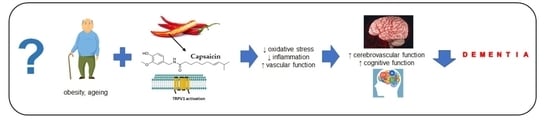
Capsaicin: A Potential Treatment to Improve Cerebrovascular Function and Cognition in Obesity and Ageing
Abstract
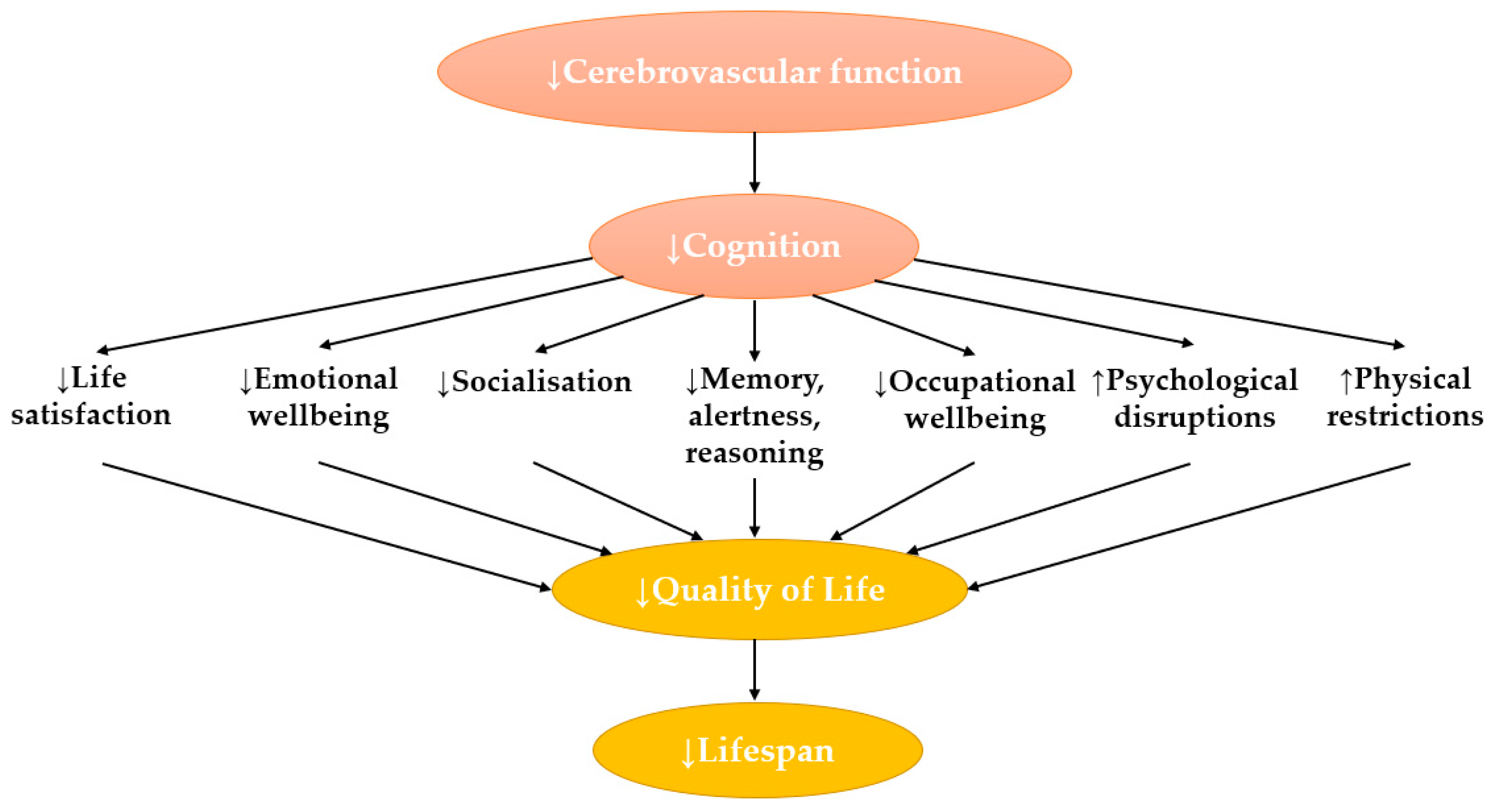
1. Introduction
2. An Overview of Cognition and Cerebrovascular Function
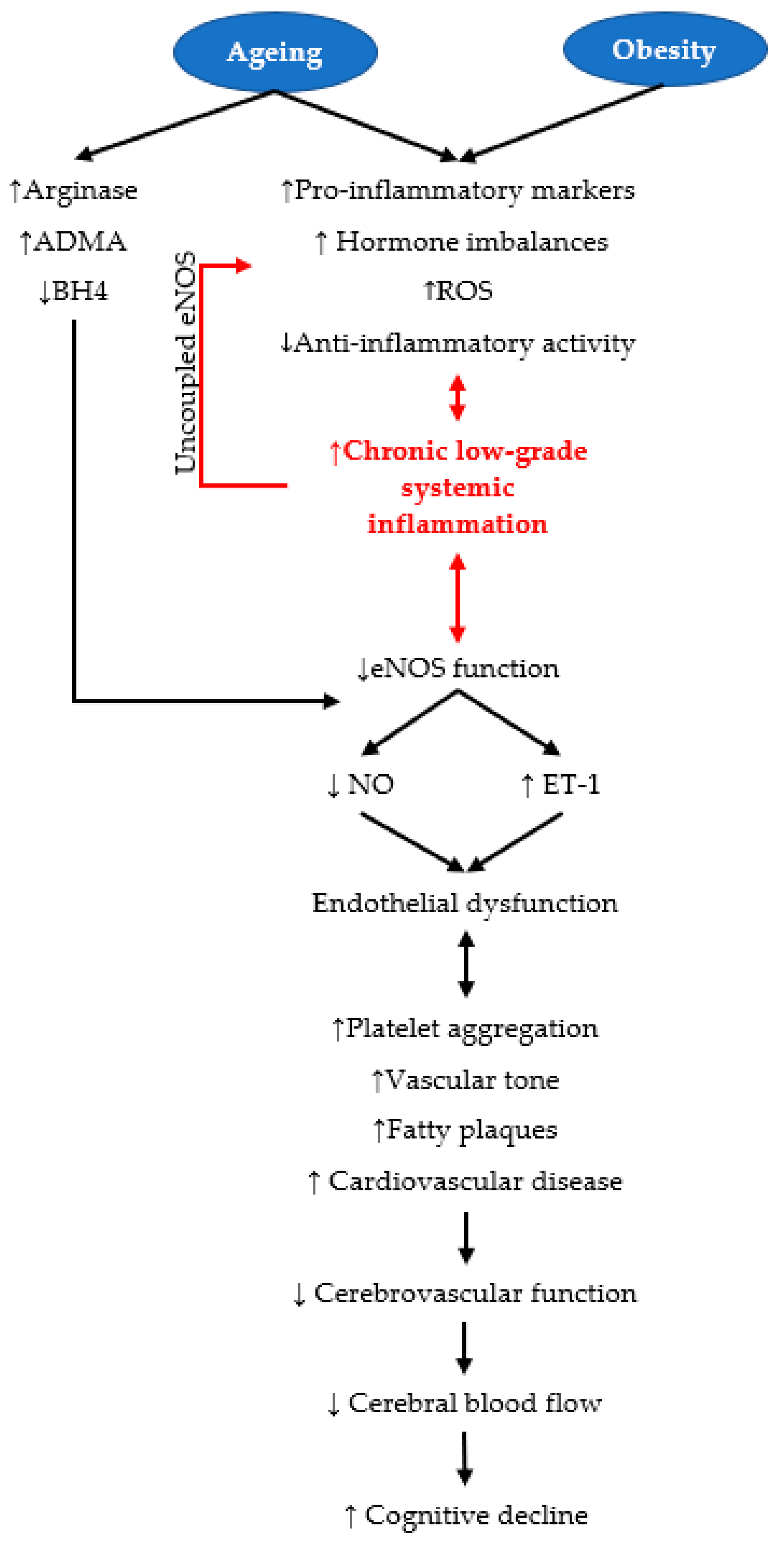
3. Ageing and Obesity Are Risk Factors for Cerebrovascular Dysregulation
3.1. Ageing
3.2. Obesity as a Risk Factor for Cognitive Decline
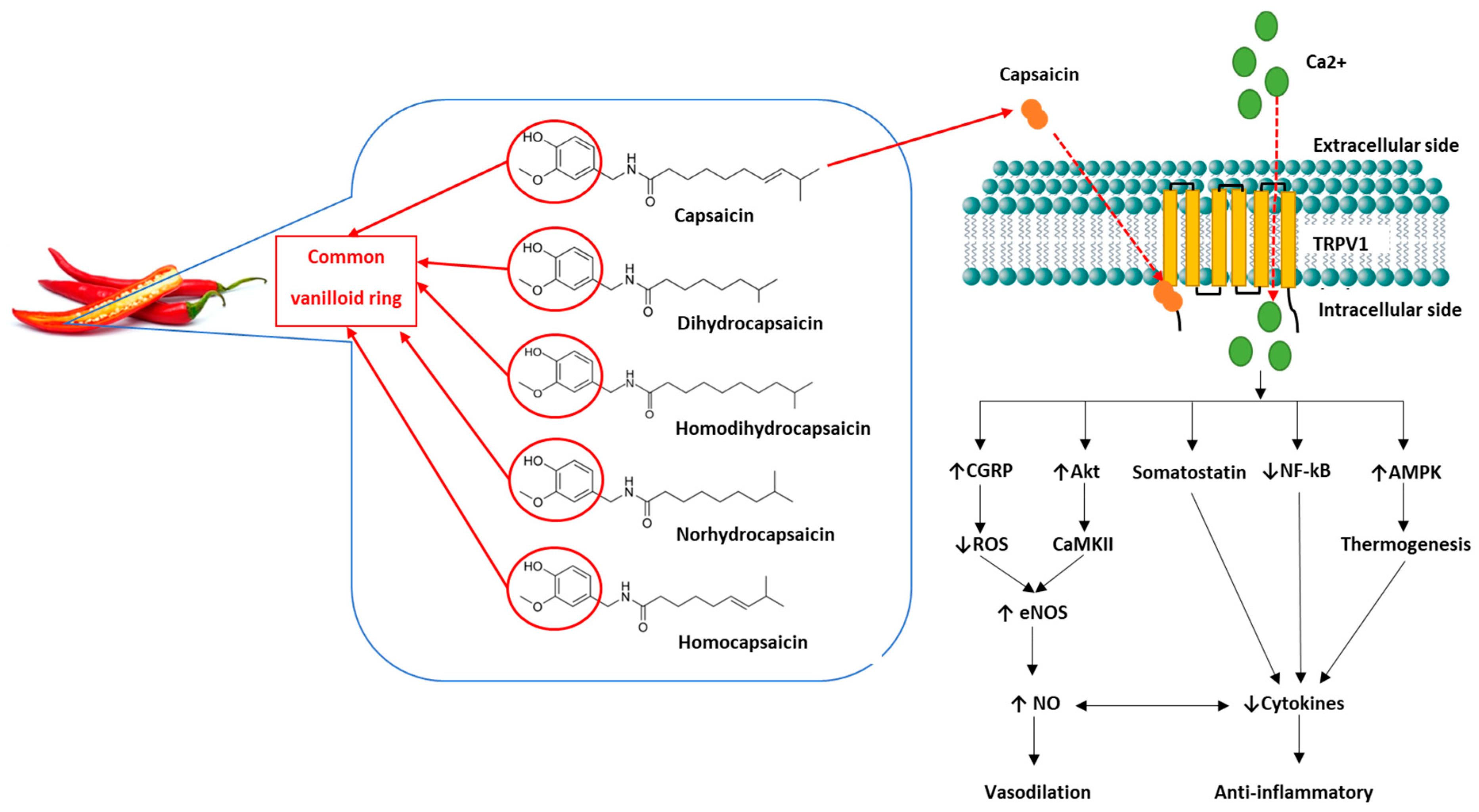
4. Capsaicin
| Disease State | Main Findings | Reference |
|---|---|---|
| Cardiovascular | ↓Blood pressure | [97] |
| Cancer | Anti-proliferative | [88] |
| Neuropathic pain | ↓Painful neuropathy | [98,99] |
| Adiposity and metabolic derangements | ↑Energy expenditure ↑Fat oxidation ↑Thermogenesis ↑Glucose tolerance ↑Insulin sensitivity ↑Resting metabolic rate ↓Body mass ↓Total cholesterol ↓Triglycerides ↓ Glucose | [83,100,101,102,103,104] |
Capsaicin: A Brief Overview of Its Role as Anti-Cardiometabolic Disease Treatment
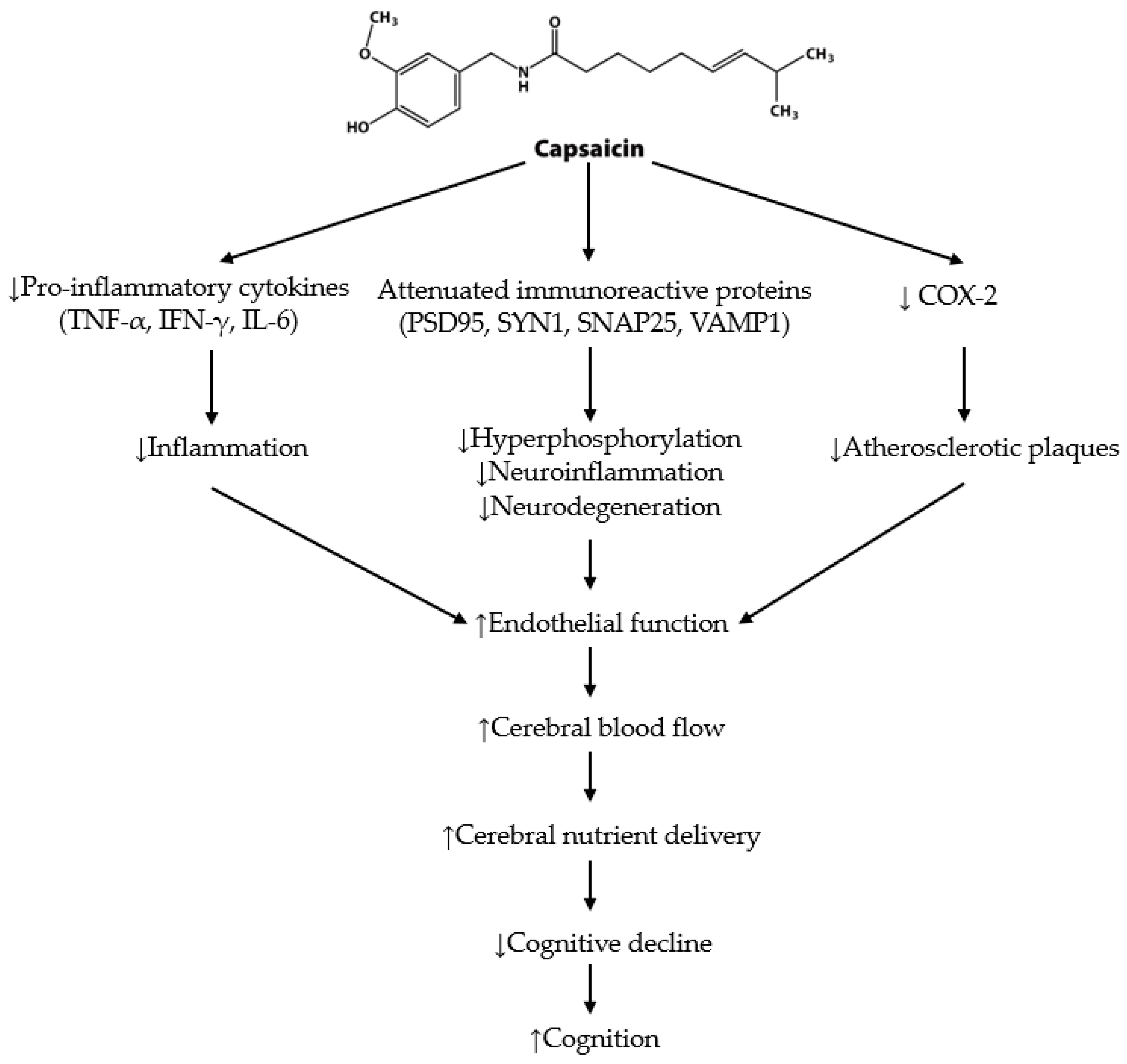
5. The Effects of Capsaicin on Cognition and Cerebrovascular Function
5.1. Cognition in Animal Studies
5.2. Cognition in Human Studies
5.3. Cerebrovascular Function in Animals and Humans
6. Capsaicin Summary
| Reference | Species and Characteristics | Capsaicin Dosage/Application (Duration) | Outcomes of Capsaicin Treatment |
|---|---|---|---|
| Effects of acute dose capsaicin on cognition in animals | |||
| [143] | Adult male Sprague Dawley rats | 125 mg/kg s.c. (3 days) | ↓BBB permeability ↓vasogenic oedema formation ↓motor and cognitive deficit ↑free magnesium |
| [22] | Ischaemia model Male Mongolian gerbils | 0.025, 0.05, 0.01, 0.2, 0.6 mg/kg s.c. | ↑cognition ↑neuronal activity |
| [145] | Young male Wistar rats | 10 mg/kg i.G. | ↑spatial memory ↑neuronal long-term potentiation |
| [132] | Female Sabra mice | 1.25 µg/kg i.p. (single dose) | ↑cognitive function ↑neurological score |
| [130] | C57BL/6 WT mice | 1 mg/kg i.v. (8 days) | ↑spatial learning ↑hippocampal CGRP ↑IGF-1 expression |
| [129] | Sprague Dawley rats (Grade II, male) | 10 mg/kg i.G. (single dose) | ↑cognitive performance ↑hippocampal CREB |
| [23] | Male Wistar mice | 10 mg/kg i.p. (single dose) | ↑cognitive performance |
| [24] | P17-30 C57BL/6 (WT) and TRPV1 KO male mice | Hippocampal slices (tissue bath) | ↓Aβ neuronal degradation |
| [131] | Male Wistar rats | 0.5, 0.3, 0.1 µg/rat (intrahippocampal injection) | ↑memory ↑TRPV1 ↑cAMP mRNA |
| [118] | APP23/PS45 double transgenic AD mice | 1 mg/kg i.p.(single dose) | ↑spatial learning ↑memory ↑TRPV1 upregulation ↓hippocampal neurotic plaques |
