Association between Late-Eating Pattern and Higher Consumption of Ultra-Processed Food among Italian Adults: Findings from the INHES Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Assessment of Dietary Data
2.3. Assessment of Meal Timing
2.4. Ascertainment of Covariates
2.5. Statistical Analysis
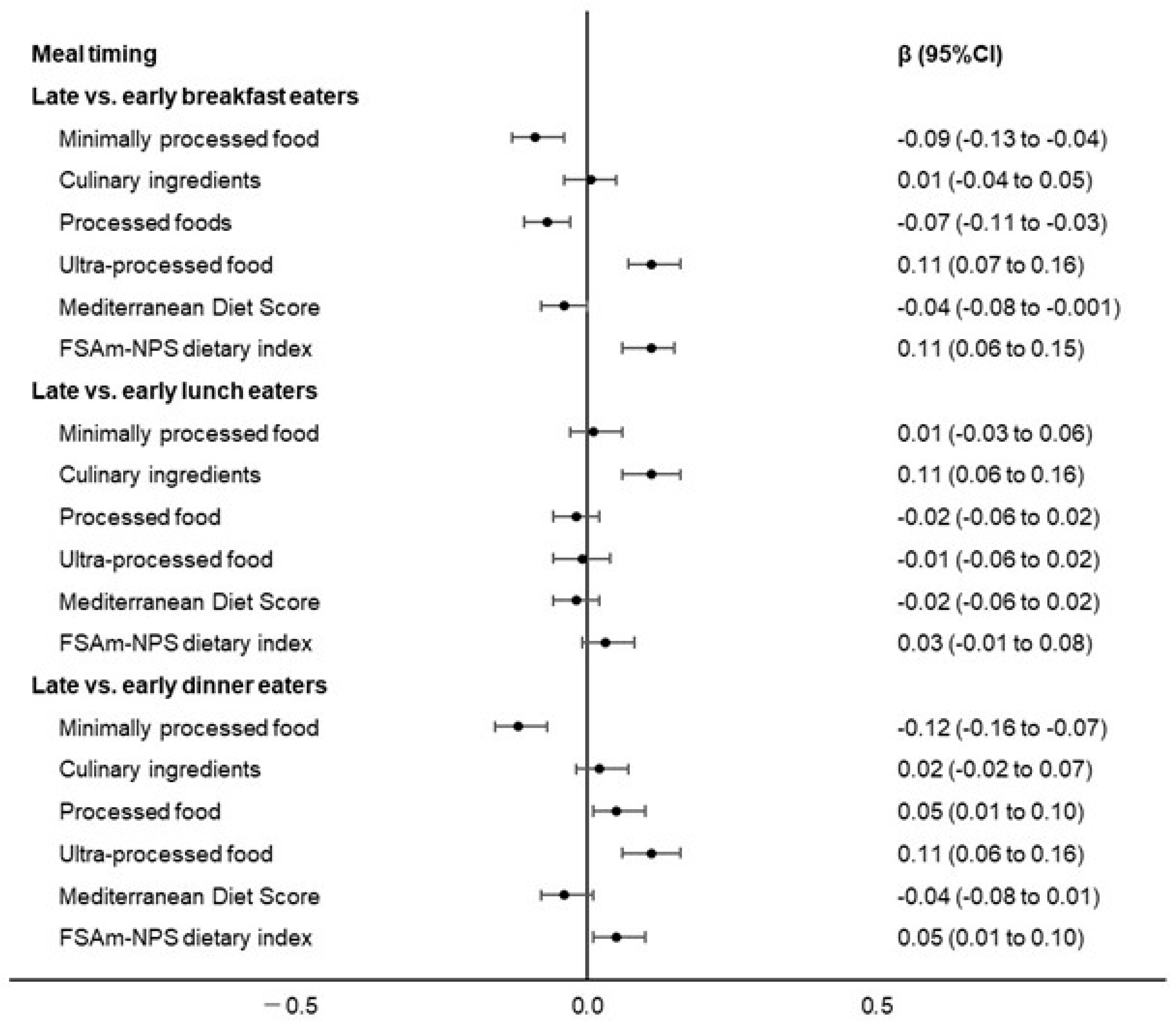
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P. GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021, Erratum in: J. Am. Coll. Cardiol. 2021, 77, 1958–1959. [Google Scholar] [CrossRef]
- Swinburn, B.A.; Kraak, V.I.; Allender, S.; Atkins, V.J.; Baker, P.I.; Bogard, J.R.; Brinsden, H.; Calvillo, A.; De Schutter, O.; Devarajan, R.; et al. The Global Syndemic of Obesity, Undernutrition, and Climate Change: The Lancet Commission report. Lancet 2019, 393, 791–846, Erratum in: Lancet 2019, 393, 746. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972, Erratum in: Lancet 2021, 397, 2466. [Google Scholar] [CrossRef]
- Waijers, P.M.; Feskens, E.J.; Ocké, M.C. A critical review of predefined diet quality scores. Br. J. Nutr. 2007, 97, 219–231. [Google Scholar] [CrossRef]
- Garaulet, M.; Gómez-Abellán, P. Timing of food intake and obesity: A novel association. Physiol. Behav. 2014, 134, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.; Rogers, M.; Coates, A.M.; Leung, G.K.W.; Bonham, M.P. The Impact of Meal Timing on Risk of Weight Gain and Development of Obesity: A Review of the Current Evidence and Opportunities for Dietary Intervention. Curr. Diab. Rep. 2022, 22, 147–155. [Google Scholar] [CrossRef]
- Xiao, Q.; Garaulet, M.; Scheer, F.A.J.L. Meal timing and obesity: Interactions with macronutrient intake and chronotype. Int. J. Obes 2019, 43, 1701–1711. [Google Scholar] [CrossRef]
- Baron, K.G.; Reid, K.J.; Horn, L.V.; Zee, P.C. Contribution of evening macronutrient intake to total caloric intake and body mass index. Appetite 2013, 60, 246–251. [Google Scholar] [CrossRef]
- Baron, K.G.; Reid, K.J.; Kern, A.S.; Zee, P.C. Role of sleep timing in caloric intake and BMI. Obesity 2011, 19, 1374–1381. [Google Scholar] [CrossRef]
- Wang, J.B.; Patterson, R.E.; Ang, A.; Emond, J.A.; Shetty, N.; Arab, L. Timing of energy intake during the day is associated with the risk of obesity in adults. J. Hum. Nutr. Diet. 2014, 27 (Suppl. 2), 255–262. [Google Scholar] [CrossRef]
- Kahleova, H.; Lloren, J.I.; Mashchak, A.; Hill, M.; Fraser, G.E. Meal Frequency and Timing Are Associated with Changes in Body Mass Index in Adventist Health Study 2. J. Nutr. 2017, 147, 1722–1728. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lozano, N.; Tvarijonaviciute, A.; Ríos, R.; Barón, I.; Scheer, F.A.J.L.; Garaulet, M. Late Eating Is Associated with Obesity, Inflammatory Markers and Circadian-Related Disturbances in School-Aged Children. Nutrients 2020, 12, 2881. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P.; Ard, J.; Baskin, M.L.; Chiuve, S.E.; Johnson, H.M.; Kris-Etherton, P.; Varady, K.; American Heart Association Obesity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; et al. Meal Timing and Frequency: Implications for Cardiovascular Disease Prevention: A Scientific Statement from the American Heart Association. Circulation 2017, 135, e96–e121. [Google Scholar] [CrossRef]
