Synbiotic Supplementation Improves Quality of Life and Inmunoneuroendocrine Response in Patients with Fibromyalgia: Influence of Codiagnosis with Chronic Fatigue Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. The Synbiotic
2.2. Participants
2.3. Procedures
2.4. Biompedance Analysis: Determination of Body Composition Measurements
2.5. Accelerometry: Determination of Objective Levels of Physical Activity, Sedentary Lifestyle and Sleep Quality
2.6. Questionnaires: Determination of Perceived Levels of Stress, Anxiety, Fatigue, Pain, Depression, Sleep Quality and Quality of Life
- The Spanish version [17] of the Beck Depression Inventory (BDI) was used to determine possible signs of depression in the past week. Higher scores indicate higher levels of depression.
- State-Trait Anxiety Inventory (STAI), to analyse the levels of anxiety presented at a specific time and in general. A Spanish version [18] was used for this purpose. Higher scores indicate higher levels of anxiety.
- The Perceived Stress Scale (PSS), to assess the frequency with which participants experience stressful situations and thoughts in the last month. Higher scores indicate higher levels of stress. Remor was used in its Spanish version [19].
- Brief Pain Inventory (BPI). Used to determine the intensity and interference of pain in daily activities. The greater the perception of pain, the higher the score obtained. A Spanish version [20] was used.
- Brief Fatigue Inventory (BFI). This questionnaire measures the intensity of fatigue in the last 24 h and its interference with daily activities and work. The higher the perception of fatigue, the higher the score obtained [21].
- Healthy Lifestyle and Personal Control Questionnaire (HLPCQ). The Healthy Lifestyle and Personal Control Questionnaire is composed of several sections referring to type of diet, organised physical exercise, as well as social and mental balance [22].
- Pittsburgh Sleep Quality Questionnaire (PSQI). This questionnaire analyses various parameters related to subjective sleep quality: latency, duration, efficiency and disturbances, as well as consumption of sleeping pills. The Spanish version of the questionnaire was used [23].
- FIQ (Fibromyalgia Impact Questionnaire). A Spanish version [24] was used to assess the impact of FM on physical and mental functions (pain, tiredness, fatigue, stiffness, anxiety and depression). Higher scores indicate a worse health condition.
- Gastrointestinal Health Questionnaire. This questionnaire provides insight into gastrointestinal function in adults by identifying the level of severity of gastrointestinal symptoms. The higher the final score, the more severe the symptoms [25].
- COVID-19 questionnaires:
- CAS (Coronavirus Anxiety Scale). The higher the score, the greater the sense of anxiety. Higher scores are related to higher anxiety towards COVID-19 [26].
