Association of Circulating Branched-Chain Amino Acids with Cardiovascular Diseases: A Mendelian Randomization Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Bidirectional Mendelian Randomization Analysis
Data Sources
2.2. Genetic Instrumental Variants for BCAAs
2.3. MR Analysis
2.4. The UK Biobank Research
2.4.1. Research Population
2.4.2. Study Endpoints
2.5. Statistical Analysis
3. Results
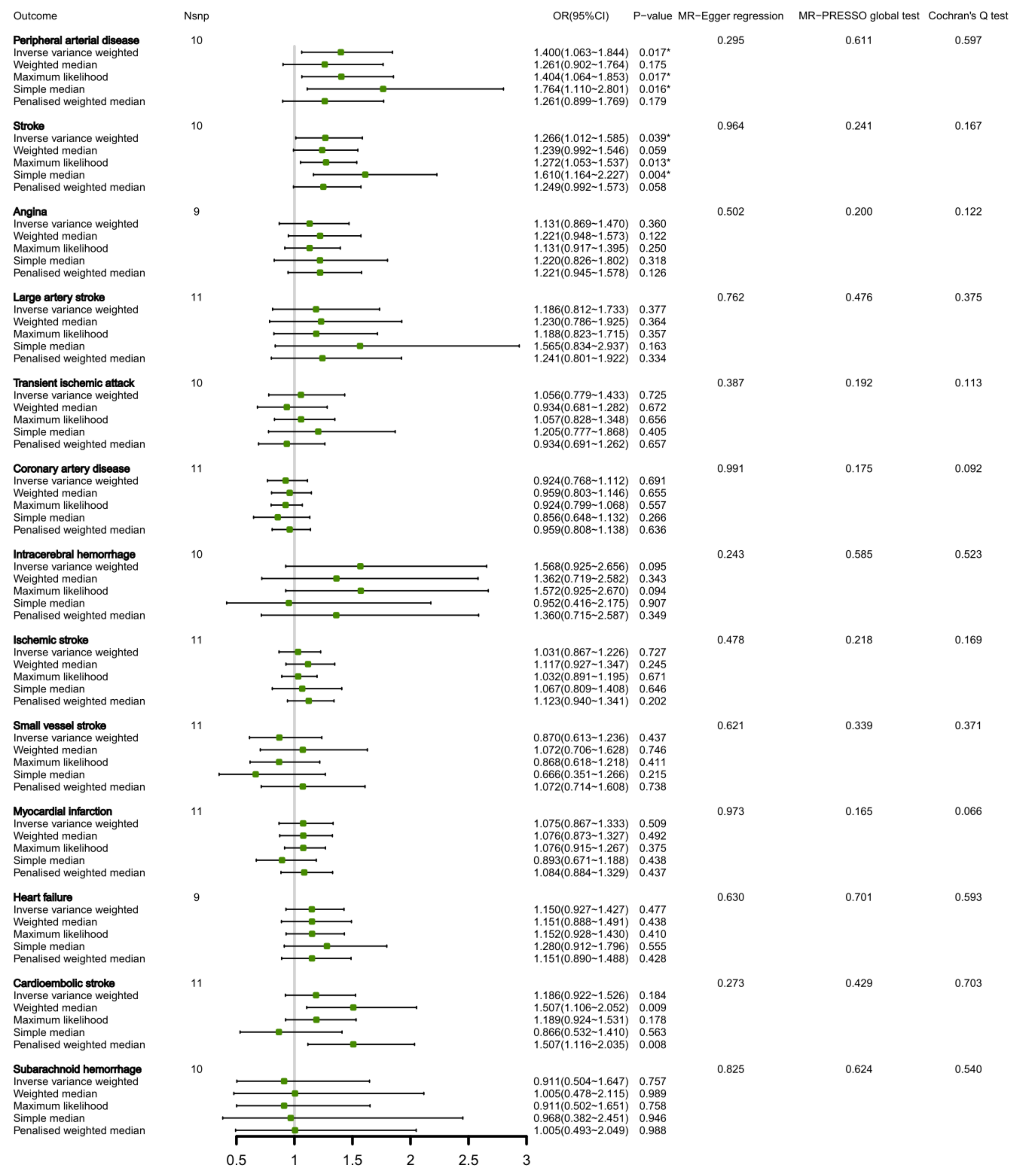
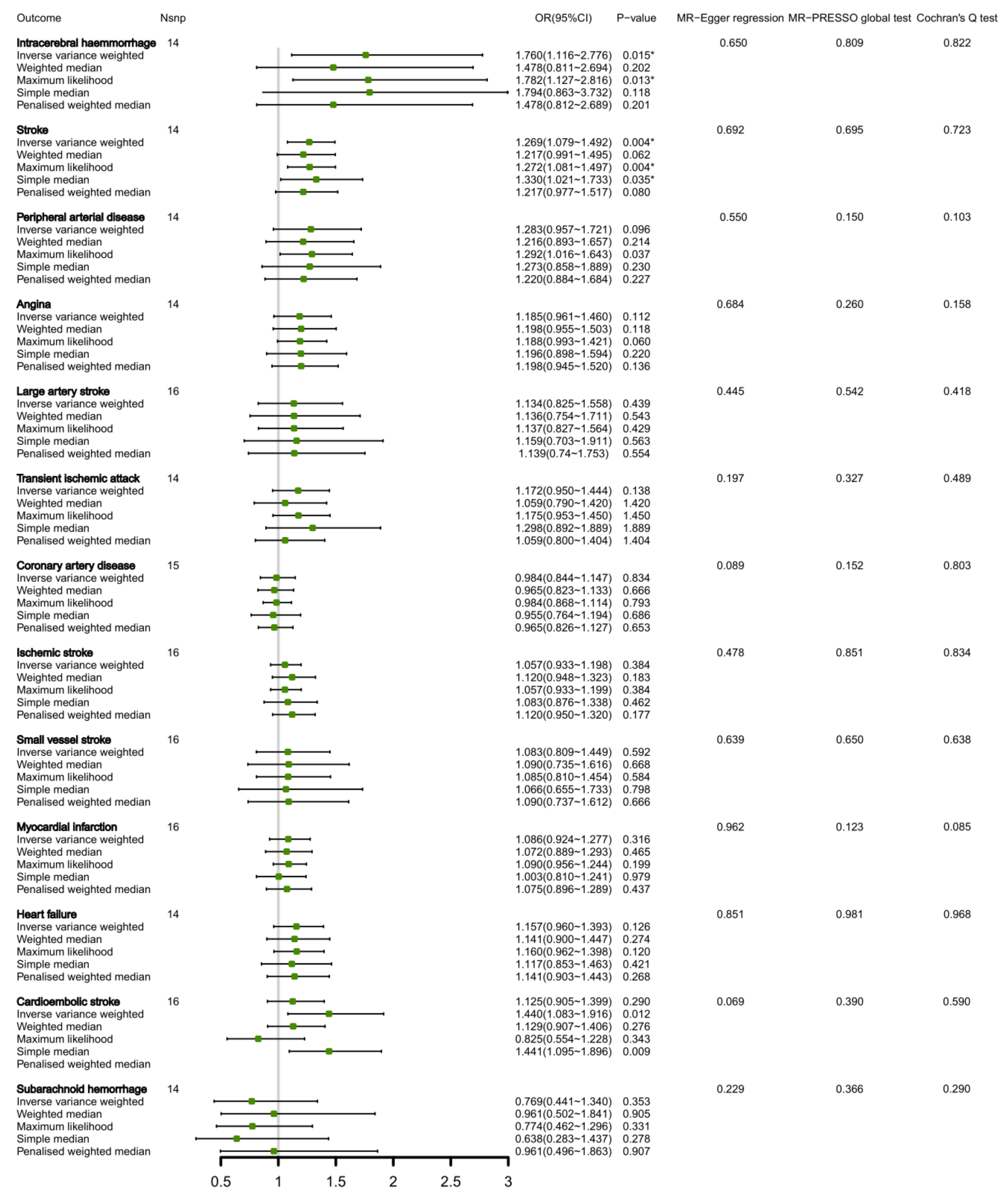
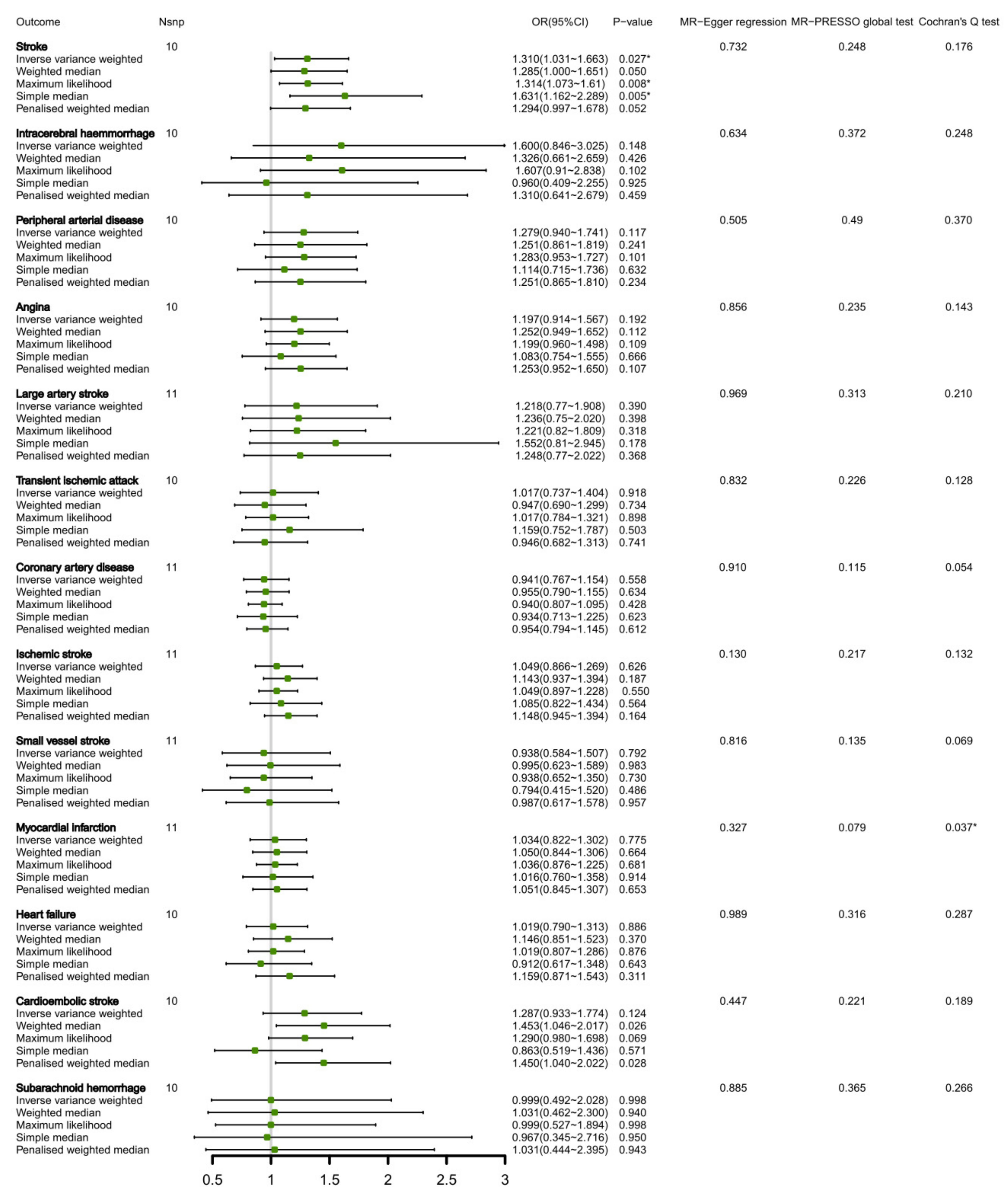
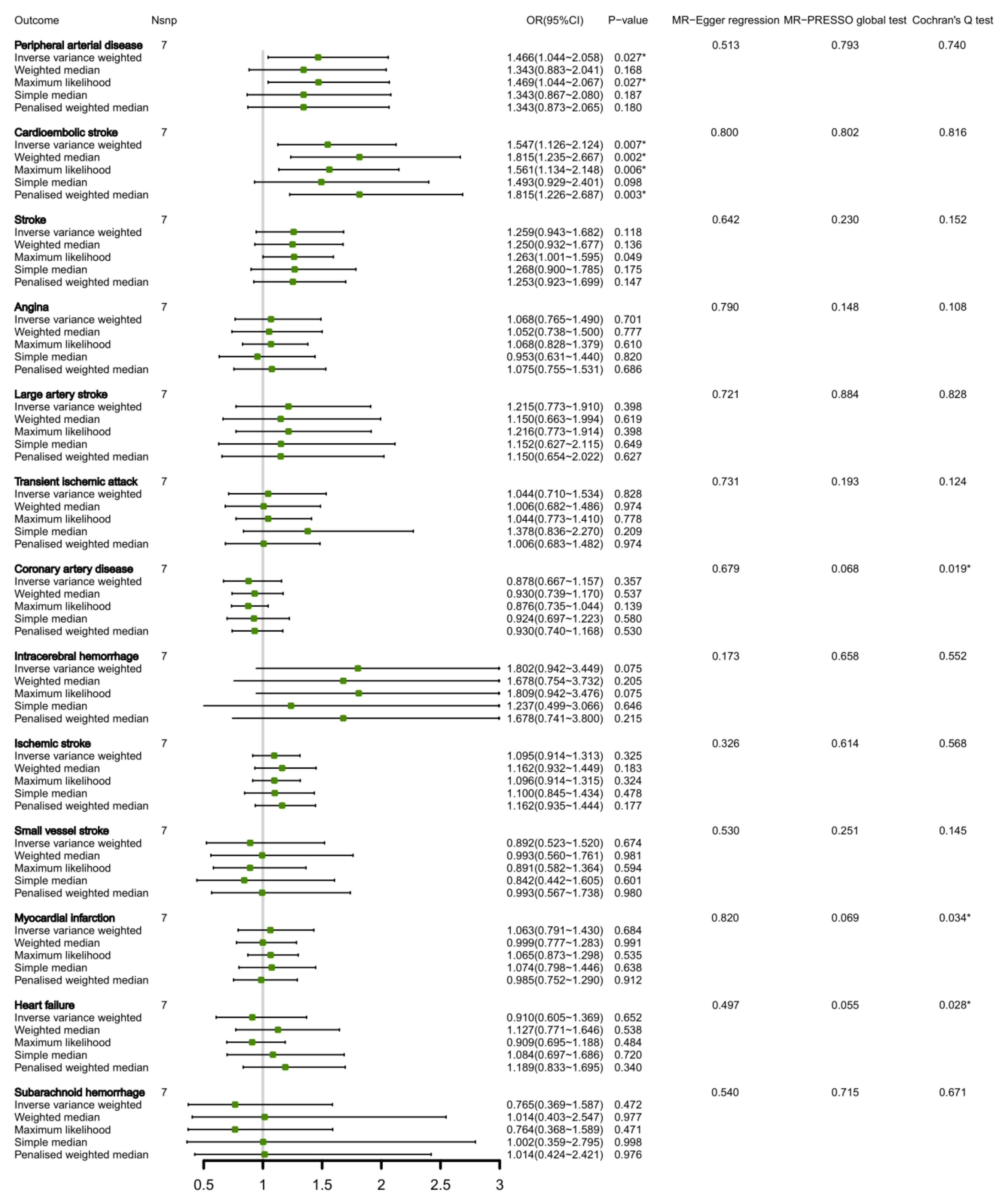
3.1. Association of Circulating Total BCAAs and CVDs in the Bi-Directional MR Analysis
3.2. Connection between Circulating Valine Levels and CVDs by Bi-Directional MR Analysis
3.3. Relationship of Circulating Leucine Levels and CVDs Inside Bi-Directional MR Analysis
3.4. Connection of Circulating Isoleucine Levels and CVDs Inside Bi-Directional MR Analysis
3.5. The UK Biobank Cohort Study of Circulating BCAAs with the Incidence of CVDs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef]
- Nakamura, H.; Jinzu, H.; Nagao, K.; Noguchi, Y.; Shimba, N.; Miyano, H.; Watanabe, T.; Iseki, K. Plasma amino acid profiles are associated with insulin, C-peptide and adiponectin levels in type 2 diabetic patients. Nutr. Diabetes 2014, 4, e133. [Google Scholar] [CrossRef] [PubMed]
- Jewell, J.L.; Russell, R.C.; Guan, K.L. Amino acid signalling upstream of mTOR. Nat. Rev. Mol. Cell Biol. 2013, 14, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Sun, L.; Gong, Y.; Zhou, Y.; Yang, P.; Ye, Z.; Fu, J.; Huang, A.; Fu, Z.; Yu, W.; et al. Relationship between Branched-Chain Amino Acids, Metabolic Syndrome, and Cardiovascular Risk Profile in a Chinese Population: A Cross-Sectional Study. Int. J. Endocrinol. 2016, 2016, 8173905. [Google Scholar] [CrossRef]