- Leung, G.K.W.; Huggins, C.E.; Bonham, M.P. Effect of meal timing on postprandial glucose responses to a low glycemic index meal: A crossover trial in healthy volunteers. Clin. Nutr. 2019, 38, 465–471. [Google Scholar] [CrossRef]
- Wehrens, S.M.T.; Christou, S.; Isherwood, C.; Middleton, B.; Gibbs, M.A.; Archer, S.N.; Skene, D.J.; Johnston, J.D. Meal Timing Regulates the Human Circadian System. Curr. Biol. 2017, 27, 1768–1775. [Google Scholar] [CrossRef]
- Gallant, A.; Lundgren, J.; Drapeau, V. Nutritional Aspects of Late Eating and Night Eating. Curr. Obes. Rep. 2014, 3, 101–107. [Google Scholar] [CrossRef]
- Gontijo, C.A.; Balieiro, L.C.T.; Teixeira, G.P.; Fahmy, W.M.; Crispim, C.A.; Maia, Y.C.P. Effects of timing of food intake on eating patterns, diet quality and weight gain during pregnancy. Br. J. Nutr. 2020, 123, 922–933. [Google Scholar] [CrossRef]
- Silva, C.M.; Teixeira, B.S.; Wright, K.P., Jr.; Maia, Y.C.P.; Crispim, C.A. Time-Related Eating Patterns Are Associated with the Total Daily Intake of Calories and Macronutrients in Day and Night Shift Workers. Nutrients 2022, 14, 2202. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Moubarac, J.C.; Levy, R.B.; Canella, D.S.; Louzada, M.L.D.C.; Cannon, G. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2018, 21, 18–26. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.C.; Louzada, M.L.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Rauber, F.; Chang, K.; Vamos, E.P.; da Costa Louzada, M.L.; Monteiro, C.A.; Millett, C.; Levy, R.B. Ultra-processed food consumption and risk of obesity: A prospective cohort study of UK Biobank. Eur. J. Nutr. 2021, 60, 2169–2180. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Insausti, H.; Jiménez-Onsurbe, M.; Donat-Vargas, C.; Rey-García, J.; Banegas, J.R.; Rodríguez-Artalejo, F.; Guallar-Castillón, P. Ultra-Processed Food Consumption Is Associated with Abdominal Obesity: A Prospective Cohort Study in Older Adults. Nutrients 2020, 12, 2368. [Google Scholar] [CrossRef] [PubMed]
- Pagliai, G.; Dinu, M.; Madarena, M.P.; Bonaccio, M.; Iacoviello, L.; Sofi, F. Consumption of ultra-processed foods and health status: A systematic review and meta-analysis. Br. J. Nutr. 2021, 125, 308–318. [Google Scholar] [CrossRef]
