Circulating Metabolomic and Lipidomic Signatures Identify a Type 2 Diabetes Risk Profile in Low-Birth-Weight Men with Non-Alcoholic Fatty Liver Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Study Design
2.3. Untargeted Serum Metabolomics
2.4. Untargeted Serum Lipidomics
2.5. Statistical Analyses
2.6. Pathway Enrichment Analysis
2.7. Correlation Network Analysis
3. Results
3.1. Clinical Characteristics
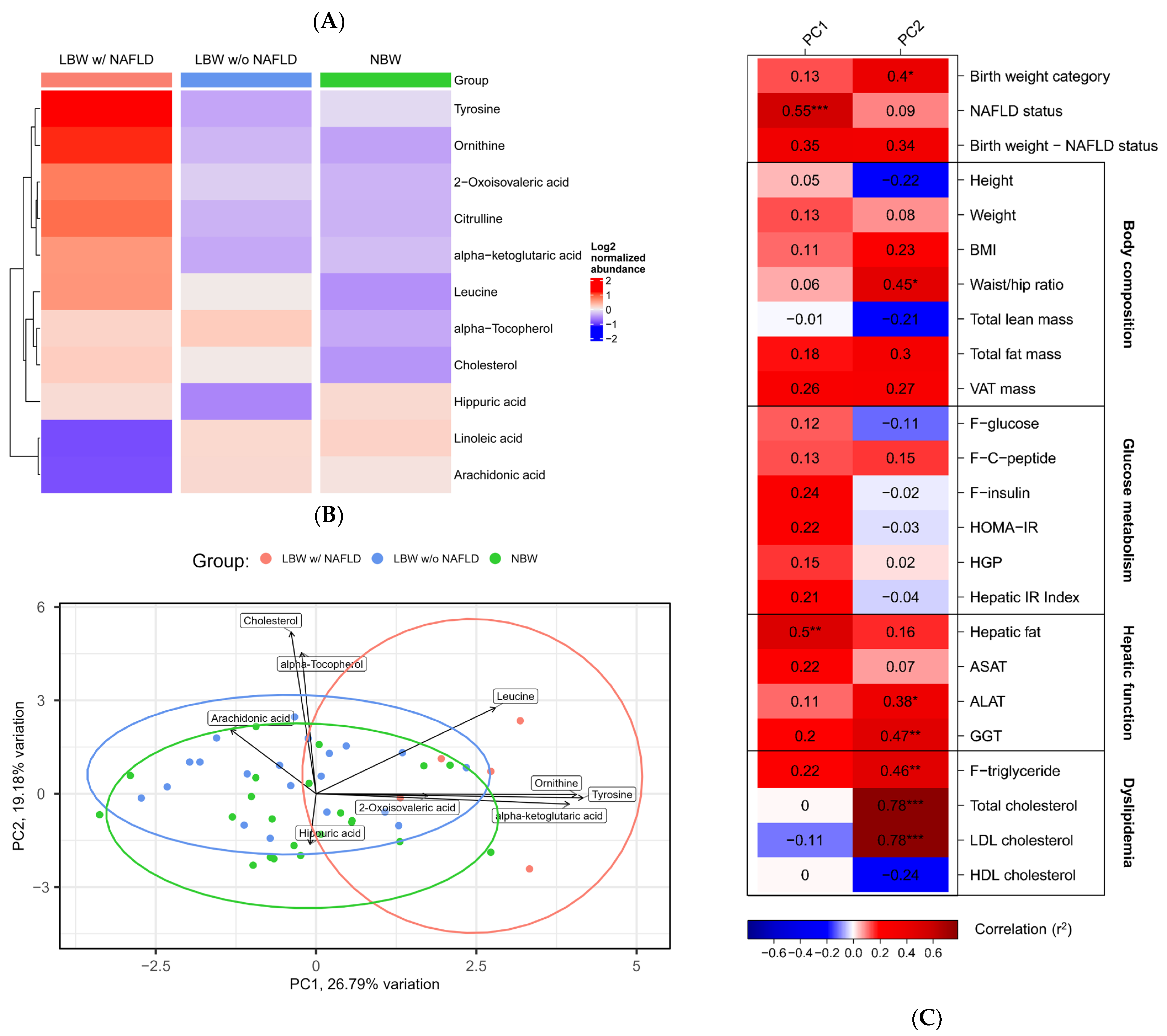
3.2. Serum Metabolomics
3.3. Pathway Enrichment Analyses
3.4. Serum Lipidomics
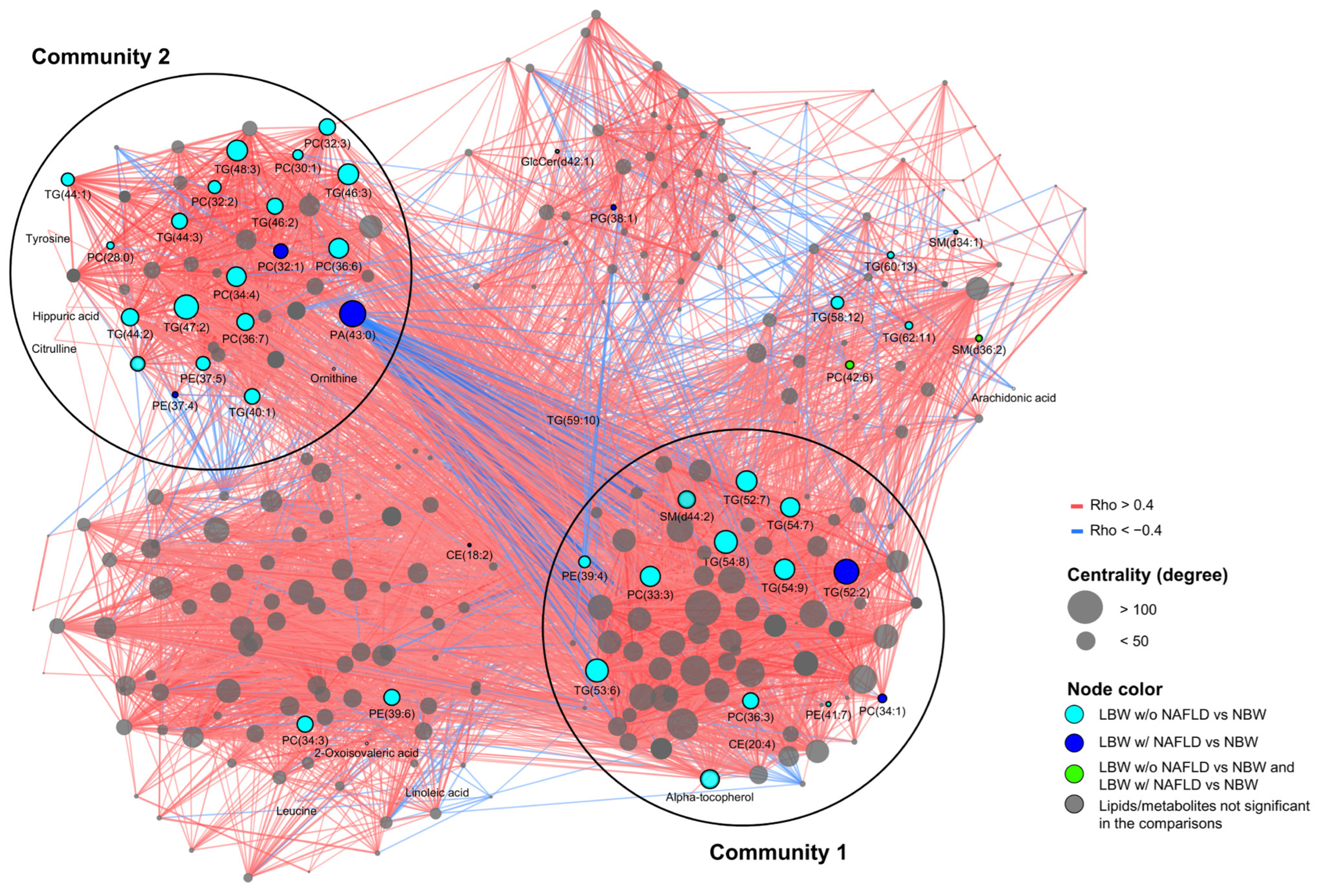
3.5. Correlation Network Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hales, C.N.; Barker, D.J.P. Type 2 (Non-Insulin-Dependent) Diabetes Mellitus: The Thrifty Phenotype Hypothesis. Diabetologia 1992, 35, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Harder, T.; Rodekamp, E.; Schellong, K.; Dudenhausen, J.W.; Plagemann, A. Birth Weight and Subsequent Risk of Type 2 Diabetes: A Meta-Analysis. Am. J. Epidemiol. 2007, 165, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Brøns, C.; Thuesen, A.C.B.; Elingaard-Larsen, L.O.; Justesen, L.; Jensen, R.T.; Henriksen, N.S.; Juel, H.B.; Størling, J.; Ried-Larsen, M.; Sparks, L.M.; et al. Increased Liver Fat Associates with Severe Metabolic Perturbations in Low Birth Weight Men. Eur. J. Endocrinol. 2022, 186, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.P.; Feldman, H.S.; Chambers, C.D.; Wilson, L.; Behling, C.; Clark, J.M.; Molleston, J.P.; Chalasani, N.; Sanyal, A.J.; Fishbein, M.H.; et al. Low and High Birth Weights Are Risk Factors for Nonalcoholic Fatty Liver Disease in Children. J. Pediatr. 2017, 187, 141–146.e1. [Google Scholar] [CrossRef]
- Nobili, V.; Marcellini, M.; Marchesini, G.; Vanni, E.; Manco, M.; Villani, A.; Bugianesi, E. Intrauterine Growth Retardation, Insulin Resistance, and Nonalcoholic Fatty Liver Disease in Children. Diabetes Care 2007, 30, 2638–2640. [Google Scholar] [CrossRef]
- Amadou, C.; Nabi, O.; Serfaty, L.; Lacombe, K.; Boursier, J.; Mathurin, P.; Ribet, C.; de Ledinghen, V.; Zins, M.; Charles, M.-A. Association between Birth Weight, Preterm Birth, and Nonalcoholic Fatty Liver Disease in a Community-Based Cohort. Hepatology 2022, 76, 1438–1451. [Google Scholar] [CrossRef]
