The Association of Specific Dietary Patterns with Cardiometabolic Outcomes in Women with a History of Gestational Diabetes Mellitus: A Scoping Review
Abstract
1. Introduction
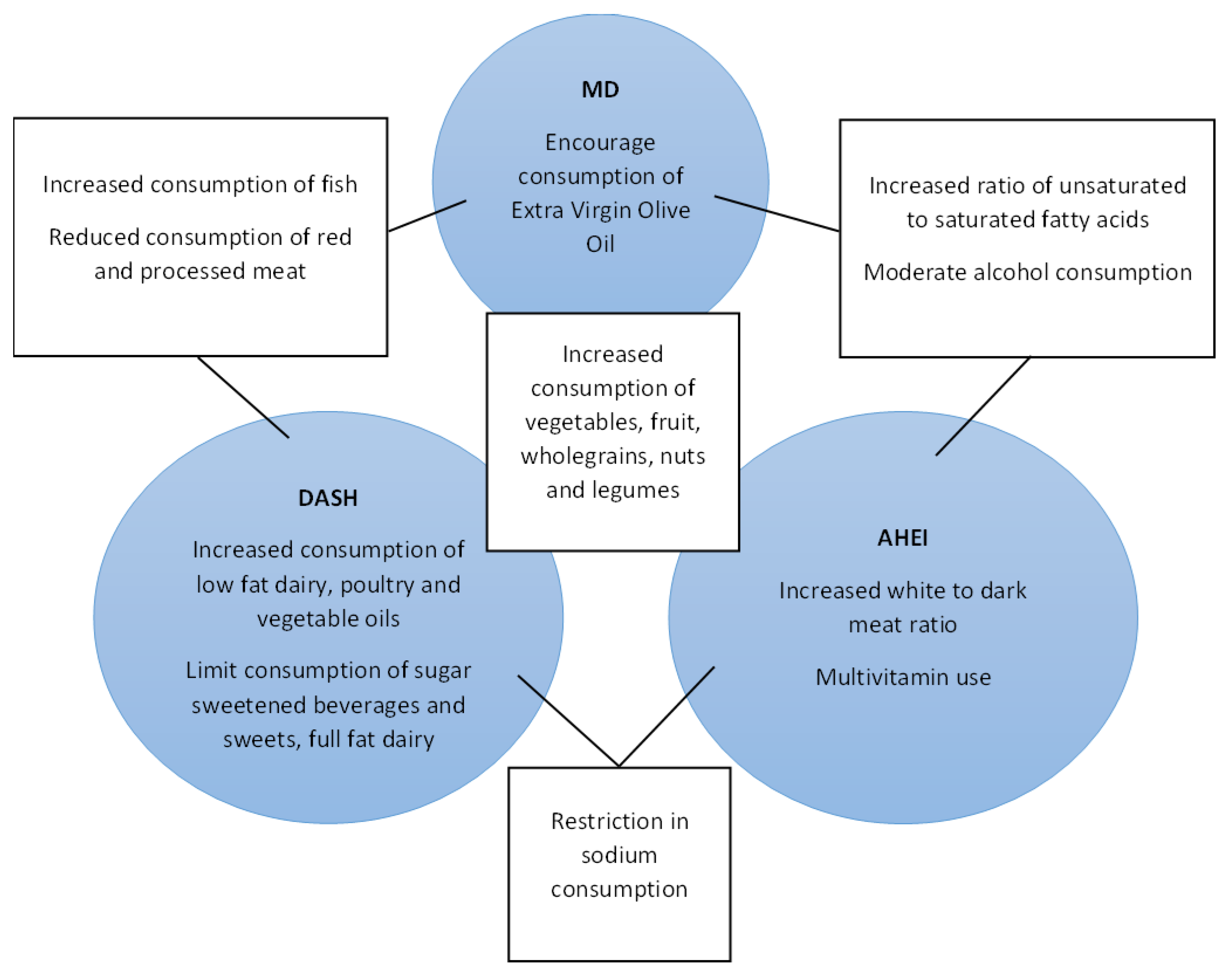
1.1. Dietary Patterns
1.2. Dietary Patterns and Prevention of Gestational Diabetes
2. Materials and Methods
2.1. Search Strategy
2.2. Data Extraction
3. Results
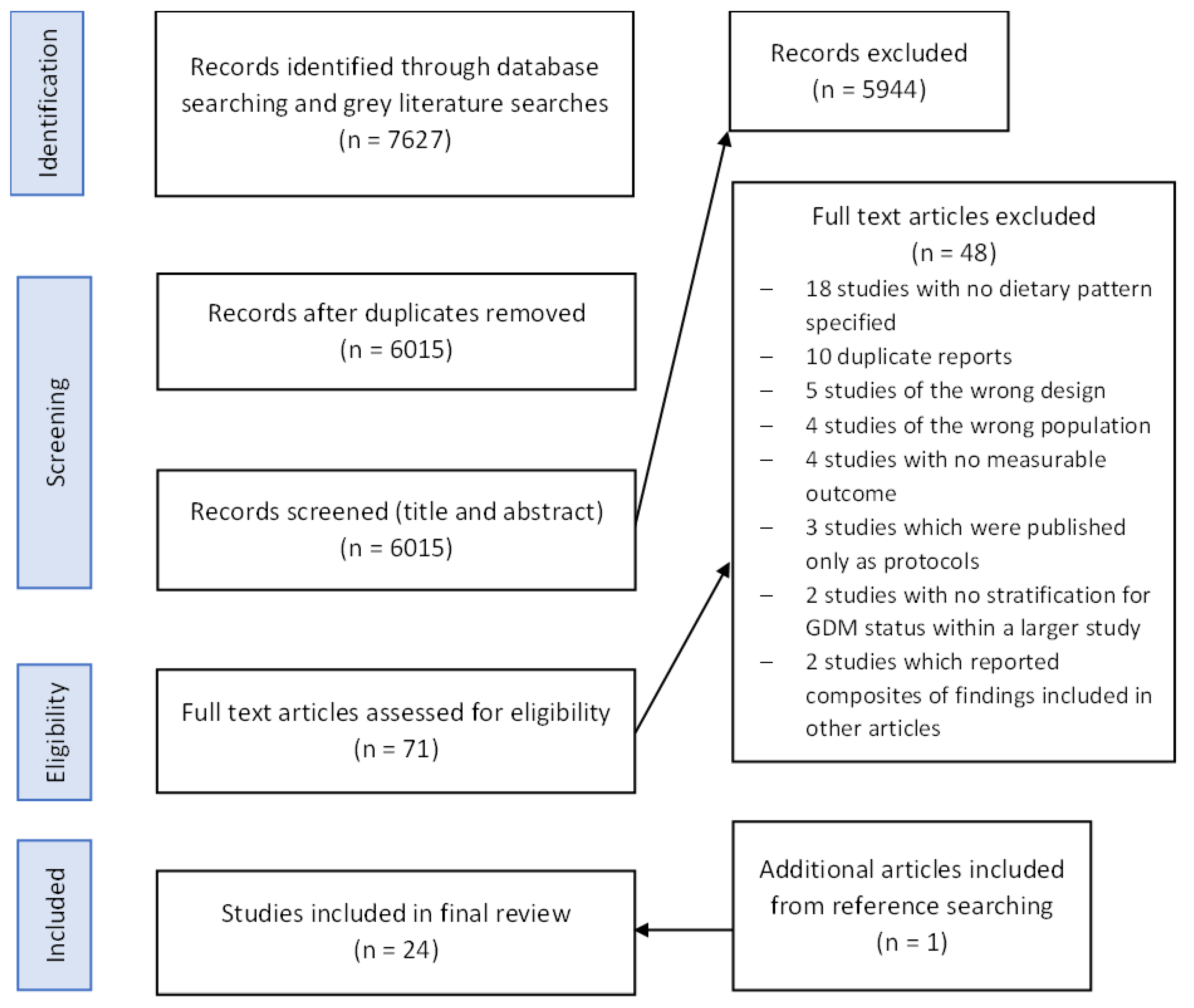
3.1. Study Selection
3.2. Overview of Studies
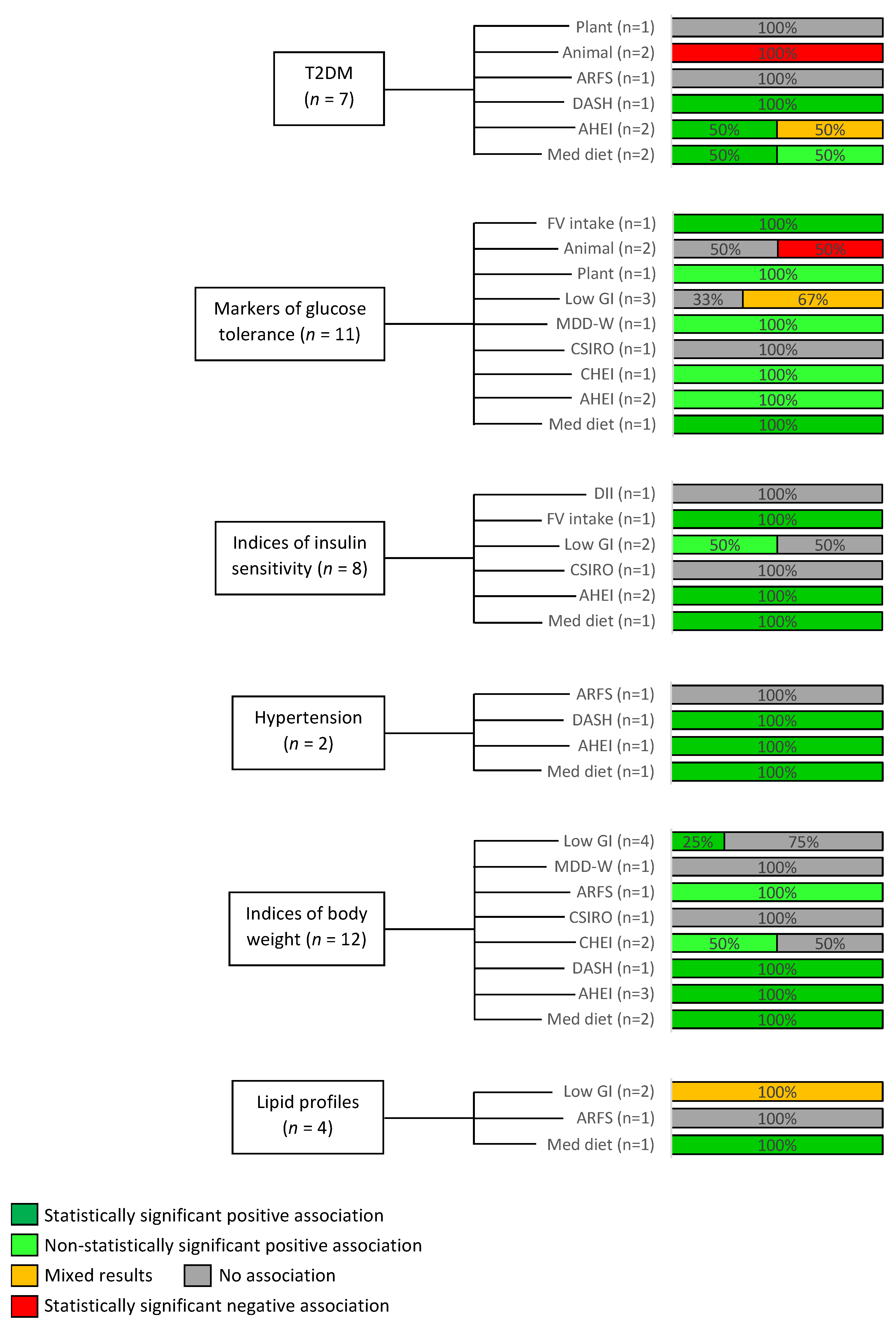
3.3. Overview of Dietary Patterns and Outcomes
3.4. Risk of Incident T2DM
3.5. Markers of Glucose Tolerance
3.6. Indices of Insulin Sensitivity
3.7. Modifiable Cardiovascular Risk Factors
4. Discussion
4.1. Strengths and Limitations
4.2. Future Work
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Search Terms Employed for Literature Searches
Appendix A.1. EMBASE and Medline Search Terms
| NUMBER | SEARCH TERM |
| 1 | Diabetes, Gestational |
| 2 | low fiber diet/or Mediterranean diet/or high-fructose diet/or lactovegetarian diet/or modified Atkins diet/or diet composition/ or vegan diet/or “high fat/high fructose diet”/or Nordic diet/or healthy diet/or cariogenic diet/or ovovegetarian diet/or lipid diet/or high-glucose diet/or lactoovovegetarian diet/or high fiber diet/or diet supplementation/or low fat diet/or protein diet/or diet therapy/or six food elimination diet/or diabetic diet/or raw food diet/or low FODMAP diet/or low calorie diet/or fruitarian diet/or very low calorie diet/or lithogenic diet/or high glycemic index diet/or atherogenic diet/or elemental diet/or gluten free diet/or gluten free casein free diet/or very low calorie ketogenic diet/or high-protein low-carbohydrate diet/or “high fat/high glucose diet”/or casein free diet/or low glycemic index diet/or obesogenic diet/or “high fat/high sucrose diet”/or pescovegetarian diet/or lactose free diet/or low iodine diet/or MIND diet/or low residue diet/or carbohydrate diet/or Atkins diet/or low carbohydrate diet/or liquid diet/or paleolithic diet/or Western diet/or experimental diet/or high methionine diet/or macrobiotic diet/or fiber free diet/or ketogenic diet/or high calorie diet/or soft diet/or high salt diet/or cafeteria diet/or artificial diet/or cereal-based diet/or high-sucrose diet/or elimination diet/or diet/or renal diet/or cholesterol diet/or full liquid diet/or diet restriction/or clear liquid diet/or vegetarian diet/or diet-induced obesity/or “methionine/choline deficient diet”/or unhealthy diet/or carbohydrate loading diet/or DASH diet/ |
| 3 | “diet” |
| 4 | Diet* |
| 5 | Low fibre diet.mp |
| 6 | “Dietary pattern” |
| 7 | Dietary pattern *.mp |
| 8 | Dietary habit |
| 9 | “Eating habit” |
| 10 | Dietary habit *.mp |
| 11 | Nutrient pattern*.mp |
| 12 | Diet, high-fat/or diet, western/or diet, healthy/ |
| 13 | “DASH diet *”.mp. |
| 14 | “Dietary Approaches to Stop Hypertension*”.mp. |
| 15 | “Low-GI Diet *”.mp. |
| 16 | “Healthy Diet *”.mp. |
| 17 | “Prudent Diet *”.mp |
| 18 | “Healthy Eating Ind * mp |
| 19 | “Alternative Healthy Eating Ind *”.mp. |
| 20 | “Inflammatory diet *”.mp. |
| 21 | “Diet* Inflammatory Ind *”.mp. |
| 22 | “Food pattern *”.mp |
| 23 | Cardiovascular disease |
| 24 | Stroke/or Embolic Stroke/or Ischemic Stroke/or Hemorrhagic Stroke/ |
| 25 | Myocardial Infarction/ |
| 26 | Coronary Disease/or Cardiovascular Diseases/or ischaemic heart disease.mp. or Myocardial Ischemia/ |
| 27 | Peripheral Vascular Diseases/ |
| 28 | Atherosclerosis |