- Du, X.; Li, Y.; Wang, Y.; You, H.; Hui, P.; Zheng, Y.; Du, J. Increased branched-chain amino acid levels are associated with long-term adverse cardiovascular events in patients with STEMI and acute heart failure. Life Sci. 2018, 209, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Wurtz, P.; Havulinna, A.S.; Soininen, P.; Tynkkynen, T.; Prieto-Merino, D.; Tillin, T.; Ghorbani, A.; Artati, A.; Wang, Q.; Tiainen, M.; et al. Metabolite profiling and cardiovascular event risk: A prospective study of 3 population-based cohorts. Circulation 2015, 131, 774–785. [Google Scholar] [CrossRef]
- Sun, H.; Olson, K.C.; Gao, C.; Prosdocimo, D.A.; Zhou, M.; Wang, Z.; Jeyaraj, D.; Youn, J.Y.; Ren, S.; Liu, Y.; et al. Catabolic Defect of Branched-Chain Amino Acids Promotes Heart Failure. Circulation 2016, 133, 2038–2049. [Google Scholar] [CrossRef]
- Le Couteur, D.G.; Ribeiro, R.; Senior, A.; Hsu, B.; Hirani, V.; Blyth, F.M.; Waite, L.M.; Simpson, S.J.; Naganathan, V.; Cumming, R.G.; et al. Branched Chain Amino Acids, Cardiometabolic Risk Factors and Outcomes in Older Men: The Concord Health and Ageing in Men Project. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1805–1810. [Google Scholar] [CrossRef]
- Shah, S.H.; Sun, J.L.; Stevens, R.D.; Bain, J.R.; Muehlbauer, M.J.; Pieper, K.S.; Haynes, C.; Hauser, E.R.; Kraus, W.E.; Granger, C.B.; et al. Baseline metabolomic profiles predict cardiovascular events in patients at risk for coronary artery disease. Am. Heart J. 2012, 163, 844–850.e841. [Google Scholar] [CrossRef]
- Kimberly, W.T.; Wang, Y.; Pham, L.; Furie, K.L.; Gerszten, R.E. Metabolite profiling identifies a branched chain amino acid signature in acute cardioembolic stroke. Stroke 2013, 44, 1389–1395. [Google Scholar] [CrossRef]
