. J. Epidemiol. 2021, 51, e295–e302. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.T.; McCrae, R.R. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI); Psychological Assessment Resources: Odessa, FL, USA, 1992. [Google Scholar]
- McCarthy, S.; Das, S.; Kretzschmar, W.; Delaneau, O.; Wood, A.R.; Teumer, A.; Kang, H.M.; Fuchsberger, C.; Danecek, P.; Sharp, K.; et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat. Genet. 2016, 48, 1279–1283. [Google Scholar] [CrossRef]
- Neustaeter, A.; Nolte, I.; Snieder, H.; Jansonius, N.M. Genetic pre-screening for glaucoma in population-based epidemiology: Protocol for a double-blind prospective screening study within Lifelines (EyeLife). BMC Ophthalmol. 2021, 21, 18. [Google Scholar] [CrossRef] [PubMed]
- Auton, A.; Brooks, L.D.; Durbin, R.M.; Garrison, E.P.; Kang, H.M.; Korbel, J.O.; Marchini, J.L.; McCarthy, S.; McVean, G.A.; Abecasis, G.R. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef]
- Coombes, B.J.; Ploner, A.; Bergen, S.E.; Biernacka, J.M. A principal component approach to improve association testing with polygenic risk scores. Genet. Epidemiol. 2020, 44, 676–686. [Google Scholar] [CrossRef]
- Vinke, P.C.; Corpeleijn, E.; Dekker, L.H.; Jacobs, D.R.; Navis, G.; Kromhout, D. Development of the food-based Lifelines Diet Score (LLDS) and its application in 129,369 Lifelines participants. Eur. J. Clin. Nutr. 2018, 72, 1111–1119. [Google Scholar] [CrossRef]
- RIVM. NEVO-Tabel. Dutch Food Composition Table—Version 3; RIVM: Bilthoven, The Netherlands, 2011. [Google Scholar]
- Schofield, W. Predicting basal metabolic rate, new standards and review of previous work. Hum. Nutr. Clin. Nutr. 1985, 39 (Suppl. S1), 5–41. [Google Scholar]
- Kromhout, D.; Spaaij, C.J.K.; De Goede, J.; Weggemans, R.M. The 2015 Dutch food-based dietary guidelines. Eur. J. Clin. Nutr. 2016, 70, 869–878. [Google Scholar] [CrossRef]
- Amine, E.K.; Baba, N.H.; Belhadj, M.; Deurenberg-Yap, M.; Djazayery, A.; Forrestre, T.; Galuska, D.A.; Herman, S.; James, W.P.T.; M’Buyamba Kabangu, J.R.; et al. Diet, Nutrition and the Prevention of Chronic Diseases. Am. J. Clin. Nutr. 2003, 60, 644–645. [Google Scholar] [CrossRef]
- Wendel-Vos, G.C.W.; Schuit, A.J.; Saris, W.H.M.; Kromhout, D. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J. Clin. Epidemiol. 2003, 56, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Byambasukh, O.; Snieder, H.; Corpeleijn, E. Relation Between Leisure Time, Commuting, and Occupational Physical Activity With Blood Pressure in 125 402 Adults: The Lifelines Cohort. J. Am. Heart Assoc. 2020, 9, e014313. [Google Scholar] [CrossRef]
- Boulos, M.I.; Jairam, T.; Kendzerska, T.; Im, J.; Mekhael, A.; Murray, B.J. Normal polysomnography parameters in healthy adults: A systematic review and meta-analysis. Lancet Respir. Med. 2019, 7, 533–543. [Google Scholar] [CrossRef]
- Schweren, L.J.S.; Haavik, J.; Li, L.; Skretting-Solberg, B.; Larsson, H.; Hartman, C.A. Drinking habits and executive functioning: A propensity score-weighted analysis of 78,832 adults. medRxiv 2021. [Google Scholar] [CrossRef]
- Akimova, E.T.; Breen, R.; Brazel, D.M.; Mills, M.C. Gene-environment dependencies lead to collider bias in models with polygenic scores. Sci. Rep. 2021, 11, 9457. [Google Scholar] [CrossRef] [PubMed]
- Jaffee, S.R.; Price, T.S. Gene–environment correlations: A review of the evidence and implications for prevention of mental illness. Mol. Psychiatry 2007, 12, 432. [Google Scholar] [CrossRef] [PubMed]
- Hunjan, A.K.; Hübel, C.; Lin, Y.; Eley, T.C.; Breen, G. Association between polygenic propensity for psychiatric disorders and nutrient intake. Commun. Biol. 2021, 4, 965. [Google Scholar] [CrossRef]
- Schweren, L.J.; Larsson, H.; Vinke, P.C.; Li, L.; Kvalvik, L.G.; Arias-Vasquez, A.; Haavik, J.; Hartman, C.A. Diet quality, stress and common mental health problems: A cohort study of 121,008 adults. Clin. Nutr. 2021, 40, 901–906. [Google Scholar] [CrossRef]
- Equivalence Factors for Disposable Household Income. Available online: https://longreads.cbs.nl/welvaartinnederland-2019/bijlagen/ (accessed on 23 February 2023).