| 29 | Glucose intolerance |
| 30 | Prediabetic State/ or impaired fasting glucose.mp. |
| 31 | Impaired glycaemia.mp |
| 32 | impaired glucose tolerance.mp. or Glucose Intolerance/ |
| 33 | Diabetes Mellitus, Type 2/or Diabetes Mellitus/ |
| 34 | Hypertension |
| 35 | Obesity associated inflammation/or morbid obesity/or diabetic obesity/or obesity |
| 36 | Body weight |
| 37 | Overweight |
| 38 | Dysglycaemia |
| 39 | Kidney injury/ or chronic kidney failure/or kidney failure/or acute kidney failure/or kidney disease/or kidney function |
| 40 | Renal impairment |
| 41 | 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 |
| 42 | 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 |
| 43 | 1 and 41 and 42 |
| 44 | Limit 43 to (humans) |
Appendix A.2. Web of Science Search Terms
| 1 | cardiovascular disease (All Fields) or stroke (All Fields) or isch$emic heart disease (All Fields) or coronary disease (All Fields) or myocardial isch$emia (All Fields) or (All Fields) or (All Fields) or atherosclerosis (All Fields) or glucose intolerance (All Fields) or prediabetes (All Fields) or impaired fasting glucose (All Fields) or impaired glucose (All Fields) or impaired glucose tolerance (All Fields) or diabetes mellitus (All Fields) or type 2 diabetes (All Fields) or hypertension (All Fields) or obesity (All Fields) or kidney failure (All Fields) or renal impairment (All Fields) or body weight (All Fields) or overweight (All Fields) |
| 2 | ALL = (diet *) |
| 3 | gestational diabetes (All fields) or “diabetes in pregnancy” (All Fields) |
| 4 | #1 AND #2 AND #3 |
Appendix B. Sample Data Extraction Form
| |
| Citation details | |
| Methods | |
| Country | |
| Study design | |
| Study date | |
| Study duration | |
| Number of study centres | |
| Setting | |
| Study aim | |
| Participants | |
| Study cohort | |
| Who participated | |
| Inclusion criteria | |
| Exclusion criteria | |
| Subgroup analyses | |
| Interventions | |
| Intervention | |
| How is adherence to dietary pattern recorded? | |
| GDM criteria used | |
| Control | |
| N numbers | |
| Outcomes | |
| Primary outcomes | |
| Secondary outcomes | |
| Additional outcomes | |
| Study details | |
| Length of follow-up | |
| Trial ID | |
| Publication details | |
| Language of publication | |
| Funding | |
| Main relevant findings | |
References
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020, 43, S14–S31. [Google Scholar] [CrossRef]
- IDF. IDF Diabetes Atlas; IDF: Brussels, Belgium, 2021. [Google Scholar]
- Vounzoulaki, E.; Khunti, K.; Abner, S.C.; Tan, B.K.; Davies, M.J.; Gillies, C.L. Progression to type 2 diabetes in women with a known history of gestational diabetes: Systematic review and meta-analysis. BMJ 2020, 369, 1361. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.K.; Campbell, S.; Retnakaran, R. Gestational diabetes and the risk of cardiovascular disease in women: A systematic review and meta-analysis. Diabetologia 2019, 62, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Cheng, Y.J.; Beckles, G.L. Cardiovascular Disease Risk Profiles in Women with Histories of Gestational Diabetes but Without Current Diabetes. Obstet. Gynecol. 2008, 112, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Galassi, A.; Reynolds, K.; He, J. Metabolic Syndrome and Risk of Cardiovascular Disease: A Meta-Analysis. Am. J. Med. 2006, 119, 812–819. [Google Scholar] [CrossRef]
