Association between Phytochemical Index and Osteoporosis in Women: A Prospective Cohort Study in Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. General Characteristics and Health Information of the Participants
2.3. Dietary Assessment
2.4. Phytochemical Index
2.5. Bone Mineral Density Measurement and Definition of Osteoporosis
2.6. Statistical Analysis
3. Results
3.1. General Characteristics of the Participants
3.2. Association between PI and Osteoporosis Incidence
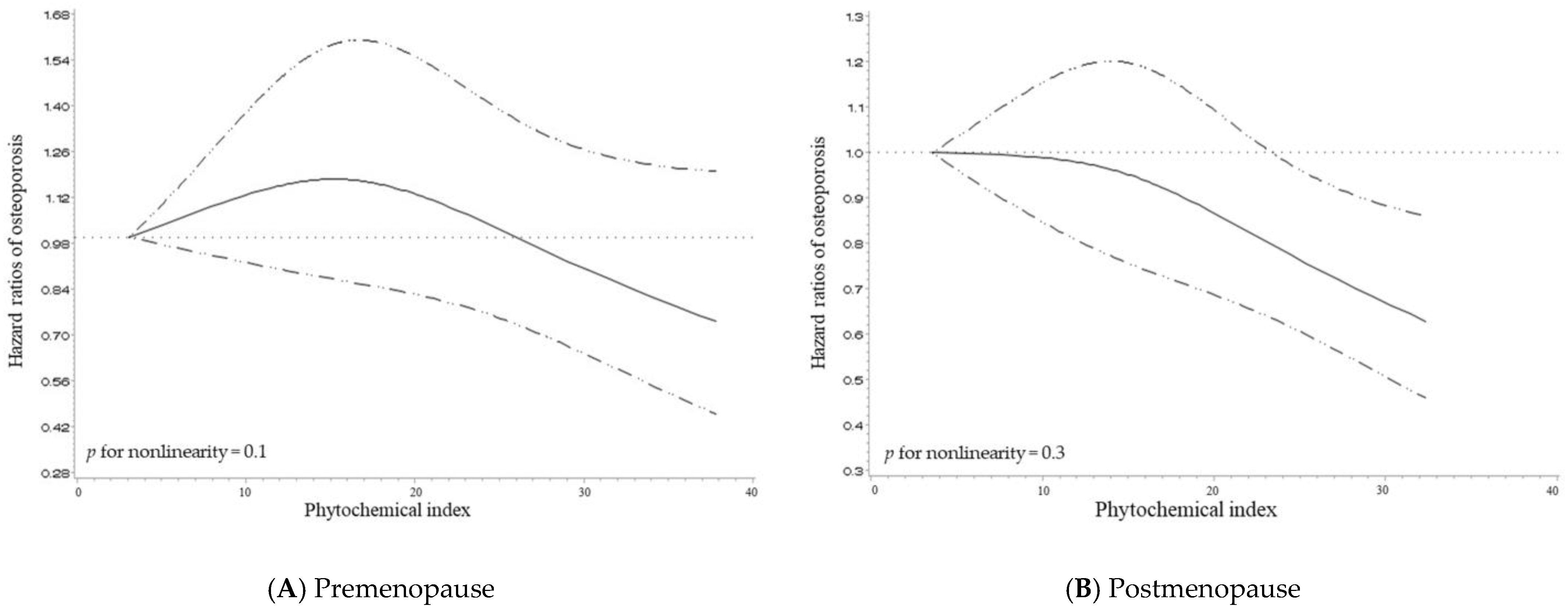
3.3. Dose-Response Relationship between PI and Osteoporosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aspray, T.J.; Hill, T.R. Osteoporosis and the Ageing Skeleton. Subcell Biochem. 2019, 91, 453–476. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis: Report of a WHO Study Group [Meeting Held in Rome from 22 to 25 June 1992]; World Health Organization: Geneva, Switzerland, 1994; pp. 2–6.
- International Osteoporosis Foundation. About Osteoporosis: Epidemiology. Available online: https://www.osteoporosis.foundation/health-professionals/about-osteoporosis/epidemiology (accessed on 24 February 2023).
