The Effect of Copper Nanoparticles and a Different Source of Dietary Fibre in the Diet on the Integrity of the Small Intestine in the Rat
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Dietary Fibre
2.2. Experimental Protocol
2.3. Sample Collection and Analyses
2.4. RNA Extraction and Quantitative Real-Time PCR
2.5. Histological Examination of Organs
2.6. Data Analysis and Statistics
3. Results
3.1. One-Way Analysis of Variance (ANOVA)
3.1.1. C vs. CN, PN, JN and SN
3.1.2. CH vs. CNH, PNH, JNH and SNH
3.2. Two-Way Analysis of Variance (ANOVA)
3.2.1. Effect of CuNPs Dose
3.2.2. Effect of Dietary Fibre Type
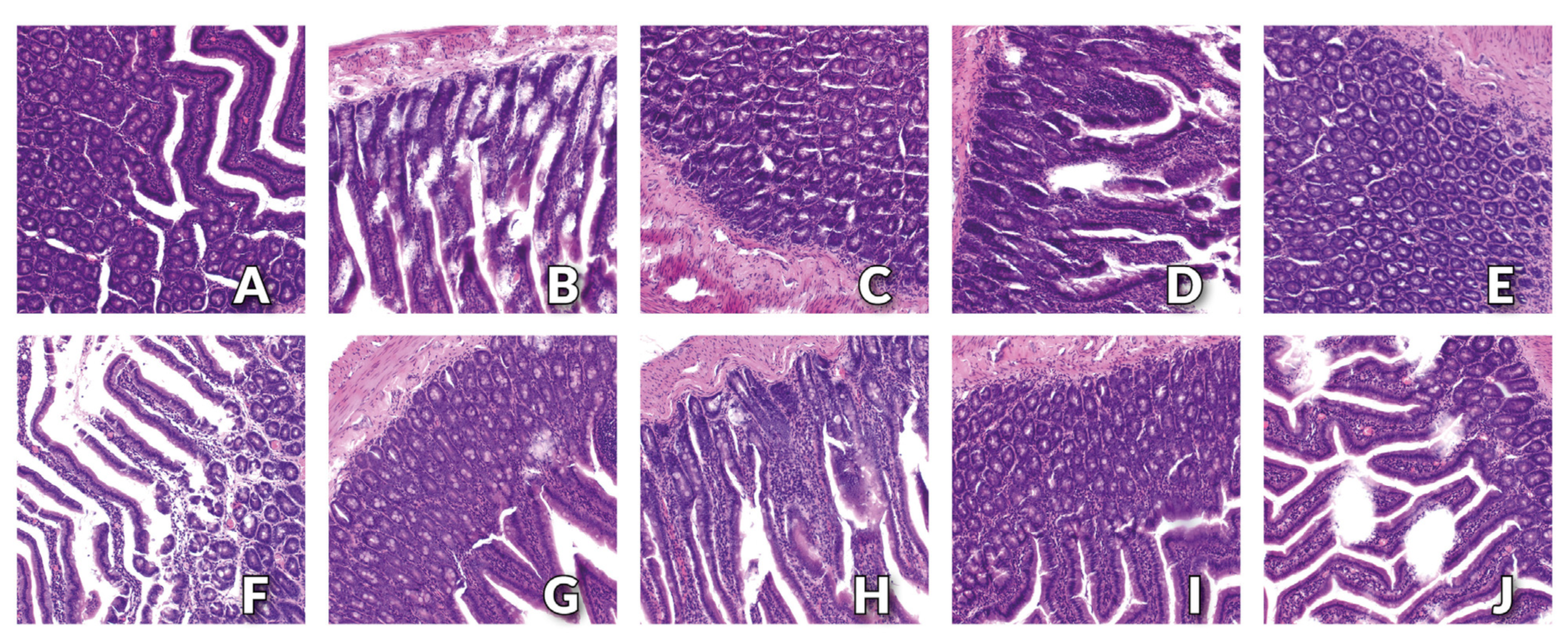
3.3. Histology Examination of Small Intestine
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brewer, G.J. Risks of copper and iron toxicity during aging in humans. Chem. Res. Toxicol. 2010, 23, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Angelova, M.; Asenova, S.; Nedkova, V.; Koleva-Kolarova, R. Copper in the human organism. Trakia J. Sci. 2011, 9, 88–98. [Google Scholar]
- Opazo, C.M.; Greenough, M.A.; Bush, A.I. Copper: From neurotransmission to neuroproteostasis. Front. Aging Neurosci. 2014, 6, 143. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kalita, J.; Misra, U.K.; Bora, H.K. A study of dose response and organ susceptibility of copper toxicity in a rat model. J. Trace Elem. Med. Biol. 2015, 29, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kalita, J.; Bora, H.K.; Misra, U.K. Temporal kinetics of organ damage in copper toxicity: A histopathological correlation in rat model. Regul. Toxicol. Pharmacol. 2016, 81, 372–380. [Google Scholar] [CrossRef]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.F.; Margaritis, I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elem. Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef]
- Tishchenko, K.I.; Beloglazkina, E.K.; Mazhuga, A.G.; Zyk, N.V. Copper-containing enzymes: Site types and low-molecular-weight model compounds. Ref. J. Chem. 2016, 6, 49–82. [Google Scholar] [CrossRef]
- Ognik, K.; Stępniowska, A.; Cholewińska, E.; Kozłowski, K. The effect of administration of copper nanoparticles to chickens in drinking water on estimated intestinal absorption of iron, zinc, and calcium. Poult. Sci. 2016, 95, 2045–2051. [Google Scholar] [CrossRef]
- Scott, A.; Vadalasetty, K.P.; Chwalibog, A.; Sawosz, E. Copper nanoparticles as an alternative feed additive in poultry diet: A review. Nanotechnol. Rev. 2018, 7, 69–93. [Google Scholar] [CrossRef]
- Sawosz, E.; Łukasiewicz, M.; Łozicki, A.; Sosnowska, M.; Jaworski, S.; Niemiec, J.; Scott, A.; Jankowski, J.; Józefiak, D.; Chwalibog, A. Effect of copper nanoparticles on the mineral content of tissues and droppings, and growth of chickens. Arch. Anim. Nutr. 2018, 72, 396–406. [Google Scholar] [CrossRef]
- Cholewińska, E.; Ognik, K.; Fotschki, B.; Zduńczyk, Z.; Juśkiewicz, J. Comparison of the effect of dietary copper nanoparticles and one copper (II) salt on the copper biodistribution and gastrointestinal and hepatic morphology and function in a rat model. PLoS ONE 2018, 13, e0197083. [Google Scholar] [CrossRef] [PubMed]