- Davey Smith, G.; Ebrahim, S. What can mendelian randomisation tell us about modifiable behavioural and environmental exposures? Bmj 2005, 330, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, J.; Langenberg, C.; Prokopenko, I.; Saxena, R.; Soranzo, N.; Jackson, A.U.; Wheeler, E.; Glazer, N.L.; Bouatia-Naji, N.; Gloyn, A.L.; et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat. Genet. 2010, 42, 105–116. [Google Scholar] [CrossRef]
- Lotta, L.A.; Scott, R.A.; Sharp, S.J.; Burgess, S.; Luan, J.; Tillin, T.; Schmidt, A.F.; Imamura, F.; Stewart, I.D.; Perry, J.R.; et al. Genetic Predisposition to an Impaired Metabolism of the Branched-Chain Amino Acids and Risk of Type 2 Diabetes: A Mendelian Randomisation Analysis. PLoS Med. 2016, 13, e1002179. [Google Scholar] [CrossRef]
- Wu, F.; Huang, Y.; Hu, J.; Shao, Z. Mendelian randomization study of inflammatory bowel disease and bone mineral density. BMC Med. 2020, 18, 312. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Zheng, J.S.; Mason, A.M.; Burgess, S.; Larsson, S.C. Genetically predicted circulating vitamin C in relation to cardiovascular disease. Eur. J. Prev. Cardiol. 2022, 28, 1829–1837. [Google Scholar] [CrossRef]
- Palmer, L.J. UK Biobank: Bank on it. Lancet 2007, 369, 1980–1982. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.A.; Woodward, M. Women’s reproductive factors and incident cardiovascular disease in the UK Biobank. Heart 2018, 104, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Li, X.; Sun, D.; Zhou, T.; Ley, S.H.; Gustat, J.; Heianza, Y.; Qi, L. Association of habitual glucosamine use with risk of cardiovascular disease: Prospective study in UK Biobank. Bmj 2019, 365, l1628. [Google Scholar] [CrossRef]
- Stamatakis, E.; Owen, K.B.; Shepherd, L.; Drayton, B.; Hamer, M.; Bauman, A.E. Is Cohort Representativeness Passé? Poststratified Associations of Lifestyle Risk Factors with Mortality in the UK Biobank. Epidemiology 2021, 32, 179–188. [Google Scholar] [CrossRef]