- FCV-19S (Fear of Coronavirus). The higher the score, the greater the sense of fear of the coronavirus [27].
2.7. Blood Sampling: Determination of Inflammatory and Stress Biomarkers
2.8. Statistics
3. Results
3.1. Effects of the Synbiotic on Body Composition Measurements Determined by Bioelectrical Impedance Analysis (BIA)
3.2. Effects of the Synbiotic on Physical Activity/Sedentarism Levels and Sleep Quality Determined by Accelerometry
3.3. Effects of the Synbiotic on Perceived Levels of Depression, Stress, Anxiety, Pain, Fatigue, Sleep Quality and Quality of Life Determined by Questionnaires
3.4. Effects of the Synbiotic on Inmunoneuroendocrine Biomarkers
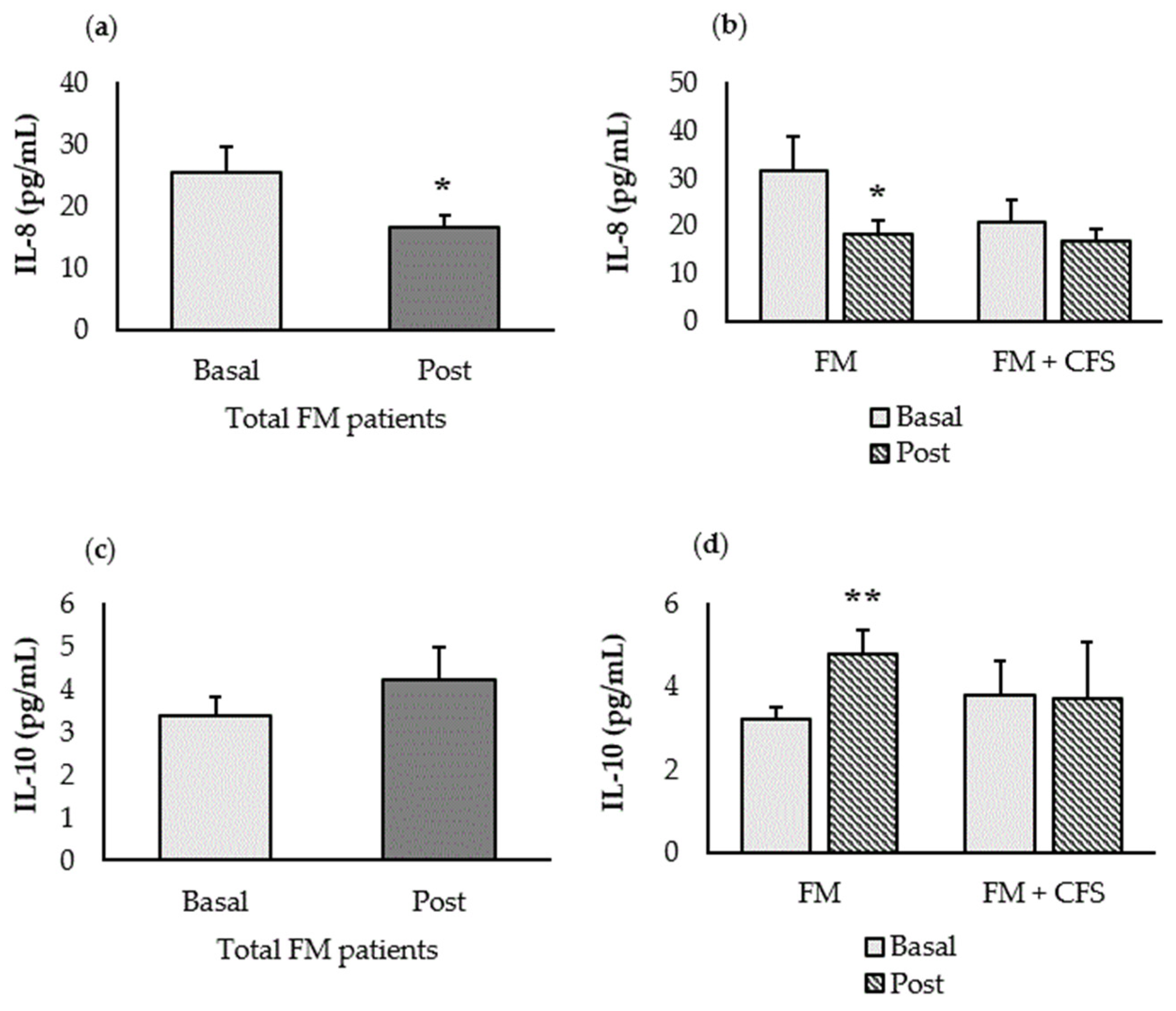
3.4.1. Inflammatory Biomarkers (IL-8 and IL-10)
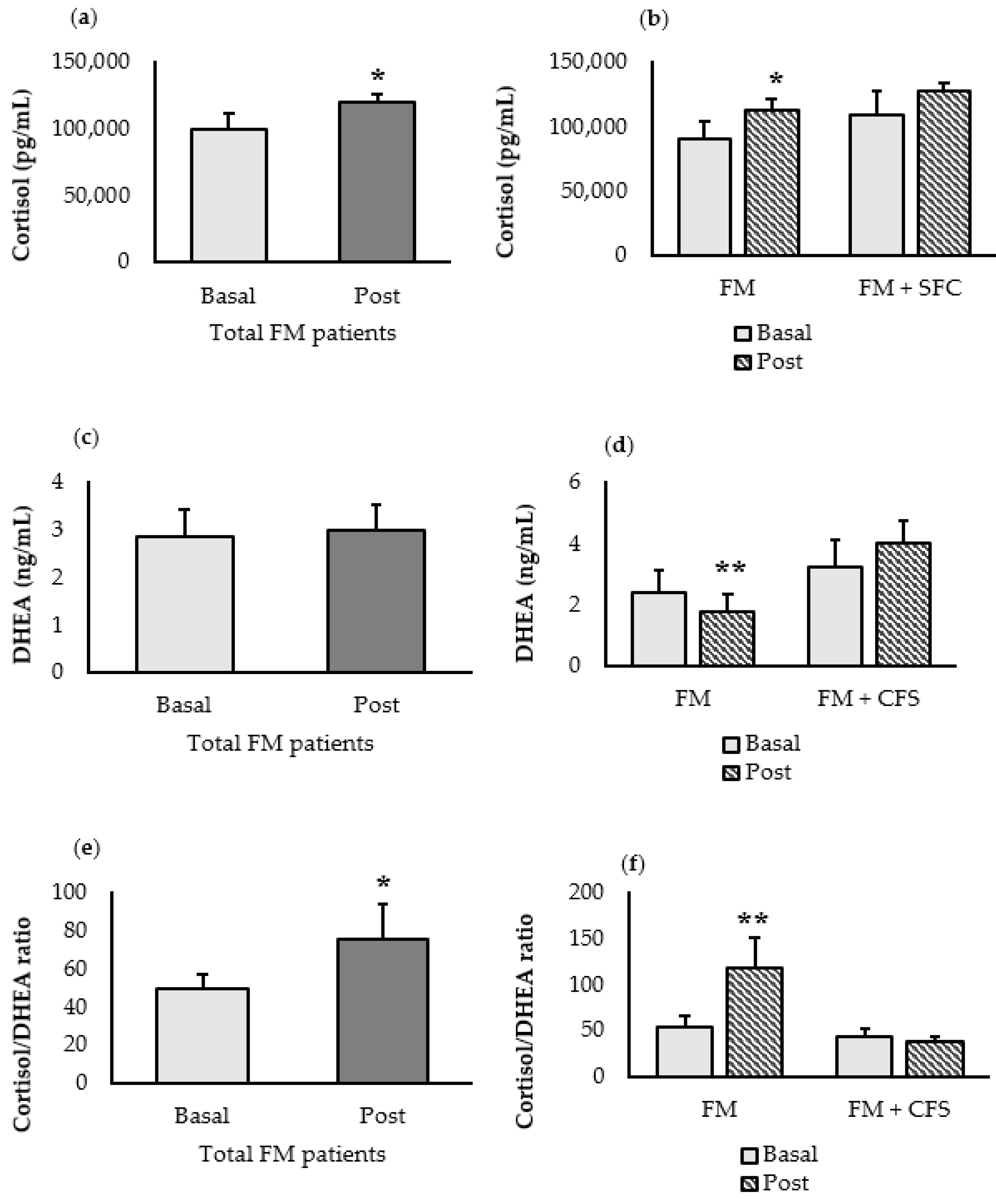
3.4.2. Stress-Related Biomarkers (Cortisol and DHEA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sañudo, B.; Galiano, D.; Carrasco, L.; De Hoyo, M. Evidencias Para La Prescripción de Ejercicio Físico En Pacientes Con Fibromialgia. Rev. Andal. Med. Deport. 2010, 3, 159–169. [Google Scholar]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.-A.; Goldenberg, D.L.; Häuser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. 2016 Revisions to the 2010/2011 Fibromyalgia Diagnostic Criteria. In Seminars in Arthritis and Rheumatism; Elsevier: Amsterdam, The Netherlands, 2016; Volume 46, pp. 319–329. [Google Scholar]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A.; International Chronic Fatigue Syndrome Study Group. The Chronic Fatigue Syndrome: A Comprehensive Approach to Its Definition and Study. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Yunus, M.B. The Prevalence of Fibromyalgia in Other Chronic Pain Conditions. Pain Res. Treat. 2012, 2012, 584573. [Google Scholar] [CrossRef]
- Rivera, J.; Alegre, C.; Ballina, F.J.; Carbonell, J.; Carmona, L.; Castel, B.; Collado, A.; Esteve, J.J.; Martínez, F.G.; Tornero, J. Documento de Consenso de La Sociedad Española de Reumatología Sobre La Fibromialgia. Reumatol. Clín. 2006, 2, S55–S66. [Google Scholar] [CrossRef]
- Ortega, E.; García, J.J.; Bote, M.E.; Martín-Cordero, L.; Escalante, Y.; Saavedra, J.M.; Northoff, H.; Giraldo, E. Exercise in Fibromyalgia and Related Inflammatory Disorders: Known Effects and Unknown Chances. Exerc. Immunol. Rev. 2009, 15, 42–65. [Google Scholar] [PubMed]
- Bote, M.E.; García, J.J.; Hinchado, M.D.; Ortega, E. Inflammatory/Stress Feedback Dysregulation in Women with Fibromyalgia. Neuroimmunomodulation 2012, 19, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Madden, J.A.J.; Hunter, J.O. A Review of the Role of the Gut Microflora in Irritable Bowel Syndrome and the Effects of Probiotics. Br. J. Nutr. 2002, 88 (Suppl. S1), s67–s72. [Google Scholar] [CrossRef]