- Cholewińska, E.; Juśkiewicz, J.; Ognik, K. Comparison of the effect of dietary copper nanoparticles and one copper (II) salt on the metabolic and immune status in a rat model. J. Trace Elem. Med. Biol. 2018, 48, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Ognik, K.; Cholewińska, E.; Juśkiewicz, J.; Zduńczyk, Z.; Tutaj, K.; Szlązak, R. The effect of copper nanoparticles and copper (II) salt on redox reactions and epigenetic changes in a rat model. J. Anim. Physiol. Anim. Nutr. 2019, 103, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Magaye, R.; Zhao, J.; Bowman, L.; Ding, M. Genotoxicity and carcinogenicity of cobalt-, nickel- and copper-based nanoparticles. Exp. Ther. Med. 2012, 4, 551–561. [Google Scholar] [CrossRef]
- Elhussainy, E.M.A.; El-Shourbagy, S. Protective effect of Multivitamin Complex on Copper Oxide nanoparticles (nanoCuO) induced toxicity in rats. BESPS 2014, 34, 404–418. [Google Scholar] [CrossRef]
- Lee, I.C.; Ko, J.W.; Park, S.H.; Shin, N.R.; Shin, I.S.; Moon, C.; Kim, J.H.; Kim, H.C.; Kim, J.C. Comparative toxicity and biodistribution assessments in rats following subchronic oral exposure to copper nanoparticles and microparticles. Part. Fibre Toxicol. 2016, 13, 56. [Google Scholar] [CrossRef]
- Lee, I.C.; Ko, J.W.; Park, S.H.; Lim, J.O.; Shin, I.S.; Moon, C.; Kim, S.H.; Heo, J.D.; Kim, J.C. Comparative toxicity and biodistribution of copper nanoparticles and cupric ions in rats. Int. J. Nanomed. 2016, 11, 2883–2900. [Google Scholar]
- Ognik, K.; Cholewińska, E.; Tutaj, K.; Cendrowska-Pinkosz, M.; Dworzański, W.; Dworzańska, A.; Juśkiewicz, J. The effect of the source and dosage of dietary Cu on redox status in rat tissues. J. Anim. Physiol. Anim. Nutr. 2020, 104, 352–361. [Google Scholar] [CrossRef]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef]
- McRorie, J.W., Jr.; McKeown, N.M. Understanding the Physics of Functional Fibers in the Gastrointestinal Tract: An Evidence-Based Approach to Resolving Enduring Misconceptions about Insoluble and Soluble Fiber. J. Acad. Nutr. Diet. 2017, 117, 251–264. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
- Gralak, M.A.; Leontowicz, M.; Morawiec, M.; Bartnikowska, E.; Kulasek, G.W. Comparison of the influence of dietary fibre sources with different proportions of soluble and insoluble fibre on Ca, Mg, Fe, Zn, Mn and Cu apparent absorption in rats. Arch. Anim. Nutr. 1996, 49, 293–299. [Google Scholar] [CrossRef] [PubMed]
- El-Zoghbi, M.; Sitohy, M.Z. Mineral absorption by albino rats as affected by some types of dietary pectins with different degrees of esterification. Nahrung 2001, 45, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Shin, H.K. The water-soluble extract of chicory reduces glucose uptake from the perfused jejunum in rats. J. Nutr. 1996, 126, 2236–2242. [Google Scholar] [CrossRef]
- Coudray, C.; Feillet-Coudray, C.; Gueux, E.; Mazur, A.; Rayssiguier, Y. Dietary inulin intake and age can affect intestinal absorption of zinc and copper in rats. J. Nutr. 2006, 136, 117–122. [Google Scholar] [CrossRef]
- Krzysik, M.; Grajeta, H.; Prescha, A. Effect of pectin and cellulose on the content of minerals in the femur of rats. Pol. J. Food Nutr. Sci. 2009, 59, 357–360. [Google Scholar]
- Coudray, C.; Demigné, C.; Rayssiguier, Y. Effects of dietary fibers on magnesium absorption in animals and humans. J. Nutr. 2003, 133, 1–4. [Google Scholar] [CrossRef]