- Bonaccio, M.; Di Castelnuovo, A.; Ruggiero, E.; Costanzo, S.; Grosso, G.; De Curtis, A.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; et al. Joint association of food nutritional profile by Nutri-Score front-of-pack label and ultra-processed food intake with mortality: Moli-sani prospective cohort study. BMJ. 2022, 378, e070688. [Google Scholar] [CrossRef] [PubMed]
- Pounis, G.; Bonanni, A.; Ruggiero, E.; Di Castelnuovo, A.; Costanzo, S.; Persichillo, M.; Bonaccio, M.; Cerletti, C.; Riccardi, G.; Donati, M.B.; et al. INHES Investigators. Food group consumption in an Italian population using the updated food classification system FoodEx2: Results from the Italian Nutrition & HEalth Survey (INHES) study. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 307–328. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, C.; Arcella, D.; Piccinelli, R.; Sette, S.; Le Donne, C.; Turrini, A.; INRAN-SCAI 2005–06 Study Group. The Italian National Food Consumption Survey INRAN-SCAI 2005–06: Main results in terms of food consumption. Public Health Nutr. 2009, 12, 2504–2532. [Google Scholar] [CrossRef] [PubMed]
- Illner, A.K.; Harttig, U.; Tognon, G.; Palli, D.; Salvini, S.; Bower, E.; Amiano, P.; Kassik, T.; Metspalu, A.; Engeset, D.; et al. Feasibility of innovative dietary assessment in epidemiological studies using the approach of combining different assessment instruments. Public Health Nutr. 2011, 14, 1055–1063. [Google Scholar] [CrossRef]
- Sette, S.; Le Donne, C.; Piccinelli, R.; Arcella, D.; Turrini, A.; Leclercq, C. INRAN-SCAI 2005–6 Study Group. The third Italian National Food Consumption Survey, INRAN-SCAI 2005–06--part 1: Nutrient intakes in Italy. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 922–932. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.; Moubarac, J.C.; Jaime, P.; Martins, A.P.; Canella, D.; Louzada, M.; Parra, D. NOVA. The star shines bright. Food classification. Public Health World Nutr. 2016, 7, 28–38. [Google Scholar]
- Srour, B.; Fezeu, L.K.; Kesse-Guyot, E.; Allès, B.; Méjean, C.; Andrianasolo, R.M.; Chazelas, E.; Deschasaux, M.; Hercberg, S.; Galan, P.; et al. Ultra-processed food intake and risk of cardiovascular disease: Prospective cohort study (NutriNet-Santé). BMJ 2019, 365, l1451. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Julia, C.; Hercberg, S. Development of a new front-of-pack nutrition label in France: The five-Colour Nutri-Score. Public Health Panorama. 2017, 3, 712–725. [Google Scholar]
- Deschasaux, M.; Huybrechts, I.; Julia, C.; Hercberg, S.; Egnell, M.; Srour, B.; Kesse-Guyot, E.; Latino-Martel, P.; Biessy, C.; Casagrande, C.; et al. Association between nutritional profiles of foods underlying Nutri-Score front-of-pack labels and mortality: EPIC cohort study in 10 European countries. BMJ 2020, 370, m3173. [Google Scholar] [CrossRef] [PubMed]
- ISTAT: Istituto Nazionale di Statistica; Atlante Statistico dei Comuni. Edizione 2014. Available online: https://www.istat.it/it/archivio/113712 (accessed on 20 January 2023).
