Nutritional Considerations in Celiac Disease and Non-Celiac Gluten/Wheat Sensitivity
Abstract
1. Introduction
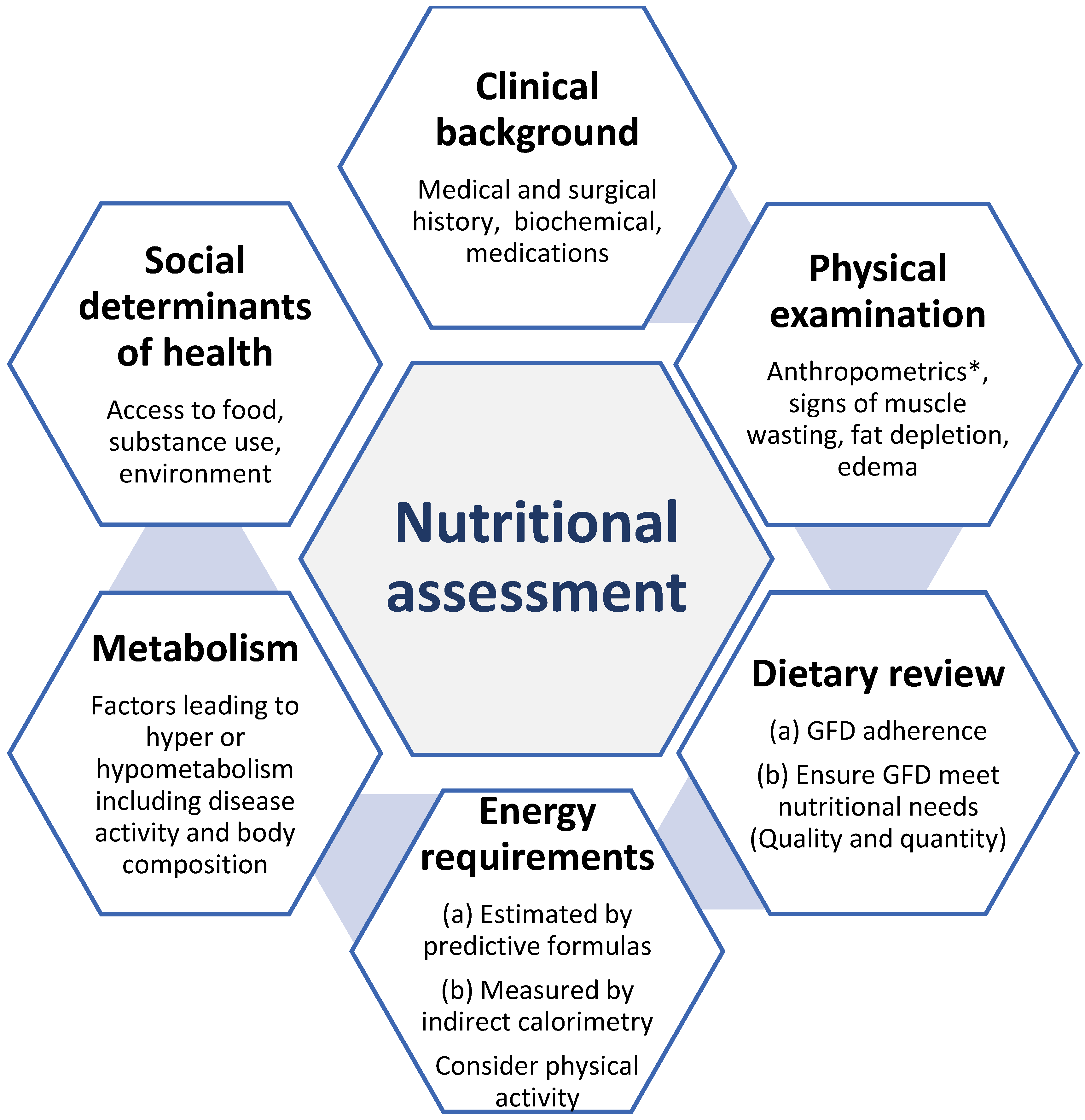
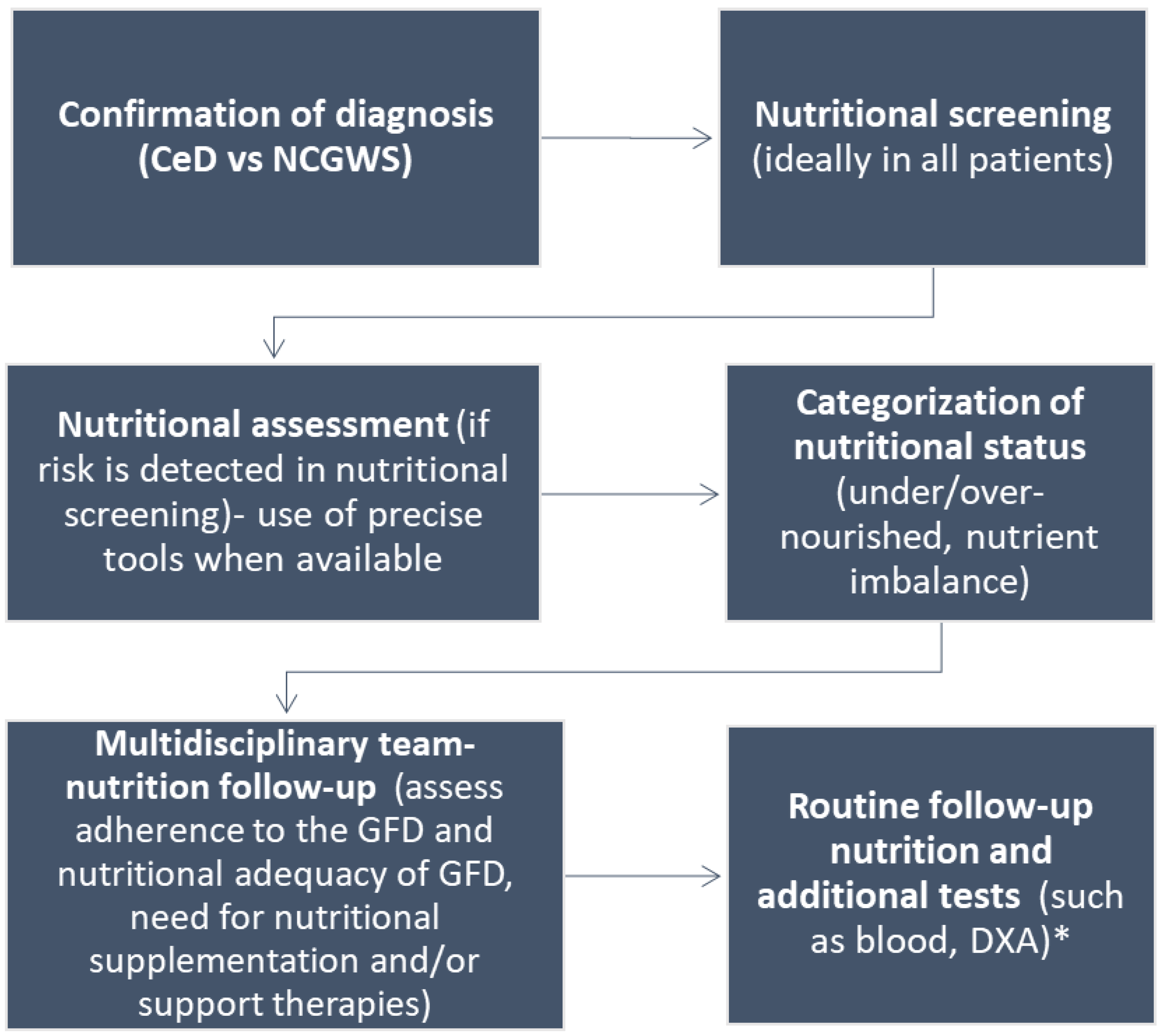
2. Nutritional Assessment Tools to Evaluate Nutritional Status
2.1. Clinical Assessment
2.2. Measurement of Body Composition
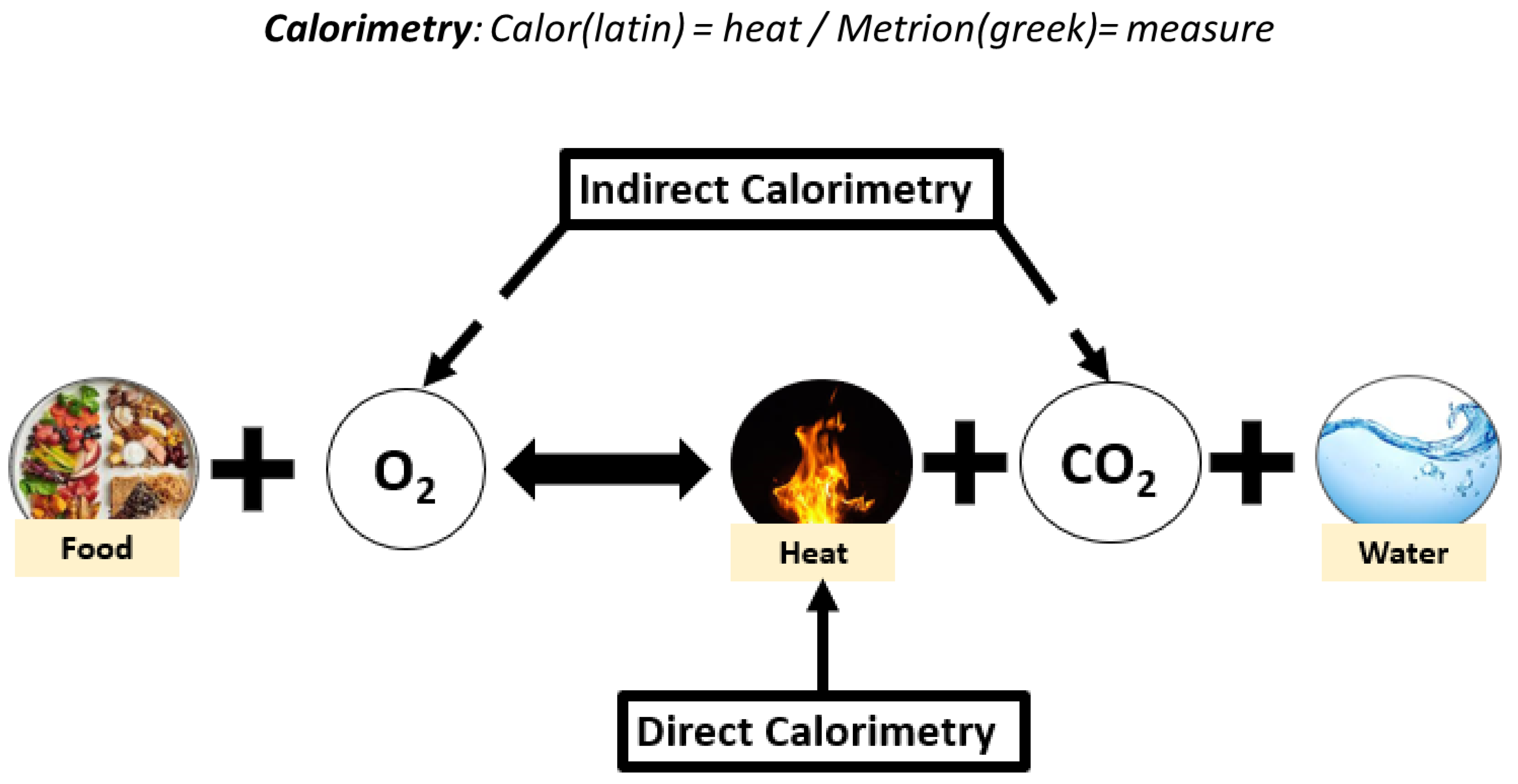
2.3. Dietary Intake and Measurements of Energy Needs
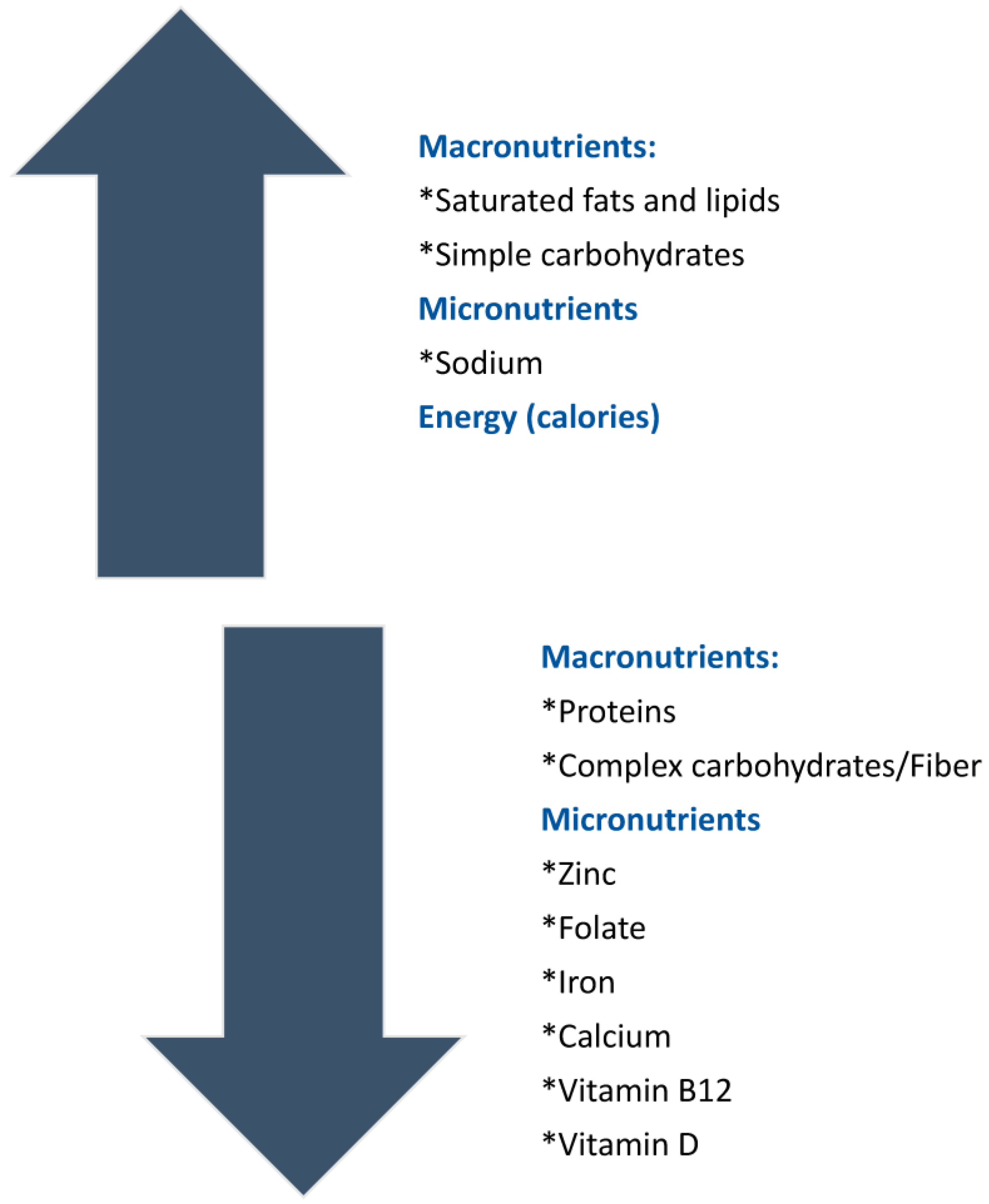
3. Nutrient Imbalance Associated with GFD
3.1. Macronutrient Imbalance
3.2. Micronutrient Deficiencies Associated with GFD
4. Nutritional Status of CeD and NCGWS Patients
5. Nutritional Therapies for Celiac Disease and NCGWS
5.1. Dietary Therapies: How to Follow a Proper Gluten-Free Diet
5.2. Challenges of Adopting a Strict GFD
5.3. Nutritional Support Therapies in CeD and NCGWS
6. Role of an Experienced Registered Dietitian as Part of Multidisciplinary Management in CeD/NCGWS