- McGlinchey, A.J.; Govaere, O.; Geng, D.; Ratziu, V.; Allison, M.; Bousier, J.; Petta, S.; de Oliviera, C.; Bugianesi, E.; Schattenberg, J.M.; et al. Metabolic Signatures across the Full Spectrum of Non-Alcoholic Fatty Liver Disease. JHEP Rep. Innov. Hepatol. 2022, 4, 100477. [Google Scholar] [CrossRef]
- Masoodi, M.; Gastaldelli, A.; Hyötyläinen, T.; Arretxe, E.; Alonso, C.; Gaggini, M.; Brosnan, J.; Anstee, Q.M.; Millet, O.; Ortiz, P.; et al. Metabolomics and Lipidomics in NAFLD: Biomarkers and Non-Invasive Diagnostic Tests. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 835–856. [Google Scholar] [CrossRef]
- Kalhan, S.C.; Guo, L.; Edmison, J.; Dasarathy, S.; McCullough, A.J.; Hanson, R.W.; Milburn, M. Plasma Metabolomic Profile in Nonalcoholic Fatty Liver Disease. Metabolism 2011, 60, 404–413. [Google Scholar] [CrossRef]
- Gaggini, M.; Carli, F.; Rosso, C.; Buzzigoli, E.; Marietti, M.; Della Latta, V.; Ciociaro, D.; Abate, M.L.; Gambino, R.; Cassader, M.; et al. Altered Amino Acid Concentrations in NAFLD: Impact of Obesity and Insulin Resistance. Hepatology 2018, 67, 145–158. [Google Scholar] [CrossRef]
- Suvitaival, T.; Bondia-Pons, I.; Yetukuri, L.; Pöhö, P.; Nolan, J.J.; Hyötyläinen, T.; Kuusisto, J.; Orešič, M. Lipidome as a Predictive Tool in Progression to Type 2 Diabetes in Finnish Men. Metabolism 2018, 78, 1–12. [Google Scholar] [CrossRef]
- Orešič, M.; Hyötyläinen, T.; Kotronen, A.; Gopalacharyulu, P.; Nygren, H.; Arola, J.; Castillo, S.; Mattila, I.; Hakkarainen, A.; Borra, R.J.H.; et al. Prediction of Non-Alcoholic Fatty-Liver Disease and Liver Fat Content by Serum Molecular Lipids. Diabetologia 2013, 56, 2266–2274. [Google Scholar] [CrossRef]
- Rhee, E.P.; Cheng, S.; Larson, M.G.; Walford, G.A.; Lewis, G.D.; McCabe, E.; Yang, E.; Farrell, L.; Fox, C.S.; O’Donnell, C.J.; et al. Lipid Profiling Identifies a Triacylglycerol Signature of Insulin Resistance and Improves Diabetes Prediction in Humans. J. Clin. Investig. 2011, 121, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Würtz, P.; Wang, Q.; Niironen, M.; Tynkkynen, T.; Tiainen, M.; Drenos, F.; Kangas, A.J.; Soininen, P.; Skilton, M.R.; Heikkila, K.; et al. Metabolic Signatures of Birthweight in 18 288 Adolescents and Adults. Int. J. Epidemiol. 