- Clos-Garcia, M.; Andrés-Marin, N.; Fernández-Eulate, G.; Abecia, L.; Lavín, J.L.; van Liempd, S.; Cabrera, D.; Royo, F.; Valero, A.; Errazquin, N. Gut Microbiome and Serum Metabolome Analyses Identify Molecular Biomarkers and Altered Glutamate Metabolism in Fibromyalgia. EBioMedicine 2019, 46, 499–511. [Google Scholar] [CrossRef]
- Bote, M.E.; Garcia, J.J.; Hinchado, M.D.; Ortega, E. Fibromyalgia: Anti-Inflammatory and Stress Responses after Acute Moderate Exercise. PLoS ONE 2013, 8, e74524. [Google Scholar] [CrossRef]
- Roman, P.; Estévez, Á.F.; Sánchez-Labraca, N.; Cañadas, F.; Miras, A.; Cardona, D. Probiotics for Fibromyalgia: Study Design for a Pilot Double-Blind, Randomized Controlled Trial. Nutr. Hosp. 2017, 34, 1246–1251. [Google Scholar] [CrossRef]
- Roman, P.; Estévez, A.F.; Miras, A.; Sánchez-Labraca, N.; Cañadas, F.; Vivas, A.B.; Cardona, D. A Pilot Randomized Controlled Trial to Explore Cognitive and Emotional Effects of Probiotics in Fibromyalgia. Sci. Rep. 2018, 8, 10965. [Google Scholar] [CrossRef] [PubMed]
- Pagliai, G.; Giangrandi, I.; Dinu, M.; Sofi, F.; Colombini, B. Nutritional Interventions in the Management of Fibromyalgia Syndrome. Nutrients 2020, 12, 2525. [Google Scholar] [CrossRef] [PubMed]
- Cardona, D.; Roman, P.; Cañadas, F.; Sánchez-Labraca, N. The Effect of Multiprobiotics on Memory and Attention in Fibromyalgia: A Pilot Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 3543. [Google Scholar] [CrossRef]
- Pareja, J.L.; Martín, F.; Berná, G.; Cáceres, O.; Blanco, M.; Prada, F.A.; Berral, F.J. Fibromyalgia: A Search for Markers and Their Evaluation throughout a Treatment. Eur. Sci. J. 2015, 426–434. Available online: https://core.ac.uk/display/236412666?utm_source=pdf&utm_medium=banner&utm_campaign=pdf-decoration-v1 (accessed on 27 February 2023).
- Lakhan, S.E.; Kirchgessner, A. Gut Inflammation in Chronic Fatigue Syndrome. Nutr. Metab. 2010, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.; Perdigón, A.L.; Vázquez, C. Adaptación Española Del Inventario Para La Depresión de Beck-II (BDI-II): 2. Propiedades Psicométricas En Población General. Clín. Salud 2003, 14, 249–280. [Google Scholar]
- Buela-Casal, G.; Guillén-Riquelme, A. Short Form of the Spanish Adaptation of the State-Trait Anxiety Inventory. Int. J. Clin. Health Psychol. 2017, 17, 261–268. [Google Scholar] [CrossRef]
- Remor, E. Psychometric Properties of a European Spanish Version of the Perceived Stress Scale (PSS). Span. J. Psychol. 2006, 9, 86–93. [Google Scholar] [CrossRef]
- Badia, X.; Muriel, C.; Gracia, A.; Núñez-Olarte, J.M.; Perulero, N.; Gálvez, R.; Carulla, J.; Cleeland, C.S. Validation of the Spanish Version of the Brief Pain Inventory in Patients with Oncological Pain. Med. Clin. 2003, 120, 52–59. [Google Scholar] [CrossRef]