- Carr, D.B.; Utzschneider, K.M.; Hull, R.L.; Tong, J.; Wallace, T.M.; Kodama, K.; Shofer, J.B.; Heckbert, S.R.; Boyko, E.J.; Fujimoto, W.Y.; et al. Gestational Diabetes Mellitus Increases the Risk of Cardiovascular Disease in Women with a Family History of Type 2 Diabetes. Diabetes Care 2006, 29, 2078–2083. [Google Scholar] [CrossRef] [PubMed]
- Bentley-Lewis, R.; Powe, C.; Ankers, E.; Wenger, J.; Ecker, J.; Thadhani, R. Effect of Race/Ethnicity on Hypertension Risk Subsequent to Gestational Diabetes Mellitus. Am. J. Cardiol. 2014, 113, 1364–1370. [Google Scholar] [CrossRef]
- O’Higgins, A.C.; O’Dwyer, V.; O’Connor, C.; Daly, S.F.; Kinsley, B.T.; Turner, M.J. Postpartum dyslipidaemia in women diagnosed with gestational diabetes mellitus. Ir. J. Med. Sci. 2016, 186, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Linné, Y.; Dye, L.; Barkeling, B.; Rössner, S. Long-Term Weight Development in Women: A 15-Year Follow-up of the Effects of Pregnancy. Obes. Res. 2004, 12, 1166–1178. [Google Scholar] [CrossRef]
- Linné, Y.; Dye, L.; Barkeling, B.; Rössner, S. Weight development over time in parous women—The SPAWN study—15 years follow-up. Int. J. Obes. 2003, 27, 1516–1522. [Google Scholar] [CrossRef]
- Lipsky, L.M.; Strawderman, M.S.; Olson, C.M. Maternal Weight Change Between 1 and 2 Years Postpartum: The Importance of 1 Year Weight Retention. Obesity 2012, 20, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Gore, S.A.; Brown, D.M.; West, D.S. The role of postpartum weight retention in obesity among women: A review of the evidence. Ann. Behav. Med. 2003, 26, 149–159. [Google Scholar] [CrossRef] [PubMed]
- McKinley, M.C.; Allen-Walker, V.; McGirr, C.; Rooney, C.; Woodside, J.V. Weight loss after pregnancy: Challenges and opportunities. Nutr. Res. Rev. 2018, 31, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Rayanagoudar, G.; Hashi, A.A.; Zamora, J.; Khan, K.S.; Hitman, G.A.; Thangaratinam, S. Quantification of the type 2 diabetes risk in women with gestational diabetes: A systematic review and meta-analysis of 95,750 women. Diabetologia 2016, 59, 1403–1411. [Google Scholar] [CrossRef]
- Rooney, B.L.; Schauberger, C.W.; Mathiason, M.A. Impact of Perinatal Weight Change on Long-Term Obesity and Obesity-Related Illnesses. Obstet. Gynecol. 2005, 106, 1349–1356. [Google Scholar] [CrossRef]
- Kirkegaard, H.; Stovring, H.; Rasmussen, K.M.; Abrams, B.; Sørensen, T.I.; Nohr, E. How do pregnancy-related weight changes and breastfeeding relate to maternal weight and BMI-adjusted waist circumference 7 y after delivery? Results from a path analysis. Am. J. Clin. Nutr. 2014, 99, 312–319. [Google Scholar] [CrossRef]
- Peters, R.; Xiang, A.; Kjos, S.; Buchanan, T. Long-term diabetogenic effect of single pregnancy in women with previous gestational diabetes mellitus. Lancet 1996, 347, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Yeung, E.; Tobias, D.K.; Hu, F.B.; Vaag, A.A.; Chavarro, J.E.; Mills, J.L.; Grunnet, L.G.; Bowers, K.; Ley, S.H.; et al. Long-term risk of type 2 diabetes mellitus in relation to BMI and weight change among women with a history of gestational diabetes mellitus: A prospective cohort study. Diabetologia 2015, 58, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Kwak, S.H.; Jung, H.S.; Choi, S.H.; Lim, S.; Cho, Y.M.; Park, K.S.; Jang, H.C.; Cho, N.H. Weight Gain and Progression to Type 2 Diabetes in Women with a History of Gestational Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2015, 100, 3548–3555. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Tobias, D.K.; Bowers, K.; Chavarro, J.; Vaag, A.; Grunnet, L.G.; Strøm, M.; Mills, J.; Liu, A.; Kiely, M.; et al. Physical Activity and Sedentary Behaviors Associated with Risk of Progression from Gestational Diabetes Mellitus to Type 2 Diabetes Mellitus. JAMA Intern. Med. 2014, 174, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Jowell, A.R.; Sarma, A.A.; Gulati, M.; Michos, E.D.; Vaught, A.J.; Natarajan, P.; Powe, C.E.; Honigberg, M.C. Interventions to Mitigate Risk of Cardiovascular Disease After Adverse Pregnancy Outcomes. JAMA Cardiol. 2022, 7, 346. [Google Scholar] [CrossRef] [PubMed]