2016, 45, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.H.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human Gut Microbes Impact Host Serum Metabolome and Insulin Sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Castillo, S.; Mattila, I.; Miettinen, J.; Orešič, M.; Hyötyläinen, T. Data Analysis Tool for Comprehensive Two-Dimensional Gas Chromatography/Time-of-Flight Mass Spectrometry. Anal. Chem. 2011, 83, 3058–3067. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Bowden, J.A.; Heckert, A.; Ulmer, C.Z.; Jones, C.M.; Koelmel, J.P.; Abdullah, L.; Ahonen, L.; Alnouti, Y.; Armando, A.M.; Asara, J.M.; et al. Harmonizing Lipidomics: NIST Interlaboratory Comparison Exercise for Lipidomics Using SRM 1950-Metabolites in Frozen Human Plasma. J. Lipid Res. 2017, 58, 2275–2288. [Google Scholar] [CrossRef]
- Tofte, N.; Suvitaival, T.; Ahonen, L.; Winther, S.A.; Theilade, S.; Frimodt-Møller, M.; Ahluwalia, T.S.; Rossing, P. Lipidomic Analysis Reveals Sphingomyelin and Phosphatidylcholine Species Associated with Renal Impairment and All-Cause Mortality in Type 1 Diabetes. Sci. Rep. 2019, 9, 16398. [Google Scholar] [CrossRef]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Oresic, M. MZmine 2: Modular Framework for Processing, Visualizing, and Analyzing Mass Spectrometry-Based Molecular Profile Data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef]
- Khoury, S.; Canlet, C.; Lacroix, M.Z.; Berdeaux, O.; Jouhet, J.; Bertrand-Michel, J. Quantification of Lipids: Model, Reality, and Compromise. Biomolecules 2018, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Lazar, C.; Meganck, S.; Taminau, J.; Steenhoff, D.; Coletta, A.; Molter, C.; Weiss-Solís, D.Y.; Duque, R.; Bersini, H.; Nowé, A. Batch Effect Removal Methods for Microarray Gene Expression Data Integration: A Survey. Brief. Bioinform. 2013, 14, 469–490. [Google Scholar] [CrossRef]
- Olund Villumsen, S.; Benfeitas, R.; Knudsen, A.D.; Gelpi, M.; Høgh, J.; Thomsen, M.T.; Murray, D.; Ullum, H.; Neogi, U.; Nielsen, S.D. Integrative Lipidomics and Metabolomics for System-Level Understanding of the Metabolic Syndrome in Long-Term Treated HIV-Infected Individuals. Front. Immunol. 2021, 12, 742736. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Traag, V.A.; Waltman, L.; van Eck, N.J. From Louvain to Leiden: Guaranteeing Well-Connected Communities. Sci. Rep. 2019, 9, 5233. [Google Scholar] [CrossRef]