- Lane, N.E. Epidemiology, etiology, and diagnosis of osteoporosis. Am. J. Obstet. Gynecol. 2006, 194, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Health Insurance Review & Assessment Service. Statistics of Diseases and Medical Practices in Life. Available online: https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA020045010000&brdScnBltNo=4&brdBltNo=2361#none (accessed on 6 February 2023).
- Health Insurance Review & Assessment Service. Statistical Disease Information. Available online: https://www.hira.or.kr/ra/stcIlnsInfm/stcIlnsInfmView.do?pgmid=HIRAA030502000000&sortSno=191 (accessed on 6 February 2023).
- Health Insurance Review & Assessment Service. Healthcare Bigdata Hub: Osteoporosis without Pathological Fractures. Available online: http://opendata.hira.or.kr/op/opc/olap3thDsInfo.do (accessed on 7 February 2023).
- Burge, R.; Dawson-Hughes, B.; Solomon, D.H.; Wong, J.B.; King, A.; Tosteson, A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J. Bone Miner Res. 2007, 22, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Varacallo, M.A.; Fox, E.J. Osteoporosis and its complications. Med. Clin. N. Am. 2014, 98, 817–831, xii–xiii. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.; Greenwood, D.C.; Cade, J.E. Risk of hip fracture in meat-eaters, pescatarians, and vegetarians: Results from the UK Women’s Cohort Study. BMC Med. 2022, 20, 275. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Cheng, L.; Jiang, W. Fruit and vegetable consumption and the risk of postmenopausal osteoporosis: A meta-analysis of observational studies. Food Funct. 2018, 9, 2607–2616. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.F.; Yang, W.Y.; Liang, G.H.; Luo, M.H.; Cao, Y.; Chen, H.Y.; Pan, J.K.; Huang, H.T.; Han, Y.H.; Zhao, D.; et al. Can increasing the prevalence of vegetable-based diets lower the risk of osteoporosis in postmenopausal subjects? A systematic review with meta-analysis of the literature. Complement. Ther. Med. 2019, 42, 302–311. [Google Scholar] [CrossRef]
- Chi, X.X.; Zhang, T. The effects of soy isoflavone on bone density in north region of climacteric Chinese women. J. Clin. Biochem. Nutr. 2013, 53, 102–107. [Google Scholar] [CrossRef]
- Chen, X.W.; Garner, S.C.; Anderson, J.J. Isoflavones regulate interleukin-6 and osteoprotegerin synthesis during osteoblast cell differentiation via an estrogen-receptor-dependent pathway. Biochem. Biophys. Res. Commun. 2002, 295, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Nicolin, V.; De Tommasi, N.; Nori, S.L.; Costantinides, F.; Berton, F.; Di Lenarda, R. Modulatory Effects of Plant Polyphenols on Bone Remodeling: A Prospective View From the Bench to Bedside. Front. Endocrinol. 2019, 10, 494. [Google Scholar] [CrossRef]
- Rao, L.G.; Rao, A.V. (Eds.) Oxidative stress and antioxidants in the risk of osteoporosis—Role of phytochemical antioxidants lycopene and polyphenol-containing nutritional supplements. In Phytochemicals—Isolation, Characterisation and Role in Human Health; Intechopen: Rijeka, Croatia, 2015; pp. 250–255. [Google Scholar] [CrossRef]
- Yamaguchi, M. Nutritional factors and bone homeostasis: Synergistic effect with zinc and genistein in osteogenesis. Mol. Cell Biochem. 2012, 366, 201–221. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.G.; Mackinnon, E.S.; Josse, R.G.; Murray, T.M.; Strauss, A.; Rao, A.V. Lycopene consumption decreases oxidative stress and bone resorption markers in postmenopausal women. Osteoporos. Int. 2007, 18, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Mackinnon, E.S.; Rao, A.V.; Rao, L.G. Dietary restriction of lycopene for a period of one month resulted in significantly increased biomarkers of oxidative stress and bone resorption in postmenopausal women. J. Nutr. Health Aging 2011, 15, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Kan, B.; Guo, D.; Yuan, B.; Vuong, A.M.; Jiang, D.; Zhang, M.; Cheng, H.; Zhao, Q.; Li, B.; Feng, L.; et al. Dietary carotenoid intake and osteoporosis: The National Health and Nutrition Examination Survey, 2005–2018. Arch. Osteoporos. 2021, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Park, K. Association between phytochemical index and metabolic syndrome. Nutr. Res. Pract. 2020, 14, 252–261. [Google Scholar] [CrossRef]