- Chasan-Taber, L. Lifestyle interventions to reduce risk of diabetes among women with prior gestational diabetes mellitus. Best Pr. Res. Clin. Obstet. Gynaecol. 2015, 29, 110–122. [Google Scholar] [CrossRef]
- Peacock, A.S.; Bogossian, F.; McIntyre, H.D.; Wilkinson, S. A review of interventions to prevent Type 2 Diabetes after Gestational Diabetes. Women Birth 2014, 27, e7–e15. [Google Scholar] [CrossRef] [PubMed]
- Balk, E.M.; Earley, A.; Raman, G.; Avendano, E.A.; Pittas, A.G.; Remington, P.L. Combined Diet and Physical Activity Promotion Programs to Prevent Type 2 Diabetes Among Persons at Increased Risk: A Systematic Review for the Community Preventive Services Task Force. Ann. Intern. Med. 2015, 163, 437–451. [Google Scholar] [CrossRef]
- Lim, S.; Versace, V.L.; O’Reilly, S.; Janus, E.; Dunbar, J. Weight Change and Cardiometabolic Outcomes in Postpartum Women with History of Gestational Diabetes. Nutrients 2019, 11, 922. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, J.M.; Rosner, B.A.; Zera, C.A.; Seely, E.W. Association Between Changes in Postpartum Weight and Waist Circumference and Changes in Cardiometabolic Risk Factors Among Women with Recent Gestational Diabetes. Prev. Chronic Dis. 2019, 16, E47. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.R.; Retnakaran, R.; Booth, G.L. Increased Risk of Cardiovascular Disease in Young Women Following Gestational Diabetes Mellitus. Diabetes Care 2008, 31, 1668–1669. [Google Scholar] [CrossRef]
- Goueslard, K.; Cottenet, J.; Mariet, A.-S.; Giroud, M.; Cottin, Y.; Petit, J.-M.; Quantin, C. Early cardiovascular events in women with a history of gestational diabetes mellitus. Cardiovasc. Diabetol. 2016, 15, 1–7. [Google Scholar] [CrossRef]
- Tobias, D.K.; Chen, M.; Manson, J.; Ludwig, D.S.; Willett, W.; Hu, F.B. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015, 3, 968–979. [Google Scholar] [CrossRef]
- Chiavaroli, L.; Viguiliouk, E.; Nishi, S.K.; Mejia, S.B.; Rahelić, D.; Kahleova, H.; Salas-Salvadó, J.; Kendall, C.W.C.; Sievenpiper, J.L. DASH Dietary Pattern and Cardiometabolic Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses. Nutrients 2019, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Jannasch, F.; Kröger, J.; Schulze, M.B. Dietary Patterns and Type 2 Diabetes: A Systematic Literature Review and Meta-Analysis of Prospective Studies. J. Nutr. 2017, 147, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.L.; Dunbar, S.A.; Jaacks, L.M.; Karmally, W.; Mayer-Davis, E.J.; Wylie-Rosett, J.; Yancy, W.S. Macronutrients, Food Groups, and Eating Patterns in the Management of Diabetes. Diabetes Care 2012, 35, 434–445. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Bulló, M.; Estruch, R.; Ros, E.; Covas, M.-I.; Ibarrola-Jurado, N.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; et al. Prevention of Diabetes with Mediterranean Diets. Ann. Intern. Med. 2014, 160, 1–10. [Google Scholar] [CrossRef]