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Catassi, C.; Verdu, E.F.; Bai, J.C.; Lionetti, E. Coeliac disease. Lancet 2022, 399, 2413–2426. [Google Scholar] [CrossRef]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.; Kurppa, K.; Mearin, M.L.; Ribes-Koninckx, C.; Shamir, R.; Troncone, R.; Auricchio, R.; Castillejo, G.; et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020. J. Parenter. Enter. Nutr. 2020, 70, 141–156. [Google Scholar] [CrossRef]
- Sergi, C.; Villanacci, V.; Carroccio, A. Non-celiac wheat sensitivity: Rationality and irrationality of a gluten-free diet in individuals affected with non-celiac disease: A review. BMC Gastroenterol. 2021, 21, 5. [Google Scholar] [CrossRef]
- Catassi, C.; Elli, L.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; Cellier, C.; Cristofori, F.; De Magistris, L.; Dolinsek, J.; et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts’ Criteria. Nutrients 2015, 7, 4966–4977. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Peters, S.L.; Newnham, E.D.; Rosella, O.; Muir, J.G.; Gibson, P.R. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 2013, 145, 320–328.e83. [Google Scholar] [CrossRef]
- Skodje, G.I.; Sarna, V.K.; Minelle, I.H.; Rolfsen, K.L.; Muir, J.G.; Gibson, P.R.; Veierød, M.B.; Henriksen, C.; Lundin, K.E. Fructan, Rather Than Gluten, Induces Symptoms in Patients With Self-Reported Non-Celiac Gluten Sensitivity. Gastroenterology 2018, 154, 529–539.e2. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Volta, U.; Salvatore, C.; Biancheri, P.; Caio, G.; De Giorgio, R.; di Stefano, M.; Corazza, G.R. Small Amounts of Gluten in Subjects With Suspected Nonceliac Gluten Sensitivity: A Randomized, Double-Blind, Placebo-Controlled, Cross-Over Trial. Clin. Gastroenterol. Hepatol. 2015, 13, 1604–1612.e3. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Murray, J.A. Epidemiology of Celiac Disease. Gastroenterol. Clin. N. Am. 2019, 48, 1–18. [Google Scholar] [CrossRef]