- Toubiana, D.; Sade, N.; Liu, L.; Rubio Wilhelmi, M.D.M.; Brotman, Y.; Luzarowska, U.; Vogel, J.P.; Blumwald, E. Correlation-Based Network Analysis Combined with Machine Learning Techniques Highlight the Role of the GABA Shunt in Brachypodium Sylvaticum Freezing Tolerance. Sci. Rep. 2020, 10, 4489. [Google Scholar] [CrossRef] [PubMed]
- Ajaz, S.; McPhail, M.J.; Gnudi, L.; Trovato, F.M.; Mujib, S.; Napoli, S.; Carey, I.; Agarwal, K. Mitochondrial Dysfunction as a Mechanistic Biomarker in Patients with Non-Alcoholic Fatty Liver Disease (NAFLD). Mitochondrion 2021, 57, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Morze, J.; Wittenbecher, C.; Schwingshackl, L.; Danielewicz, A.; Rynkiewicz, A.; Hu, F.B.; Guasch-Ferré, M. Metabolomics and Type 2 Diabetes Risk: An Updated Systematic Review and Meta-Analysis of Prospective Cohort Studies. Diabetes Care 2022, 45, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.A.; Smith, G.D.; Ben-Shlomo, Y.; Litchfield, P. Low Birth Weight Is Associated with Higher Adult Total Cholesterol Concentration in Men: Findings from an Occupational Cohort of 25,843 Employees. Circulation 2004, 110, 1258–1262. [Google Scholar] [CrossRef]
- Ribel-Madsen, A.; Hellgren, L.I.; Brøns, C.; Ribel-Madsen, R.; Newgard, C.B.; Vaag, A.A. Plasma Amino Acid Levels Are Elevated in Young, Healthy Low Birth Weight Men Exposed to Short-Term High-Fat Overfeeding. Physiol. Rep. 2016, 4, e13044. [Google Scholar] [CrossRef]
- De Chiara, F.; Heebøll, S.; Marrone, G.; Montoliu, C.; Hamilton-Dutoit, S.; Ferrandez, A.; Andreola, F.; Rombouts, K.; Grønbæk, H.; Felipo, V.; et al. Urea Cycle Dysregulation in Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2018, 69, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Iwakiri, Y.; Kim, M.Y. Nitric Oxide in Liver Diseases. Trends Pharmacol. Sci. 2015, 36, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Lake, A.D.; Novak, P.; Shipkova, P.; Aranibar, N.; Robertson, D.G.; Reily, M.D.; Lehman-Mckeeman, L.D.; Vaillancourt, R.R.; Cherrington, N.J. Branched Chain Amino Acid Metabolism