- Hernán, M.A.; Hernández-Díaz, S.; Werler, M.M.; Mitchell, A.A. Causal knowledge as a prerequisite for confounding evaluation: An application to birth defects epidemiology. Am. J. Epidemiol. 2002, 155, 176–184. [Google Scholar] [CrossRef]
- Jakubowicz, D.; Barnea, M.; Wainstein, J.; Froy, O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity 2013, 21, 2504–2512. [Google Scholar] [CrossRef]
- Arble, D.M.; Bass, J.; Laposky, A.D.; Vitaterna, M.H.; Turek, F.W. Circadian timing of food intake contributes to weight gain. Obesity 2009, 17, 2100–2102. [Google Scholar] [CrossRef]
- Garaulet, M.; Gómez-Abellán, P.; Alburquerque-Béjar, J.J.; Lee, Y.-C.; Ordovás, J.M.; Scheer, F.A.J.L. Timing of food intake predicts weight loss effectiveness. Int. J. Obes. 2013, 37, 604–611. [Google Scholar] [CrossRef]
- Dashti, H.S.; Gómez-Abellán, P.; Qian, J.; Esteban, A.; Morales, E.; Scheer, F.A.J.L.; Garaulet, M. Late eating is associated with cardiometabolic risk traits, obesogenic behaviors, and impaired weight loss. Am. J. Clin. Nutr. 2020, 113, 154–161. [Google Scholar] [CrossRef]
- Dashti, H.S.; Scheer, F.A.J.L.; Saxena, R.; Garaulet, M. Timing of Food Intake: Identifying Contributing Factors to Design Effective Interventions. Adv. Nutr. 2019, 10, 606–620. [Google Scholar] [CrossRef]
- Morris, C.J.; Yang, J.N.; Garcia, J.I.; Myers, S.; Bozzi, I.; Wang, W.; Buxton, O.M.; Shea, S.A.; Scheer, F.A. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc. Natl. Acad. Sci. USA 2015, 112, E2225–E2234. [Google Scholar] [CrossRef]
- Lopez-Minguez, J.; Gómez-Abellán, P.; Garaulet, M. Timing of Breakfast, Lunch, and Dinner. Effects on Obesity and Metabolic Risk. Nutrients 2019, 11, 2624. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.T.M.; Nunes, F.S.M.; Custódio, I.D.D.; Carvalho, K.P.; Canto, P.P.L.; Paiva, C.E.; Crispim, C.A.; Paiva Maia, Y.C. Eating Earlier and More Frequently Is Associated With Better Diet Quality in Female Brazilian Breast Cancer Survivors Using Tamoxifen. J. Acad. Nutr. Diet. 2022, 122, 1688–1702. [Google Scholar] [CrossRef] [PubMed]
- de Castro, J.M. The time of day of food intake influences overall intake in humans. J. Nutr. 2004, 134, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, L.G.; Martinez Steele, E.; Canella, D.S.; Monteiro, C.A. Consumption of ultra-processed foods and associated sociodemographic factors in the USA between 2007 and 2012: Evidence from a nationally representative cross-sectional study. BMJ Open 2018, 8, e020574. [Google Scholar] [CrossRef]
- Rauber, F.; da Costa Louzada, M.L.; Steele, E.M.; Millett, C.; Monteiro, C.A.; Levy, R.B. Ultra-Processed Food Consumption and Chronic Non-Communicable Diseases-Related Dietary Nutrient Profile in the UK (2008–2014). Nutrients 2018, 10, 587. [Google Scholar] [CrossRef]
- Polsky, J.Y.; Moubarac, J.C.; Garriguet, D. Consumption of ultra-processed foods in Canada. Health Rep. 2020, 31, 3–15. [Google Scholar] [CrossRef]
- Ruggiero, E.; Esposito, S.; Costanzo, S.; Di Castelnuovo, A.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; Bonaccio, M. INHES Study Investigators. Ultra-processed food consumption and its correlates among Italian children, adolescents and adults from the Italian Nutrition & Health Survey (INHES) cohort study. Public Health Nutr. 2021, 24, 6258–6271. [Google Scholar] [CrossRef]