- Ganzeboom, H.B.G. A New International Socio-Economic Index (ISEI) of Occupational Status for the International Standard Classification of Occupation 2008 (ISCO 08). Constructed with Data from the ISSP 2002–2007. In Proceedings of the Paper presented at Annual Conference of International Social, Lisbon, Portugal, 1 May 2010. [Google Scholar]
- van Buuren, S.; Groothuis-Oudshoorn, K. Mice: Multivariate imputation by chained equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- Turley, P.; Walters, R.K.; Maghzian, O.; Okbay, A.; Lee, J.J.; Fontana, M.A.; Nguyen-Viet, T.A.; Wedow, R.; Zacher, M.; Furlotte, N.A.; et al. Multi-trait analysis of genome-wide association summary statistics using MTAG. Nat. Genet. 2018, 50, 229–237. [Google Scholar] [CrossRef]
- Grotzinger, A.D.; Rhemtulla, M.; de Vlaming, R.; Ritchie, S.J.; Mallard, T.T.; Hill, W.D.; Ip, H.F.; Marioni, R.E.; McIntosh, A.M.; Deary, I.J.; et al. Genomic structural equation modelling provides insights into the multivariate genetic architecture of complex traits. Nat. Hum. Behav. 2019, 3, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Kale, D.; Stautz, K.; Cooper, A. Impulsivity related personality traits and cigarette smoking in adults: A meta-analysis using the UPPS-P model of impulsivity and reward sensitivity. Drug Alcohol Depend. 2018, 185, 149–167. [Google Scholar] [CrossRef] [PubMed]
- Stautz, K.; Cooper, A. Impulsivity-related personality traits and adolescent alcohol use: A meta-analytic review. Clin. Psychol. Rev. 2013, 33, 574–592. [Google Scholar] [CrossRef] [PubMed]
- Gillett, G.; Watson, G.; Saunders, K.E.; McGowan, N.M. Sleep and circadian rhythm actigraphy measures, mood instability and impulsivity: A systematic review. J. Psychiatr. Res. 2021, 144, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Selinus, E.N.; Durbeej, N.; Zhan, Y.; Lichtenstein, P.; Lundström, S.; Ekblom, M. Inattention and hyperactivity symptoms in childhood predict physical activity in adolescence. BMC Psychiatry 2021, 2, 629. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.E.; Lavender, J.M.; Leventhal, A.M.; Mason, T.B. Facets of Impulsivity in Relation to Diet Quality and Physical Activity in Adolescence. Int. J. Environ. Res. Public Health 2021, 18, 613. [Google Scholar] [CrossRef] [PubMed]
- Monroe, S.M.; Simons, A.D. Diathesis-stress theories in the context of life stress research: Implications for the depressive disorders. Psychol. Bull. 1991, 110, 406–425. [Google Scholar] [CrossRef]