- Wang, X.; Fang, X.; Zheng, W.; Zhou, J.; Song, Z.; Xu, M.; Min, J.; Wang, F. Genetic Support of A Causal Relationship Between Iron Status and Type 2 Diabetes: A Mendelian Randomization Study. J. Clin. Endocrinol. Metab. 2021, 106, e4641–e4651. [Google Scholar] [CrossRef]
- Ferguson, J.F.; Wang, T.J. Branched-Chain Amino Acids and Cardiovascular Disease: Does Diet Matter? Clin. Chem. 2016, 62, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Hruby, A.; Toledo, E.; Clish, C.B.; Martínez-González, M.A.; Salas-Salvadó, J.; Hu, F.B. Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-analysis. Diabetes Care 2016, 39, 833–846. [Google Scholar] [CrossRef]
- Menni, C.; Migaud, M.; Glastonbury, C.A.; Beaumont, M.; Nikolaou, A.; Small, K.S.; Brosnan, M.J.; Mohney, R.P.; Spector, T.D.; Valdes, A.M. Metabolomic profiling to dissect the role of visceral fat in cardiometabolic health. Obesity 2016, 24, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Flores-Guerrero, J.L.; Groothof, D.; Connelly, M.A.; Otvos, J.D.; Bakker, S.J.L.; Dullaart, R.P.F. Concentration of Branched-Chain Amino Acids Is a Strong Risk Marker for Incident Hypertension. Hypertension 2019, 74, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; McGarrah, R.W.; Grimsrud, P.A.; Tso, S.C.; Yang, W.H.; Haldeman, J.M.; Grenier-Larouche, T.; An, J.; Lapworth, A.L.; Astapova, I.; et al. The BCKDH Kinase and Phosphatase Integrate BCAA and Lipid Metabolism via Regulation of ATP-Citrate Lyase. Cell Metab. 2018, 27, 1281–1293.e1287. [Google Scholar] [CrossRef]
- Yang, R.; Dong, J.; Zhao, H.; Li, H.; Guo, H.; Wang, S.; Zhang, C.; Wang, S.; Wang, M.; Yu, S.; et al. Association of branched-chain amino acids with carotid intima-media thickness and coronary artery disease risk factors. PLoS ONE 2014, 9, e99598. [Google Scholar] [CrossRef]