- McCarty, M.F. Proposal for a dietary “phytochemical index”. Med. Hypotheses 2004, 63, 813–817. [Google Scholar] [CrossRef]
- Kim, C.; Park, K. Association between Phytochemical Index and Inflammation in Korean Adults. Antioxidants 2022, 11, 348. [Google Scholar] [CrossRef]
- Jo, U.; Park, K. Phytochemical index and hypertension in Korean adults using data from the Korea National Health and Nutrition Examination Survey in 2008–2019. Eur. J. Clin. Nutr. 2022, 76, 1594–1599. [Google Scholar] [CrossRef]
- Abshirini, M.; Mahaki, B.; Bagheri, F.; Siassi, F.; Koohdani, F.; Sotoudeh, G. Higher Intake of Phytochemical-Rich Foods is Inversely Related to Prediabetes: A Case-Control Study. Int. J. Prev. Med. 2018, 9, 64. [Google Scholar] [CrossRef]
- Rigi, S.; Mousavi, S.M.; Shakeri, F.; Keshteli, A.H.; Benisi-Kohansal, S.; Saadatnia, M.; Esmaillzadeh, A. Dietary phytochemical index in relation to risk of stroke: A case-control study. Nutr. Neurosci. 2022, 25, 2239–2246. [Google Scholar] [CrossRef]
- Wei, C.; Liu, L.; Liu, R.; Dai, W.; Cui, W.; Li, D. Association between the Phytochemical Index and Overweight/Obesity: A Meta-Analysis. Nutrients 2022, 14, 1429. [Google Scholar] [CrossRef] [PubMed]
- Korea Disease Control and Prevention Agency. Korean Genome and Epidemiology Study Integrated Data User Guide. Available online: https://nih.go.kr/ko/main/contents.do?menuNo=300584 (accessed on 14 February 2023).
- Kim, Y.; Han, B.G.; Ko, G.E.S.g. Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int. J. Epidemiol. 2017, 46, e20. [Google Scholar] [CrossRef] [PubMed]
- Korea Disease Control and Prevention Agency. Manual of Korean Genome and Epidemiology Study: Baseline Survey and Examination; Korea Disease Control and Prevention Agency: Cheongju, Republic of Korea, 2011.
- Ainsworth, B.E.; Haskell, W.L.; Leon, A.S.; Jacobs, D.R., Jr.; Montoye, H.J.; Sallis, J.F.; Paffenbarger, R.S., Jr. Compendium of physical activities: Classification of energy costs of human physical activities. Med. Sci. Sports Exerc. 1993, 25, 71–80. [Google Scholar] [CrossRef]
- Ahn, Y.; Kwon, E.; Shim, J.E.; Park, M.K.; Joo, Y.; Kimm, K.; Park, C.; Kim, D.H. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur. J. Clin. Nutr. 2007, 61, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Korea Disease Control and Prevention Agency. Manual of Korean Genome and Epidemiology Study: Food Frequency Questionnaire; Korea Disease Control and Prevention Agency: Cheongju, Republic of Korea, 2019.
- Korea Disease Control and Prevention Agency. Manual of Korean Genome and Epidemiology Study: Nutritional Survey; Korea Disease Control and Prevention Agency: Cheongju, Republic of Korea, 2011.
- Chae, M.J.; Jang, J.Y.; Park, K. Association between dietary calcium intake and the risk of cardiovascular disease among Korean adults. Eur. J. Clin. Nutr. 2020, 74, 834–841. [Google Scholar] [CrossRef]
- National Institute of Agricultural Sciences. 10th Revision Korean Food Composition Table. Available online: http://koreanfood.rda.go.kr/kfi/fct/fctIntro/list?menuId=PS03562# (accessed on 23 January 2023).
- The Korean Nutrition Society. Computer Aided Nutritional Analysis Program. Available online: http://canpro5.kns.or.kr/EgovContent.do (accessed on 14 February 2023).