- Mahadev, S.; Simpson, S.; Lebwohl, B.; Lewis, S.K.; Tennyson, C.A.; Green, P.H. Is dietitian use associated with celiac disease outcomes? Nutrients 2013, 5, 1585–1594. [Google Scholar] [CrossRef]
- Theethira, T.G.; Dennis, M.; Leffler, D.A. Nutritional consequences of celiac disease and the gluten-free diet. Expert Rev. Gastroenterol. Hepatol. 2014, 8, 123–129. [Google Scholar] [CrossRef]
- Theethira, T.G.; Dennis, M. Celiac disease and the gluten-free diet: Consequences and recommendations for improvement. Dig. Dis. 2015, 33, 175–182. [Google Scholar] [CrossRef]
- Di Nardo, G.; Villa, M.P.; Conti, L.; Ranucci, G.; Pacchiarotti, C.; Principessa, L.; Raucci, U.; Parisi, P. Nutritional Deficiencies in Children with Celiac Disease Resulting from a Gluten-Free Diet: A Systematic Review. Nutrients 2019, 11, 1588. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/malnutrition (accessed on 4 October 2022).
- Correia, M. Nutrition Screening vs Nutrition Assessment: What’s the Difference? Nutr. Clin. Pract. 2018, 33, 62–72. [Google Scholar] [CrossRef]
- Mueller, C.; Compher, C.; Ellen, D.M. American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors. A.S.P.E.N. clinical guidelines: Nutrition screening, assessment, and intervention in adults. JPEN J. Parenter. Enter. Nutr. 2011, 35, 16–24. [Google Scholar] [CrossRef]
- Lochs, H.; Allison, S.P.; Meier, R.; Pirlich, M.; Kondrup, J.; Schneider, S.; van den Berghe, G.; Pichard, C. Introductory to the ESPEN Guidelines on Enteral Nutrition: Terminology, definitions and general topics. Clin. Nutr. 2006, 25, 180–186. [Google Scholar] [CrossRef]
- Academy of Nutrition and Dietetics Evidence Analysis Library Celiac Disease Evidence-Based Nutrition Practice Guideline [Internet]. EAL. Available online: https://www.andeal.org/default.cfm?auth=1 (accessed on 4 October 2022).