Profiles in Progressive Human Nonalcoholic Fatty Liver Disease. Amino Acids 2015, 47, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Banton, S.; Tran, V.T.; Konomi, J.V.; Li, S.; Jones, D.P.; Vos, M.B. Amino Acid Metabolism Is Altered in Adolescents with Nonalcoholic Fatty Liver Disease—An Untargeted, High Resolution Metabolomics Study. J. Pediatr. 2015, 47, 603–615. [Google Scholar] [CrossRef]
- Miwa Kawanaka, M.; Nishino, K.; Oka, T.; Urata, N.; Nakamura, J.; Suehiro, M.; Kawamoto, H.; Chiba, Y.; Yamada, G. Tyrosine Levels Are Associated with Insulin Resistance in Patients with Nonalcoholic Fatty Liver Disease. Hepatic Med. Evid. Res. 2015, 7, 29–35. [Google Scholar] [CrossRef]
- Ji, M.; Jo, Y.; Choi, S.J.; Kim, S.M.; Kim, K.K.; Oh, B.-C.; Ryu, D.; Paik, M.-J.; Lee, D.H. Plasma Metabolomics and Machine Learning-Driven Novel Diagnostic Signature for Non-Alcoholic Steatohepatitis. Biomedicines 2022, 10, 1669. [Google Scholar] [CrossRef]
- Vangipurapu, J.; Stancáková, A.; Smith, U.; Kuusisto, J.; Laakso, M. Nine Amino Acids Are Associated With Decreased Insulin Secretion and Elevated Glucose Levels in a 7.4-Year Follow-up Study of 5,181 Finnish Men. Diabetes 2019, 68, 1353–1358. [Google Scholar] [CrossRef]
- Gobeil, É.; Maltais-Payette, I.; Taba, N.; Brière, F.; Ghodsian, N.; Abner, E.; Bourgault, J.; Gagnon, E.; Manikpurage, H.D.; Couture, C.; et al. Mendelian Randomization Analysis Identifies Blood Tyrosine Levels as a Biomarker of Non-Alcoholic Fatty Liver Disease. Metabolites 2022, 12, 440. [Google Scholar] [CrossRef]
- Arroyo, M.N.; Green, J.A.; Cnop, M.; Igoillo-Esteve, M. Trna Biology in the Pathogenesis of Diabetes: Role of Genetic and Environmental Factors. Int. J. Mol. Sci. 2021, 22, 496. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, B.; Yu, D.; Bian, M. Roles of TRNA Metabolism in Aging and Lifespan. Cell Death Dis. 2021, 12, 548. [Google Scholar] [CrossRef]
- Ozanne, S.E.; Jensen, C.B.; Tingey, K.J.; Martin-Gronert, M.S.; Grunnet, L.; Brons, C.; Storgaard, H.; Vaag, A.A. Decreased Protein Levels of Key Insulin Signalling Molecules in Adipose Tissue from Young Men with a Low Birthweight–Potential Link to Increased Risk of Diabetes? Diabetologia 2006, 49, 2993–2999. [Google Scholar] [CrossRef] [PubMed]