- Han, M.-R.; Park, Y.; Paik, H.-Y.; Song, Y. An iodine database for common Korean foods and the association between iodine intake and thyroid disease in Korean adults. Int. J. Thyroidol. 2015, 8, 170–182. [Google Scholar] [CrossRef]
- World Health Organization. Prevention and Management of Osteoporosis: Report of a WHO Scientific Group; WHO: Geneva, Switzerland, 2003.
- Bijelic, R.; Milicevic, S.; Balaban, J. Risk Factors for Osteoporosis in Postmenopausal Women. Med. Arch. 2017, 71, 25–28. [Google Scholar] [CrossRef]
- Kelsey, J.L. Risk factors for osteoporosis and associated fractures. Public Health Rep. 1989, 104, 14–20. [Google Scholar] [PubMed]
- Roh, Y.H.; Lee, E.S.; Ahn, J.; Kim, H.S.; Gong, H.S.; Baek, K.H.; Chung, H.Y. Factors affecting willingness to get assessed and treated for osteoporosis. Osteoporos. Int. 2019, 30, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- McMillan, L.B.; Zengin, A.; Ebeling, P.R.; Scott, D. Prescribing Physical Activity for the Prevention and Treatment of Osteoporosis in Older Adults. Healthcare 2017, 5, 85. [Google Scholar] [CrossRef]
- Management of Osteoporosis in Postmenopausal Women: The Position Statement of The North American Menopause Society; Editorial, P. Management of osteoporosis in postmenopausal women: The 2021 position statement of The North American Menopause Society. Menopause 2021, 28, 973–997. [Google Scholar] [CrossRef]
- Korean Society for Bone and Mineral Research. Fracture Liaison Services Guidebook; Korean Society for Bone and Mineral Research: Seoul, Republic of Korea, 2019. [Google Scholar]
- Salaffi, F.; Malavolta, N.; Cimmino, M.A.; Di Matteo, L.; Scendoni, P.; Carotti, M.; Stancati, A.; Mule, R.; Frigato, M.; Gutierrez, M.; et al. Validity and reliability of the Italian version of the ECOS-16 questionnaire in postmenopausal women with prevalent vertebral fractures due to osteoporosis. Clin. Exp. Rheumatol. 2007, 25, 390–403. [Google Scholar] [PubMed]
- Morabito, N.; Crisafulli, A.; Vergara, C.; Gaudio, A.; Lasco, A.; Frisina, N.; D’Anna, R.; Corrado, F.; Pizzoleo, M.A.; Cincotta, M.; et al. Effects of genistein and hormone-replacement therapy on bone loss in early postmenopausal women: A randomized double-blind placebo-controlled study. J. Bone Miner Res. 2002, 17, 1904–1912. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.S.; Zhao, Y. The effects of beta-carotene on osteoporosis: A systematic review and meta-analysis of observational studies. Osteoporos. Int. 2022. [Google Scholar] [CrossRef]
- Brown, J.P.; Albert, C.; Nassar, B.A.; Adachi, J.D.; Cole, D.; Davison, K.S.; Dooley, K.C.; Don-Wauchope, A.; Douville, P.; Hanley, D.A.; et al. Bone turnover markers in the management of postmenopausal osteoporosis. Clin. Biochem. 2009, 42, 929–942. [Google Scholar] [CrossRef]
- Sugiura, M.; Nakamura, M.; Ogawa, K.; Ikoma, Y.; Ando, F.; Yano, M. Bone mineral density in post-menopausal female subjects is associated with serum antioxidant carotenoids. Osteoporos. Int. 2008, 19, 211–219. [Google Scholar] [CrossRef]