- Baye, K.; Guyot, J.-P.; Mouquet-Rivier, C. The unresolved role of dietary fibers on mineral absorption. Crit. Rev. Food Sci. Nutr. 2017, 57, 949–957. [Google Scholar] [CrossRef]
- Turnlund, J.R.; King, J.C.; Gong, B.; Keyes, W.R.; Michel, M.C. A stable isotope study of copper absorption in young men: Effect of phytate and α-cellulose. Am. J. Clin. Nutr. 1985, 42, 18–23. [Google Scholar] [CrossRef]
- Adams, S.; Sello, C.T.; Qin, G.-X.; Che, D.; Han, R. Does Dietary Fiber Affect the Levels of Nutritional Components after Feed Formulation? Fibers 2018, 6, 29. [Google Scholar] [CrossRef]
- Cholewińska, E.; Juśkiewicz, J.; Majewski, M.; Smagieł, R.; Listos, P.; Fotschki, B.; Godycka-Kłos, I.; Ognik, K. Effect of Copper Nanoparticles in the Diet of WKY and SHR Rats on the Redox Profile and Histology of the Heart, Liver, Kidney, and Small Intestine. Antioxidants 2022, 11, 910. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. OJEU 2010, L276, 33–79. [Google Scholar]
- Reeves, P.G. Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr. 1997, 127, 838–841. [Google Scholar] [CrossRef] [PubMed]
- Coudray, C.; Rambeau, M.; Feillet-Coudray, C.; Tressol, J.C.; Demigne, C.; Gueux, E.; Mazur, A.; Rayssiguier, Y. Dietary inulin intake and age can significantly affect intestinal absorption of calcium and magnesium in rats: A stable isotope approach. Nutr. J. 2005, 4, 29. [Google Scholar] [CrossRef]
- Kang, D.H.; Jung, E.Y.; Chang, U.J.; Bae, S.H.; Suh, H.J. Psyllium husk combined with hydroxycitrate reduces body weight gain and body fat in diet-induced obese rats. Nutr. Res. 2007, 27, 349–355. [Google Scholar] [CrossRef]
- Suriyasomboon, A.; Suriyasomboon, A.; Chantip, S.; Petchsom, A. Effect of Dietary Fiber, Ocimumcanum and Psyllium Seed, in High Cholesterol-diet Fed Rats. Thai J. Vet. Med. 2011, 41, 479–485. [Google Scholar]
- Adam, C.L.; Williams, P.A.; Dalby, M.J.; Garden, K.; Thomson, L.M.; Richardson, A.J.; Gratz, S.W.; Ross, A.W. Different types of soluble fermentable dietary fibre decrease food intake, body weight gain and adiposity in young adult male rats. Nutr. Metab. 2014, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Regalado-Rentería, E.; Aguirre-Rivera, J.R.; Godínez-Hernández, C.I.; García-López, J.C.; Oros-Ovalle, A.C.; Martínez-Gutiérrez, F.; Martinez-Martinez, M.; Ratering, S.; Schnell, S.; Ruíz-Cabrera, M.Á.; et al. Effects of Agave Fructans, Inulin, and Starch on Metabolic Syndrome Aspects in Healthy Wistar Rats. ACS Omega 2020, 5, 10740–10749. [Google Scholar] [CrossRef]
- Huwiler, V.V.; Schönenberger, K.A.; Segesser von Brunegg, A.; Reber, E.; Mühlebach, S.; Stanga, Z.; Balmer, M.L. Prolonged Isolated Soluble Dietary Fibre Supplementation in Overweight and Obese Patients: A Systematic Review with Meta-Analysis of Randomised Controlled Trials. Nutrients 2022, 14, 2627. [Google Scholar] [CrossRef]
- Chen, C.; Shang, C.; Xin, L.; Xiang, M.; Wang, Y.; Shen, Z.; Jiao, L.; Ding, F.; Cui, X. Beneficial effects of psyllium on the prevention and treatment of cardiometabolic diseases. Food Funct. 2022, 13, 7473–7486. [Google Scholar] [CrossRef]