- Detsky, A.S.; McLaughlin, J.R.; Baker, J.P.; Johnston, N.; Whittaker, S.; Mendelson, R.A.; Jeejeebhoy, K.N. What is subjective global assessment of nutritional status? JPEN J. Parenter. Enter. Nutr. 1987, 11, 8–13. [Google Scholar] [CrossRef]
- Duerksen, D.R.; Laporte, M.; Jeejeebhoy, K. Evaluation of Nutrition Status Using the Subjective Global Assessment: Malnutrition, Cachexia, and Sarcopenia. Nutr. Clin. Pract. 2021, 36, 942–956. [Google Scholar] [CrossRef]
- Reber, E.; Gomes, F.; Vasiloglou, M.F.; Schuetz, P.; Stanga, Z. Nutritional Risk Screening and Assessment. J. Clin. Med. Res. 2019, 8, 1065. [Google Scholar] [CrossRef]
- Weekes, C.E.; Elia, M.; Emery, P.W.; Elizabethweekes, C. The development, validation and reliability of a nutrition screening tool based on the recommendations of the British Association for Parenteral and Enteral Nutrition (BAPEN). Clin. Nutr. 2004, 23, 1104–1112. [Google Scholar] [CrossRef]
- Guigoz, Y.; Vellas, B.; Garry, P.J. Assessing the nutritional status of the elderly: The Mini Nutritional Assessment as part of the geriatric evaluation. Nutr. Rev. 1996, 54 1 Pt 2, S59–S65. [Google Scholar] [CrossRef]
- Rubenstein, L.Z.; Harker, J.O.; Salvà, A.; Guigoz, Y.; Vellas, B. Screening for undernutrition in geriatric practice: Developing the short-form mini-nutritional assessment (MNA-SF). J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M366–M372. [Google Scholar] [CrossRef]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M. Educational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN). ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef]
- Balstad, T.R.; Bye, A.; Jenssen, C.R.; Solheim, T.S.; Thoresen, L.; Sand, K. Patient interpretation of the Patient-Generated Subjective Global Assessment (PG-SGA) Short Form. Patient Prefer. Adherence 2019, 13, 1391–1400. [Google Scholar] [CrossRef]
- Allard, J.P.; Keller, H.; Gramlich, L.; Jeejeebhoy, K.N.; Laporte, M.; Duerksen, D.R. GLIM criteria has fair sensitivity and specificity for diagnosing malnutrition when using SGA as comparator. Clin. Nutr. 2020, 39, 2771–2777. [Google Scholar] [CrossRef]
- Davidson, I.; Smith, S. Nutritional screening: Pitfalls of nutritional screening in the injured obese patient. Proc. Nutr. Soc. 2004, 63, 421–425. [Google Scholar] [CrossRef]
- Sharma, A.M.; Kushner, R.F. A proposed clinical staging system for obesity. Int. J. Obes. 2009, 33, 289–295. [Google Scholar] [CrossRef]
- Pelkowski, T.D.; Viera, A.J. Celiac disease: Diagnosis and management. Am. Fam. Physician 2014, 89, 99–105. [Google Scholar]
- Mansueto, P.; Soresi, M.; La Blasca, F.; Fayer, F.; D’Alcamo, A.; Carroccio, A. Body Mass Index and Associated Clinical Variables in Patients with Non-Celiac Wheat Sensitivity. Nutrients 2019, 11, 1220. [Google Scholar] [CrossRef]
- Varma, S.; Krishnareddy, S. Uncomplicated celiac disease. Refract. Celiac. Dis. 2022, 5–19. [Google Scholar] [CrossRef]
- Zanchetta, M.B.; Longobardi, V.; Bai, J.C. Bone and Celiac Disease. Curr. Osteoporos. Rep. 2016, 14, 43–48. [Google Scholar] [CrossRef]
- Camargo Pereira, C.; Pagotto, V.; de Oliveira, C.; Silveira, E.A. Low muscle mass and mortality risk later in life: A 10-year follow-up study. PLoS ONE 2022, 17, e0271579. [Google Scholar] [CrossRef]
- Padilla, C.J.; Ferreyro, F.A.; Arnold, W.D. Anthropometry as a readily accessible health assessment of older adults. Exp. Gerontol. 2021, 153, 111464. [Google Scholar] [CrossRef]