- Ozanne, S.E.; Jensen, C.B.; Tingey, K.J.; Storgaard, H.; Madsbad, S.; Vaag, A.A. Low Birthweight Is Associated with Specific Changes in Muscle Insulin-Signalling Protein Expression. Diabetologia 2005, 48, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wang, T.; Zhuang, X.; Sun, Q.; Wang, X.; Lin, H.; Feng, M.; Zhang, J.; Cao, Q.; Jiang, Y. Metabolic Analysis of Early Nonalcoholic Fatty Liver Disease in Humans Using Liquid Chromatography-Mass Spectrometry. J. Transl. Med. 2021, 19, 152. [Google Scholar] [CrossRef]
- Zhu, L.; Li, J.; Gong, Y.; Wu, Q.; Tan, S.; Sun, D.; Xu, X.; Zuo, Y.; Zhao, Y.; Wei, Y.-Q.; et al. Exosomal TRNA-Derived Small RNA as a Promising Biomarker for Cancer Diagnosis. Mol. Cancer 2019, 18, 74. [Google Scholar] [CrossRef]
- Pirola, C.J.; Sookoian, S. Multiomics Biomarkers for the Prediction of Nonalcoholic Fatty Liver Disease Severity. World J. Gastroenterol. 2018, 24, 1601–1615. [Google Scholar] [CrossRef]
- Gosis, B.S.; Wada, S.; Thorsheim, C.; Li, K.; Jung, S.; Rhoades, J.H.; Yang, Y.; Brandimarto, J.; Li, L.; Uehara, K.; et al. Inhibition of Nonalcoholic Fatty Liver Disease in Mice by Selective Inhibition of MTORC1. Science 2022, 376, eabf8271. [Google Scholar] [CrossRef]
- Gorden, D.L.; Myers, D.S.; Ivanova, P.T.; Fahy, E.; Maurya, M.R.; Gupta, S.; Min, J.; Spann, N.J.; McDonald, J.G.; Kelly, S.L.; et al. Biomarkers of NAFLD Progression: A Lipidomics Approach to an Epidemic 1. J. Lipid Res. 2015, 56, 722–736. [Google Scholar] [CrossRef]
- Flores, Y.N.; Amoon, A.T.; Su, B.; Velazquez-Cruz, R.; Ramírez-Palacios, P.; Salmerón, J.; Rivera-Paredez, B.; Sinsheimer, J.S.; Lusis, A.J.; Huertas-Vazquez, A.; et al. Serum Lipids Are Associated with Nonalcoholic Fatty Liver Disease: A Pilot Case-Control Study in Mexico. Lipids Health Dis. 2021, 20, 136. [Google Scholar] [CrossRef] [PubMed]
- Alibegovic, A.C.; Højbjerre, L.; Sonne, M.P.; Van Hall, G.; Alsted, T.J.; Kiens, B.; Stallknecht, B.; Dela, F.; Vaag, A. Increased Rate of Whole Body Lipolysis before and after 9 Days of Bed Rest in Healthy Young Men Born with Low Birth Weight. Am. J. Physiol.-Endocrinol. Metab. 2010, 298, E555–E564. [Google Scholar] [CrossRef] [PubMed]