| Effects of chronic dose capsaicin in animals | |||
| [117] | Adult C57B1/6 mice | 1 mg/kg/day i.p. (2 weeks) | ↑spatial learning ↑memory ↑PSD95 expression ↓synapse loss |
| [25] | Adult male Sprague Dawley rats | AlCl3 + 25, 50 mg/kg/day i.p. capsicum extract 50 mg/kg/day i.p. capsicum extract (1.2% capsaicin) (30 days) | ↓neuro and systemic inflammation ↓oxidative stress ↓Aβ-peptide accumulation ↓cerebral cortex, substantia nigra and hippocampal neurodegeneration ↑ brain NO concentration and memory |
| [125] | Adult albino mice | 10 mg/kg i.G. (47 days) | ↓behavioural impairments ↓Aβ1-42 ↓tau proteins |
| [123] | Male Wistar rats | Scopolamine + 50 mg/kg oral chilli oleoresin (13 days) | ↓Acetylcholinesterase (−50%) ↑locomotion activity ↓escape latency time |
| [141] | Male, female C57BL/6J littermate ApoE4 mice | 1 mg/kg i.p./day (1 month) | ↓memory impairment ↓tau pathology ↓neuronal autophagy ↓microglial phagocytosis |
| [27] | APP/PS1 transgenic mice on C57BL/6 background | 0.1% capsaicin-rich chow (approx. 30 mg/kg capsaicin) (6 months) | ↑memory ↑spatial learning ↓Aβ plaque density ↓Aβ vessel deposition ↓Aβ42, ↓Aβ40 ↓hyperphosphorylation and tau ↓neuroinflammation ↓neurodegeneration ↓proinflammatory cytokines ↑synapse related proteins |
| [124] | C47BL6/J mice; APP/PSI mice | 2 mg/kg, i.p. (4 weeks) | ↑spatial learning ↑memory ↓Aβ42/Aβ40 ratio ↑basal synaptic activity ↓hippocampal oxidative stress ↓hippocampal Neuroinflammation |
| Effect of capsaicin on animal cerebrovasculature | |||
| [134] | Adult felines | 10−7–10−5 M (tissue bath) | ↑vasodilation |
| [137] | C57BL/6J mice; TRPV1 KO mice; Wistar-Kyoto rats; Stroke-prone, hypertensive male mice | chow + 0.01% dietary capsaicin (0.01% mice; 0.02% rats) (6 months) | ↑phosphorylated eNOS ↑eNOS expression Delayed stroke onset TRPV1 function ↑cerebrovascular activity |
| [135] | Male Sprague Dawley rats | 100 nM (low dose), 10 µM (high dose) (in vivo and dural application) (20 weeks) | ↑meningeal blood flow ↑CGRP release |
| Effect of capsaicin on human cerebrovasculature | |||
| [138] | Healthy male adults (n = 30) 21 ± 5 years old | 33, 66, 99, 132, 165 µM dose escalation Hemi-palate (20 min) | ↑MCA velocity |
| Effect of capsaicin on cognition in humans | |||
| [133] | Long-term community dwelling adults (n = 338) ≥40 years | Capsaicin dietary intake assessed by food frequency questionnaire | ↑cognition Improved Aβ42/Aβ40 ratio |
7. Capsimax
8. Summary
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Risk Reduction of Cognitive Decline and Dementia: WHO Guildelines; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Grossman, S.C.; Porth, C.M. Porth’s Pathophysiology: Concepts of Altered Health States, 9th ed.; Wolters Kluwer/Lippincott Williams & Wilkins: London, UK, 2014. [Google Scholar]