- Blanco-Rojo, R.; Sandoval-Insausti, H.; López-Garcia, E.; Graciani, A.; Ordovás, J.M.; Banegas, J.R.; Rodríguez-Artalejo, F.; Guallar-Castillón, P. Consumption of Ultra-Processed Foods and Mortality: A National Prospective Cohort in Spain. Mayo. Clin. Proc. 2019, 94, 2178–2188. [Google Scholar] [CrossRef]
- Santos, F.S.D.; Dias, M.D.S.; Mintem, G.C.; Oliveira, I.O.; Gigante, D.P. Food processing and cardiometabolic risk factors: A systematic review. Rev. Saude. Publica. 2020, 54, 70. [Google Scholar] [CrossRef]
- Mignogna, C.; Costanzo, S.; Di Castelnuovo, A.; Ruggiero, E.; Shivappa, N.; Hebert, J.R.; Esposito, S.; De Curtis, A.; Persichillo, M.; Cerletti, C.; et al. Moli-sani Study Investigators. The inflammatory potential of the diet as a link between food processing and low-grade inflammation: An analysis on 21,315 participants to the Moli-sani study. Clin. Nutr. 2022, 41, 2226–2234. [Google Scholar] [CrossRef]
- Fonken, L.K.; Lieberman, R.A.; Weil, Z.M.; Nelson, R.J. Dim light at night exaggerates weight gain and inflammation associated with a high-fat diet in male mice. Endocrinology 2013, 154, 3817–3825. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.J.; Purvis, T.E.; Hu, K.; Scheer, F.A. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc. Natl. Acad. Sci. USA 2016, 113, E1402–E1411. [Google Scholar] [CrossRef] [PubMed]
- Juul, F.; Vaidean, G.; Parekh, N. Ultra-processed Foods and Cardiovascular Diseases: Potential Mechanisms of Action. Adv. Nutr. 2021, 12, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A. Minimally processed foods are more satiating and less hyperglycemic than ultra-processed foods: A preliminary study with 98 ready-to-eat foods. Food Funct. 2016, 7, 2338–2346. [Google Scholar] [CrossRef]
- Fardet, A. Characterization of the Degree of Food Processing in Relation With Its Health Potential and Effects. Adv. Food Nutr. Res. 2018, 85, 79–129. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Dehghan, M.; Mente, A.; Bangdiwala, S.I.; Rangarajan, S.; Srichaikul, K.; Mohan, V.; Avezum, A.; Díaz, R.; Rosengren, A. PURE Study Investigators. Glycemic Index, Glycemic Load, and Cardiovascular Disease and Mortality. N. Engl. J. Med. 2021, 384, 1312–1322. [Google Scholar] [CrossRef]
- Bonaccio, M.; Costanzo, S.; Di Castelnuovo, A.; Persichillo, M.; Magnacca, S.; De Curtis, A.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L. Ultra-processed food intake and all-cause and cause-specific mortality in individuals with cardiovascular disease: The Moli-sani Study. Eur. Heart J. 2022, 43, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Romero Ferreiro, C.; Lora Pablos, D.; Gómez de la Cámara, A. Two Dimensions of Nutritional Value: Nutri-Score and NOVA. Nutrients 2021, 13, 2783. [Google Scholar] [CrossRef] [PubMed]
- Rico-Campà, A.; Martínez-González, M.A.; Alvarez-Alvarez, I.; Mendonça, R.D.; de la Fuente-Arrillaga, C.; Gómez-Donoso, C.; Bes-Rastrollo, M. Association between consumption of ultra-processed foods and all cause mortality: SUN prospective cohort study. BMJ 2019, 365, l1949. [Google Scholar] [CrossRef]