- Brouwer-Brolsma, E.M.; Perenboom, C.; Sluik, D.; van de Wiel, A.; Geelen, A.; Feskens, E.J.; de Vries, J.H. Development and external validation of the “Flower-FFQ”: A FFQ designed for the Lifelines Cohort Study. Public Health Nutr. 2022, 25, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Siebelink, E.; Geelen, A.; De Vries, J.H.M. Self-reported energy intake by FFQ compared with actual energy intake to maintain body weight in 516 adults. Br. J. Nutr. 2011, 106, 274–281. [Google Scholar] [CrossRef]
- Müller, M.; Kersten, S. Nutrigenomics: Goals and strategies. Nat. Rev. Genet. 2003, 4, 315–322. [Google Scholar] [CrossRef]
- Mondal, S.; Panda, D. Nutrigenomics: An Interface of Gene-Diet-Disease Interaction. In Mineral Deficiencies - Electrolyte Disturbances, Genes, Diet and Disease Interface; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Bartke, T.; Schneider, R. You are what you eat—How nutrition and metabolism shape the genome through epigenetics. Mol. Metab. 2020, 38, 100987. [Google Scholar] [CrossRef] [PubMed]
- Vucetic, Z.; Carlin, J.L.; Totoki, K.; Reyes, T.M. Epigenetic dysregulation of the dopamine system in diet-induced obesity. J. Neurochem. 2012, 120, 891. [Google Scholar] [CrossRef] [PubMed]
- Alsiö, J.; Olszewski, P.; Norbäck, A.; Gunnarsson, Z.; Levine, A.; Pickering, C.; Schiöth, H. Dopamine D1 receptor gene expression decreases in the nucleus accumbens upon long-term exposure to palatable food and differs depending on diet-induced obesity phenotype in rats. Neuroscience 2010, 171, 779–787. [Google Scholar] [CrossRef] [PubMed]

| Overall | Male | Female | |
|---|---|---|---|
| N | 33,047 | 13,280 | 19,767 |
| Age (years) | 42.12 (12.35) | 42.40 (12.22) | 41.93 (12.43) |
| Impulsivity score b | 0.01 (0.99) | −0.04 (0.98) | 0.04 (0.99) |
| ADHD PRS c | −0.01 (1.00) | −0.02 (0.99) | 0.00 (1.00) |
| Diet | |||
| Overall diet quality (LLDS_I) | 23.22 (5.99) | 24.73 (5.57) | 22.20 (6.04) |
| Intake of energy (KCAL) | 1.28 [1.08, 1.50] | 1.28 [1.08, 1.51] | 1.27 [1.08, 1.49] |
| Intake of sugar (SUGAR) | 1.06 [1.00, 1.43] | 1.10 [1.00, 1.48] | 1.04 [1.00, 1.39] |
| Intake of fat (FAT) | 1.18 [1.08, 1.28] | 1.19 [1.08, 1.29] | 1.18 [1.07, 1.28] |
| Lifestyles | |||
| MVPA (minutes per week) | 200.00 [75.00, 370.00] | 210.00 [60.00, 395.00] | 195.00 [90.00, 360.00] |
| Sleep duration (hours) | 7.49 (0.83) | 7.32 (0.79) | 7.60 (0.84) |
| Sleep duration group (n, %) | |||
| Normal | 26,355 (80.0) | 10,636 (80.4) | 15,719 (79.8) |