- Shkembi, B.; Huppertz, T. Calcium Absorption from Food Products: Food Matrix Effects. Nutrients 2021, 14, 180. [Google Scholar] [CrossRef]
- Schlemmer, U.; Frølich, W.; Prieto, R.M.; Grases, F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. 2009, 53, S330–S375. [Google Scholar] [CrossRef]
- Wolf, R.L.; Cauley, J.A.; Baker, C.E.; Ferrell, R.E.; Charron, M.; Caggiula, A.W.; Salamone, L.M.; Heaney, R.P.; Kuller, L.H. Factors associated with calcium absorption efficiency in pre- and perimenopausal women. Am. J. Clin. Nutr. 2000, 72, 466–471. [Google Scholar] [CrossRef]
| Quartiles of PI | p-Value | ||||
|---|---|---|---|---|---|
| Characteristics | Q1 | Q2 | Q3 | Q4 | |
| Premenopausal | |||||
| No. of participants | 688 | 689 | 689 | 689 | |
| PI, median (range) | 7.45 (0.27–10.27) | 12.90 (10.28–15.24) | 17.44 (15.25–19.70) | 23.33 (19.71–64.22) | |
| Age (years) | 47.65 ± 0.29 | 46.36 ± 0.25 | 47.48 ± 0.28 | 50.46 ± 0.34 | <0.001 |
| Household income | <0.001 | ||||
| Low or mid–low | 346 (51.49) | 283 (41.80) | 250 (36.71) | 332 (49.04) | |
| Mid–high or high | 326 (48.51) | 394 (58.20) | 431 (63.29) | 345 (50.96) | |
| Education level | 0.03 | ||||
| High school graduation or lower | 647 (94.45) | 625 (91.11) | 621 (90.39) | 624 (91.50) | |
| College graduation or higher | 38 (5.55) | 61 (8.89) | 66 (9.61) | 58 (8.50) | |
| Alcohol consumption | <0.001 | ||||
| Non-drinkers | 465 (67.78) | 457 (66.62) | 470 (68.61) | 521 (75.95) | |
| Drinkers | 221 (32.22) | 229 (33.38) | 215 (31.39) | 165 (24.05) | |
| Smoking status | 0.4 | ||||
| Non-smokers | 629 (93.46) | 644 (95.55) | 653 (96.03) | 651 (95.32) | |
| Former smokers | 9 (1.34) | 8 (1.19) | 8 (1.18) | 8 (1.17) | |
| Current Smokers | 35 (5.20) | 22 (3.26) | 19 (2.79) | 24 (3.51) | |
| Physical activity ¹ | 0.04 | ||||
| Low | 257 (38.02) | 209 (30.69) | 242 (35.38) | 211 (30.85) | |
| Mid | 203 (30.03) | 227 (33.33) | 223 (32.60) | 245 (35.82) | |
| High | 216 (31.95) | 245 (35.98) | 219 (32.02) | 228 (33.33) | |
| Body mass index (kg/m2) | 24.75 ± 0.12 | 24.82 ± 0.12 | 24.57 ± 0.12 | 24.75 ± 0.13 | 0.7 |
| Calcium intake (mg/day) | 382.56 ± 7.36 | 478.82 ± 8.64 | 503.36 ± 9.26 | 529.41 ± 9.63 | <0.001 |
| Total energy intake (kcal/day) | 1723.23 ± 20.15 | 1930.91 ± 22.43 | 1899.72 ± 21.51 | 1832.15 ± 21.64 | 0.003 |
| Vitamin D intake (μg/day) | 1.63 ± 0.05 | 2.18 ± 0.05 | 2.22 ± 0.06 | 2.23 ± 0.07 | <0.001 |
| Postmenopausal | |||||
| No. of participants | 461 | 461 | 462 | 461 | |
| PI, median (range) | 8.00 (0.67–10.63) | 12.99 (10.63–15.03) | 17.02 (15.05–19.15) | 22.04 (19.17–46.42) | |
| Age (years) | 58.38 ± 0.32 | 57.62 ± 0.32 | 58.22 ± 0.32 | 58.32 ± 0.32 | 0.8 |
| Household income | 0.01 | ||||
| Low or mid–low | 351 (77.65) | 342 (75.50) | 310 (69.35) | 320 (70.64) | |
| Mid–high or high | 101 (22.35) | 111 (24.50) | 137 (30.65) | 133 (29.36) | |
| Education level | <0.001 | ||||