- Valenzuela, J.O.; Gning, I.; Irarrazaval, M.E.; Fasce, G.; Marin, L.; Mendoza, T.R.; Palos, G.; Reynolds, R.; Wang, X.S.; Cleeland, C.S. Psychometric Validation of the Spanish Version of the Brief Fatigue Inventory; The University of Texas MD Anderson Cancer Center, Division of Internal Medicine Research Retreat: Houston, TX, USA, 2012. [Google Scholar]
- Darviri, C.; Alexopoulos, E.C.; Artemiadis, A.K.; Tigani, X.; Kraniotou, C.; Darvyri, P.; Chrousos, G.P. The Healthy Lifestyle and Personal Control Questionnaire (HLPCQ): A Novel Tool for Assessing Self-Empowerment through a Constellation of Daily Activities. BMC Public Health 2014, 14, 995. [Google Scholar] [CrossRef]
- Hita-Contreras, F.; Martínez-López, E.; Latorre-Román, P.A.; Garrido, F.; Santos, M.A.; Martínez-Amat, A. Reliability and Validity of the Spanish Version of the Pittsburgh Sleep Quality Index (PSQI) in Patients with Fibromyalgia. Rheumatol. Int. 2014, 34, 929–936. [Google Scholar] [CrossRef]
- Rivera, J.; González, T. The Fibromyalgia Impact Questionnaire: A Validated Spanish Version to Assess the Health Status in Women with Fibromyalgia. Clin. Exp. Rheumatol. 2004, 22, 554–560. [Google Scholar] [PubMed]
- Cháidez, Y.L.B.; Guadarrama, R.G.; Hernández, G.A.; Merino, M.V.F.; Alpizar, E.J.; Barretero, D.Y.R.; García, M.V.D. Construcción y Validación de Un Cuestionario Para Medir Función Gastrointestinal En Adultos. Nutr. Clín. Diet. Hosp. 2020, 40, 26–35. [Google Scholar] [CrossRef]
- Caycho-Rodríguez, T.; Tomás, J.M.; Barboza-Palomino, M.; Ventura-León, J.; Gallegos, M.; Reyes-Bossio, M.; Vilca, L.W. Assessment of Fear of COVID-19 in Older Adults: Validation of the Fear of COVID-19 Scale. Int. J. Ment. Health Addict. 2022, 20, 1231–1245. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Teruel, D.; Robles-Bello, M.A. The COVID-19 Fear Scale (FCV-19S): Psychometric Properties and Invariance of the Measure in the Spanish Version. Actas Esp. Psiquiatr. 2021, 49, 96–105. [Google Scholar]
- Hinchado, M.D.; Otero, E.; Navarro, M.D.C.; Martín-Cordero, L.; Gálvez, I.; Ortega, E. Influence of Codiagnosis of Chronic Fatigue Syndrome and Habitual Physical Exercise on the Psychological Status and Quality of Life of Patients with Fibromyalgia. J. Clin. Med. 2022, 11, 5735. [Google Scholar] [CrossRef]
- Minerbi, A.; Gonzalez, E.; Brereton, N.J.B.; Anjarkouchian, A.; Dewar, K.; Fitzcharles, M.-A.; Chevalier, S.; Shir, Y. Altered Microbiome Composition in Individuals with Fibromyalgia. Pain 2019, 160, 2589–2602. [Google Scholar] [CrossRef]
- König, R.S.; Albrich, W.C.; Kahlert, C.R.; Bahr, L.S.; Löber, U.; Vernazza, P.; Scheibenbogen, C.; Forslund, S.K. The Gut Microbiome in Myalgic Encephalomyelitis (ME)/Chronic Fatigue Syndrome (CFS). Front. Immunol. 2022, 12, 628741. [Google Scholar] [CrossRef]
- Freidin, M.B.; Stalteri, M.A.; Wells, P.M.; Lachance, G.; Baleanu, A.-F.; Bowyer, R.C.E.; Kurilshikov, A.; Zhernakova, A.; Steves, C.J.; Williams, F.M.K. An Association between Chronic Widespread Pain and the Gut Microbiome. Rheumatology 2021, 60, 3727–3737. [Google Scholar] [CrossRef]