- Farness, P.L.; Schneeman, B.O. Effects of dietary cellulose, pectin and oat bran on the small intestine in the rat. J. Nutr. 1982, 112, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Dongowski, G.; Lorenz, A.; Proll, J. The degree of methylation influences the degradation of pectin in the intestinal tract of rats and in vitro. J. Nutr. 2002, 132, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Arjmandi, B.H.; Sohn, E.; Juma, S.; Murthy, S.R.; Daggy, B.P. Native and partially hydrolyzed psyllium have comparable effects on cholesterol metabolism in rats. J. Nutr. 1997, 127, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, M.; Knudsen, K.E.B.; Jørgensen, H.; Oomah, D.; Bügel, S.; Toubro, S.; Tetens, I.; Astrup, A. Linseed Dietary Fibers Reduce Apparent Digestibility of Energy and Fat and Weight Gain in Growing Rats. Nutrients 2013, 5, 3287–3298. [Google Scholar] [CrossRef]
- Krupa-Kozak, U.; Markiewicz, L.H.; Lamparski, G.; Juśkiewicz, J. Administration of Inulin-Supplemented Gluten-Free Diet Modified Calcium Absorption and Caecal Microbiota in Rats in a Calcium-Dependent Manner. Nutrients 2017, 9, 702. [Google Scholar] [CrossRef]
- Pirman, T.; Patureau Mirand, P.; Oresnik, A.; Salobir, J. Effects of dietary pectin on protein digestion and metabolism in growing rats. Acta Agric. Slov. 2009, 94, 111–119. [Google Scholar]
- Tan, Z.; Ou, Y.; Cai, W.; Zheng, Y.; Li, H.; Mao, Y.; Zhou, S.; Tu, J. Advances in the Clinical Application of Histamine and Diamine Oxidase (DAO) Activity: A Review. Catalysts 2023, 13, 48. [Google Scholar] [CrossRef]
- Şahin, M.; Buluş, H.; Yavuz, A.; Turhan, V.B.; Öztürk, B.; Kılıç, N.A.; Babayiğit, M.; Öztürk, D. The role of the lactate level in determining the risk rates of small bowel resection in incarcerated hernias. Ulus Travma Acil Cerrahi Derg 2020, 26, 593–599. [Google Scholar]
- Iraporda, C.; Romanin, D.E.; Bengoa, A.A.; Errea, A.J.; Cayet, D.; Foligné, B.; Sirard, J.C.; Garrote, G.L.; Abraham, A.G.; Rumbo, M. Local Treatment with Lactate Prevents Intestinal Inflammation in the TNBS-Induced Colitis Model. Front. Immunol. 2016, 7, 651. [Google Scholar]
- Louis, P.; Duncan, S.; Sheridan, P.; Walker, A.; Flint, H. Microbial lactate utilisation and the stability of the gut microbiome. Gut Microbiome 2022, 3, e3. [Google Scholar] [CrossRef]
- Okada, T.; Fukuda, S.; Hase, K.; Nishiumi, S.; Izumi, Y.; Yoshida, M.; Hagiwara, T.; Kawashima, R.; Yamazaki, M.; Oshio, T. Microbiota-derived lactate accelerates colon epithelial cell turnover in starvation-refed mice. Nat. Commun. 2013, 4, 1654. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, G.; MacDonald, K.; Reid, G.; Burton, J.P. The influence of the human microbiome and probiotics on cardiovascular health. Gut Microbes 2014, 5, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Solovyev, M.M.; Kashinskaya, E.N.; Izvekova, G.I.; Glupov, V.V. pH values and activity of digestive enzymes in the gastrointestinal tract of fish in Lake Chany (West Siberia). J. Ichthyol. 2015, 55, 251–258. [Google Scholar] [CrossRef]
- Stewart, A.S.; Pratt-Phillips, S.; Gonzalez, L.M. Alterations in intestinal permeability: The role of the “Leaky Gut” in health and disease. J. Equine Vet. Sci. 2017, 52, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Dworzański, W.; Cholewińska, E.; Fotschki, B.; Juśkiewicz, J.; Ognik, K. Oxidative, epigenetic changes and fermentation processes in the intestine of rats fed high-fat diets supplemented with various chromium forms. Sci. Rep. 2022, 12, 9817. [Google Scholar] [CrossRef] [PubMed]