- Tobias, D.K.; Lawler, P.R.; Harada, P.H.; Demler, O.V.; Ridker, P.M.; Manson, J.E.; Cheng, S.; Mora, S. Circulating Branched-Chain Amino Acids and Incident Cardiovascular Disease in a Prospective Cohort of US Women. Circ. Genom. Precis. Med. 2018, 11, e002157. [Google Scholar] [CrossRef]
- Hu, F.B.; Martínez-González, M.A.; Serra-Majem, L.; Lapetra, J.; Fiol, M.; Arós, F.; Gómez-Gracia, E.; Fitó, M.; Ros, E.; Estruch, R.; et al. Plasma Branched-Chain Amino Acids and Incident Cardiovascular Disease in the PREDIMED Trial. Clin. Chem. 2016, 62, 582–592. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, H.; Li, L.; Chen, F.; Liu, Y.; Zhou, M.; Wang, J.; Jiang, J.; Li, X.; Fan, X.; et al. Branched-Chain Amino Acid Catabolism Promotes Thrombosis Risk by Enhancing Tropomodulin-3 Propionylation in Platelets. Circulation 2020, 142, 49–64. [Google Scholar] [CrossRef]
- Aguirre, M.; Eck, A.; Koenen, M.E.; Savelkoul, P.H.; Budding, A.E.; Venema, K. Diet drives quick changes in the metabolic activity and composition of human gut microbiota in a validated in vitro gut model. Res. Microbiol. 2016, 167, 114–125. [Google Scholar] [CrossRef]
- Szczuko, M.; Kikut, J.; Maciejewska, D.; Kulpa, D.; Celewicz, Z.; Ziętek, M. The Associations of SCFA with Anthropometric Parameters and Carbohydrate Metabolism in Pregnant Women. Int. J. Mol. Sci. 2020, 21, 9212. [Google Scholar] [CrossRef]
- Russell, W.R.; Gratz, S.W.; Duncan, S.H.; Holtrop, G.; Ince, J.; Scobbie, L.; Duncan, G.; Johnstone, A.M.; Lobley, G.E.; Wallace, R.J.; et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am. J. Clin. Nutr. 2011, 93, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- François, I.E.; Lescroart, O.; Veraverbeke, W.S.; Marzorati, M.; Possemiers, S.; Hamer, H.; Windey, K.; Welling, G.W.; Delcour, J.A.; Courtin, C.M.; et al. Effects of wheat bran extract containing arabinoxylan oligosaccharides on gastrointestinal parameters in healthy preadolescent children. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Rios-Covian, D.; González, S.; Nogacka, A.M.; Arboleya, S.; Salazar, N.; Gueimonde, M.; de Los Reyes-Gavilán, C.G. An Overview on Fecal Branched Short-Chain Fatty Acids Along Human Life and as Related with Body Mass Index: Associated Dietary and Anthropometric Factors. Front. Microbiol. 2020, 11, 973. [Google Scholar] [CrossRef]
- Granado-Serrano, A.B.; Martín-Garí, M.; Sánchez, V.; Riart Solans, M.; Berdún, R.; Ludwig, I.A.; Rubió, L.; Vilaprinyó, E.; Portero-Otín, M.; Serrano, J.C.E. Faecal bacterial and short-chain fatty acids signature in hypercholesterolemia. Sci. Rep. 2019, 9, 1772. [Google Scholar] [CrossRef]
- Heimann, E.; Nyman, M.; Pålbrink, A.K.; Lindkvist-Petersson, K.; Degerman, E. Branched short-chain fatty acids modulate glucose and lipid metabolism in primary adipocytes. Adipocyte 2016, 5, 359–368. [Google Scholar] [CrossRef]
- Ziętek, M.; Celewicz, Z.; Kikut, J.; Szczuko, M. Implications of SCFAs on the Parameters of the Lipid and Hepatic Profile in Pregnant Women. Nutrients 2021, 13, 1749. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Granger, C.B.; Craig, D.; Haynes, C.; Bain, J.; Stevens, R.D.; Hauser, E.R.; Newgard, C.B.; Kraus, W.E.; Newby, L.K.; et al. Validation of the association between a branched chain amino acid metabolite profile and extremes of coronary artery disease in patients referred for cardiac catheterization. Atherosclerosis 2014, 232, 191–196. [Google Scholar] [CrossRef]
- Cheng, S.; Shah, S.H.; Corwin, E.J.; Fiehn, O.; Fitzgerald, R.L.; Gerszten, R.E.; Illig, T.; Rhee, E.P.; Srinivas, P.R.; Wang, T.J.; et al. Potential Impact and Study Considerations of Metabolomics in Cardiovascular Health and Disease: A Scientific Statement From the American Heart Association. Circ. Cardiovasc. Genet. 2017, 10, e000032. [Google Scholar] [CrossRef]