- Minerbi, A.; Fitzcharles, M.-A. Gut Microbiome: Pertinence in Fibromyalgia. Clin. Exp. Rheumatol. 2020, 38, 99–104. [Google Scholar]
- Wallace, D.J.; Hallegua, D.S. Fibromyalgia: The Gastrointestinal Link. Curr. Pain Headache Rep. 2004, 8, 364–368. [Google Scholar] [CrossRef]
- Ortega, E.; Bote, M.E.; Giraldo, E.; Garcia, J.J. Aquatic Exercise Improves the Monocyte Pro-and Anti-inflammatory Cytokine Production Balance in Fibromyalgia Patients. Scand. J. Med. Sci. Sport. 2012, 22, 104–112. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, J.F.; da Rocha Araújo, F.A.G.; da Mota, L.M.A.; Aires, R.B.; de Araujo, R.P. Vitamin D Supplementation Seems to Improve Fibromyalgia Symptoms: Preliminary Results. Isr. Med. Assoc. J. 2018, 20, 379–381. [Google Scholar] [PubMed]
- Boomershine, C.S.; Koch, T.A.; Morris, D. A Blinded, Randomized, Placebo-Controlled Study to Investigate the Efficacy and Safety of Ferric Carboxymaltose in Iron-Deficient Patients with Fibromyalgia. Rheumatol. Ther. 2018, 5, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Bagis, S.; Karabiber, M.; As, I.; Tamer, L.; Erdogan, C.; Atalay, A. Is Magnesium Citrate Treatment Effective on Pain, Clinical Parameters and Functional Status in Patients with Fibromyalgia? Rheumatol. Int. 2013, 33, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Calero, C.D.Q.; Rincón, E.O.; Marqueta, P.M. Probiotics, Prebiotics and Synbiotics: Useful for Athletes and Active Individuals? A Systematic Review. Benef. Microbes 2020, 11, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Quero, C.D.; Manonelles, P.; Fernández, M.; Abellán-Aynés, O.; López-Plaza, D.; Andreu-Caravaca, L.; Hinchado, M.D.; Gálvez, I.; Ortega, E. Differential Health Effects on Inflammatory, Immunological and Stress Parameters in Professional Soccer Players and Sedentary Individuals after Consuming a Synbiotic. A Triple-Blinded, Randomized, Placebo-Controlled Pilot Study. Nutrients 2021, 13, 1321. [Google Scholar] [CrossRef]
- Sullivan, Å.; Nord, C.E.; Evengård, B. Effect of Supplement with Lactic-Acid Producing Bacteria on Fatigue and Physical Activity in Patients with Chronic Fatigue Syndrome. Nutr. J. 2009, 8, 4. [Google Scholar] [CrossRef]
- Singh, P.K.; Chopra, K.; Kuhad, A.; Kaur, I.P. Role of Lactobacillus Acidophilus Loaded Floating Beads in Chronic Fatigue Syndrome: Behavioral and Biochemical Evidences. Neurogastroenterol. Motil. 2012, 24, 366-e170. [Google Scholar] [CrossRef]
- Haddad, H.W.; Mallepalli, N.R.; Scheinuk, J.E.; Bhargava, P.; Cornett, E.M.; Urits, I.; Kaye, A.D. The Role of Nutrient Supplementation in the Management of Chronic Pain in Fibromyalgia: A Narrative Review. Pain Ther. 2021, 10, 827–848. [Google Scholar] [CrossRef]