- Alkerwi, A.; Vernier, C.; Sauvageot, N.; Crichton, G.E.; Elias, M.F. Demographic and socioeconomic disparity in nutrition: Application of a novel Correlated Component Regression approach. BMJ Open 2015, 5, e006814. [Google Scholar] [CrossRef]
- Papadaki, A.; Wood, L.; Sebire, S.J.; Jago, R. Adherence to the Mediterranean diet among employees in South West England: Formative research to inform a web-based, work-place nutrition intervention. Prev. Med. Rep. 2015, 2, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Park, S.; Kim, J.Y. Comparison of dietary share of ultra-processed foods assessed with a FFQ against a 24-h dietary recall in adults: Results from KNHANES 2016. Public Health Nutr. 2022, 25, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Gibney, M.J. Ultra-processed foods: Definitions and policy issues. Curr. Dev. Nutr. 2018, 3, nzy077. [Google Scholar] [CrossRef] [PubMed]
| NOVA Food Category | Food Items |
|---|---|
| Group 1: Unprocessed or minimally processed foods | Water; fresh, squeezed or dried fruits and leafy and root vegetables; nuts; fresh legumes; wheat; rice; pasta; flour; potatoes; meat; poultry; fish and seafood; milk; plain yogurts without added sugar; eggs; spices; tea and coffee. |
| Group 2: Processed culinary ingredients | Vinegars; creams; vegetable oils; butter; lard; sugar and honey. |
| Group 3: Processed foods | Jam; cured traditional ham; olives; canned fruits; salted or sugared nuts; canned or bottled vegetables and legumes; breads; artisanal pizza; smoked and canned fish; cheese; wine and beer. |
| Group 4: Ultra-processed food | Processed meat (e.g., salami, mortadella, sausages, hamburger, chicken nuggets); fish products (e.g., fish sticks); packaged breads and buns; bread substitutes (e.g., crackers, rusks, breadstick); breakfast cereals and bars; fruit yogurt; fruit drinks; carbonated soft drinks; cocoa drinks; alcoholic drinks (e.g., rum, gin, whisky); energy drinks and bars; milk substitutes (e.g., soy drinks); margarine; mayonnaise and similar; sliced cheese; sweet packaged snacks; plant-based meat alternatives (e.g., veggie burgers); non-sugar sweeteners; sweet biscuits; cakes, croissant and other non-handmade pastries; ice-cream; chocolate; candies and gums; non-sugar sweeteners; baby food. |
| Meal Timing Pattern | ||||
|---|---|---|---|---|
| All | Early Eaters | Late Eaters | p-Value | |
| N of subjects, % | 8688 (100.0) | 5781 (66.5) | 2907 (33.5) | - |
| Sex | 0.44 | |||
| Men | 4053 (46.7) | 2680 (46.4) | 1373 (47.3) | |
| Women | 4635 (53.3) | 3101 (53.6) | 1534 (52.8) | |
| Age (years; mean ± SD) | 56.9 ± 14.6 | 58.9 ± 14.5 | 52.9 ± 13.9 | <0.0001 |
| Age groups, years | <0.0001 | |||
| 19–50 | 2967 (34.2) | 1718 (29.7) | 1249 (43.0) | |
| 51–65 | 2863 (32.9) | 1799 (31.1) | 1064 (36.6) | |
| 66–97 | 2858 (32.9) | 2264 (39.2) | 594 (20.4) | |
| Geographical area | <0.0001 | |||
| Northern | 3556 (40.9) | 2932 (50.7) | 624 (21.5) | |
| Centre | 1407 (16.2) | 886 (15.3) | 521 (17.9) | |
