Association between the Inflammatory Potential of the Diet and Biological Aging: A Cross-Sectional Analysis of 4510 Adults from the Moli-Sani Study Cohort
Abstract
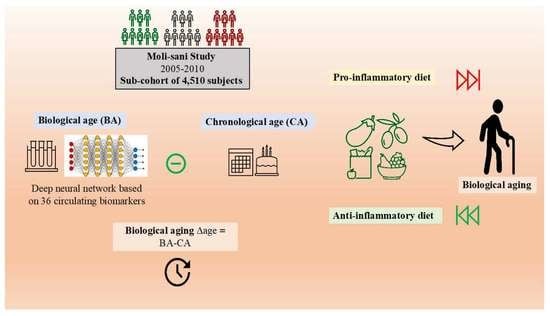
:1. Introduction
2. Methods
2.1. Study Population
2.2. Computation of Biological Age
2.3. Dietary Assessment
2.4. Computation of DII and E-DII Scores
2.5. Ascertainment of Covariates
2.6. Statistical Analysis
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 11 October 2022).
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. A Ser. Biol. Sci. Med. Sci. 2014, 69 (Suppl. 1), S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Hodes, R.J.; Sierra, F.; Austad, S.N.; Epel, E.; Neigh, G.N.; Erlandson, K.M.; Schafer, M.J.; LeBrasseur, N.K.; Wiley, C.; Campisi, J.; et al. Disease drivers of aging. Ann. N. Y. Acad. Sci. 2016, 1386, 45–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Witkowski, J.M.; Olivieri, F.; Larbi, A. The integration of inflammaging in age-related diseases. Semin. Immunol. 2018, 40, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Galvin, A.E.; Friedman, D.B.; Hébert, J.R. Focus on disability-free life expectancy: Implications for health-related quality of life. Qual. Life Res. 2021, 30, 2187–2195. [Google Scholar] [CrossRef]
- Franceschi, C.; Valensin, S.; Bonafè, M.; Paolisso, G.; Yashin, A.; Monti, D.; De Benedictis, G. The network and the remodeling theories of aging: Historical background and new perspectives. Exp. Gerontol. 2000, 35, 879–896. [Google Scholar] [CrossRef]
- Biagi, E.; Candela, M.; Franceschi, C.; Brigidi, P. The aging gut microbiota: New perspectives. Ageing Res. Rev. 2011, 10, 428–429. [Google Scholar] [CrossRef]
- Campisi, J.; d’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef]
- Grant, R.W.; Dixit, V.D. Mechanisms of disease: Inflammasome activation and the development of type 2 diabetes. Front. Immunol. 2013, 4, 50. [Google Scholar] [CrossRef] [Green Version]
- Lowsky, D.J.; Olshansky, S.J.; Bhattacharya, J.; Goldman, D.P. Heterogeneity in Healthy Aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 69, 640–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gialluisi, A.; di Castelnuovo, A.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; Castelnuovo, A.; Donati, M.B.; Gaetano, G.; Iacoviello, L.; Investigators, M.S. Learning Approaches for the Estimation of Biological Aging: The Road Ahead for Population Studies. Front. Med. 2019, 6, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanhooren, V.; Dewaele, S.; Libert, C.; Engelborghs, S.; De Deyn, P.P.; Toussaint, O.; Debacq-Chainiaux, F.; Poulain, M.; Glupczynski, Y.; Franceschi, C.; et al. Serum N-glycan profile shift during human ageing. Exp. Gerontol. 2010, 45, 738–743. [Google Scholar] [CrossRef] [Green Version]
- Levine, M.E. Modeling the Rate of Senescence: Can Estimated Biological Age Predict Mortality More Accurately Than Chronological Age? J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012, 68, 667–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gialluisi, A.; Di Castelnuovo, A.; Costanzo, S.; Bonaccio, M.; Persichillo, M.; Magnacca, S.; De Curtis, A.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; et al. Exploring domains, clinical implications and environmental associations of a deep learning marker of biological ageing. Eur. J. Epidemiol. 