- Calandre, E.P.; Hidalgo-Tallon, J.; Molina-Barea, R.; Rico-Villademoros, F.; Molina-Hidalgo, C.; Garcia-Leiva, J.M.; Carrillo-Izquierdo, M.D.; Slim, M. The Probiotic VSL# 3® Does Not Seem to Be Efficacious for the Treatment of Gastrointestinal Symptomatology of Patients with Fibromyalgia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Pharmaceuticals 2021, 14, 1063. [Google Scholar]
- Rao, A.V.; Bested, A.C.; Beaulne, T.M.; Katzman, M.A.; Iorio, C.; Berardi, J.M.; Logan, A.C. A Randomized, Double-Blind, Placebo-Controlled Pilot Study of a Probiotic in Emotional Symptoms of Chronic Fatigue Syndrome. Gut Pathog. 2009, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A Novel Class of Psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Borchers, A.T.; Gershwin, M.E. Fibromyalgia: A Critical and Comprehensive Review. Clin. Rev. Allergy Immunol. 2015, 49, 100–151. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.J.; Linker-Israeli, M.; Hallegua, D.; Silverman, S.; Silver, D.; Weisman, M.H. Cytokines Play an Aetiopathogenetic Role in Fibromyalgia: A Hypothesis and Pilot Study. Rheumatology 2001, 40, 743–749. [Google Scholar] [CrossRef] [PubMed]
- García, J.J.; Cidoncha, A.; Bote, M.E.; Hinchado, M.D.; Ortega, E. Altered Profile of Chemokines in Fibromyalgia Patients. Ann. Clin. Biochem. 2014, 51, 576–581. [Google Scholar] [CrossRef]
- Mendieta, D.; De la Cruz-Aguilera, D.L.; Barrera-Villalpando, M.I.; Becerril-Villanueva, E.; Arreola, R.; Hernández-Ferreira, E.; Pérez-Tapia, S.M.; Pérez-Sánchez, G.; Garcés-Alvarez, M.E.; Aguirre-Cruz, L. IL-8 and IL-6 Primarily Mediate the Inflammatory Response in Fibromyalgia Patients. J. Neuroimmunol. 2016, 290, 22–25. [Google Scholar] [CrossRef]
- Ahrens, C.; Schiltenwolf, M.; Wang, H. Cytokines in Psychoneuroendocrine Immunological Context of Nonspecific Musculoskeletal Pain. Schmerz 2012, 26, 383–388. [Google Scholar] [CrossRef]
- Kiguchi, N.; Kobayashi, Y.; Kishioka, S. Chemokines and Cytokines in Neuroinflammation Leading to Neuropathic Pain. Curr. Opin. Pharmacol. 2012, 12, 55–61. [Google Scholar] [CrossRef]
- Ang, D.C.; Moore, M.N.; Hilligoss, J.; Tabbey, R. MCP-1 and IL-8 as Pain Biomarkers in Fibromyalgia: A Pilot Study. Pain Med. 2011, 12, 1154–1161. [Google Scholar] [CrossRef]
- Bote, M.E.; Garcia, J.J.; Hinchado, M.D.; Ortega, E. An Exploratory Study of the Effect of Regular Aquatic Exercise on the Function of Neutrophils from Women with Fibromyalgia: Role of IL-8 and Noradrenaline. Brain Behav. Immun. 2014, 39, 107–112. [Google Scholar] [CrossRef]
- Kumbhare, D.; Hassan, S.; Diep, D.; Duarte, F.C.K.; Hung, J.; Damodara, S.; West, D.W.D.; Selvaganapathy, P.R. Potential Role of Blood Biomarkers in Patients with Fibromyalgia: A Systematic Review with Meta-Analysis. Pain 2022, 163, 1232–1253. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, H.O.; Del Rey, A. Physiology of Psychoneuroimmunology: A Personal View. Brain Behav. Immun. 2007, 21, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Ortega, E. The “Bioregulatory Effect of Exercise” on the Innate/Inflammatory Responses. J. Physiol. Biochem. 2016, 72, 361–369. [Google Scholar] [CrossRef] [PubMed]