- Simon, H.; Vartanian, V.; Wong, M.H.; Nakabeppu, Y.; Sharma, P.; Lloyd, R.S.; Sampath, H. OGG1 deficiency alters the intestinal microbiome and increases intestinal inflammation in a mouse model. PLoS ONE 2020, 15, e0227501. [Google Scholar] [CrossRef]
- Thakur, S.; Sarkar, B.; Cholia, R.; Gautam, N.; Dhiman, M.; Mantha, A.K. APE1/Ref-1 as an emerging therapeutic target for various human diseases: Phytochemical modulation of its functions. Exp. Mol. Med. 2014, 46, e106. [Google Scholar] [CrossRef]
- Hussar, P. Apoptosis Regulators Bcl-2 and Caspase-3. Encyclopedia 2022, 2, 111. [Google Scholar] [CrossRef]
- Liu, W.; Mi, S.; Ruan, Z.; Li, J.; Shu, X.; Yao, K.; Jiang, M.; Deng, Z. Dietary Tryptophan Enhanced the Expression of Tight Junction Protein ZO-1 in Intestine. J. Food Sci. 2017, 82, 562–567. [Google Scholar] [CrossRef]
- Tian, S.; Guo, R.; Wei, S.; Kong, Y.; Wei, X.; Wang, W.; Shi, X.; Jiang, H. Curcumin protects against the intestinal ischemia-reperfusion injury: Involvement of the tight junction protein ZO-1 and TNF-α related mechanism. Korean J. Physiol. Pharm. 2016, 20, 147–152. [Google Scholar] [CrossRef]
- Hwang, I.; An, B.S.; Yang, H.; Kang, H.S.; Jung, E.M.; Jeung, E.B. Tissue-specific expression of occludin, zona occludens-1, and junction adhesion molecule A in the duodenum, ileum, colon, kidney, liver, lung, brain, and skeletal muscle of C57BL mice. J. Physiol. Pharmacol. 2013, 64, 11–18. [Google Scholar] [PubMed]
- Shen, Z.Y.; Zhang, J.; Song, H.L.; Zheng, W.P. Bone-marrow mesenchymal stem cells reduce rat intestinal ischemia-reperfusion injury, ZO-1 downregulation and tight junction disruption via a TNF-α-regulated mechanism. World J. Gastroenterol. 2013, 19, 3583–3595. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.; Chi, A.L.; Lim, S.; Cui, G.; Dubeykovskaya, Z.; Ai, W.; Fleming, J.V.; Takaishi, S.; Wang, T.C. Gastrin regulates the TFF2 promoter through gastrin-responsive cis-acting elements and multiple signaling pathways. Am. J. Physiol. -Gastrointest. Liver Physiol. 2007, 292, G1726–G1737. [Google Scholar] [CrossRef] [PubMed]
- Henson, T.E.; Navratilova, J.; Tennant, A.H.; Bradham, K.D.; Rogers, K.R.; Hughes, M.F. In Vitro intestinal toxicity of copper oxide nanoparticles in rat and human cell models. Nanotoxicology 2019, 13, 795–811. [Google Scholar] [CrossRef] [PubMed]
- Asvarujanon, P.; Ishizuka, S.; Hara, H. Inhibitory effects of psyllium on rat mineral absorption were abolished by reduction of viscosity with partial hydrolysis. Biosci. Biotechnol. Biochem. 2004, 68, 1737–1742. [Google Scholar] [CrossRef]
- Stark, A.H.; Madar, Z. In vitro production of short-chain fatty acids by bacterial fermentation of dietary fiber compared with effects of those fibers on hepatic sterol synthesis in rats. J. Nutr. 1993, 123, 2166–2173. [Google Scholar]
- Spiller, G.A. Handbook of Dietary Fiber in Human Nutrition, 3rd ed.; CRC Press LLC: Boca Raton, FL, USA, 2001. [Google Scholar]
| C | CH | CN | CNH | PN | PNH | JN | JNH | SN | SNH | |
|---|---|---|---|---|---|---|---|---|---|---|