2021, 37, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Mamoshina, P.; Kochetov, K.; Putin, E.; Cortese, F.; Aliper, A.; Lee, W.-S.; Ahn, S.-M.; Uhn, L.; Skjodt, N.; Kovalchuk, O.; et al. Population Specific Biomarkers of Human Aging: A Big Data Study Using South Korean, Canadian, and Eastern European Patient Populations. J. Gerontol. Ser. A 2018, 73, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Putin, E.; Mamoshina, P.; Aliper, A.; Korzinkin, M.; Moskalev, A.; Kolosov, A.; Ostrovskiy, A.; Cantor, C.; Vijg, J.; Zhavoronkov, A. Deep biomarkers of human aging: Application of deep neural networks to biomarker development. Aging 2016, 8, 1021–1033. [Google Scholar] [CrossRef] [Green Version]
- Bae, C.-Y.; Im, Y.; Lee, J.; Park, C.-S.; Kim, M.; Kwon, H.; Kim, B.; Park, H.R.; Lee, C.-K.; Kim, I.; et al. Comparison of Biological Age Prediction Models Using Clinical Biomarkers Commonly Measured in Clinical Practice Settings: AI Techniques Vs. Traditional Statistical Methods. Front. Anal. Sci. 2021, 1, 8. [Google Scholar] [CrossRef]
- Nie, C.; Li, Y.; Li, R.; Yan, Y.; Zhang, D.; Li, T.; Li, Z.; Sun, Y.; Zhen, H.; Ding, J.; et al. Distinct biological ages of organs and systems identified from a multi-omics study. Cell Rep. 2022, 38, 110459. [Google Scholar] [CrossRef]
- Yeung, S.S.Y.; Kwan, M.; Woo, J.; Barchitta, M.; Cicero, A.; Fogacci, F.; Borghi, C. Healthy Diet for Healthy Aging. Nutrients 2021, 13, 4310. [Google Scholar] [CrossRef]
- Kim, S.; Myers, L.; Wyckoff, J.; Cherry, K.E.; Jazwinski, S.M. The frailty index outperforms DNA methylation age and its derivatives as an indicator of biological age. Geroscience 2017, 39, 83–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shivappa, N.; Bonaccio, M.; Hebert, J.R.; Di Castelnuovo, A.; Costanzo, S.; Ruggiero, E.; Pounis, G.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; et al. Association of proinflammatory diet with low-grade inflammation: Results from the Moli-sani study. Nutrition 2018, 54, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef] [PubMed]
- Stromsnes, K.; Correas, A.; Lehmann, J.; Gambini, J.; Olaso-Gonzalez, G. Anti-Inflammatory Properties of Diet: Role in Healthy Aging. Biomedicines 2021, 9, 922. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [Green Version]
- Hébert, J.R.; Shivappa, N.; Wirth, M.D.; Hussey, J.R.; Hurley, T.G. Perspective: The Dietary Inflammatory Index (DII)—Lessons Learned, Improvements Made, and Future Directions. Adv. Nutr. Int. Rev. J. 2019, 10, 185–195. [Google Scholar] [CrossRef]
- Mazidi, M.; Shivappa, N.; Wirth, M.D.; Hebert, J.R.; Mikhailidis, D.P.; Kengne, A.P.; Banach, M. Dietary inflammatory index and cardiometabolic risk in US adults. Atherosclerosis 2018, 276, 23–27. [Google Scholar] [CrossRef]
- Tabung, F.; Steck, S.E.; Ma, Y.; Liese, A.D.; Zhang, J.; Caan, B.; Hou, L.; Johnson, K.C.; Mossavar-Rahmani, Y.; Shivappa, N.; et al. The association between dietary inflammatory index and risk of colorectal cancer among postmenopausal women: Results from the Women’s Health Initiative. Cancer Causes Control 2014, 26, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Ramallal, R.; Toledo, E.; Martinez-Gonzalez, M.; Hernandez-Hernandez, A.; Garcia-Arellano, A.; Shivappa, N.; Hebert, J.R.; Ruiz-Canela, M. Dietary Inflammatory Index and Incidence of Cardiovascular Disease in the SUN Cohort. PLoS ONE 2015, 10, e0135221. [Google Scholar] [CrossRef]