- World Health Organization. Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 11 March 2023).
- Jessen, F.; Amariglio, R.E.; Buckley, R.F.; Van Der Flier, W.M.; Han, Y.; Molinuevo, J.L.; Rabin, L.; Rentz, D.M.; Rodriguez-Gomez, O.; Saykin, A.J.; et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020, 19, 271–278. [Google Scholar] [CrossRef]
- Testa, M.A.; Nackley, J.F. Methods for quality-of-life studies. Annu. Rev. Public Health 1994, 15, 535–559. [Google Scholar] [CrossRef] [PubMed]
- Leist, A.K.; Kulmala, J.; Nyqvist, F. (Eds.) Health and Cognition in Old Age: From a Biomedical and Life Course Factors to Policy and Practice; Springer: New York, NY, USA, 2014; Volume 10. [Google Scholar]
- Kivipelto, M.; Mangialasche, F.; Ngandu, T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Exalto, L.G.; Quesenberry, C.P.; Barnes, D.; Kivipelto, M.; Biessels, G.J.; Whitmer, R.A. Midlife risk score for the prediction of dementia four decades later. Alzheimers Dement 2014, 10, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Reitz, C.; Luchsinger, J.A.; Mayeux, R. Vascular disease and cognitive impairment. Expert Rev. Neurother. 2008, 8, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Stanimirovic, D.B.; Friedman, A. Pathophysiology of the neurovascular unit: Disease cause or consequence? J. Cereb. Blood Flow Metab. 2012, 32, 1207–1221. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.R.; Zuelsdorff, M.; Norton, D.; Johnson, S.C.; Wyman, M.F.; Hancock, L.M.; Carlsson, C.M.; Asthana, S.; Flowers-Benton, S.; Gleason, C.E.; et al. Association of cardiovascular risk factors with cerebral perfusion in whites and African Americans. J. Alzheimers Dis. 2020, 75, 649–660. [Google Scholar] [CrossRef]
- World Health Organization. Obesity. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 28 September 2022).
- Audureau, E.; Pouchot, J.; Coste, J. Gender-related differential effects of obesity on health-related quality of life via obesity-related comorbidities: A mediation analysis of a french nationwide survey. Circ. Cardiovasc. Qual. Outcomes 2016, 9, 246–256. [Google Scholar] [CrossRef]
- Caballero, B. Humans against obesity: Who will win? Adv. Nutr. 2019, 10, S4–S9. [Google Scholar] [CrossRef]
- Knight, S.P.; Laird, E.; Williamson, W.; O’Connor, J.; Newman, L.; Carey, D.; De Looze, C.; Fagan, A.J.; Chappell, M.A.; Meaney, J.F.; et al. Obesity is associated with reduced cerebral blood flow-modified by physical activity. Neurobiol. Aging 2021, 105, 35–47. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 28 September 2022).