- Bray, G.A.; Heisel, W.E.; Afshin, A.; Jensen, M.D.; Dietz, W.H.; Long, M.; Kushner, R.F.; Daniels, S.R.; Wadden, T.A.; Tsai, A.G.; et al. The Science of Obesity Management: An Endocrine Society Scientific Statement. Endocr. Rev. 2018, 39, 79–132. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196. [Google Scholar] [CrossRef]
- Dinu, M.; Pagliai, G.; Casini, A.; Sofi, F. Mediterranean diet and multiple health outcomes: An umbrella review of meta-analyses of observational studies and randomised trials. Eur. J. Clin. Nutr. 2017, 72, 30–43. [Google Scholar] [CrossRef]
- Salehi-Abargouei, A.; Maghsoudi, Z.; Shirani, F.; Azadbakht, L. Effects of Dietary Approaches to Stop Hypertension (DASH)-style diet on fatal or nonfatal cardiovascular diseases—Incidence: A systematic review and meta-analysis on observational prospective studies. Nutrition 2013, 29, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Shirani, F.; Salehi-Abargouei, A.; Azadbakht, L. Effects of Dietary Approaches to Stop Hypertension (DASH) diet on some risk for developing type 2 diabetes: A systematic review and meta-analysis on controlled clinical trials. Nutrition 2013, 29, 939–947. [Google Scholar] [CrossRef]
- Liese, A.D.; Nichols, M.; Sun, X.; D’Agostino, R.B.; Haffner, S.M. Adherence to the DASH Diet Is Inversely Associated with Incidence of Type 2 Diabetes: The Insulin Resistance Atherosclerosis Study. Diabetes Care 2009, 32, 1434–1436. [Google Scholar] [CrossRef]
- Kong, N.W.; Ning, H.; Zhong, V.W.; Paluch, A.; Wilkins, J.T.; Lloyd-Jones, D.; Allen, N.B. Association between diet quality and incident cardiovascular disease stratified by body mass index. Am. J. Prev. Cardiol. 2021, 8, 100298. [Google Scholar] [CrossRef]
- Ibsen, D.B.; Christiansen, A.H.; Olsen, A.; Tjønneland, A.; Overvad, K.; Wolk, A.; Mortensen, J.K.; Dahm, C.C. Adherence to the EAT-Lancet Diet and Risk of Stroke and Stroke Subtypes: A Cohort Study. Stroke 2022, 53, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, K.L.; Milone, G.F.; Grobman, W.A.; Haas, D.M.; Mercer, B.M.; Simhan, H.N.; Saade, G.R.; Silver, R.M.; Chung, J.H. Periconceptional diet quality is associated with gestational diabetes risk and glucose concentrations among nulliparous gravidas. Front. Endocrinol. 2022, 13, 870. [Google Scholar] [CrossRef] [PubMed]
- Merino, J.; Guasch-Ferré, M.; Li, J.; Chung, W.; Hu, Y.; Ma, B.; Li, Y.; Kang, J.H.; Kraft, P.; Liang, L.; et al. Polygenic scores, diet quality, and type 2 diabetes risk: An observational study among 35,759 adults from 3 US cohorts. PLoS Med. 2022, 19, e1003972. [Google Scholar] [CrossRef]
- Livesey, G.; Taylor, R.; Livesey, H.F.; Buyken, A.E.; Jenkins, D.J.A.; Augustin, L.S.A.; Sievenpiper, J.L.; Barclay, A.W.; Liu, S.; Wolever, T.M.S.; et al. Dietary Glycemic Index and Load and the Risk of Type 2 Diabetes: Assessment of Causal Relations. Nutrients 2019, 11, 1436. [Google Scholar] [CrossRef]
- Zafar, M.I.; Mills, K.; Zheng, J.; Regmi, A.; Hu, S.Q.; Gou, L.; Chen, L.-L. Low-glycemic index diets as an intervention for diabetes: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2019, 110, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ye, S. Influence of low-glycemic index diet for gestational diabetes: A meta-analysis of randomized controlled trials. J. Matern. Neonatal Med. 2018, 33, 687–692. [Google Scholar] [CrossRef]
- Tonstad, S.; Butler, T.; Yan, R.; Fraser, G.E. Type of Vegetarian Diet, Body Weight, and Prevalence of Type 2 Diabetes. Diabetes Care 2009, 32, 791–796. [Google Scholar] [CrossRef]