- Kivimäki, M.; Shipley, M.J.; Batty, G.D.; Hamer, M.; Akbaraly, T.N.; Kumari, M.; Jokela, M.; Virtanen, M.; Lowe, G.D.; Ebmeier, K.P.; et al. Long-term inflammation increases risk of common mental disorder: A cohort study. Mol. Psychiatry 2014, 19, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Resciniti, N.V.; Lohman, M.C.; Wirth, M.D.; Shivappa, N.; Hebert, J.R. Dietary Inflammatory Index, Pre-Frailty and Frailty Among Older US Adults: Evidence from the National Health and Nutrition Examina-tion Survey, 2007–2014. J. Nutr. Health Aging 2019, 23, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, P.; Shivappa, N.; Hébert, J.R.; Nasab, S.J.; Bahrami, A.; Hekmatdoost, A.; Rashidkhani, B.; Sadeghi, A.; Houshyari, M.; Hejazi, E. Dietary Inflammatory Index and Odds of Colorectal Cancer and Colorectal Adenomatous Polyps in a Case-Control Study from Iran. Nutrients 2019, 11, 1213. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, D.R.; Tapsell, L.C. Food, Not Nutrients, Is the Fundamental Unit in Nutrition. Nutr. Rev. 2007, 65, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Byrd, D.A.; Judd, S.E.; Flanders, W.D.; Hartman, T.J.; Fedirko, V.; Bostick, R.M. Development and Validation of Novel Dietary and Lifestyle Inflammation Scores. J. Nutr. 2019, 149, 2206–2218. [Google Scholar] [CrossRef]
- Li, Z.; Gao, Y.; Byrd, D.A.; Gibbs, D.C.; E Prizment, A.; Lazovich, D.; Bostick, R.M. Novel Dietary and Lifestyle Inflammation Scores Directly Associated with All-Cause, All-Cancer, and All-Cardiovascular Disease Mortality Risks Among Women. J. Nutr. 2021, 151, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Byrd, D.A.; E Judd, S.; Flanders, W.D.; Hartman, T.J.; Fedirko, V.; Agurs-Collins, T.; Bostick, R.M. Associations of Novel Dietary and Lifestyle Inflammation Scores With Incident Colorectal Cancer in the NIH-AARP Diet and Health Study. JNCI Cancer Spectr. 2020, 4, pkaa009. [Google Scholar] [CrossRef] [Green Version]
- Iacoviello, L.; Bonanni, A.; Costanzo, S.; Curtis, A.; Castelnuovo, A.; Olivieri, M.; Zito, F.; Donati, M.B.; Gaetano, G. The Moli-Sani Project, a Randomized, Prospective Cohort Study in the Molise Region in Italy; Design, Rationale and Objectives. Ital. J. Public Health 2007, 4, 110–118. [Google Scholar]
- Pisani, P.; Faggiano, F.; Krogh, V.; Palli, D.; Vineis, P.; Berrino, F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int. J. Epidemiol. 1997, 26 (Suppl. 1), 152S–160S. [Google Scholar] [CrossRef] [Green Version]
- Pala, V.; Sieri, S.; Palli, D.; Salvini, S.; Berrino, F.; Bellegotti, M.; Frasca, G.; Tumino, R.; Sacerdote, C.; Fiorini, L.; et al. Diet in the Italian Epic Cohorts: Presentation of Data and Methodological Issues. Tumori J. 2003, 89, 594–607. [Google Scholar] [CrossRef]
- Salvini, S.; Parpinel, M.; Gnagnarella, P.; Maissoneuve, P.; Turrini, A. Banca dati Composizione degli Alimenti per Studi Epidemiologici in Italia; European Institute of Oncology: Milano, Italy, 1988. [Google Scholar]
- ISTAT: Istituto Nazionale di Statistica. Atlante Statistic dei Comuni. 2014. Available online: https://www.istat.it/it/archivio/113712 (accessed on 15 September 2020).