- Burgess, S.; Butterworth, A.; Thompson, S.G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 2013, 37, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Dudbridge, F.; Thompson, S.G. Combining information on multiple instrumental variables in Mendelian randomization: Comparison of allele score and summarized data methods. Stat. Med. 2016, 35, 1880–1906. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, Y.; Jonsson, A.; Have, C.T.; Allin, K.H.; Witte, D.R.; Jørgensen, M.E.; Grarup, N.; Pedersen, O.; Kilpeläinen, T.O.; Hansen, T. Genetic evidence of a causal effect of insulin resistance on branched-chain amino acid levels. Diabetologia 2017, 60, 873–878. [Google Scholar] [CrossRef]
| Serum Total BCAA Levels (mmol/L) | ||||
| Tertile 1 (<0.346) | Tertile 2 (0.346~0.416) | Tertile 3 (>0.416) | ||
| Prevalence ratio | Prevalence ratio | Prevalence ratio | p-trend | |
| Stroke | (95% CI) | (95% CI) | (95% CI) | |
| Model 1 | Reference | 1.188 (0.935~1.510) | 1.290 (1.017~1.636) | 0.038 * |
| Model 2 | Reference | 1.190 (0.935~1.516) | 1.293 (1.106~1.646) | 0.039 * |
| Model 3 | Reference | 1.188 (0.933~1.514) | 1.285 (1.009~1.636) | 0.044 * |
| Serum valine levels (mmol/L) | ||||
| Tertile 1 (<0.203) | Tertile 2 (0.203~0.238) | Tertile 3 (>0.238) | ||
| Prevalence ratio | Prevalence ratio | Prevalence ratio | p-trend | |
| Stroke | (95% CI) | (95% CI) | (95% CI) | |
| Model 1 | Reference | 1.244 (0.981~1.577) | 1.278 (1.007~1.623) | 0.047 * |
| Model 2 | Reference | 1.244 (0.978~1.581) | 1.284 (1.006~1.638) | 0.049 * |
| Model 3 | Reference | 1.245 (0.979~1.584) | 1.287 (1.009~1.642) | 0.047 * |
| Serum leucine levels (mmol/L) | ||||
| Tertile 1 (<0.096) | Tertile 2 (0.096~0.119) | Tertile 3 (>0.119) | ||
| Prevalence ratio | Prevalence ratio | Prevalence ratio | p-trend | |
| Stroke | (95% CI) | (95% CI) | (95% CI) | |