| Southern | 3725 (42.9) | 1963 (34.0) | 1762 (60.6) | |
| Place of residence | <0.0001 | |||
| Rural | 1178 (13.6) | 861 (14.9) | 317 (10.9) | |
| Urban | 7510 (86.4) | 4920 (85.1) | 2590 (89.1) | |
| Educational level | <0.0001 | |||
| Up to elementary | 1540 (17.7) | 1252 (21.7) | 288 (9.9) | |
| Lower secondary | 2268 (26.1) | 1589 (27.5) | 679 (23.4) | |
| Upper secondary | 3430 (39.5) | 2142 (37.0) | 1288 (44.3) | |
| Postsecondary | 1385 (15.9) | 747 (12.9) | 638 (21.9) | |
| Missing data | 65 (0.8) | 51 (0.9) | 14 (0.5) | |
| Occupation | 0.0001 | |||
| Non-manual workers | 2658 (30.6) | 1525 (26.4) | 1133 (39.0) | |
| Manual workers | 1537 (17.7) | 1006 (17.4) | 531 (18.3) | |
| Housewife | 958 (11.0) | 623 (10.9) | 325 (11.2) | |
| Retired | 3129 (36.0) | 2406 (41.6) | 723 (24.9) | |
| Student | 142 (1.6) | 61 (1.1) | 81 (2.7) | |
| Unemployed | 251 (2.9) | 145 (2.5) | 106 (3.6) | |
| Missing data | 13 (0.2) | 5 (0.1) | 8 (0.3) | |
| Marital status | 0.19 | |||
| Married/in couple | 6533 (75.2) | 4382 (75.8) | 2151 (74.0) | |
| Single | 1244 (14.3) | 707 (12.2) | 537 (18.5) | |
| Separated/divorced | 270 (3.1) | 185 (3.2) | 85 (2.9) | |
| Widowed | 616 (7.1) | 492 (8.5) | 124 (4.3) | |
| Missing data | 25 (0.3) | 15 (0.3) | 10 (0.3) | |
| Smoking habit | <0.0001 | |||
| No | 5180 (59.6) | 3533 (61.1) | 1647 (56.7) | |
| Current | 1390 (16.0) | 888 (15.4) | 502 (17.3) | |
| Ex | 1925 (22.2) | 1244 (21.5) | 681 (23.4) | |
| Occasional | 163 (1.9) | 96 (1.7) | 67 (2.3) | |
| Missing data | 30 (0.3) | 20 (0.3) | 10 (0.3) | |
| Sport activity | 0.067 | |||
| No | 7096 (81.7) | 4835 (83.6) | 2261 (77.8) | |
| Yes | 1585 (18.2) | 943 (16.3) | 642 (22.1) | |
| Missing data | 7 (0.1) | 3 (0.1) | 4 (0.1) | |
| Cardiovascular disease | 0.095 | |||
| No | 8397 (96.7) | 5576 (96.6) | 2821 (97.0) | |
| Yes | 291 (3.3) | 205 (3.4) | 86 (3.0) | |
| Cancer | 0.73 | |||
| No | 8397 (96.6) | 5570 (96.3) | 2827 (97.2) | |
| Yes | 291 (3.4) | 211 (3.7) | 80 (2.8) | |
| Hypertension | 0.026 | |||
| No | 5859 (67.4) | 3762 (65.1) | 2097 (72.1) | |
| Yes | 2809 (32.4) | 2008 (34.7) | 801 (27.6) | |
| Missing data | 20 (0.2) | 11 (0.2) | 9 (0.3) | |
| Hyperlipidaemia | 0.010 | |||
| No | 6756 (77.8) | 4456 (77.2) | 2300 (79.0) | |
| Yes | 1902 (21.9) | 1307 (22.5) | 595 (20.6) | |
| Missing data | 30 (0.3) | 18 (0.3) | 12 (0.4) | |
| Diabetes | 0.33 | |||
| No | 7997 (92.1) | 5281 (91.4) | 2716 (93.4) | |
| Yes | 661 (7.6) | 482 (8.3) | 179 (6.2) | |
| Missing data | 30 (0.3) | 18 (0.3) | 12 (0.4) | |
| Body mass index | 0.13 | |||
| Normal weight | 4168 (48.0) | 2727 (47.2) | 1441 (49.6) | |
| Overweight | 3333 (38.3) | 2250 (38.9) | 1083 (37.2) | |
| Obese | 1172 (13.5) | 795 (13.8) | 377 (13.0) | |
| Missing data | 15 (0.2) | 9 (0.1) | 6 (0.2) | |
| Meal Timing Pattern | |||
|---|---|---|---|
| Early Eaters | Late Eaters | p-Value | |
| Energy intake (kcal/d) | 1889 ± 578 | 1913 ± 592 | 0.093 |
| Alcohol intake (g/d) | 9.0 ± 14.5 | 8.9 ± 13.9 | 0.87 |
| Carbohydrate (% TEI) | 49.1 ± 9.9 | 48.5 ± 9.7 | 0.018 |
| Sugar (g/d) | 70.1 ± 29.9 | 69.4 ± 30.2 | 0.30 |
| Fibre intake (g/d) | 18.0 ± 7.8 | 18.1 ± 8.1 | 0.48 |