- Hernán, M.A.; Hernández-Díaz, S.; Werler, M.M.; Mitchell, A.A. Causal Knowledge as a Prerequisite for Confounding Evaluation: An Application to Birth Defects Epidemiology. Am. J. Epidemiol. 2002, 155, 176–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hornedo-Ortega, R.; Cerezo, A.B.; Pablos, R.M.; Krisa, S.; Richard, T.; Garcia-Parrilla, M.C.; Troncoso, A.M.; Ceruti, S.; Silva, R.F.M.; Troncoso, A.M.; et al. Phenolic Compounds Characteristic of the Mediterranean Diet in Mitigating Microglia-Mediated Neuroinflammation. Front. Cell. Neurosci. 2018, 12, 373. [Google Scholar] [CrossRef]
- Okada, E.; Shirakawa, T.; Shivappa, N.; Wakai, K.; Suzuki, K.; Date, C.; Iso, H.; Hébert, J.R.; Tamakoshi, A. Dietary Inflammatory Index Is Associated with Risk of All-Cause and Cardiovascular Disease Mortality but Not with Cancer Mortality in Middle-Aged and Older Japanese Adults. J. Nutr. 2019, 149, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Gialluisi, A.; Santoro, A.; Tirozzi, A.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Franceschi, C.; Iacoviello, L. Epidemiological and genetic overlap among biological aging clocks: New challenges in biogerontology. Ageing Res. Rev. 2021, 72, 101502. [Google Scholar] [CrossRef]
- Esposito, S.; Gialluisi, A.; Costanzo, S.; Di Castelnuovo, A.; Ruggiero, E.; De Curtis, A.; Persichillo, M.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; et al. Mediterranean diet and other dietary patterns in association with biological aging in the Moli-sani Study cohort. Clin. Nutr. 2022, 41, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Gialluisi, A.; Costanzo, S.; Di Castelnuovo, A.; Ruggiero, E.; De Curtis, A.; Persichillo, M.; Cerletti, C.; Donati, M.; de Gaetano, G.; et al. Dietary Polyphenol Intake Is Associated with Biological Aging, a Novel Predictor of Cardiovascular Disease: Cross-Sectional Findings from the Moli-Sani Study. Nutrients 2021, 13, 1701. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Hebert, J.R.; Marcos, A.; Diaz, L.-E.; Gomez, S.; Nova, E.; Michels, N.; Arouca, A.; González-Gil, E.; Frederic, G.; et al. Association between dietary inflammatory index and inflammatory markers in the HELENA study. Mol. Nutr. Food Res. 2017, 61, 1600707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Z.; Zhao, A.; Wang, Y.; Meng, L.; Szeto, I.M.-Y.; Li, T.; Gong, H.; Tian, Z.; Zhang, Y.; Wang, P. Association between Dietary Inflammatory Index, C-Reactive Protein and Metabolic Syndrome: A Cross-Sectional Study. Nutrients 2018, 10, 831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Kang, D.; Lee, S.-A. Effect of dietary patterns on serum C-reactive protein level. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1004–1011. [Google Scholar] [CrossRef]
- García-Calzón, S.; Zalba, G.; Ruiz-Canela, M.; Shivappa, N.; Hébert, J.R.; Martínez, J.A.; Fitó, M.; Gómez-Gracia, E.; A Martínez-González, M.; Marti, A. Dietary inflammatory index and telomere length in subjects with a high cardiovascular disease risk from the PREDIMED-NAVARRA study: Cross-sectional and longitudinal analyses over 5 y. Am. J. Clin. Nutr. 2015, 102, 897–904. [Google Scholar] [CrossRef] [Green Version]