- Melby-Lervag, M.; Hulme, C. Is working memory training effective? a meta-analytic review. Dev. Psychol. 2013, 49, 270–291. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-T. Cognitive reserve and the prevention of dementia: The role of physical and cognitive activities. Curr. Psychiatry Rep. 2016, 18, 85. [Google Scholar] [CrossRef] [PubMed]
- Seals, D.R.; Kaplon, R.E.; Gioscia-Ryan, R.A.; LaRocca, T.J. You’re only as old as your arteries: Translational strategies for preserving vascular endothelial function with aging. Physiology 2014, 29, 250–264. [Google Scholar] [CrossRef] [PubMed]
- Blaikie, L.; Kay, G.; Kong Thoo Lin, P. Current and emerging therapeutic targets of alzheimer’s disease for the design of multi-target directed ligands. Medchemcomm 2019, 10, 2052–2072. [Google Scholar] [CrossRef]
- Pegorini, S.; Braida, D.; Verzoni, C.; Guerini-Rocco, C.; Consalez, G.G.; Croci, L.; Sala, M. Capsaicin exhibits neuroprotective effects in a model of transient global cerebral ischemia in Mongolian gerbils. Br. J. Pharmacol. 2005, 144, 727–735. [Google Scholar] [CrossRef]
- Shiri, M.; Komaki, A.; Oryan, S.; Taheri, M.; Komaki, H.; Etaee, F. Effects of cannabinoid and vanilloid receptor agonists and their interaction on learning and memory in rats. Can. J. Physiol. Pharmacol. 2017, 95, 382–387. [Google Scholar] [CrossRef]
- Balleza-Tapia, H.; Crux, S.; Andrade-Talavera, Y.; Dolz-Gaiton, P.; Papadia, D.; Chen, G.; Johansson, J.; Fisahn, A. TrpV1 receptor activation rescues neuronal function and network gamma oscillations from Aβ-induced impairment in mouse hippocampus in vitro. eLife 2018, 7, e37703. [Google Scholar] [CrossRef]
- Abdel-Salam, O.M.E.; El-Sayed El-Shamarka, M.; Youness, E.R.; Shaffie, N. Protective effect of hot peppers against amyloid beta peptide and brain injury in AlCl(3)-induced Alzheimer’s disease in rats. Iran. J. Basic Med. Sci. 2023, 26, 335–342. [Google Scholar] [CrossRef]
- Tyagi, S.; Shekhar, N.; Thakur, A.K. Protective role of capsaicin in neurological disorders: An overview. Neurochem. Res. 2022, 47, 1513–1531. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.L.; Xiang, Y.; Tian, D.Y.; Zhu, C.; Li, W.W.; Liu, Y.H.; Bu, X.L.; Shen, L.L.; Jin, W.S.; et al. Capsaicin consumption reduces brain amyloid-beta generation and attenuates Alzheimer’s disease-type pathology and cognitive deficits in APP/PS1 mice. Transl. Psychiatry 2020, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.R. ACT: A simple theory of complex cognition. Am. Psychol. 1996, 51, 355–365. [Google Scholar] [CrossRef]
- Caplan, L.R. Primer on Cerebrovascaular Diseases; Academic Press: London, UK, 2017. [Google Scholar]
- Salthouse, T.A. Selective review of cognitive aging. J. Int. Neuropsychol. Soc. 2010, 16, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.Y.; Tarumi, T.; Liu, J.; Zhang, Y.; Turner, M.; Riley, J.; Tinajero, C.D.; Yuan, L.J.; Zhang, R. Distribution of cardiac output to the brain across the adult lifespan. J. Cereb. Blood Flow Metab. 2017, 37, 2848–2856. [Google Scholar] [CrossRef] [PubMed]
- Toth, P.; Tarantini, S.; Csiszar, A.; Ungvari, Z. Functional vascular contributions to cognitive impairment and dementia: Mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H1–H20. [Google Scholar] [CrossRef] [PubMed]
- Armstead, W.M. Cerebral bood flow autoregulation and dysautoregulation. Anesthesiol. Clin. 2016, 34, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Schmid, F.; Barrett, M.J.P.; Patrick, J.; Weber, B. Vascular density and distribution in neocortex. NeuroImage 2019, 197, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, Y.-C.; Ainslie, P.N. Blood pressure regulation IX: Cerebral autoregulation under blood pressure challenges. Eur. J. Appl. Physiol. 2014, 114, 545–559. [Google Scholar] [CrossRef]
- Gyawali, P.; Lillicrap, T.P.; Tomari, S.; Bivard, A.; Holliday, E.; Parsons, M.; Levi, C.; Garcia-Esperon, C.; Spratt, N. Whole blood viscosity is associated with baseline cerebral perfusion in acute ischemic stroke. Neurol. Sci. 2022, 43, 2375–2381. [Google Scholar] [CrossRef]
- Duchemin, S.; Boily, M.; Sadekova, N.; Girouard, H. The complex contribution of NOS interneurons in the physiology of cerebrovascular regulation. Front. Neural Circuits 2012, 6, 51. [Google Scholar] [CrossRef]
- Drake, C.T.; Iadecola, C. The role of neuronal signaling in controlling cerebral blood flow. Brain Lang. 2007, 102, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Song, W.; Li, L.; Fan, X. Endothelial nitric oxide synthase: A potential therapeutic target for cerebrovascular diseases. Mol. Brain 2016, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Triggle, C.R.; Samuel, S.M.; Ravishankar, S.; Marei, I.; Arunachalam, G.; Ding, H. The endothelium: Influencing vascular smooth muscle in many ways. Can. J. Physiol. Pharmacol. 2012, 90, 713–738. [Google Scholar] [CrossRef] [PubMed]
- Toda, N. Age-related changes in endothelial function and blood flow regulation. Pharmacol. Ther. 2012, 133, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Toda, N.; Ayajiki, K.; Okamura, T. Cerebral blood flow regulation by nitric oxide: Recent advances. Pharmacol. Rev. 2009, 61, 62–97. [Google Scholar] [CrossRef]
- Fantini, S.; Sassaroli, A.; Tgavalekos, K.T.; Kornbluth, J. Cerebral blood flow and autoregulation: Current measurement techniques and prospects for noninvasive optical methods. Neurophotonics 2016, 3, 031411. [Google Scholar] [CrossRef]
- Heinzel, S.; Metzger, F.G.; Ehlis, A.C.; Korell, R.; Alboji, A.; Haeussinger, F.B.; Wurster, I.; Brockmann, K.; Suenkel, U.; Eschweiler, G.W.; et al. Age and vascular burden determinants of cortical hemodynamics underlying verbal fluency. PLoS ONE 2015, 10, e0138863. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Kiss, T.; Tarantini, S.; Nyul-Toth, A.; Ahire, C.; Yabluchanskiy, A.; Csipo, T.; Lipecz, A.; Tabak, A.; Institoris, A.; et al. Obesity-induced cognitive impairment in older adults: A microvascular perspective. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H740–H761. [Google Scholar] [CrossRef]
- Guix, F.X.; Uribesalgo, I.; Coma, M.; Muñoz, F.J. The physiology and pathophysiology of nitric oxide in the brain. Prog. Neurobiol. 2005, 76, 126–152. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Cremers, L.G.M.; Huizinga, W.; Niessen, W.J.; Krestin, G.P.; Poot, D.H.J.; Ikram, M.A.; Lötjönen, J.; Klein, S.; Vernooij, M.W. Predicting global cognitive decline in the general population using the Disease State Index. Front. Aging Neurosci. 2020, 11, 379. [Google Scholar] [CrossRef] [PubMed]
- Love, S.; Miners, J.S. Cerebrovascular disease in ageing and Alzheimer’s disease. Acta Neuropathol. 2016, 131, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; Liu, R.; Travis, O.K.; Roman, R.J.; Fan, F. Cerebral autoregulation in hypertension and ischemic stroke: A mini review. J. Pharm. Sci. Exp. Pharmacol. 2017, 2017, 21–27. [Google Scholar] [PubMed]
- Veldsman, M.; Tai, X.Y.; Nichols, T.; Smith, S.; Peixoto, J.; Manohar, S.; Husain, M. Cerebrovascular risk factors impact frontoparietal network integrity and executive function in healthy ageing. Nat. Commun. 2020, 11, 4340. [Google Scholar] [CrossRef] [PubMed]
- Kielstein, J.T.; Donnerstag, F.; Gasper, S.; Menne, J.; Kielstein, A.; Martens-Lobenhoffer, J.; Scalera, F.; Cooke, J.P.; Fliser, D.; Bode-Böger, S.M. ADMA increases arterial stiffness and decreases cerebral blood flow in humans. Stroke 2006, 37, 2024–2029. [Google Scholar] [CrossRef]
- Berkowitz, D.E.; White, R.; Li, D.; Minhas, K.M.; Cernetich, A.; Kim, S.; Burke, S.; Shoukas, A.A.; Nyhan, D.; Champion, H.C.; et al. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation 2003, 108, 2000–2006. [Google Scholar] [CrossRef]