| Short | 3,283 (10.0) | 1,374 (10.4) | 1909 (9.7) |
| Long | 3,289 (10.0) | 1215 (9.2) | 2074 (10.5) |
| Alcohol intake (grams) | 4.73 [1.24, 10.80] | 7.96 [2.92, 15.81] | 2.70 [0.63, 6.92] |
| Alcohol intake group (n, %) | |||
| Abstinent | 5186 (15.7) | 1000 (7.5) | 4186 (21.2) |
| Occasional | 6809 (20.6) | 1836 (13.8) | 4973 (25.2) |
| Light | 15,672 (47.4) | 6871 (51.7) | 8801 (44.5) |
| Moderate | 4462 (13.5) | 2800 (21.1) | 1662 (8.4) |
| Heavy | 918 (2.8) | 773 (5.8) | 145 (0.7) |
| Smoke (n, %) | |||
| Current | 6329 (19.3) | 2827 (21.5) | 3502 (17.9) |
| Never | 16,071 (49.1) | 6277 (47.7) | 9794 (50.0) |
| Past | 10,330 (31.6) | 4044 (30.8) | 6286 (32.1) |
| Covariates | |||
| Lifetime diagnosis of non-communicable diseases (n, %) | 10,434 (31.6) | 4051 (30.5) | 6383 (32.3) |
| Current diagnosis of depression (n, %) | 835 (2.5) | 239 (1.8) | 596 (3.0) |
| Current diagnosis of anxiety (n, %) | 2354 (7.1) | 675 (5.1) | 1679 (8.5) |
| Past year number of stressful life events | 1.00 [0.00, 2.00] | 1.00 [0.00, 2.00] | 1.00 [0.00, 2.00] |
| Past year number of life-term difficulties | 2.00 [1.00, 4.00] | 2.00 [1.00, 3.00] | 2.00 [1.00, 4.00] |
| BMI (kg/m2) | 25.51 (4.04) | 25.91 (3.49) | 25.24 (4.34) |
| Educational attainment (n, %) | |||
| Low | 8362 (25.5) | 3349 (25.4) | 5013 (25.6) |
| Middle | 13,374 (40.8) | 5139 (38.9) | 8235 (42.0) |
| High | 11,068 (33.7) | 4708 (35.7) | 6360 (32.4) |
| Occupational status | 43.88 (13.08) | 45.41 (12.47) | 42.85 (13.37) |
| Neighbourhood socioeconomic status | −0.61 (1.08) | −0.59 (1.08) | −0.62 (1.08) |
| Disposable household income (EUR) | 1641.75 (517.12) | 1689.11 (507.71) | 1608.97 (521.04) |
| B (95% CI) | p Value | Beta | |
|---|---|---|---|
| ADHD PRS | 0.03 (0.02, 0.04) | 2.61 × 10−9 | 0.031 |
| Age (years) | −0.021 (−0.022, −0.02) | 0 | −0.258 |
| Sex = female | 0.036 (0.016, 0.057) | 4.71 × 10−4 | 0.018 |
| BMI (kg/m2) | 0.03 (0.028, 0.033) | 5.68 × 10−116 | 0.123 |
| Neighbourhood socioeconomic status | −0.011 (−0.021, −0.002) | 0.019 | −0.012 |
| Education attainment (ref = low) | |||
| Middle | −0.086 (−0.112, −0.06) | 1.50 × 10−10 | −0.043 |
| High | −0.185 (−0.217, −0.153) | 1.29 × 10−29 | −0.088 |
| Disposable household income (euros) | 2.25×10−5 (1.16 × 10−6, 4.39 × 10−5) | 0.039 | 0.011 |
| Occupational status | −0.002 (−0.003, −0.001) | 2.37 × 10−6 | −0.029 |
| Lifetime diagnosis of non-communicable diseases | −0.016 (−0.039, 0.007) | 0.181 | −0.007 |