- Crous-Bou, M.; Molinuevo, J.-L.; Sala-Vila, A. Plant-Rich Dietary Patterns, Plant Foods and Nutrients, and Telomere Length. Adv. Nutr. 2019, 10 (Suppl. 4), S296–S303. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Huan, T.; Joehanes, R.; McKeown, N.M.; Horvath, S.; Levy, D.; Ma, J. Higher diet quality relates to decelerated epigenetic aging. Am. J. Clin. Nutr. 2021, 115, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.N.; Hodges, R.; Hanes, D.; Stack, E.; Cheishvili, D.; Szyf, M.; Henkel, J.; Twedt, M.W.; Giannopoulou, D.; Herdell, J.; et al. Potential reversal of epigenetic age using a diet and lifestyle intervention: A pilot randomized clinical trial. Aging 2021, 13, 9419–9432. [Google Scholar] [CrossRef] [PubMed]
- Casati, M.; Ferri, E.; Azzolino, D.; Cesari, M.; Arosio, B. Gut microbiota and physical frailty through the mediation of sarcopenia. Exp. Gerontol. 2019, 124, 110639. [Google Scholar] [CrossRef] [PubMed]
- Mangiola, F.; Nicoletti, A.; Gasbarrini, A.; Ponziani, F.R. Gut Microbiota and Aging. Eur. Rev. Med. Pharmacol. Sci. 2015, 350, 1214–1215. [Google Scholar] [CrossRef]
- Sonnenburg, J.L.; Bäckhed, F. Diet–microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [Green Version]
- Leeming, E.R.; Louca, P.; Gibson, R.; Menni, C.; Spector, T.D.; Le Roy, C.I. The complexities of the diet-microbiome relationship: Advances and perspectives. Genome Med. 2021, 13, 10. [Google Scholar] [CrossRef]
- Picca, A.; Fanelli, F.; Calvani, R.; Mule, G.; Pesce, V.; Sisto, A.; Pantanelli, C.; Bernabei, R.; Landi, F.; Marzetti, E. Gut Dysbiosis and Muscle Aging: Searching for Novel Targets against Sarcopenia. Mediat. Inflamm. 2018, 2018, 7026198. [Google Scholar] [CrossRef]
- Beam, A.; Clinger, E.; Hao, L. Effect of Diet and Dietary Components on the Composition of the Gut Microbiota. Nutrients 2021, 13, 2795. [Google Scholar] [CrossRef]
- Santoro, A.; Pini, E.; Scurti, M.; Palmas, G.; Berendsen, A.; Brzozowska, A.; Pietruszka, B.; Szczecinska, A.; Cano, N.; Meunier, N.; et al. Combating inflammaging through a Mediterranean whole diet approach: The NU-AGE project’s conceptual framework and design. Mech. Ageing Dev. 2014, 136, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, A.; Hermes, G.; Berendsen, A.; Van De Rest, O.; Zoetendal, E.; Fuentes, S.; Santoro, A.; Franceschi, C.; De Groot, L.; De Vos, W. Associations between Pro- and Anti-Inflammatory Gastro-Intestinal Microbiota, Diet, and Cognitive Functioning in Dutch Healthy Older Adults: The NU-AGE Study. Nutrients 2020, 12, 3471. [Google Scholar] [CrossRef] [PubMed]
- Senizza, A.; Rocchetti, G.; Mosele, J.I.; Patrone, V.; Callegari, M.L.; Morelli, L.; Lucini, L. Lignans and Gut Microbiota: An Interplay Revealing Potential Health Implications. Molecules 2020, 25, 5709. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Wirth, M.D.; Murphy, E.A.; Hurley, T.G.; Hébert, J.R. Association between the Dietary Inflammatory Index (DII) and urinary enterolignans and C-reactive protein from the National Health and Nutrition Examination Survey-2003–2008. Eur. J. Nutr. 2018, 58, 797–805. [Google Scholar] [CrossRef]
- Markle, J.G.M.; Frank, D.N.; Mortin-Toth, S.; Robertson, C.E.; Feazel, L.M.; Rolle-Kampczyk, U.; von Bergen, M.; McCoy, K.D.; Macpherson, A.J.; Danska, J.S. Sex Differences in the Gut Microbiome Drive Hormone-Dependent Regulation of Autoimmunity. Science 2013, 339, 1084–1088. [Google Scholar] [CrossRef] [Green Version]