- Pikula, A.; Böger, R.H.; Beiser, A.S.; Maas, R.; DeCarli, C.; Schwedhelm, E.; Himali, J.J.; Schulze, F.; Au, R.; Kelly-Hayes, M.; et al. Association of plasma ADMA levels with MRI markers of vascular brain injury: Framingham offspring study. Stroke 2009, 40, 2959–2964. [Google Scholar] [CrossRef]
- Masodsai, K.; Lin, Y.Y.; Lin, S.Y.; Su, C.T.; Lee, S.D.; Yang, A.L. Aging additively influences insulin and insulin-like growth factor-1 mediated endothelial dysfunction and antioxidant deficiency in spontaneously hypertensive rats. Biomedicines 2021, 9, 676. [Google Scholar] [CrossRef]
- Vaynman, S.; Ying, Z.; Gomez-Pinilla, F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur. J. Neurosci. 2004, 20, 2580–2590. [Google Scholar] [CrossRef]
- Ding, Q.; Vaynman, S.; Akhavan, M.; Ying, Z.; Gomez-Pinilla, F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience 2006, 140, 823–833. [Google Scholar] [CrossRef]
- Amidfar, M.; de Oliveira, J.; Kucharska, E.; Budni, J.; Kim, Y.K. The role of CREB and BDNF in neurobiology and treatment of Alzheimer’s disease. Life Sci. 2020, 257, 118020. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M.; Huang, A.; Kaley, G.; Sun, D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1829–H1836. [Google Scholar] [CrossRef] [PubMed]
- Capuron, L.; Geisler, S.; Kurz, K.; Leblhuber, F.; Sperner-Unterweger, B.; Fuchs, D. Activated immune system and inflammation in healthy ageing: Relevance for tryptophan and neopterin metabolism. Curr. Pharm. Des. 2014, 20, 6048–6057. [Google Scholar] [CrossRef] [PubMed]
- Landmesser, U.; Dikalov, S.; Price, S.R.; McCann, L.; Fukai, T.; Holland, S.M.; Mitch, W.E.; Harrison, D.G. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Investig. 2003, 111, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Pinosanu, L.R.; Capitanescu, B.; Glavan, D.; Godeanu, S.; Cadenas, I.F.; Doeppner, T.R.; Hermann, D.M.; Balseanu, A.-T.; Bogdan, C.; Popa-Wagner, A. Neuroglia cells transcriptomic in brain development, aging and neurodegenerative diseases. Aging Dis. 2023, 14, 63–83. [Google Scholar] [CrossRef] [PubMed]
- Perry, V.H.; Holmes, C. Microglial priming in neurodegenerative disease. Nat. Reviews. Neurol. 2014, 10, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s disease. J. Cell Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef]
- Villa, V.; Thellung, S.; Bajetto, A.; Gatta, E.; Robello, M.; Novelli, F.; Tasso, B.; Tonelli, M.; Florio, T. Novel celecoxib analogues inhibit glial production of prostaglandin E2, nitric oxide, and oxygen radicals reverting the neuroinflammatory responses induced by misfolded prion protein fragment 90-231 or lipopolysaccharide. Pharmacol. Res. 2016, 113, 500–514. [Google Scholar] [CrossRef]
- Rawji, K.S.; Mishra, M.K.; Michaels, N.J.; Rivest, S.; Stys, P.K.; Yong, V.W. Immunosenescence of microglia and macrophages: Impact on the ageing central nervous system. Brain 2016, 139, 653–661. [Google Scholar] [CrossRef]
- Donato, A.J.; Gano, L.B.; Eskurza, I.; Silver, A.E.; Gates, P.E.; Jablonski, K.; Seals, D.R. Vascular endothelial dysfunction with aging: Endothelin-1 and endothelial nitric oxide synthase. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H425–H432. [Google Scholar] [CrossRef]
- Seals, D.R.; Jablonski, K.L.; Donato, A.J. Aging and vascular endothelial function in humans. Clin. Sci. 2011, 120, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Kleniewska, P.; Kolodziejczyk, M.; Skibska, B.; Goraca, A. The role of endothelin-1 and endothelin receptor antagonists in inflammatory response and sepsis. Arch. Immunol. Ther. Exp. 2015, 63, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Austin, S.A.; Santhanam, A.V.; Hinton, D.J.; Choi, D.S.; Katusic, Z.S. Endothelial nitric oxide deficiency promotes Alzheimer’s disease pathology. J. Neurochem. 2013, 127, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.M.; Sink, K.M. Hypertension and its role in cognitive function: Current evidence and challenges for the future. Am. J. Hypertens. 2015, 29, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Pedditzi, E.; Peters, R.; Beckett, N. The risk of overweight/obesity in mid-life and late life for the development of dementia: A systematic review and meta-analysis of longitudinal studies. Age Ageing 2016, 45, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Willeumier, K.C.; Taylor, D.V.; Amen, D.G. Elevated BMI is associated with decreased blood flow in the prefrontal cortex using SPECT imaging in healthy adults. Obesity 2011, 19, 1095–1097. [Google Scholar] [CrossRef]
- Nguyen, J.C.D.; Killcross, A.S.; Jenkins, T.A. Obesity and cognitive decline: Role of inflammation and vascular changes. Front. Neurosci. 2014, 8, 375. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Llorens, S.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Talebi, M.; Shakibaei, M.; Samarghandian, S. An overview of the role of adipokines in cardiometabolic diseases. Molecules 2020, 25, 5218. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Khaza’ai, H.; Rahmat, A.; Patimah, I.; Abed, Y. Obesity can predict and promote systemic inflammation in healthy adults. Int. J. Cardiol. 2016, 215, 318–324. [Google Scholar] [CrossRef]
- Ouchi, N.; Kihara, S.; Funahashi, T.; Matsuzawa, Y.; Walsh, K. Obesity, adiponectin and vascular inflammatory disease. Curr. Opin. Lipidol. 2003, 14, 561–566. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Honjo, K.; Black, S.E.; Verhoeff, N.P. Alzheimer’s disease, cerebrovascular disease, and the beta-amyloid cascade. Can. J. Neurol. Sci. 2012, 39, 712–728. [Google Scholar] [CrossRef] [PubMed]
- Koyama, A.; O’Brien, J.; Weuve, J.; Blacker, D.; Metti, A.L.; Yaffe, K. The role of peripheral inflammatory markers in dementia and Alzheimer’s disease: A meta-analysis. J. Gerontol. Med. Sci. 2013, 68, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Anstey, K.J.; Wood, J. Chronological age and age-related cognitive deficits are associated with an increase in multiple types of driving errors in late life. Neuropsychology 2011, 25, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Xiong, S.; Zhu, Z. Dietary capsaicin protects cardiometabolic organs from dysfunction. Nutrients 2016, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Bliss, E.; Brown, L. Capsaicin in metabolic syndrome. Nutrients 2018, 10, 630. [Google Scholar] [CrossRef] [PubMed]
- Fatehi-Hassanabad, Z.; Chan, C.B.; Furman, B.L. Reactive oxygen species and endothelial function in diabetes. Eur. J. Pharmacol. 2010, 636, 8–17. [Google Scholar] [CrossRef]
- Bliss, E.S.; Wong, R.H.X.; Howe, P.R.C.; Mills, D.E. Benefits of exercise training on cerebrovascular and cognitive function in ageing. J. Cereb. Blood Flow Metab. 2021, 41, 447–470. [Google Scholar] [CrossRef]
- Gaber El-Saber, B.; Alqahtani, A.; Oluwafemi Adeleke, O.; Shaheen, H.M.; Wasef, L.; Elzeiny, M.; Ismail, M.; Shalaby, M.; Murata, T.; Zaragoza-Bastida, A.; et al. Biological properties, bioactive constituents, and pharmacokinetics of some Capsicum spp. and capsaicinoids. Int. J. Mol. Sci. 2020, 21, 5179. [Google Scholar] [CrossRef]
- Rollyson, W.D.; Stover, C.A.; Brown, K.C.; Perry, H.E.; Stevenson, C.D.; McNees, C.A.; Ball, J.G.; Valentovic, M.A.; Dasgupta, P. Bioavailability of capsaicin and its implications for drug delivery. J. Control. Release 2014, 196, 96–105. [Google Scholar] [CrossRef]
- Adetunji, T.L.; Olawale, F.; Olisah, C.; Adetunji, A.E.; Aremu, A.O. Capsaicin: A two-decade systematic review of global research output and recent advances against human cancer. Front. Oncol. 2022, 12, 908487. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, P.R.; Ravishankar, G.A. Casaicin: A promising multifaceted drug from Capsicum spp. Evid. Based Integr. Med. 2005, 1, 147–166. [Google Scholar] [CrossRef]