| Model 1 | Reference | 1.126 (0.888~1.428) | 1.155 (0.910~1.466) | 0.242 |
| Model 2 | Reference | 1.119 (0.882~1.421) | 1.155 (0.908~1.469) | 0.247 |
| Model 3 | Reference | 1.111 (0.875~1.411) | 1.146 (0.901~1.458) | 0.272 |
| Serum isoleucine levels (mmol/L) | ||||
| Tertile 1 (<0.046) | Tertile 2 (0.046~0.060) | Tertile 3 (>0.060) | ||
| Prevalence ratio | Prevalence ratio | Prevalence ratio | p-trend | |
| Stroke | (95% CI) | (95% CI) | (95% CI) | |
| Model 1 | Reference | 1.209 (0.949~1.539) | 1.365 (1.077~1.731) | 0.010 * |
| Model 2 | Reference | 1.204 (0.944~1.534) | 1.367 (1.076~1.737) | 0.011 * |
| Model 3 | Reference | 1.202 (0.943~1.532) | 1.352 (1.064~1.718) | 0.014 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Wang, X.; Geng, G.; Xu, X.; Liu, L.; Zhang, Y.; Wang, Z.; Wang, L.; Li, Y. Association of Circulating Branched-Chain Amino Acids with Cardiovascular Diseases: A Mendelian Randomization Study. Nutrients 2023, 15, 1580. https://doi.org/10.3390/nu15071580
Xu H, Wang X, Geng G, Xu X, Liu L, Zhang Y, Wang Z, Wang L, Li Y. Association of Circulating Branched-Chain Amino Acids with Cardiovascular Diseases: A Mendelian Randomization Study. Nutrients. 2023; 15(7):1580. https://doi.org/10.3390/nu15071580
Chicago/Turabian StyleXu, Huan, Xuanyang Wang, Guannan Geng, Xiaoqing Xu, Lin Liu, Yuntao Zhang, Ziqi Wang, Lulu Wang, and Ying Li. 2023. "Association of Circulating Branched-Chain Amino Acids with Cardiovascular Diseases: A Mendelian Randomization Study" Nutrients 15, no. 7: 1580. https://doi.org/10.3390/nu15071580
APA StyleXu, H., Wang, X., Geng, G., Xu, X., Liu, L., Zhang, Y., Wang, Z., Wang, L., & Li, Y. (2023). Association of Circulating Branched-Chain Amino Acids with Cardiovascular Diseases: A Mendelian Randomization Study. Nutrients, 15(7), 1580. https://doi.org/10.3390/nu15071580