- Hold, G.L.; Smith, M.; Grange, C.; Watt, E.R.; El-Omar, E.M.; Mukhopadhya, I. Role of the gut microbiota in inflammatory bowel disease pathogenesis: What have we learnt in the past 10 years? World J. Gastroenterol. 2014, 20, 1192–1210. [Google Scholar] [CrossRef]
- Rosano, G.M.C.; Spoletini, I.; Vitale, C. Cardiovascular disease in women, is it different to men? The role of sex hormones. Climacteric 2017, 20, 125–128. [Google Scholar] [CrossRef]
- Franconi, F.; Brunelleschi, S.; Steardo, L.; Cuomo, V. Gender differences in drug responses. Pharmacol. Res. 2007, 55, 81–95. [Google Scholar] [CrossRef]
- Haro, C.; Rangel-Zuñiga, O.A.; Alcala-Diaz, J.F.; Delgado, F.G.; Pérez-Martínez, P.; Delgado-Lista, J.; Quintana-Navarro, G.M.; Landa, B.B.; Navas-Cortes, J.; Tena-Sempere, M.; et al. Intestinal Microbiota Is Influenced by Gender and Body Mass Index. PLoS ONE 2016, 11, e0154090. [Google Scholar] [CrossRef] [Green Version]
- Kankaanpää, A.; Tolvanen, A.; Heikkinen, A.; Kaprio, J.; Ollikainen, M.; Sillanpää, E. The role of adolescent lifestyle habits in biological aging: A prospective twin study. Elife 2022, 11, e80729. [Google Scholar] [CrossRef]
- Liu, F.-H.; Liu, C.; Gong, T.-T.; Gao, S.; Sun, H.; Jiang, Y.-T.; Zhang, J.-Y.; Zhang, M.; Gao, C.; Li, X.-Y.; et al. Dietary Inflammatory Index and Health Outcomes: An Umbrella Review of Systematic Review and Meta-Analyses of Observational Studies. Front. Nutr. 2021, 8, 647122. [Google Scholar] [CrossRef] [PubMed]
- Denova-Gutiérrez, E.; Muñoz-Aguirre, P.; Shivappa, N.; Hébert, J.R.; Tolentino-Mayo, L.; Batis, C.; Barquera, S. Dietary Inflammatory Index and Type 2 Diabetes Mellitus in Adults: The Diabetes Mellitus Survey of Mexico City. Nutrients 2018, 10, 385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, D.E.; Xiang, J. The Dietary Inflammatory Index Is Associated With Diabetes Severity. J. Am. Board Fam. Med. 2019, 32, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Muriach, M.; Flores-Bellver, M.; Romero, F.J.; Barcia, J.M. Diabetes and the Brain: Oxidative Stress, Inflammation, and Autophagy. Oxidative Med. Cell. Longev. 2014, 2014, 102158. [Google Scholar] [CrossRef] [Green Version]
- Byrd, D.A.; Judd, S.; Flanders, W.D.; Hartman, T.J.; Fedirko, V.; Bostick, R.M. Associations of Novel Dietary and Lifestyle Inflammation Scores with Incident, Sporadic Colorectal Adenoma. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2300–2308. [Google Scholar] [CrossRef]
- Ferrer, R.A.; Klein, W.M. Risk perceptions and health behavior. Curr. Opin. Psychol. 2015, 5, 85–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kipnis, V.; Subar, A.F.; Midthune, D.; Freedman, L.S.; Ballard-Barbash, R.; Troiano, R.P.; Bingham, S.; Schoeller, D.A.; Schatzkin, A.; Carroll, R.J. Structure of Dietary Measurement Error: Results of the OPEN Biomarker Study. Am. J. Epidemiol. 2003, 158, 14–21, discussion 16–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhee, J.J.; Sampson, L.; Cho, E.; Hughes, M.D.; Hu, F.B.; Willett, W.C. Comparison of Methods to Account for Implausible Reporting of Energy Intake in Epidemiologic Studies. Am. J. Epidemiol. 2015, 181, 225–233. [Google Scholar] [CrossRef]
- Palmieri, L.; Panico, S.; Vanuzzo, D.; Ferrario, M.; Pilotto, L.; Sega, R.; Cesana, G.; per il Gruppo di ricerca del Progetto CUORE. La valutazione del rischio cardiovascolare globale assoluto: Il punteggio individuale del Progetto CUORE. Ann. Ist. Super Sanità 2004, 40, 393–399. [Google Scholar]