- Luo, X.J.; Peng, J.; Li, Y.J. Recent advances in the study on capsaicinoids and capsinoids. Eur. J. Pharmacol. 2011, 650, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Hosokawa, M.; Otsu, K.; Watanabe, T.; Yazawa, S. Assessment of capsiconinoid composition, nonpungent capsaicinoid analogues, in capsicum cultivars. J. Agric. Food Chem. 2009, 57, 5407–5412. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Luo, Z.; Ma, S.; Wong, W.T.; Ma, L.; Zhong, J.; He, H.; Zhao, Z.; Cao, T.; Yan, Z.; et al. Activation of TRPV1 by dietary capsaicin improves endothelium-dependent vasorelaxation and prevents hypertension. Cell Metab. 2010, 12, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Ching, L.C.; Kou, Y.R.; Shyue, S.K.; Su, K.H.; Wei, J.; Cheng, L.C.; Yu, Y.B.; Pan, C.C.; Lee, T.S. Molecular mechanisms of activation of endothelial nitric oxide synthase mediated by transient receptor potential vanilloid type 1. Cardiovasc. Res. 2011, 91, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Patowary, P.; Pathak, M.P.; Zaman, K.; Raju, P.S.; Chattopadhyay, P. Research progress of capsaicin responses to various pharmacological challenges. Biomed. Pharmacother. 2017, 96, 1501–1512. [Google Scholar] [CrossRef]
- Yao, X.; Garland, C.J. Recent developments in vascular endothelial cell transient receptor potential channels. Circ. Res. 2005, 97, 853–863. [Google Scholar] [CrossRef]
- McCarty, M.F.; DiNicolantonio, J.J.; O’Keefe, J.H. Capsaicin may have important potential for promoting vascular and metabolic health. Open Heart 2015, 2, e000262. [Google Scholar] [CrossRef]
- Amini, M.R.; Sheikhhossein, F.; Bazshahi, E.; Hajiaqaei, M.; Shafie, A.; Shahinfar, H.; Azizi, N.; Gharehgheshlaghi, H.E.; Naghshi, S.; Fathipour, R.B.; et al. The effects of capsinoids and fermented red pepper paste supplementation on blood pressure: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2021, 40, 1767–1775. [Google Scholar] [CrossRef]
- Derry, S.; Rice, A.S.; Cole, P.; Tan, T.; Moore, R.A. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2017, 1, 1465–1858. [Google Scholar] [CrossRef]
- D’Souza, R.S.; Barman, R.; Joseph, A.; Abd-Elsayed, A. Evidence-based treatment of painful diabetic neuropathy: A systematic review. Curr. Pain Headache Rep. 2022, 26, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Ludy, M.J.; Moore, G.E.; Mattes, R.D. The effects of capsaicin and capsiate on energy balance: Critical review and meta-analyses of studies in humans. Chem. Senses 2012, 37, 103–121. [Google Scholar] [CrossRef]
- Whiting, S.; Derbyshire, E.; Tiwari, B.K. Capsaicinoids and capsinoids. A potential role for weight management? A systematic review of the evidence. Appetite 2012, 59, 341–348. [Google Scholar] [CrossRef]
- Irandoost, P.; Lotfi Yagin, N.; Namazi, N.; Keshtkar, A.; Farsi, F.; Mesri Alamdari, N.; Vafa, M. The effect of Capsaicinoids or Capsinoids in red pepper on thermogenesis in healthy adults: A systematic review and meta-analysis. Phytother. Res. 2021, 35, 1358–1377. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Qu, H.; Lin, G.; Shi, D.; Chen, K.; Gao, Z. Lipid-lowering efficacy of the capsaicin in patients with metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. Front. Nutr. 2022, 9, 812294. [Google Scholar] [CrossRef] [PubMed]
- Catalfamo, L.M.; Marrone, G.; Basilicata, M.; Vivarini, I.; Paolino, V.; Della-Morte, D.; De Ponte, F.S.; Di Daniele, F.; Quattrone, D.; De Rinaldis, D.; et al. The utility of Capsicum annuum L. in internal medicine and in dentistry: A comprehensive review. Int. J. Environ. Res. Public Health 2022, 19, 11187. [Google Scholar] [CrossRef] [PubMed]
- Szallasi, A. Capsaicin for weight control: “exercise in a pill” (or just another fad)? Pharmaceuticals 2022, 15, 851. [Google Scholar] [CrossRef]
- Li, B.H.; Yin, Y.W.; Liu, Y.; Pi, Y.; Guo, L.; Cao, X.J.; Gao, C.Y.; Zhang, L.L.; Li, J.C. TRPV1 activation impedes foam cell formation by inducing autophagy in oxLDL-treated vascular smooth muscle cells. Cell Death Dis. 2014, 5, e1182. [Google Scholar] [CrossRef]
- Belza, A.; Jessen, A.B. Bioactive food stimulants of sympathetic activity: Effect on 24-h energy expenditure and fat oxidation. Eur. J. Clin. Nutr. 2005, 59, 733–741. [Google Scholar] [CrossRef]
- Sahin, K.; Orhan, C.; Tuzcu, M.; Sahin, N.; Erten, F.; Juturu, V. Capsaicinoids improve consequences of physical activity. Toxicol. Rep. 2018, 15, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhong, J.; Zhao, Z.; Luo, Z.; Ma, S.; Sun, J.; He, H.; Zhu, T.; Liu, D.; Zhu, Z.; et al. Activation of TRPV1 reduces vascular lipid accumulation and attenuates atherosclerosis. Cardiovasc. Res. 2011, 92, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, L.; Xu, H.; Liu, S.; Zhu, F.; Yan, F.; Shen, S.; Zhu, M. TRPV1 agonism inhibits endothelial cell inflammation via activation of eNOS/NO pathway. Atherosclerosis 2017, 260, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Li, S.; Meng, Y.; Zhao, Q.; Zhang, Y.; Suonan, Z.; Sun, Y.; Shen, Q.; Liao, X.; Xue, Y. Capsaicin ameliorates high-fat diet-induced atherosclerosis in ApoE(-/-) mice via remodeling gut microbiota. Nutrients 2022, 14, 4334. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tian, W.; Wang, S.; Wei, W.; Wu, D.; Wang, H.; Wang, L.; Yang, R.; Ji, A.; Li, Y. Anti-inflammatory and retinal protective effects of capsaicin on ischaemia-induced injuries through the release of endogenous somatostatin. Clin. Exp. Pharmacol. Physiol. 2017, 44, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Ebenebe, O.V.; Heather, A.; Erickson, J.R. CaMKII in vascular signalling: “friend or foe”? Heart Lung Circ. 2018, 27, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Hwang, J.T.; Park, H.S.; Kwon, D.Y.; Kim, M.S. Capsaicin stimulates glucose uptake in C2C12 muscle cells via the reactive oxygen species (ROS)/AMPK/p38 MAPK pathway. Biochem. Biophys. Res. Commun. 2013, 439, 66–70. [Google Scholar] [CrossRef]
- Kovacs, G.G. Tauopathies. Handb. Clin. Neurol. 2017, 145, 355–368. [Google Scholar] [CrossRef]
- Mietelska-Porowska, A.; Wasik, U.; Goras, M.; Filipek, A.; Niewiadomska, G. Tau protein modifications and interactions: Their role in function and dysfunction. Int. J. Mol. Sci. 2014, 15, 4671–4713. [Google Scholar] [CrossRef]
- Chen, L.; Huang, Z.; Du, Y.; Fu, M.; Han, H.; Wang, Y.; Dong, Z. Capsaicin attenuates amyloid-beta-Induced synapse loss and cognitive impairments in mice. J. Alzheimers Dis. 2017, 59, 683–694. [Google Scholar] [CrossRef]
- Du, Y.; Fu, M.; Huang, Z.; Tian, X.; Li, J.; Pang, Y.; Song, W.; Tian Wang, Y.; Dong, Z. TRPV1 activation alleviates cognitive and synaptic plasticity impairments through inhibiting AMPAR endocytosis in APP23/PS45 mouse model of Alzheimer’s disease. Aging Cell 2020, 19, e13113. [Google Scholar] [CrossRef] [PubMed]
- Claassen, J.A.; Jansen, R.W. Cholinergically mediated augmentation of cerebral perfusion in Alzheimer’s disease and related cognitive disorders: The cholinergic-vascular hypothesis. J. Gerontol. Med. Sci. 2006, 61, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Herholz, K. Acetylcholine esterase activity in mild cognitive impairment and Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, S25–S29. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Scouten, A.; Schwindt, G.; Janik, R.; Lee, W.; Sled, J.G.; Black, S.E.; Stefanovic, B. Hemodynamic effects of cholinesterase inhibition in mild Alzheimer’s disease. J. Magn. Reson. Imaging 2013, 38, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Nalivaeva, N.N.; Turner, A.J. AChE and the amyloid precursor protein (APP)-cross-talk in Alzheimer’s disease. Chem.-Biol. Interact. 2016, 259, 301–306. [Google Scholar] [CrossRef]