| Past year number of stressful life events | 0.021 (0.012, 0.029) | 6.96 × 10−7 | 0.027 |
| Past year number of life-term difficulties | 0.091 (0.086, 0.096) | 2.61 × 10−292 | 0.215 |
| Current diagnosis of depression | 0.259 (0.192, 0.326) | 3.83 × 10−14 | 0.041 |
| Current diagnosis of anxiety | 0.156 (0.115, 0.197) | 1.13 × 10−13 | 0.040 |
| B (95% CI) | p Value | Beta | |
|---|---|---|---|
| Overall diet quality (LLDS_I) (ref = Q1) b | |||
| LLDS_I_Q2 | 0.058 (0.033, 0.083) | 5.36 × 10−6 | 0.027 |
| LLDS_I_Q3 | 0.14 (0.115, 0.166) | 6.91 × 10−27 | 0.068 |
| ADHD PRS | 0.025 (0.008, 0.043) | 0.004 | 0.025 |
| ADHD PRS × LLDS_I_Q2 | 0.009 (−0.016, 0.034) | 0.467 | 0.005 |
| ADHD PRS × LLDS_I_Q3 | 0.005 (−0.019, 0.029) | 0.674 | 0.003 |
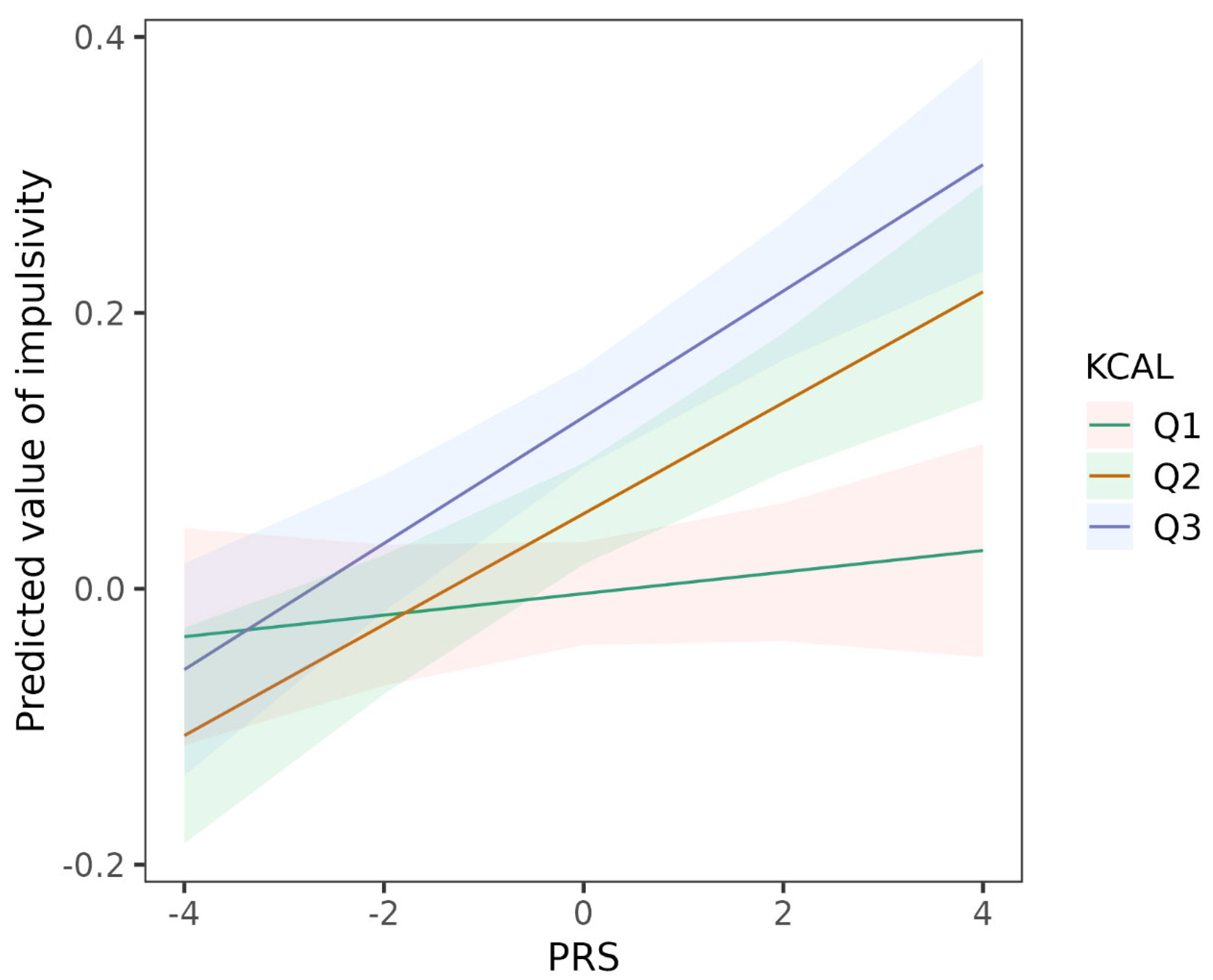
| Intake of energy (KCAL) (ref = Q1) b | |||
| KCAL_Q2 | 0.058 (0.034, 0.082) | 3.08 × 10−6 | 0.028 |
| KCAL_Q3 | 0.128 (0.103, 0.153) | 2.81 × 10−23 | 0.061 |
| ADHD PRS | 0.008 (−0.009, 0.025) | 0.371 | 0.008 |
| ADHD PRS × KCAL_Q2 | 0.032 (0.008, 0.057) | 0.009 | 0.019 |
| ADHD PRS × KCAL_Q3 | 0.038 (0.014, 0.062) | 0.002 | 0.022 |
| Intake of fat (FAT) (ref = Q1) b | |||
| FAT_Q2 | 0.065 (0.041, 0.089) | 1.32 × 10−7 | 0.031 |
| FAT_Q3 | 0.145 (0.12, 0.169) | 9.50 × 10−32 | 0.069 |
| ADHD PRS | 0.019 (0.002, 0.036) | 0.030 | 0.019 |
| ADHD PRS × FAT_Q2 | 0.017 (−0.007, 0.041) | 0.161 | 0.010 |
| ADHD PRS × FAT_Q3 | 0.016 (−0.008, 0.04) | 0.192 | 0.009 |
| Intake of sugar (SUGAR) (ref = Q1) c | |||
| SUGAR_Q2 | 0.001 (−0.02, 0.021) | 0.940 | 0.0004 |
| ADHD PRS | 0.031 (0.018, 0.045) | 4.42 × 10−6 | 0.032 |
| ADHD PRS × SUGAR_Q2 | −0.002 (−0.022, 0.018) | 0.833 | −0.001 |
| B (95% CI) | p Value | Beta | |
|---|---|---|---|
| Sleep duration (ref = middle sleep duration) | |||
| Short sleep duration | 0.078 (0.045, 0.112) | 4.19 × 10−6 | 0.024 |
| Long sleep duration | 0.049 (0.016, 0.082) | 0.004 | 0.015 |
| ADHD PRS | 0.027 (0.015, 0.038) | 3.10 × 10−6 | 0.027 |
| ADHD PRS × short sleep duration | 0.022 (−0.011, 0.055) | 0.187 | 0.007 |
| ADHD PRS × long sleep duration | 0.011 (−0.022, 0.044) | 0.508 | 0.004 |
| Smoking (ref = never smoking) | |||
| Current smoking | 0.302 (0.275, 0.328) | 6.70 × 10−107 | 0.120 |
| Past smoking | 0.208 (0.185, 0.232) | 7.50 × 10−67 | 0.098 |
| ADHD PRS | 0.03 (0.016, 0.044) | 3.55 × 10−5 | 0.030 |
| ADHD PRS × current smoking | −0.012 (−0.038, 0.015) | 0.382 | −0.005 |
| ADHD PRS × past smoking | −0.014 (−0.036, 0.009) | 0.227 | −0.008 |