| Variable | Total FM Patients (n = 15) | FM (n = 8) | FM + CFS (n = 7) |
|---|---|---|---|
| Gender (%) | Women (100%) | Women (100%) | Women (100%) |
| Ethnic group (%) | Caucasian (100%) | Caucasian (100%) | Caucasian (100%) |
| Duration of FM/CFS diagnosed (years) | >2 | >2 | >2 |
| Age (years) | 59.38 ± 2.35 | 63.00 ± 3.35 | 55.75 ± 3.18 |
| BMI (kg/m2) | 29.19 ± 1.50 | 29.78 ± 1.35 | 28.60 ± 2.82 |
| Total FM Patients (n = 15) | FM (n = 8) | FM + CFS (n = 7) | ||||
|---|---|---|---|---|---|---|
| Basal | Post | Basal | Post | Basal | Post | |
| Weight (kg) | 76.93 ± 3.76 | 76.72 ± 3.76 | 75.33 ± 2.47 | 74.56 ± 2.47 | 78.76 ± 7.91 | 79.19 ± 7.62 |
| Body fat mass (%) | 40.15 ± 1.55 | 40.43 ± 1.54 | 41.25 ± 1.55 | 41.50 ± 1.53 | 39.05 ± 2.80 | 39.36 ± 2.79 |
| Bone mass (kg) | 2.30 ± 0.05 | 2.30 ± 0.06 | 2.33 ± 0.03 | 2.30 ± 0.05 | 2.28 ± 0.11 | 2.30 ± 0.11 |
| Body water (%) | 41.25 ± 1.03 | 41.34 ± 1.05 | 40.29 ± 1.42 | 40.29 ± 1.44 | 42.21 ± 1.59 | 42.40 ± 1.62 |
| Muscle mass (kg) | 42.78 ± 1.09 | 42.83 ± 1.07 | 42.99 ± 0.87 | 42.61 ± 1.08 | 42.56 ± 2.10 | 43.05 ± 1.98 |
| Visceral fat index | 9.53 ± 0.87 | 9.56 ± 0.75 | 10.31 ± 0.80 | 10.13 ± 0.81 * | 8.75 ± 1.45 | 9.00 ± 1.33 |
| Total FM Patients (n = 15) | FM (n = 8) | FM + CFS (n = 7) | ||||
|---|---|---|---|---|---|---|
| Basal | Post | Basal | Post | Basal | Post | |
| METs (mL O2/kg·min) | 1.43 ± 0.04 | 1.41 ± 0.04 | 1.47 ± 0.06 | 1.44 ± 0.05 | 1.37 ± 0.04 | 1.36 ± 0.05 |
| Activity bouts (<1 min) | 58.04 ± 7.59 | 49.10 ± 7.19 | 58.71 ± 11.08 | 54.70 ± 10.62 | 58.25 ± 9.81 | 39.25 ± 6.22 |
| Total Time in Activity bouts (min) | 974 ± 166.38 | 759.36 ± 123.17 * | 1004.71 ± 251.04 | 847.86 ± 179.17 | 921.75 ± 178.188 | 604.5 ± 118.98 |
| Average Time per Activity bout (min) | 15.55 ± 0.98 | 14.87 ± 0.62 | 16.05 ± 1.53 | 15.00 ± 0.74 | 14.84 ± 1.12 | 14.68 ± 1.19 |
| Sedentary bouts (<1 min) | 125.01 ± 7.95 | 120.67 ± 8.17 | 124.28 ± 12.58 | 119.14 ± 11.02 | 126.01 ± 9.19 | 122.8 ± 13.57 |
| Total Time in Sedentary bouts (min) | 2885.67 ± 235.55 | 2599.91 ± 155.15 | 2860.85 ± 335.14 | 2561.57 ± 219.28 | 2920.41 ± 360.31 | 2653.61 ± 238.06 |
| Average Time per Sedentary bout (min) | 23.22 ± 1.43 | 21.84 ± 0.72 | 22.95 ± 1.02 | 21.80 ± 0.92 | 23.61 ± 3.36 | 21.90 ± 1.23 |
| Sleep latency (min) | 0.70 ± 0.14 | 0.75 ± 0.14 | 1.14 ± 0.56 | 1.14 ± 0.86 | 0.91 ± 0.25 | 0.72 ± 0.22 |
| Sleep efficiency (%) | 87.71 ± 1.37 | 87.09 ± 1.37 | 88.72 ± 1.73 | 87.79 ± 2.03 | 86.29 ± 2.30 | 86.1 ± 1.49 |
| WASO (min) | 49.18 ± 5.25 | 53.37 ± 6.21 | 45.36 ± 6.15 | 49.95 ± 9.44 | 54.54 ± 9.50 | 58.16 ±7.61 |
| Total FM Patients (n = 15) | FM (n = 8) | FM + CFS (n = 7) | ||||