- Pan, A.; Sun, Q.; Bernstein, A.M.; Manson, J.E.; Willett, W.C.; Hu, F.B. Changes in Red Meat Consumption and Subsequent Risk of Type 2 Diabetes Mellitus. JAMA Intern. Med. 2013, 173, 1328–1335. [Google Scholar] [CrossRef]
- Chen, Z.; Zuurmond, M.G.; van der Schaft, N.; Nano, J.; Wijnhoven, H.A.H.; Ikram, M.A.; Franco, O.H.; Voortman, T. Plant versus animal based diets and insulin resistance, prediabetes and type 2 diabetes: The Rotterdam Study. Eur. J. Epidemiol. 2018, 33, 883–893. [Google Scholar] [CrossRef]
- Mijatovic-Vukas, J.; Capling, L.; Cheng, S.; Stamatakis, E.; Louie, J.; Cheung, N.W.; Markovic, T.; Ross, G.; Senior, A.; Brand-Miller, J.C.; et al. Associations of Diet and Physical Activity with Risk for Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 698. [Google Scholar] [CrossRef]
- Izadi, V.; Tehrani, H.G.; Haghighatdoost, F.; Dehghan, A.; Surkan, P.J.; Azadbakht, L. Adherence to the DASH and Mediterranean diets is associated with decreased risk for gestational diabetes mellitus. Nutrition 2016, 32, 1092–1096. [Google Scholar] [CrossRef]
- Aromatis, E.; Munn, Z. JBI Manual for Evidence Synthesis; Joanna Briggs Institute: Adelaide, Australia, 2020. [Google Scholar]
- PRISMA-P Group; Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015 statement. Syst. Rev. 2015, 4, g7647. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lin, Q.; Shi, J.; Xi, Y.; Xiang, C.; Yong, C.; Guo, J. The Impact of Lifestyle Intervention on Dietary Quality among Rural Women with Previous Gestational Diabetes Mellitus—A Randomized Controlled Study. Nutrients 2021, 13, 2642. [Google Scholar] [CrossRef]
- Li, M.; Shi, J.; Luo, J.; Long, Q.; Yang, Q.; OuYang, Y.; Liu, H.; Lin, Q.; Guo, J. Diet Quality among Women with Previous Gestational Diabetes Mellitus in Rural Areas of Hunan Province. Int. J. Environ. Res. Public Health 2020, 17, 5942. [Google Scholar] [CrossRef]
- Chen, Y.; Zhong, Q.; Luo, J.; Tang, Y.; Li, M.; Lin, Q.; Willey, J.A.; Chen, J.-L.; Whittemore, R.; Guo, J. The 6-Month Efficacy of an Intensive Lifestyle Modification Program on Type 2 Diabetes Risk Among Rural Women with Prior Gestational Diabetes Mellitus: A Cluster Randomized Controlled Trial. Prev. Sci. 2022, 23, 1156–1168. [Google Scholar] [CrossRef] [PubMed]
- Gray, K.L.; Clifton, P.M.; Keogh, J.B. Weight Loss Barriers and Dietary Quality of Intermittent and Continuous Dieters in Women with a History of Gestational Diabetes. Int. J. Environ. Res. Public Health 2021, 18, 10243. [Google Scholar] [CrossRef]
- Gray, K.L.; Clifton, P.M.; Keogh, J.B. The effect of intermittent energy restriction on weight loss and diabetes risk markers in women with a history of gestational diabetes: A 12-month randomized control trial. Am. J. Clin. Nutr. 2021, 114, 794–803. [Google Scholar] [CrossRef]
- Yang, J.; Qian, F.; Chavarro, J.; Ley, S.H.; Tobias, D.K.; Yeung, E.; Hinkle, S.N.; Bao, W.; Li, M.; Liu, A.; et al. Modifiable risk factors and long-term risk of type 2 diabetes among individuals with a history of gestational diabetes mellitus: Prospective cohort study. BMJ 2022, 378, e070312. [Google Scholar] [CrossRef] [PubMed]
- Tobias, D.K.; Hu, F.B.; Chavarro, J.; Rosner, B.; Mozaffarian, D.; Zhang, C. Healthful Dietary Patterns and Type 2 Diabetes Mellitus Risk Among Women with a History of Gestational Diabetes Mellitus. Arch. Intern. Med. 2012, 172, 1566–1572. [Google Scholar] [CrossRef]
- Bao, W.; Li, S.; Chavarro, J.E.; Tobias, D.K.; Zhu, Y.; Hu, F.B. Adherence to low-carbohydrate dietary pattern and long-term risk of type 2 diabetes among women with a history of gestational diabetes: A prospective cohort study. Diabetes 2015, 64, A423. [Google Scholar]