- Rajashri, K.; Mudhol, S.; Serva Peddha, M.; Borse, B.B. Neuroprotective effect of spice oleoresins on memory and cognitive impairment associated with scopolamine-induced Alzheimer’s disease in rats. Am. Chem. Soc. Omega 2020, 5, 30898–30905. [Google Scholar] [CrossRef]
- Viayna, E.; Coquelle, N.; Cieslikiewicz-Bouet, M.; Cisternas, P.; Oliva, C.A.; Sánchez-López, E.; Ettcheto, M.; Bartolini, M.; De Simone, A.; Ricchini, M.; et al. Discovery of a potent dual inhibitor of acetylcholinesterase and butyrylcholinesterase with antioxidant activity that alleviates Alzheimer-like pathology in old APP/PS1 mice. J. Med. Chem. 2021, 64, 812–839. [Google Scholar] [CrossRef]
- Shalaby, M.A.; Nounou, H.A.; Deif, M.M. The potential value of capsaicin in modulating cognitive functions in a rat model of streptozotocin-induced Alzheimer’s disease. Egypt. J. Neurol. Psychiatry Neurosurg. 2019, 55, 48. [Google Scholar] [CrossRef]
- Dworkin, S.; Mantamadiotis, T. Targeting CREB signalling in neurogenesis. Expert Opin. Ther. Targets 2010, 14, 869–879. [Google Scholar] [CrossRef]
- Patanè, S.; Marte, F.; Bella, G.D.; Cerrito, M.; Coglitore, S. Capsaicin, arterial hypertensive crisis and acute myocardial infarction associated with high levels of thyroid stimulating hormone. Int. J. Cardiol. 2009, 134, 130–132. [Google Scholar] [CrossRef]
- Singh, Y.; Gupta, G.; Shrivastava, B.; Dahiya, R.; Tiwari, J.; Ashwathanarayana, M.; Sharma, R.K.; Agrawal, M.; Mishra, A.; Dua, K. Calcitonin gene-related peptide (CGRP): A novel target for Alzheimer’s disease. CNS Neurosci. Ther. 2017, 23, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Jia, L.-W.; Li, X.-H.; Cheng, X.-S.; Xie, J.-Z.; Ma, Z.-W.; Xu, W.-J.; Liu, Y.; Yao, Y.; Du, L.-L.; et al. Capsaicin ameliorates stress-induced Alzheimer’s disease-like pathological and cognitive impairments in rats. J. Alzheimers Dis. 2013, 33, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Harada, N.; Narimatsu, N.; Kurihara, H.; Nakagata, N.; Okajima, K. Stimulation of sensory neurons improves cognitive function by promoting the hippocampal production of insulin-like growth factor-I in mice. Transl. Res. 2009, 154, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Bashiri, H.; Hosseini-Chegeni, H.; Alsadat Sharifi, K.; Sahebgharani, M.; Salari, A.A. Activation of TRPV1 receptors affects memory function and hippocampal TRPV1 and CREB mRNA expression in a rat model of biliary cirrhosis. Neurol. Res. 2018, 40, 938–947. [Google Scholar] [CrossRef]
- Avraham, Y.; Grigoriadis, N.C.; Magen, I.; Poutahidis, T.; Vorobiav, L.; Zolotarev, O.; Ilan, Y.; Mechoulam, R.; Berry, E.M. Capsaicin affects brain function in a model of hepatic encephalopathy associated with fulminant hepatic failure in mice. Br. J. Pharmacol. 2009, 158, 896–906. [Google Scholar] [CrossRef]
- Liu, C.-H.; Bu, X.-L.; Wang, J.; Zhang, T.; Xiang, Y.; Shen, L.-L.; Wang, Q.-H.; Deng, B.; Wang, X.; Zhu, C.; et al. The associations between a capsaicin-rich diet and blood amyloid-β levels and cognitive function. J. Alzheimers Dis. 2016, 52, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L.; Jansen, I.; Kingman, T.A.; McCulloch, J. Cerebrovascular responses to capsaicin in vitro and in situ. Br. J. Pharmacol. 1990, 100, 312–318. [Google Scholar] [CrossRef]
- Marics, B.; Peitl, B.; Pazmandi, K.; Bacsi, A.; Nemeth, J.; Oszlacs, O.; Jancso, G.; Dux, M. Diet-induced obesity enhances TRPV1-mediated neurovascular reactions in the dura mater. Headache 2017, 57, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.; Willows, J. Sensory nerve neuropeptide calcitonin gene–related peptide (CGRP) in adipose tissue changes according to metabolic status. Diabetes 2022, 71, 208-LB. [Google Scholar] [CrossRef]
- Xu, X.; Wang, P.; Zhao, Z.; Cao, T.; He, H.; Luo, Z.; Zhong, J.; Gao, F.; Zhu, Z.; Li, L.; et al. Activation of transient receptor potential vanilloid 1 by dietary capsaicin delays the onset of stroke in stroke-prone spontaneously hypertensive rats. Stroke 2011, 42, 3245–3251. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Romero, J.M.; Huerta-Franco, M.R.; Vargas-Luna, M.; Madrigal-Gutierrez, C.A.; Esparza-Hernandez, J.M.; Velazquez-Barcena, M.G. Dose escalation and safety of capsaicin for cerebral perfusion augmentation: A pilot study. Stroke 2021, 52, 2203–2209. [Google Scholar] [CrossRef] [PubMed]
- Laolob, T.; Bunyapraphatsara, N.; Waranuch, N.; Pongcharoen, S.; Punyain, W.; Chancharunee, S.; Sakchaisri, K.; Pratuangdejkul, J.; Chongruchiroj, S.; Kielar, F.; et al. Enhancement of lipolysis in 3T3-L1 adipocytes by nitroarene capsaicinoid analogs. Nat. Prod. Commun. 2021, 16, 1–13. [Google Scholar] [CrossRef]
- Jamornwan, S.; Chokpanuwat, T.; Uppakara, K.; Laorob, T.; Wichai, U.; Ketsawatsomkron, P.; Saengsawang, W. Nitro capsaicin suppressed microglial activation and TNF-alpha-Induced brain microvascular endothelial cell damage. Biomedicines 2022, 10, 2680. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lu, J.; Sha, X.; Qiu, Y.; Chen, H.; Yu, Z. TRPV1 regulates ApoE4-disrupted intracellular lipid homeostasis and decreases synaptic phagocytosis by microglia. Exp. Mol. Med. 2023, 55, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Pasierski, M.; Szulczyk, B. Beneficial effects of capsaicin in disorders of the central nervous system. Molecules 2022, 27, 2484. [Google Scholar] [CrossRef] [PubMed]
- Nimmo, A.J.; Cernak, I.; Heath, D.L.; Hu, X.; Bennett, C.J.; Vink, R. Neurogenic inflammation is associated with development of edema and functional deficits following traumatic brain injury in rats. Neuropeptides 2004, 38, 40–47. [Google Scholar] [CrossRef]
- Jørgensen, M.R.; Pedersen, A.M.L. Analgesic effect of topical oral capsaicin gel in burning mouth syndrome. Acta Odontol. Scand. 2017, 75, 130–136. [Google Scholar] [CrossRef]
- Li, H.B.; Mao, R.R.; Zhang, J.C.; Yang, Y.; Cao, J.; Xu, L. Antistress effect of TRPV1 channel on synaptic plasticity and spatial memory. Biol. Psychiatry 2008, 64, 286–292. [Google Scholar] [CrossRef]
- Liang, Y.; Tian, X.-Y.; Chen, J.; Peng, C.; Ma, K.; Zuo, Y.; Jiao, R.; Lu, Y.; Huang, Y.; Chen, Z.-Y. Capsaicinoids lower plasma cholesterol and improve endothelial function in hamsters. Eur. J. Nutr. 2013, 52, 379–388. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, P.; Xia, F.; Tang, H.; Chen, J.; Zhang, J.; Liu, D.; Zhu, Y.; Liu, Y.; Gu, L.; et al. Capsaicin ameliorates inflammation in a TRPV1-independent mechanism by inhibiting PKM2-LDHA-mediated Warburg effect in sepsis. Cell Chem. Biol. 2022, 29, 1248–1259. [Google Scholar] [CrossRef]
- Deshpande, J.; Jeyakodi, S.; Juturu, V. Tolerability of capsaicinoids from capsicum extract in a beadlet form: A pilot study. J. Toxicol. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.J.; Canale, R.E.; Shastri, S.; Suvarnapathki, S. Effect of oral intake of capsaicinoid beadlets on catecholamine secretion and blood markers of lipolysis in healthy adults: A randomized, placebo controlled, double-blind, cross-over study. Lipids Health Dis. 2010, 9, 72. [Google Scholar] [CrossRef] [PubMed]
- Manca, C.; Lacroix, S.; Perusse, F.; Flamand, N.; Chagnon, Y.; Drapeau, V.; Tremblay, A.; Marzo, V.D.; Silvestri, C. Oral capsaicinoid administration alters the plasma endocannabinoidome and fecal microbiota of reproductive-aged women living with overweight and obesity. Biomedicines 2021, 9, 1246. [Google Scholar] [CrossRef] [PubMed]
- OmniActive Health Technologies. Capsimax. Available online: https://omniactives.com/product/capsimax/#science-overview (accessed on 31 March 2022).