- Tiwari-Heckler, S.; Gan-Schreier, H.; Stremmel, W.; Chamulitrat, W.; Pathil, A. Circulating Phospholipid Patterns in NAFLD Patients Associated with a Combination of Metabolic Risk Factors. Nutrients 2018, 10, 649. [Google Scholar] [CrossRef]
- Westerbacka, J.; Kotronen, A.; Fielding, B.A.; Wahren, J.; Hodson, L.; Perttilä, J.; Seppänen-Laakso, T.; Suortti, T.; Arola, J.; Hultcrantz, R.; et al. Splanchnic Balance of Free Fatty Acids, Endocannabinoids, and Lipids in Subjects with Nonalcoholic Fatty Liver Disease. Gastroenterology 2010, 139, 1961–1971.e1. [Google Scholar] [CrossRef] [PubMed]

| NBW | LBW | ANOVA | ||
|---|---|---|---|---|
| (n = 22) | w/o NAFLD (n = 21) | w/NAFLD (n = 5) | p-Value | |
| Birth weight (g) | 3804 (±172) | 2787 (±176) £ | 2800 (±187) # | <0.001 |
| Age (years) | 37.6 (±1.1) | 37.8 (±0.9) | 37.5 (±1.3) | 0.83 |
| Height (cm) | 184.0 (±6.3) | 178.2 (±5.7) £ | 181.0 (±5.0) | 0.009 |
| Weight (kg) | 84.64 (±10.63) | 76.15 (±7.32) £ | 91.22 (±6.58) * | 0.001 |
| BMI (kg/m2) | 25.0 (±2.8) | 24.0 (±2.2) | 27.9 (±2.4) * | 0.01 |
| Total lean mass (DXA) (kg) | 60.64 (±6.52) | 55.16 (±5.18) £ | 58.46 (±3.71) | 0.01 |
| Total fat mass (DXA) (kg) | 20.64 (±7.97) | 18.23 (±3.99) | 29.48 (±6.29) # * | 0.004 |
| Total fat mass (DXA) (%) | 24.83 (±7.46) | 24.73 (±4.03) | 33.32 (±5.48) # * | 0.02 |
| Hepatic fat (MRS) (%) a | 0.78 (0.58–0.90) | 0.80 (0.51–1.34) | 9.45 (7.44–9.54) # * | <0.001 |
| F-glucose (mmol/L) a | 5.1 (4.9–5.2) | 5.1 (4.8–5.2) | 5.2 (5.1–5.8) | 0.17 |
| F-insulin (pmol/L) a | 53.3 (33.9–87.2) | 40.7 (31.6–61.0) | 101.0 (96.0–132.3) * | 0.008 |
| F-C-peptide (pmol/L) | 702.3 (±264.7) | 604.5 (±156.7) | 943.6 (±353.0) * | 0.019 |
| F-triglyceride (mmol/L) a | 0.92 (0.60–1.27) | 0.91 (0.73–1.12) | 2.25 (1.29–5.36) # * | 0.03 |
| F-total cholesterol (mmol/L) | 4.27 (±0.76) | 4.67 (±5.39) | 5.28 (±1.61) | 0.05 |
| HOMA-IR a | 1.62 (1.07–3.08) | 1.38 (0.98–1.96) | 3.30 (2.95–4.43) * | 0.006 |
| HGP (µmol/kg FFM/min) b | 6.8 (5.4–8.7) | 5.7 (4.4–7.6) | 6.2 (6.0–8.6) | 0.53 |
| Hepatic IR Index (insulin*HGP) a | 356 (214–669) | 254 (166–438) | 610 (591–742) * | 0.02 |
| Group 1 | Group 2 | Metabolite | logFC | p-Value |
| LBW | NBW | Leucine | 0.68 | 0.02 |
| Alpha-tocopherol | 0.63 | 0.03 | ||
| Hippuric acid | −0.61 | 0.04 | ||