- Kim, S.-H.; Kim, M.-Y.; Yang, J.-H.; Park, S.-Y.; Yim, C.H.; Han, K.O.; Yoon, H.K.; Park, S. Nutritional risk factors of early development of postpartum prediabetes and diabetes in women with gestational diabetes mellitus. Nutrition 2011, 27, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Morrison, M.K.; Koh, D.; Lowe, J.M.; Miller, Y.D.; Marshall, A.L.; Colyvas, K.; Collins, C. Postpartum diet quality in Australian women following a gestational diabetes pregnancy. Eur. J. Clin. Nutr. 2012, 66, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ferre, N.; Del Valle, L.; Torrejón, M.J.; Barca, I.; Calvo, M.I.; Matía, P.; Rubio, M.A.; Calle-Pascual, A.L. Diabetes mellitus and abnormal glucose tolerance development after gestational diabetes: A three-year, prospective, randomized, clinical-based, Mediterranean lifestyle interventional study with parallel groups. Clin. Nutr. 2014, 34, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Franzago, M.; Fraticelli, F.; Di Nicola, M.; Bianco, F.; Marchetti, D.; Celentano, C.; Liberati, M.; De Caterina, R.; Stuppia, L.; Vitacolonna, E. Early Subclinical Atherosclerosis in Gestational Diabetes: The Predictive Role of Routine Biomarkers and Nutrigenetic Variants. J. Diabetes Res. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tobias, D.K.; Zhang, C.; Chavarro, J.; Bowers, K.; Rich-Edwards, J.; Rosner, B.; Mozaffarian, D.; Hu, F.B. Prepregnancy adherence to dietary patterns and lower risk of gestational diabetes mellitus. Am. J. Clin. Nutr. 2012, 96, 289–295. [Google Scholar] [CrossRef]
- Li, M.; Rahman, M.L.; Wu, J.; Ding, M.; Chavarro, J.; Lin, Y.; Ley, S.H.; Bao, W.; Grunnet, L.G.; Hinkle, S.N.; et al. Genetic factors and risk of type 2 diabetes among women with a history of gestational diabetes: Findings from two independent populations. BMJ Open Diabetes Res. Care 2020, 8, e000850. [Google Scholar] [CrossRef]
- Shyam, S.; Arshad, F.; Ghani, R.A.; Wahab, N.; Safii, N.S.; Nisak, M.Y.B.; Chinna, K.; Kamaruddin, N.A. Low glycaemic index diets improve glucose tolerance and body weight in women with previous history of gestational diabetes: A six months randomized trial. Nutr. J. 2013, 12, 68. [Google Scholar] [CrossRef]
- Ghani, R.; Shyam, S.; Arshad, F.; Wahab, N.; Chinna, K.; Safii, N.S.; Nisak, M.Y.B.; Kamaruddin, N. The influence of fasting insulin level in post-gestational diabetes mellitus women receiving low-glycaemic-index diets. Nutr. Diabetes 2014, 4, e107. [Google Scholar] [CrossRef]
- Louie, J.C.Y.; Markovic, T.; Ross, G.P.; Foote, D.; Brand-Miller, J.C. Effect of a low glycaemic index diet in gestational diabetes mellitus on post-natal outcomes after 3 months of birth: A pilot follow-up study. Matern. Child Nutr. 2013, 11, 409–414. [Google Scholar] [CrossRef]
- Gingras, V.; Paradis, A.-M.; Tchernof, A.; Weisnagel, S.J.; Robitaille, J. Relationship between the adoption of preventive practices and the metabolic profile of women with prior gestational diabetes mellitus. Appl. Physiol. Nutr. Metab. 2012, 37, 1232–1238. [Google Scholar] [CrossRef]
- Ferranti, E.P.; Dunbar, S.B.; Reilly, C.M.; Foster, J.W.; Guo, Y. Diet quality and cardiometabolic risk status of women within five years following gestational diabetes. Circulation 2013, 128, 22. Available online: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed14&NEWS=N&AN=71338210 (accessed on 20 November 2022).