- Mariwala, J.K.; Rai, D.; Padigaru, M.; Ashok Morde, A.; Maddox, E.; Maalouf, S.; Smith, K.; Vanden Heuvel, J.P. Accumulating evidence to support the safe and efficacious use of a proprietary blend of capsaicinoids in mediating risk factors for obesity. Food Sci. Nutr. 2021, 9, 2823–2835. [Google Scholar] [CrossRef]
- Sahin, K.; Orhan, C.; Tuzcu, M.; Sahin, N.; Ozdemir, O.; Juturu, V. Ingested capsaicinoids can prevent low-fat-high-carbohydrate diet and high-fat diet-induced obesity by regulating the NADPH oxidase and Nrf2 pathways. J. Inflamm. Res. 2017, 10, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Chen, F.; Forzani, E.; Juturu, V. Capsaicinoids enhance metabolic rate in normal healthy individuals using a novel metabolic tracker breezing device-an open label placebo controlled acute study. Obes. Open Access 2017, 3, 1–5. [Google Scholar] [CrossRef]
- Rigamonti, A.E.; Casnici, C.; Marelli, O.; De Col, A.; Tamini, S.; Lucchetti, E.; Tringali, G.; De Micheli, R.; Abbruzzese, L.; Bortolotti, M.; et al. Acute administration of capsaicin increases resting energy expenditure in young obese subjects without affecting energy intake, appetite, and circulating levels of orexigenic/anorexigenic peptides. Nutr. Res. 2018, 52, 71–79. [Google Scholar] [CrossRef]
- Rogers, J.; Urbina, S.L.; Taylor, L.W.; Wilborn, C.D.; Purpura, M.; Jager, R.; Juturu, V. Capsaicinoids supplementation decreases percent body fat and fat mass: Adjustment using covariates in a post hoc analysis. Biomed Cent. Obes. 2018, 5, 22. [Google Scholar] [CrossRef]
- Urbina, S.L.; Roberts, M.D.; Kephart, W.C.; Villa, K.B.; Santos, E.N.; Olivencia, A.M.; Bennett, H.M.; Lara, M.D.; Foster, C.A.; Purpura, M.; et al. Effects of twelve weeks of capsaicinoid supplementation on body composition, appetite and self-reported caloric intake in overweight individuals. Appetite 2017, 113, 264–273. [Google Scholar] [CrossRef]
- Liu, D.; Huang, Y.; Huang, C.; Yang, S.; Wei, X.; Zhang, P.; Guo, D.; Lin, J.; Xu, B.; Li, C.; et al. Calorie restriction with or without time-restricted eating in weight Loss. N. Engl. J. Med. 2022, 386, 1495–1504. [Google Scholar] [CrossRef]
| Reference | Study Design | Species/Participants/Sex | Study Duration | Capsaicin Dosage | Primary Outcome/s |
|---|---|---|---|---|---|
| Effects of Capsimax in animal studies | |||||
| [152] | Random allocation | C57BL/6J male mice (n = 24) | 52 days | 0.84 mg/kg/day + high fat diet | ↑thermogenesis ↓body mass ↓lipogenesis |
| [153] | Random allocation | Male Wistar rats (n = 42) | 8 weeks | 10 mg/kg/day | ↓Inflammatory markers ↓body mass |
| [108] | Random allocation | Male albino Wistar rats (n = 28) | 8 weeks | 10 mg/kg/day | ↑antioxidants ↓inflammation ↑time to exhaustion |
| Effects of Capsimax in human studies | |||||
| [154] | Single-blind, crossover | Healthy adults (n = 40) 17F/23M | 3 h | 100 mg single dose | ↑resting energy expenditure |
| [149] | Double-blind, crossover, randomised control trial | Healthy adults (n = 20) 10F/10M | 3 h | 100 mg single dose | ↑free fatty acids ↑glycerol |
| [155] | Single blind, crossover, randomised control trial | Obese, hospitalised 15–34 years in weight reduction program (n = 10) 4F/6M | 6 h | 100 mg single dose | ↑resting energy expenditure ↑satiety ↓hunger |
| [148] | Open-label, dose-finding, adaptive study | Healthy overweight 25–55 years women (n = 12) | 6 weeks | 100 mg/day +100 mg/day weekly escalating until 500 mg/day/week | Tolerable up to 500 mg/day No adverse events No dropouts |
| [150] | Randomised control trial | Overweight or obese 18–51 years women (n = 61) | 12 weeks | 200 mg/day + diet restriction | ↓fat mass ↓body mass ↓waist circumference ↑free fat mass |
| [156] | Parallel double-blind, randomised control trial | Heathy 18–56 years (n = 77) 47F; 30M | 12 weeks | 100 mg/day 200 mg/day | ↓fat mass (−6.68%) ↓body mass (−5.91%) |
| [157] | Double-blind, randomised control trial | Healthy adults (n = 77) | 12 weeks | 100 mg/day 200 mg/day | ↓kJ intake ↓waist-to-hip ratio ↓appetite ↓HDL cholesterol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thornton, T.; Mills, D.; Bliss, E. Capsaicin: A Potential Treatment to Improve Cerebrovascular Function and Cognition in Obesity and Ageing. Nutrients 2023, 15, 1537. https://doi.org/10.3390/nu15061537
Thornton T, Mills D, Bliss E. Capsaicin: A Potential Treatment to Improve Cerebrovascular Function and Cognition in Obesity and Ageing. Nutrients. 2023; 15(6):1537. https://doi.org/10.3390/nu15061537
Chicago/Turabian StyleThornton, Tammy, Dean Mills, and Edward Bliss. 2023. "Capsaicin: A Potential Treatment to Improve Cerebrovascular Function and Cognition in Obesity and Ageing" Nutrients 15, no. 6: 1537. https://doi.org/10.3390/nu15061537
APA StyleThornton, T., Mills, D., & Bliss, E. (2023). Capsaicin: A Potential Treatment to Improve Cerebrovascular Function and Cognition in Obesity and Ageing. Nutrients, 15(6), 1537. https://doi.org/10.3390/nu15061537