| Casein 1 | 14.8 | 14.8 | 14.8 | 14.8 | 14.8 | 14.8 | 14.8 | 14.8 | 14.8 | 14.8 |
| DL-methionine | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Cellulose 2 | 8.0 | 8.0 | 8.0 | 8.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Pectin | 6 | 6 | ||||||||
| Inulin | 6 | 6 | ||||||||
| Psyllium | 6 | 6 | ||||||||
| Choline chloride | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Rapeseed oil | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 |
| Cholesterol | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Vitamin mix 3 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Mineral mix 4 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 |
| Maize starch 5 | 64.0 | 64.0 | 64.0 | 64.0 | 64.0 | 64.0 | 64.0 | 64.0 | 64.0 | 64.0 |
| Calculation: | ||||||||||
| Cu from, mg/kg | ||||||||||
| CuCO3 | 6.5 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cu-NPs | 0 | 0 | 6.5 | 13 | 6.5 | 13 | 6.5 | 13 | 6.5 | 13 |
| Gene | Primer | Sequence (5′–3′) | Tm (°C) | Product Size (nt) | Gen Bank Access No. |
|---|---|---|---|---|---|
| ACTB | Forward | CCCGCGAGTACAACCTTCTTG | 61,27 | 71 | NM_031144.3 |
| Reverse | GTCATCCATGGCGAACTGGTG | 61,61 | |||
| GAPDH | Forward | CCGCATCTTCTTGTGCAGTG | 59.83 | 79 | NM_017008.4 |
| Reverse | CGATACGGCCAAATCCGTTC | 59.42 | |||
| ZO-1 | Forward | GGAGCGGGGACAAGATGAAG | 60.46 | 123 | XM_039105296.1 |
| Reverse | AGGATGGAGTTACCCACAGC | 59.09 | |||
| OCLN | Forward | GGGGCGCAGCAGGTCT | 61.91 | 181 | NM_031329.3 |
| Reverse | GTGCATCTCTCCGCCATACA | 59.90 | |||
| TFF2 | Forward | ACGCCCTCCAACAGAAAGAA | 59.53 | 140 | NM_053844.2 |
| Reverse | CATTGTTCCGACGCTTGGTT | 59.41 | |||
| OGG1 | Forward | GACATCGCACCCTAACCTCC | 60.18 | 118 | NM_030870.1 |
| Reverse | CTTTGCTCCCTCCACCGGAA | 62.12 |
| Initial BW | Final BW | BW Gain | BW Gain | Intake | Heart | Spleen | Kidneys | Body Fat ^ | Body Lean ^ | Body Fluids ^ | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| g | g | g | g/day | g/day | g/100 g BW | g/100 g BW | g/100 g BW | % | % | % | |
| Control C | 278 | 396 | 118 | 2.91 | 19.0 | 0.245 | 0.183 | 0.547 | 13.4 | 61.6 | 25.1 |
| Control CH | 278 | 394 | 116 | 2.86 | 18.8 | 0.243 | 0.175 | 0.557 | 12.7 | 61.2 | 24.2 |
| 2-way ANOVA: | |||||||||||
| CN | 278 | 398 | 121 | 2.97 | 19.1 | 0.247 | 0.184 | 0.542 | 12.2 | 62.9 | 24.9 |
| CNH | 278 | 393 | 116 | 2.83 | 19.1 | 0.246 | 0.190 & | 0.542 | 12.9 | 62.4 | 24.7 |
| PN | 278 | 395 | 118 | 2.86 | 18.7 | 0.252 | 0.181 | 0.556 | 12.6 | 61.9 | 25.5 |
| PNH | 278 | 398 | 120 | 2.92 | 18.9 | 0.243 | 0.181 | 0.558 | 13.0 | 63.0 | 24.0 |
| JN | 278 | 379 | 101 | 2.46 # | 17.5 # | 0.255 | 0.202 | 0.578 # | 13.0 | 62.4 | 24.6 |
| JNH | 278 | 389 | 112 | 2.72 | 18.3 | 0.246 | 0.189 & | 0.562 | 12.8 | 62.5 | 24.6 |
| SN | 278 | 385 | 107 | 2.58 # | 18.4 | 0.250 | 0.194 | 0.573 | 12.8 | 63.5 | 24.1 |
| SNH | 278 | 386 | 108 | 2.61 | 18.2 | 0.247 | 0.180 | 0.564 | 12.8 | 62.2 | 25.0 |
| SEM | 1.047 | 1.725 | 1.851 | 0.042 | 0.110 | 0.002 | 0.002 | 0.004 | 0.193 | 0.426 | 0.503 |
| Cu-NP dose (D) | |||||||||||
| L (6.5 mg/kg) | 277 | 389 | 112 | 2.72 | 18.4 | 0.251 | 0.190 | 0.562 | 12.6 | 62.7 | 24.8 |
| H (13 mg/kg) | 277 | 392 | 114 | 2.76 | 18.6 | 0.246 | 0.185 | 0.556 | 12.9 | 62.5 | 24.6 |