| E-DIITM Quartile | DIS Quartile | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics 2 | All (n = 4510) | 1 (n = 1127) | 4 (n = 1128) | p-value * | 1 (n = 1128) | 4 (n = 1127) | p-value ** |
| Chronological age, y | 55.6 (11.6) | 58.4 (11.4) | 51.9 (10.9) | <0.0001 | 57.4 (11.0) | 52.0 (11.1) | <0.0001 |
| Biological age, y | 54.8 (8.6) | 56.3 (8.6) | 53.1 (8.3) | 0.21 | 55.2 (8.3) | 52.9 (8.3) | 0.0012 |
| Δage (BA-CA) | −0.77 (7.7) | −2.1 (7.6) | 1.1 (7.4) | 0.21 | −2.1 (7.4) | 0.8 (7.6) | 0.0012 |
| Men, % | 48.0 | 37.7 | 54.0 | <0.0001 | 47.4 | 47.3 | 0.003 |
| Education, % | 0.11 | <0.0001 | |||||
| Lower secondary | 52.8 | 54.1 | 48.3 | 49.2 | 52.7 | ||
| Upper secondary | 35.0 | 33.1 | 38.3 | 35.0 | 35.6 | ||
| Post-secondary | 12.1 | 12.8 | 13.4 | 15.8 | 11.7 | ||
| Missing data | 0.02 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| Housing tenure, % | 0.19 | 0.0007 | |||||
| Rent | 9.2 | 8.8 | 10.8 | 7.9 | 12.0 | ||
| One dwelling ownership | 81.2 | 80.5 | 81.9 | 80.1 | 80.7 | ||
| >1 dwelling ownership | 9.5 | 10.7 | 7.1 | 12.0 | 7.4 | ||
| Missing data | 0.1 | 0.0 | 0.2 | 0.0 | 0.0 | ||
| Place of residence, % | 0.04 | 0.002 | |||||
| Urban | 32.7 | 70.5 | 65.7 | 71.9 | 65.2 | ||
| Rural | 67.3 | 29.5 | 24.3 | 28.1 | 34.8 | ||
| Smoking status, % | 0.04 | 0.0002 | |||||
| Non-smoker | 49.8 | 52.1 | 45.5 | 47.9 | 49.1 | ||
| Smokers | 22.4 | 17.5 | 29.0 | 18.7 | 28.7 | ||
| Former | 27.7 | 30.3 | 25.5 | 33.1 | 22.2 | ||
| Missing data | 0.1 | 0.1 | 0.0 | 0.3 | 0.1 | ||
| Body mass index, kg/m2 | 28.2 (4.8) | 28.6 (4.9) | 27.7 (4.7) | 0.02 | 28.4 (4.6) | 27.8 (5.0) | 0.16 |
| Body weight status, kg/m2 | 0.003 | 0.02 | |||||
| Normal weight (≤25 kg/m²), % | 26.7 | 24.5 | 31.6 | 24.6 | 32.6 | ||
| Overweight (25–30 kg/m²), % | 42.0 | 40.3 | 42.1 | 42.9 | 38.1 | ||
| Obesity (≥30 kg/m²), % | 31.2 | 35.0 | 26.2 | 32.4 | 29.3 | ||
| Missing data | 0.1 | 0.2 | 0.1 | 0.2 | 0.0 | ||
| Leisure-time physical activity, METS hr/d | 3.5 (4.0) | 3.8 (4.2) | 3.1 (3.6) | <0.0001 | 4.2 (4.4) | 2.9 (3.4) | <0.0001 |
| Post-menopausal, % | 59.5 | 70.1 | 48.2 | 0.38 | 68.2 | 46.7 | 0.74 |
| Postmenopausal hormone therapy, % | 1.4 | 4.9 | 1.8 | 0.02 | 3.7 | 1.9 | 0.03 |
| Comorbidities, % | |||||||
| Cardiovascular disease | 5.5 | 8.3 | 4.5 | <0.0001 | 6.6 | 4.1 | 0.53 |
| Cancer | 3.2 | 4.4 | 2.8 | 0.63 | 3.7 | 2.7 | 0.93 |
| Diabetes | 4.9 | 9.0 | 2.7 | <0.0001 | 7.3 | 2.0 | 0.003 |
| Hypertension | 29.6 | 35.2 | 22.8 | 0.54 | 31.7 | 21.9 | 0.11 |
| Hyperlipidemia | 7.9 | 12.1 | 4.4 | <0.0001 | 11.0 | 3.8 | 0.0002 |
| Comorbidity | 0.0004 | 0.01 | |||||
| Without comorbidity | 63.1 | 53.4 | 71.9 | 57.8 | 73.6 | ||
| 1 or more comorbidities | 36.9 | 46.6 | 28.1 | 42.2 | 26.4 | ||
| Dietary characteristics | |||||||
| Energy intake/d, kcal | 2083.7 (576.5) | 1843.9 (529.9) | 2274.7 (574.7) | <0.0001 | 2018.2 (580.7) | 2233.6 (593.0) | <0.0001 |
| Energy of carbohydrates; % | 48.5 (6.9) | 48.5 (6.8) | 48.7 (7.0) | 0.35 | 47.9 (7.0) | 49.8 (7.0) | <0.0001 |
| Energy of fats, % | 33.2 (5.6) | 34.3 (5.3) | 33.2 (5.8) | <0.0001 | 34.4 (5.6) | 32.3 (5.7) | <0.0001 |
| Vegetables; g/d | 159.5 (71.6) | 198.8 (86.8) | 128.9 (51.8) | <0.0001 | 210.1 (87.9) | 122.8 (52.2) | <0.0001 |