| Protein (% TEI) | 16.0 ± 3.8 | 16.1 ± 3.8 | 0.27 |
| Fat (% TEI) | 34.6 ± 7.9 | 35.1 ± 7.8 | 0.0079 |
| Saturated fat (% TEI) | 10.1 ± 3.8 | 10.3 ± 3.7 | 0.085 |
| Saturated fat (g/d) | 21.6 ± 11.4 | 21.9 ± 11.5 | 0.11 |
| MUFA (% TEI) | 10.1 ± 3.8 | 10.3 ± 3.7 | 0.085 |
| PUFA (% TEI) | 4.2 ± 1.6 | 4.2 ± 1.6 | 0.26 |
| Dietary cholesterol (mg/d) | 235.7 ± 168.4 | 232.1 ± 168.9 | 0.34 |
| Sodium (mg/d) | 1620 ± 1095 | 1600 ± 1063 | 0.36 |
| Minimally processed food (Group 1) | 74.0 ± 11.8 | 72.7 ± 12.2 | <0.0001 |
| Culinary ingredients (Group 2) | 2.6 ±1.2 | 2.6 ± 1.2 | 0.12 |
| Processed food (Group 3) | 15.9 ± 10.6 | 16.4 ± 10.7 | 0.033 |
| Ultra-processed food (Group 4) | 7.5 ± 6.7 | 8.3 ± 7.3 | <0.0001 |
| Meal Timing Pattern | |
|---|---|
| Late vs. Early Eaters | |
| NOVA Groups | β (95% CI) |
| Minimally processed food (Group 1) | |
| Model 1 | −0.11 (−0.15 to −0.07) |
| Model 2 | −0.10 (−0.14 to −0.06) |
| Culinary ingredients (Group 2) | |
| Model 1 | 0.03 (−0.01 to 0.08) |
| Model 2 | 0.05 (0.01 to 0.10) |
| Processed food (Group 3) | |
| Model 1 | 0.04 (0.003 to 0.08) |
| Model 2 | 0.02 (−0.02 to 0.06) |
| Ultra-processed food (Group 4) | |
| Model 1 | 0.11 (0.07 to 0.15) |
| Model 2 | 0.13 (0.09 to 0.18) |
| Mediterranean Diet Score | |
| Model 1 | −0.03 (−0.07 to 0.01) |
| Model 2 | −0.07 (−0.12 to −0.03) |
| FSAm-NPS dietary index | |
| Model 1 | 0.05 (0.01 to 0.10) |
| Model 2 | 0.10 (0.05 to 0.14) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonaccio, M.; Ruggiero, E.; Di Castelnuovo, A.; Martínez, C.F.; Esposito, S.; Costanzo, S.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L., on behalf of the INHES Study Investigators. Association between Late-Eating Pattern and Higher Consumption of Ultra-Processed Food among Italian Adults: Findings from the INHES Study. Nutrients 2023, 15, 1497. https://doi.org/10.3390/nu15061497
Bonaccio M, Ruggiero E, Di Castelnuovo A, Martínez CF, Esposito S, Costanzo S, Cerletti C, Donati MB, de Gaetano G, Iacoviello L on behalf of the INHES Study Investigators. Association between Late-Eating Pattern and Higher Consumption of Ultra-Processed Food among Italian Adults: Findings from the INHES Study. Nutrients. 2023; 15(6):1497. https://doi.org/10.3390/nu15061497
Chicago/Turabian StyleBonaccio, Marialaura, Emilia Ruggiero, Augusto Di Castelnuovo, Claudia Francisca Martínez, Simona Esposito, Simona Costanzo, Chiara Cerletti, Maria Benedetta Donati, Giovanni de Gaetano, and Licia Iacoviello on behalf of the INHES Study Investigators. 2023. "Association between Late-Eating Pattern and Higher Consumption of Ultra-Processed Food among Italian Adults: Findings from the INHES Study" Nutrients 15, no. 6: 1497. https://doi.org/10.3390/nu15061497
APA StyleBonaccio, M., Ruggiero, E., Di Castelnuovo, A., Martínez, C. F., Esposito, S., Costanzo, S., Cerletti, C., Donati, M. B., de Gaetano, G., & Iacoviello, L., on behalf of the INHES Study Investigators. (2023). Association between Late-Eating Pattern and Higher Consumption of Ultra-Processed Food among Italian Adults: Findings from the INHES Study. Nutrients, 15(6), 1497. https://doi.org/10.3390/nu15061497