| High school graduation or lower | 448 (99.12) | 446 (97.38) | 429 (93.46) | 439 (95.85) | |
| College graduation or higher | 4 (0.88) | 12 (2.62) | 30 (6.54) | 19 (4.15) | |
| Alcohol consumption | 0.008 | ||||
| Non-drinkers | 342 (74.67) | 365 (79.52) | 368 (80.17) | 382 (83.77) | |
| Drinkers | 116 (25.33) | 94 (20.48) | 91 (19.83) | 74 (16.23) | |
| Smoking status | 0.08 | ||||
| Non-smokers | 429 (93.87) | 437 (95.62) | 430 (94.51) | 430 (94.30) | |
| Former smokers | 3 (0.66) | 5 (1.09) | 12 (2.64) | 7 (1.54) | |
| Current Smokers | 25 (5.47) | 15 (3.28) | 13 (2.86) | 19 (4.17) | |
| Physical activity ¹ | <0.001 | ||||
| Low | 138 (30.20) | 138 (30.13) | 162 (35.37) | 165 (35.95) | |
| Mid | 130 (28.45) | 161 (35.15) | 152 (33.19) | 173 (37.69) | |
| High | 189 (41.36) | 159 (34.72) | 144 (31.44) | 121 (26.36) | |
| Body mass index (kg/m2) | 24.87 ± 0.15 | 25.25 ± 0.15 | 25.32 ± 0.16 | 24.94 ± 0.16 | 0.7 |
| Calcium intake (mg/day) | 336.16 ± 8.11 | 428.50 ± 9.00 | 465.40 ± 10.30 | 506.26 ± 11.71 | <0.001 |
| Total energy intake (kcal/day) | 1611.82 ± 21.13 | 1790.18 ± 21.81 | 1780.44 ± 21.90 | 1751.72 ± 23.90 | <0.001 |
| Vitamin D intake (μg/day) | 1.15 ± 0.05 | 1.58 ± 0.06 | 1.71 ± 0.06 | 1.78 ± 0.07 | <0.001 |
| Quartiles of PI | |||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | p for Trend | |
| Premenopause | |||||
| No. of participants | 688 | 689 | 689 | 689 | |
| No. of cases (%) | 164 (23.84) | 193 (28.01) | 182 (26.42) | 168 (24.38) | |
| Model 1 | Ref | 0.99 (0.81–1.22) | 0.99 (0.80–1.22) | 1.12 (0.90–1.39) | 0.3 |
| Model 2 | Ref | 1.12 (0.91–1.38) | 1.00 (0.81–1.23) | 0.84 (0.68–1.05) | 0.9 |
| Model 3 | Ref | 1.15 (0.92–1.44) | 1.16 (0.93–1.46) | 0.98 (0.78–1.24) | 0.8 |
| Postmenopause | |||||
| No. of participants | 461 | 461 | 462 | 461 | |
| No. of cases (%) | 348 (75.49) | 335 (72.67) | 329 (71.21) | 321 (69.63) | |
| Model 1 | Ref | 0.95 (0.82–1.10) | 0.89 (0.76–1.03) | 0.85 (0.73–0.99) | 0.03 |
| Model 2 | Ref | 1.02 (0.87–1.18) | 0.90 (0.77–1.05) | 0.85 (0.73–0.99) | 0.01 |
| Model 3 | Ref | 1.02 (0.87–1.19) | 0.92 (0.78–1.08) | 0.84 (0.71–0.99) | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, H.; Park, K. Association between Phytochemical Index and Osteoporosis in Women: A Prospective Cohort Study in Korea. Nutrients 2023, 15, 1605. https://doi.org/10.3390/nu15071605
Yoo H, Park K. Association between Phytochemical Index and Osteoporosis in Women: A Prospective Cohort Study in Korea. Nutrients. 2023; 15(7):1605. https://doi.org/10.3390/nu15071605
Chicago/Turabian StyleYoo, Hyeonji, and Kyong Park. 2023. "Association between Phytochemical Index and Osteoporosis in Women: A Prospective Cohort Study in Korea" Nutrients 15, no. 7: 1605. https://doi.org/10.3390/nu15071605
APA StyleYoo, H., & Park, K. (2023). Association between Phytochemical Index and Osteoporosis in Women: A Prospective Cohort Study in Korea. Nutrients, 15(7), 1605. https://doi.org/10.3390/nu15071605