| Fruits; g/d | 357.7 (204.9) | 492.3 (254.8) | 227.2 (121.0) | <0.0001 | 528.2 (255.2) | 242.6 (129.5) | <0.0001 |
| Fiber; g/d | 20.4 (6.6) | 23.3 (7.7) | 17.7 (5.3) | <0.0001 | 24.6 (7.7) | 18.5 (5.8) | <0.0001 |
| Biological Aging (Δage) 3 | ||
|---|---|---|
| E-DIITM | DIS 4 | |
| Age and sex adjusted | 0.14 (−0.03, 0.31) | 0.26 (0.09, 0.43) |
| Multivariable 5 | 0.22 (0.05, 0.38) | 0.27 (0.10, 0.44) |
| Biological Aging (Δage) 3 | |||||
|---|---|---|---|---|---|
| E-DIITM 4 | DIS 4,5 | ||||
| n | β (95%CI) | p-Value for Interaction | β (95%CI) | p-Value for Interaction | |
| Sex | |||||
| Men | 2164 | 0.22 (−0.04, 0.48) | 0.81 | 0.08 (−0.17, 0.33) | 0.03 |
| Women | 2346 | 0.23 (0.02, 0.45) | 0.43 (0.21, 0.65) | ||
| Age groups, y | |||||
| ≤54.3 | 2255 | 0.36 (0.11, 0.61) | 0.91 | 0.48 (0.25, 0.72) | 0.39 |
| >54.3 | 2255 | 0.46 (0.17, 0.74) | 0.33 (0.03, 0.63) | ||
| Body weight status, Kg/m² | |||||
| Normal weight (BMI ≤ 25) | 1210 | 0.27 (−0.05, 0.60) | 0.01 | 0.37 (0.05, 0.69) | 0.33 |
| Overweight (25 < BMI < 30) | 1891 | 0.25 (−0.01, 0.51) | 0.22 (−0.04, 0.49) | ||
| Obesity (BMI ≥ 30) | 1409 | 0.11 (−0.18, 0.41) | 0.16 (−0.14, 0.45) | ||
| Smoking status | |||||
| Non- smokers | 2248 | 0.15 (−0.08, 0.38) | 0.79 | 0.29 (0.06, 0.53) | 0.17 |
| Smokers | 1011 | 0.55 (0.20, 0.91) | 0.58 (0.24, 0.93) | ||
| Former | 1251 | 0.09 (−0.24, 0.42) | −0.03 (−0.36, 0.30) | ||
| Comorbidities | |||||
| Without | 2848 | 0.29 (0.08, 0.49) | 0.11 | 0.32 (0.12, 0.51) | 0.36 |
| 1 or more | 1662 | 0.05 (−0.25, 0.34) | 0.17 (−0.14, 0.49) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, C.F.; Esposito, S.; Di Castelnuovo, A.; Costanzo, S.; Ruggiero, E.; De Curtis, A.; Persichillo, M.; Hébert, J.R.; Cerletti, C.; Donati, M.B.; et al. Association between the Inflammatory Potential of the Diet and Biological Aging: A Cross-Sectional Analysis of 4510 Adults from the Moli-Sani Study Cohort. Nutrients 2023, 15, 1503. https://doi.org/10.3390/nu15061503
Martínez CF, Esposito S, Di Castelnuovo A, Costanzo S, Ruggiero E, De Curtis A, Persichillo M, Hébert JR, Cerletti C, Donati MB, et al. Association between the Inflammatory Potential of the Diet and Biological Aging: A Cross-Sectional Analysis of 4510 Adults from the Moli-Sani Study Cohort. Nutrients. 2023; 15(6):1503. https://doi.org/10.3390/nu15061503
Chicago/Turabian StyleMartínez, Claudia F., Simona Esposito, Augusto Di Castelnuovo, Simona Costanzo, Emilia Ruggiero, Amalia De Curtis, Mariarosaria Persichillo, James R. Hébert, Chiara Cerletti, Maria Benedetta Donati, and et al. 2023. "Association between the Inflammatory Potential of the Diet and Biological Aging: A Cross-Sectional Analysis of 4510 Adults from the Moli-Sani Study Cohort" Nutrients 15, no. 6: 1503. https://doi.org/10.3390/nu15061503
APA StyleMartínez, C. F., Esposito, S., Di Castelnuovo, A., Costanzo, S., Ruggiero, E., De Curtis, A., Persichillo, M., Hébert, J. R., Cerletti, C., Donati, M. B., de Gaetano, G., Iacoviello, L., Gialluisi, A., & Bonaccio, M., on behalf of the Moli-sani Study Investigators. (2023). Association between the Inflammatory Potential of the Diet and Biological Aging: A Cross-Sectional Analysis of 4510 Adults from the Moli-Sani Study Cohort. Nutrients, 15(6), 1503. https://doi.org/10.3390/nu15061503