|---|---|---|---|---|---|---|
| Basal | Post | Basal | Post | Basal | Post | |
| Healthy Life and Personal Control Score | 63.27 ± 3.38 | 65.40 ± 3.21 | 66.13 ± 3.42 | 69.00 ± 3.99 | 60.00 ± 6.19 | 61.29 ± 4.99 |
| Beck’s Depression Score | 18.67 ± 2.77 | 15.67 ± 2.82 * | 18.13 ± 3.30 | 14.75 ± 3.24 * | 19.29 ± 4.86 | 16.71 ± 5.03 |
| Perceived Stress Score | 31.27 ± 3.02 | 26.87 ± 2.89 * | 31.75 ± 3.40 | 25.75 ± 3.26 * | 30.71 ± 5.48 | 28.14 ± 5.19 |
| Trait-Anxiety Score | 33.93 ± 3.74 | 31.40 ± 3.51 * | 34.38 ± 4.98 | 31.88 ± 4.78 | 33.43 ± 6.06 | 30.86 ± 5.59 * |
| State-Anxiety Score | 33.80 ± 4.33 | 31.53 ± 4.10 | 34.25 ± 5.23 | 31.25 ± 5.25 | 33.29 ± 7.55 | 31.86 ± 6.89 |
| Brief Pain Inventory Score | 6.24 ± 0.46 | 5.97 ± 0.46 | 6.57 ± 0.41 | 6.22 ± 0.37 | 5.88 ± 0.87 | 5.68 ± 0.91 |
| Brief Fatigue Inventory Score | 7.28 ± 0.40 | 6.52 ± 0.57 * | 6.95 ± 0.51 | 6.66 ± 0.41 | 7.65 ± 0.65 | 6.35 ± 1.18 * |
| Pittsburgh Sleep Quality Score | 12.60 ± 1.02 | 11.73 ± 0.81 | 11.13 ± 0.81 | 10.25 ± 0.41 | 14.29 ± 1.86 | 13.43 ± 1.46 |
| Coronavirus Anxiety Score | 2.07 ± 0.85 | 1.47 ± 0.60 | 2.88 ± 1.44 | 1.88 ± 0.97 | 1.14 ± 0.77 | 1.00 ± 0.69 |
| Fear of COVID-19 Score | 13.85 ± 2.06 | 13.73 ± 1.73 | 14.33 ± 4.01 | 15.50 ± 2.77 | 13.43 ± 2.03 | 11.71 ± 1.84 * |
| Fibromyalgia Impact Questionnaire Score | 55.37 ± 3.09 | 50.51 ± 3.31 * | 54.55 ± 3.98 | 51.35 ± 4.01 | 56.30 ± 5.13 | 49.56 ± 5.74 * |
| Gastrointestinal Health Score | 9.73 ± 1.39 | 9.53 ± 1.41 | 11.13 ± 1.88 | 10.88 ± 2.29 | 8.14 ± 2.04 | 8.00 ± 1.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hinchado, M.D.; Quero-Calero, C.D.; Otero, E.; Gálvez, I.; Ortega, E. Synbiotic Supplementation Improves Quality of Life and Inmunoneuroendocrine Response in Patients with Fibromyalgia: Influence of Codiagnosis with Chronic Fatigue Syndrome. Nutrients 2023, 15, 1591. https://doi.org/10.3390/nu15071591
Hinchado MD, Quero-Calero CD, Otero E, Gálvez I, Ortega E. Synbiotic Supplementation Improves Quality of Life and Inmunoneuroendocrine Response in Patients with Fibromyalgia: Influence of Codiagnosis with Chronic Fatigue Syndrome. Nutrients. 2023; 15(7):1591. https://doi.org/10.3390/nu15071591
Chicago/Turabian StyleHinchado, María Dolores, Carmen Daniela Quero-Calero, Eduardo Otero, Isabel Gálvez, and Eduardo Ortega. 2023. "Synbiotic Supplementation Improves Quality of Life and Inmunoneuroendocrine Response in Patients with Fibromyalgia: Influence of Codiagnosis with Chronic Fatigue Syndrome" Nutrients 15, no. 7: 1591. https://doi.org/10.3390/nu15071591
APA StyleHinchado, M. D., Quero-Calero, C. D., Otero, E., Gálvez, I., & Ortega, E. (2023). Synbiotic Supplementation Improves Quality of Life and Inmunoneuroendocrine Response in Patients with Fibromyalgia: Influence of Codiagnosis with Chronic Fatigue Syndrome. Nutrients, 15(7), 1591. https://doi.org/10.3390/nu15071591