| Cholesterol | 0.59 | 0.04 | ||
| LBW w/NAFLD | NBW | Ornithine | 1.59 | 0.001 |
| Tyrosine | 1.33 | 0.007 | ||
| Citrulline | 1.19 | 0.02 | ||
| Leucine | 1.15 | 0.02 | ||
| Linoleic acid | −1.12 | 0.02 | ||
| 2-Oxoisovaleric acid | 1.09 | 0.03 | ||
| Arachidonic acid | −0.99 | 0.05 | ||
| LBW w/o NAFLD | NBW | Hippuric acid | −0.75 | 0.01 |
| Alpha-tocopherol | 0.64 | 0.04 | ||
| LBW w/o NAFLD | NBW | Tyrosine | 1.63 | 0.001 |
| Ornithine | 1.47 | 0.003 | ||
| Citrulline | 1.19 | 0.02 | ||
| Linoleic acid | −1.07 | 0.03 | ||
| Arachidonic acid | −1.06 | 0.03 | ||
| Alpha-ketoglutaric acid | 0.99 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elingaard-Larsen, L.O.; Villumsen, S.O.; Justesen, L.; Thuesen, A.C.B.; Kim, M.; Ali, M.; Danielsen, E.R.; Legido-Quigley, C.; van Hall, G.; Hansen, T.; et al. Circulating Metabolomic and Lipidomic Signatures Identify a Type 2 Diabetes Risk Profile in Low-Birth-Weight Men with Non-Alcoholic Fatty Liver Disease. Nutrients 2023, 15, 1590. https://doi.org/10.3390/nu15071590
Elingaard-Larsen LO, Villumsen SO, Justesen L, Thuesen ACB, Kim M, Ali M, Danielsen ER, Legido-Quigley C, van Hall G, Hansen T, et al. Circulating Metabolomic and Lipidomic Signatures Identify a Type 2 Diabetes Risk Profile in Low-Birth-Weight Men with Non-Alcoholic Fatty Liver Disease. Nutrients. 2023; 15(7):1590. https://doi.org/10.3390/nu15071590
Chicago/Turabian StyleElingaard-Larsen, Line O., Sofie O. Villumsen, Louise Justesen, Anne Cathrine B. Thuesen, Min Kim, Mina Ali, Else R. Danielsen, Cristina Legido-Quigley, Gerrit van Hall, Torben Hansen, and et al. 2023. "Circulating Metabolomic and Lipidomic Signatures Identify a Type 2 Diabetes Risk Profile in Low-Birth-Weight Men with Non-Alcoholic Fatty Liver Disease" Nutrients 15, no. 7: 1590. https://doi.org/10.3390/nu15071590
APA StyleElingaard-Larsen, L. O., Villumsen, S. O., Justesen, L., Thuesen, A. C. B., Kim, M., Ali, M., Danielsen, E. R., Legido-Quigley, C., van Hall, G., Hansen, T., Ahluwalia, T. S., Vaag, A. A., & Brøns, C. (2023). Circulating Metabolomic and Lipidomic Signatures Identify a Type 2 Diabetes Risk Profile in Low-Birth-Weight Men with Non-Alcoholic Fatty Liver Disease. Nutrients, 15(7), 1590. https://doi.org/10.3390/nu15071590