- Tang, N.; Wu, Y.; Chen, Y.; Chen, Q.; Wu, W.; Jing, J.; Cai, L. Association between postpartum low-carbohydrate-diet scores and glucose levels in Chinese women. Nutrition 2021, 89, 111305. [Google Scholar] [CrossRef]
- Mercier, R.; Perron, J.; Weisnagel, S.J.; Robitaille, J. Associations between fruit and vegetables intake and abnormal glucose tolerance among women with prior gestational diabetes mellitus. Eur. J. Nutr. 2018, 58, 689–696. [Google Scholar] [CrossRef]
- Shin, D.; Lee, K.W.; Tande, D.L.; Hur, J.; Shivappa, N.; Wirth, M.D.; Hébert, J.R. Effects of Dietary Inflammatory Index and History of Gestational Diabetes Mellitus on Insulin Resistance. J. Fed. Am. Soc. Exp. Biol. 2018, 31, 789. [Google Scholar]
- Li, S.; Zhu, Y.; Bao, W.; Mills, J.; Zhang, C.; Liu, A.; Chavarro, J.E.; Hu, F.B.; Ley, S.H.; Tobias, D.K.; et al. Healthful dietary patterns and the risk of hypertension among women with a history of gestational diabetes mellitus: A prospective cohort study. Hypertension 2016, 67, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Flore, R.; Ponziani, F.R.; Tinelli, G.; Arena, V.; Fonnescu, C.; Nesci, A.; Santorio, L.; Tondi, P.; Santoliquido, A. New modalities of ultrasound-based intima-media thickness, arterial stiffness and non-coronary vascular calcifications detection to assess cardiovascular risk. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1430–1441. [Google Scholar]
- Ott, R.; Pawlow, X.; Weiß, A.; Hofelich, A.; Herbst, M.; Hummel, N.; Prehn, C.; Adamski, J.; Römisch-Margl, W.; Kastenmüller, G.; et al. Intergenerational metabolomic analysis of mothers with a history of gestational diabetes mellitus and their offspring. Int. J. Mol. Sci. 2020, 21, 9647. [Google Scholar] [CrossRef]
- Nicklas, J.M.; Zera, C.A.; Seely, E.W. Predictors of very early postpartum weight loss in women with recent gestational diabetes mellitus. J. Matern. -Fetal Neonatal Med. 2020, 33, 120–126. [Google Scholar] [CrossRef]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M.; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Ashra, N.B.; Spong, R.; Carter, P.; Davies, M.J.; Dunkley, A.; Gillies, C.; Greaves Co Khunti, K.; Sutton, S.; Yates, T.; Youssef, D.; et al. A Systematic Review and Meta-Analysis Assessing the Effectiveness of Pragmatic Lifestyle Interventions for the Prevention of Type 2 Diabetes Mellitus in Routine Practice. Public Health England. 2015. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/733053/PHE_Evidence_Review_of_diabetes_prevention_programmes-_FINAL.pdf (accessed on 2 December 2022).
- Aroda, V.R.; Christophi, C.A.; Edelstein, S.L.; Zhang, P.; Herman, W.H.; Barrett-Connor, E.; Delahanty, L.M.; Montez, M.G.; Ackermann, R.T.; Zhuo, X.; et al. The Effect of Lifestyle Intervention and Metformin on Preventing or Delaying Diabetes Among Women with and Without Gestational Diabetes: The Diabetes Prevention Program Outcomes Study 10-Year Follow-Up. J. Clin. Endocrinol. Metab. 2015, 100, 1646–1653. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Diabetes in pregnancy: Management from preconception to the postnatal period. In National Collaborating Centre for Women’s and Children’s Health (UK); RCOG Press: London, UK, 2015. [Google Scholar]
- Halton, T.L.; Liu, S.; Manson, J.; Hu, F.B. Low-carbohydrate-diet score and risk of type 2 diabetes in women. Am. J. Clin. Nutr. 2008, 87, 339–346. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Type 2 Diabetes in Adults: Management. 2015. Available online: www.nice.org.uk/guidance/ng28 (accessed on 20 November 2022).
- Rintamäki, R.; Rautio, N.; Peltonen, M.; Jokelainen, J.; Keinänen-Kiukaanniemi, S.; Oksa, H.; Saaristo, T.; Puolijoki, H.; Saltevo, J.; Tuomilehto, J.; et al. Long-term outcomes of lifestyle intervention to prevent type 2 diabetes in people at high risk in primary health care. Prim. Care Diabetes 2021, 15, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Pelkman, C.L. Effects of the glycemic index of foods on serum concentrations of high-density lipoprotein cholesterol and triglycerides. Curr. Atheroscler. Rep. 2001, 3, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Sundarapperuma, T.D.; Wijesinghe, C.J.; Hettiarachchi, P.; Wasalathanthri, S. Perceptions on Diet and Dietary Modifications during Postpartum Period Aiming at Attenuating Progression of GDM to DM: A Qualitative Study of Mothers and Health Care Workers. J. Diabetes Res. 2018, 2018, 1–6. [Google Scholar] [CrossRef]
- Nicklas, J.M.; Zera, C.A.; Seely, E.W.; Abdul-Rahim, Z.S.; Rudloff, N.D.; Levkoff, S.E. Identifying postpartum intervention approaches to prevent type 2 diabetes in women with a history of gestational diabetes. BMC Pregnancy Childbirth 2011, 11, 23. [Google Scholar] [CrossRef]
- Bolou, A.; Lanz, D.; Drymoussi, Z.; Carreras, F.J.G.; Austin, F.; Dodds, J.; Mehay, A.; Pizzo, E.; Thomas, A.; Heighway, J.; et al. Acceptability and adherence to a Mediterranean diet in the postnatal period to prevent type 2 diabetes in women with gestational diabetes in the UK: A protocol for a single-arm feasibility study (MERIT). BMJ Open 2021, 11, e050099. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Hara, H.; Taylor, J.; Woodside, J.V. The Association of Specific Dietary Patterns with Cardiometabolic Outcomes in Women with a History of Gestational Diabetes Mellitus: A Scoping Review. Nutrients 2023, 15, 1613. https://doi.org/10.3390/nu15071613
O’Hara H, Taylor J, Woodside JV. The Association of Specific Dietary Patterns with Cardiometabolic Outcomes in Women with a History of Gestational Diabetes Mellitus: A Scoping Review. Nutrients. 2023; 15(7):1613. https://doi.org/10.3390/nu15071613
Chicago/Turabian StyleO’Hara, Hannah, Josh Taylor, and Jayne V. Woodside. 2023. "The Association of Specific Dietary Patterns with Cardiometabolic Outcomes in Women with a History of Gestational Diabetes Mellitus: A Scoping Review" Nutrients 15, no. 7: 1613. https://doi.org/10.3390/nu15071613
APA StyleO’Hara, H., Taylor, J., & Woodside, J. V. (2023). The Association of Specific Dietary Patterns with Cardiometabolic Outcomes in Women with a History of Gestational Diabetes Mellitus: A Scoping Review. Nutrients, 15(7), 1613. https://doi.org/10.3390/nu15071613