| Alcohol intake (ref = no alcohol intake) | |||
| Occasional alcohol intake | 0.091 (0.058, 0.124) | 5.37 × 10−8 | 0.037 |
| Light alcohol intake | 0.267 (0.238, 0.296) | 1.06 × 10−71 | 0.135 |
| Moderate alcohol intake | 0.435 (0.397, 0.472) | 4.44 × 10−113 | 0.150 |
| Heavy alscohol intake | 0.498 (0.433, 0.563) | 6.13 × 10−51 | 0.083 |
| ADHD PRS | 0.025 (0.001, 0.049) | 0.045 | 0.025 |
| ADHD PRS × occasional alcohol intake | 0.018 (−0.015, 0.05) | 0.287 | 0.008 |
| ADHD PRS × light alcohol intake | −0.001 (−0.029, 0.028) | 0.965 | 0.000 |
| ADHD PRS × moderate alcohol intake | 0.001 (−0.035, 0.037) | 0.944 | 0.000 |
| ADHD PRS × heavy alcohol intake | 0.042 (−0.022, 0.105) | 0.196 | 0.007 |
| Physical activity | |||
| MVPAQ | 0.019 (0.012, 0.026) | 3.87 × 10−7 | 0.027 |
| ADHD PRS | 0.03 (0.006, 0.054) | 0.016 | 0.030 |
| ADHD PRS × MVPAQ | −0.001 (−0.008, 0.007) | 0.880 | −0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, T.; Schweren, L.J.S.; Larsson, H.; Li, L.; Du Rietz, E.; Haavik, J., on behalf of Lifelines Cohort Study; Grimstvedt Kvalvik, L.; Solberg, B.S.; Klungsøyr, K.; Snieder, H.; et al. Do Poor Diet and Lifestyle Behaviors Modify the Genetic Susceptibility to Impulsivity in the General Population? Nutrients 2023, 15, 1625. https://doi.org/10.3390/nu15071625
Xie T, Schweren LJS, Larsson H, Li L, Du Rietz E, Haavik J on behalf of Lifelines Cohort Study, Grimstvedt Kvalvik L, Solberg BS, Klungsøyr K, Snieder H, et al. Do Poor Diet and Lifestyle Behaviors Modify the Genetic Susceptibility to Impulsivity in the General Population? Nutrients. 2023; 15(7):1625. https://doi.org/10.3390/nu15071625
Chicago/Turabian StyleXie, Tian, Lizanne J. S. Schweren, Henrik Larsson, Lin Li, Ebba Du Rietz, Jan Haavik on behalf of Lifelines Cohort Study, Liv Grimstvedt Kvalvik, Berit Skretting Solberg, Kari Klungsøyr, Harold Snieder, and et al. 2023. "Do Poor Diet and Lifestyle Behaviors Modify the Genetic Susceptibility to Impulsivity in the General Population?" Nutrients 15, no. 7: 1625. https://doi.org/10.3390/nu15071625
APA StyleXie, T., Schweren, L. J. S., Larsson, H., Li, L., Du Rietz, E., Haavik, J., on behalf of Lifelines Cohort Study, Grimstvedt Kvalvik, L., Solberg, B. S., Klungsøyr, K., Snieder, H., & Hartman, C. A. (2023). Do Poor Diet and Lifestyle Behaviors Modify the Genetic Susceptibility to Impulsivity in the General Population? Nutrients, 15(7), 1625. https://doi.org/10.3390/nu15071625