| p value | 0.888 | 0.559 | 0.648 | 0.626 | 0.346 | 0.243 | 0.352 | 0.544 | 0.487 | 0.895 | 0.882 |
| Fibre type (F) | |||||||||||
| C (cellulose) | 277 | 395 | 118 | 2.88 a | 19.1 a | 0.249 | 0.187 | 0.542 | 12.5 | 62.5 | 24.8 |
| P (pectin) | 278 | 397 | 119 | 2.89 a | 18.8 ab | 0.247 | 0.181 | 0.557 | 12.8 | 62.4 | 24.8 |
| J (inulin) | 278 | 384 | 107 | 2.59 b | 17.9 c | 0.251 | 0.196 | 0.570 | 12.9 | 62.5 | 24.6 |
| S (psyllium) | 279 | 386 | 108 | 2.59 b | 18.3 bc | 0.248 | 0.187 | 0.569 | 12.6 | 62.8 | 24.5 |
| p value | 0.998 | 0.051 | 0.078 | 0.028 | 0.004 | 0.951 | 0.322 | 0.110 | 0.925 | 0.991 | 0.998 |
| Interaction D × F | |||||||||||
| p value | 0.999 | 0.561 | 0.575 | 0.475 | 0.479 | 0.778 | 0.534 | 0.884 | 0.917 | 0.861 | 0.913 |
| Small Intestine with Contents | Ileal Viscosity | Ileal DM | Ileal pH | |
|---|---|---|---|---|
| g/100 g BW | mPa·s | % | ||
| Control C | 1.37 | 1.50 | 19.3 | 7.14 |
| Control CH | 1.38 | 1.55 | 19.4 | 7.15 |
| 2-way ANOVA: | ||||
| CN | 1.33 | 1.54 | 19.3 | 7.22 |
| CNH | 1.34 | 1.51 | 19.7 | 7.23 |
| PN | 1.49 # | 2.72 # | 14.3 # | 7.01 # |
| PNH | 1.52 & | 2.76 & | 15.0 & | 7.06 |
| JN | 1.49 # | 1.59 | 18.1 # | 7.15 |
| JNH | 1.57 & | 1.66 | 18.0 & | 7.09 |
| SN | 1.82 # | 2.88 # | 18.5 | 7.25 # |
| SNH | 1.78 & | 2.95 & | 17.6 & | 7.23 & |
| SEM | 0.019 | 0.068 | 0.217 | 0.012 |
| Cu-NP dose (D) | ||||
| L (6.5 mg/kg) | 1.53 | 2.18 | 17.5 | 7.15 |
| H (13 mg/kg) | 1.55 | 2.22 | 17.6 | 7.15 |
| p value | 0.436 | 0.581 | 0.937 | 0.891 |
| Fibre type (F) | ||||
| C (cellulose) | 1.34 c | 1.53 b | 19.5 a | 7.22 a |
| P (pectin) | 1.50 b | 2.74 a | 14.6 c | 7.03 c |
| J (inulin) | 1.53 b | 1.63 b | 18.0 b | 7.12 b |
| S (psyllium) | 1.80 a | 2.91 a | 18.0 b | 7.24 a |
| p value | <0.001 | <0.001 | <0.001 | <0.001 |
| Interaction D × F | ||||
| p value | 0.323 | 0.924 | 0.234 | 0.463 |
| DAO | Lactic Acid | |
|---|---|---|
| mIU/mL | ng/mL | |
| Control C | 8.63 | 26.2 |
| Control CH | 10.5 | 22.9 |
| 2-way ANOVA: | ||
| CN | 8.03 | 17.3 b# |
| CNH | 9.86 | 21.6 a |
| PN | 9.20 | 21.8 a# |
| PNH | 10.2 | 20.1 a& |
| JN | 9.85 # | 21.2 a# |
| JNH | 10.7 | 20.2 a& |
| SN | 9.67 | 21.4 a# |
| SNH | 10.9 | 20.8 a |
| SEM | 0.177 | 0.314 |
| Cu-NP dose (D) | ||
| L (6.5 mg/kg) | 9.19 b | 20.5 |
| H (13 mg/kg) | 10.4 a | 20.7 |
| p value | 0.001 | 0.669 |
| Fibre type (F) | ||
| C (cellulose) | 8.94 b | 19.5 |
| P (pectin) | 9.70 ab | 21.0 |
| J (inulin) | 10.3 a | 20.7 |
| S (psyllium) | 10.3 a | 21.1 |
| p value | 0.036 | 0.128 |
| Interaction D × F | ||
| p value | 0.808 | <0.001 |
| APE-1 | OGG-1 | DAO | 8-OHdG | Caspase-3 | Caspase-8 | Lactic Acid | |
|---|---|---|---|---|---|---|---|
| ng/g | ng/g | mIU/g | ng/g | ng/g | ng/g | ng/g | |
| Control C | 202 | 85.4 | 128 | 21.2 | 20.9 | 47.1 | 5.17 |
| Control CH | 195 | 74.0 | 121 | 25.5 | 16.1 | 36.2 | 4.48 |
| 2-way ANOVA: | |||||||
| CN | 219 a | 89.2 | 138 | 28.2# | 17.0 # | 35.1 # | 3.56 c# |
| CNH | 205 ab | 78.9 | 131 | 25.6 | 16.3 | 30.4 & | 3.81 c |
| PN | 104 d# | 77.5 | 130 | 22.4 | 12.3 # | 35.7 # | 6.24 a |
| PNH | 188 bc | 92.1 & | 132 | 23.8 | 15.6 | 32.6 | 4.39 c |
| JN | 142 cd | 94.4 | 133 | 28.2 # | 17.5 # | 33.6 # | 5.48 ab |
| JNH | 161 bc& | 99.7 & | 124 | 27.5 | 16.0 | 33.9 | 5.78 a& |
| SN | 169 bc | 96.3 | 158 | 25.3 | 14.5 # | 33.5 # | 5.32 ab |
| SNH | 196 ab | 94.2 & | 118 | 26.9 | 15.1 | 39.2 | 4.13 c |
| SEM | 5.792 | 1.805 | 3.779 | 0.672 | 0.417 | 0.942 | 0.151 |
| Cu-NP dose (D) | |||||||
| L (6.5 mg/kg) | 159 | 89.4 | 140 | 26.0 | 15.3 | 34.5 | 5.15 |
| H (13 mg/kg) | 188 | 91.2 | 127 | 25.9 | 15.8 | 34.0 | 4.53 |
| p value | 0.010 | 0.634 | 0.151 | 0.948 | 0.584 | 0.820 | 0.032 |
| Fibre type (F) | |||||||
| C (cellulose) | 212 | 84.1 b | 135 | 26.9 | 16.6 a | 32.7 | 3.69 |
| P (pectin) | 146 | 84.8 b | 131 | 23.1 | 14.0 b | 34.2 | 5.31 |
| J (inulin) | 152 | 97.1 a | 129 | 27.9 | 16.8 a | 33.8 | 5.63 |
| S (psyllium) | 182 | 95.3 ab | 138 | 26.1 | 14.8 ab | 36.3 | 4.72 |
| p value | <0.001 | 0.035 | 0.898 | 0.062 | 0.043 | 0.642 | <0.001 |
| Interaction D × F | |||||||
| p value | 0.020 | 0.151 | 0.409 | 0.606 | 0.186 | 0.294 | 0.019 |
| OCLN | OGG1 | TFF2 | ZO-1 | |
|---|---|---|---|---|
| Control C | 1.34 | 1.92 | 1.15 | 1.05 |
| Control CH | 0.543 | 0.279 | 0.306 | 0.215 |
| 2-way ANOVA: | ||||
| CN | 0.729 | 0.087 b# | 0.326 # | 0.407 # |
| CNH | 0.947 | 0.279 ab | 0.306 | 0.311 |
| PN | 0.651 | 0.719 a | 0.458 # | 0.468 # |
| PNH | 0.902 | 0.110 b | 0.402 & | 0.518 & |
| JN | 0.966 | 0.346 ab# | 0.750 | 0.337 # |
| JNH | 0.672 | 0.379 ab | 0.361 | 0.605 & |
| SN | 0.958 | 0.139 b# | 0.305 # | 0.761 |
| SNH | 1.13 & | 0.240 b& | 0.511 & | 0.646 & |
| SEM | 0.073 | 0.093 | 0.052 | 0.039 |
| Cu-NP dose (D) | ||||
| L (6.5 mg/kg) | 0.826 | 0.323 | 0.460 | 0.493 |
| H (13 mg/kg) | 0.912 | 0.252 | 0.395 | 0.520 |
| p value | 0.552 | 0.501 | 0.501 | 0.713 |
| Fibre type (F) | ||||
| C (cellulose) | 0.838 | 0.183 | 0.316 | 0.359 b |
| P (pectin) | 0.776 | 0.414 | 0.430 | 0.493 b |
| J (inulin) | 0.819 | 0.362 | 0.556 | 0.471 b |
| S (psyllium) | 1.04 | 0.190 | 0.408 | 0.704 a |
| p value | 0.567 | 0.289 | 0.370 | 0.013 |
| Interaction D × F | ||||
| p value | 0.504 | 0.037 | 0.189 | 0.228 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cholewińska, E.; Marzec, A.; Sołek, P.; Fotschki, B.; Listos, P.; Ognik, K.; Juśkiewicz, J. The Effect of Copper Nanoparticles and a Different Source of Dietary Fibre in the Diet on the Integrity of the Small Intestine in the Rat. Nutrients 2023, 15, 1588. https://doi.org/10.3390/nu15071588
Cholewińska E, Marzec A, Sołek P, Fotschki B, Listos P, Ognik K, Juśkiewicz J. The Effect of Copper Nanoparticles and a Different Source of Dietary Fibre in the Diet on the Integrity of the Small Intestine in the Rat. Nutrients. 2023; 15(7):1588. https://doi.org/10.3390/nu15071588
Chicago/Turabian StyleCholewińska, Ewelina, Aleksandra Marzec, Przemysław Sołek, Bartosz Fotschki, Piotr Listos, Katarzyna Ognik, and Jerzy Juśkiewicz. 2023. "The Effect of Copper Nanoparticles and a Different Source of Dietary Fibre in the Diet on the Integrity of the Small Intestine in the Rat" Nutrients 15, no. 7: 1588. https://doi.org/10.3390/nu15071588
APA StyleCholewińska, E., Marzec, A., Sołek, P., Fotschki, B., Listos, P., Ognik, K., & Juśkiewicz, J. (2023). The Effect of Copper Nanoparticles and a Different Source of Dietary Fibre in the Diet on the Integrity of the Small Intestine in the Rat. Nutrients, 15(7), 1588. https://doi.org/10.3390/nu15071588