- Klein, S.; Allison, D.B.; Heymsfield, S.B.; E Kelley, D.; Leibel, R.L.; Nonas, C.; Kahn, R. Waist circumference and cardiometabolic risk: A consensus statement from Shaping America’s Health: Association for Weight Management and Obesity Prevention; NAASO, The Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Am. J. Clin. Nutr. 2007, 85, 1197–1202. [Google Scholar] [CrossRef]
- Guo, C.B.; Zhang, W.; Ma, D.Q.; Zhang, K.H.; Huang, J.Q. Hand grip strength: An indicator of nutritional state and the mix of postoperative complications in patients with oral and maxillofacial cancers. Br. J. Oral. Maxillofac. Surg. 1996, 34, 325–327. [Google Scholar] [CrossRef]
- Pieterse, S.; Manandhar, M.; Ismail, S. The association between nutritional status and handgrip strength in older Rwandan refugees. Eur. J. Clin. Nutr. 2002, 56, 933–939. [Google Scholar] [CrossRef]
- Kurt, C.; Sağiroğlu, İ.; Kurt Ömürlü, İ.; Çatikkaş, F. Associations among handgrip strength, dietary pattern, and physical activity level in Physical Education students. Int. J. Sport Exerc. Train Sci. 2017, 3, 33. [Google Scholar] [CrossRef]
- Capristo, E.; Addolorato, G.; Mingrone, G.; De Gaetano, A.; Greco, A.V.; A Tataranni, P.; Gasbarrini, G. Changes in body composition, substrate oxidation, and resting metabolic rate in adult celiac disease patients after a 1-y gluten-free diet treatment. Am. J. Clin. Nutr. 2000, 72, 76–81. [Google Scholar] [CrossRef]
- Sobhiyeh, S.; Dunkel, A.; Dechenaud, M.; Mehrnezhad, A.; Kennedy, S.; Shepherd, J.; Wolenski, P.; Heymsfield, S.B. Digital anthropometric volumes: Toward the development and validation of a universal software. Med. Phys. 2021, 48, 3654–3664. [Google Scholar] [CrossRef]
- Marra, M.; Sammarco, R.; De Lorenzo, A.; Iellamo, F.; Siervo, M.; Pietrobelli, A.; Donini, L.M.; Santarpia, L.; Cataldi, M.; Pasanisi, F.; et al. Assessment of Body Composition in Health and Disease Using Bioelectrical Impedance Analysis (BIA) and Dual Energy X-Ray Absorptiometry (DXA): A Critical Overview. Contrast Media Mol. Imaging 2019, 2019, 3548284. [Google Scholar] [CrossRef]
- Pasdar, Y.; Moradi, S.; Abdollahzad, H.; Hamzeh, B.; Najafi, F.; Nachvak, S.M.; Mostafai, R. Accuracy of Waist to Hip Ratio Calculated by Bioelectric Impedance Device in the Ravansar Non-Communicable Disease Cohort Study. Top Clin. Nutr. 2019, 34, 269. [Google Scholar] [CrossRef]
- Shim, J.S.; Oh, K.; Kim, H.C. Dietary assessment methods in epidemiologic studies. Epidemiol. Health. 2014, 36, e2014009. [Google Scholar] [CrossRef]
- Prentice, R.L.; Mossavar-Rahmani, Y.; Huang, Y.; Van Horn, L.; Beresford, S.A.A.; Caan, B.; Tinker, L.; Schoeller, D.; Bingham, S.; Eaton, C.; et al. Evaluation and comparison of food records, recalls, and frequencies for energy and protein assessment by using recovery biomarkers. Am. J. Epidemiol. 2011, 174, 591–603. [Google Scholar] [CrossRef]
- de Salud, C. Valoración del Estado nutricional de la Comunidad Autónoma de Andalucía. Available online: https://repositoriosalud.es/bitstream/10668/1215/5/ValoracionNutricional_2000.pdf (accessed on 5 October 2022).
- Crespo Escobar, P.; Calvo Lerma, J.; Hervas Marin, D.; Aliaga, E.D.; Simó, E.M.; Miquel, B.P.; Koninckx, C.R. Development and Validation of Two Food Frequency Questionnaires to Assess Gluten Intake in Children up to 36 Months of Age. Nutr. Hosp. 2015, 32, 2080–2090. [Google Scholar] [CrossRef]
- Mazzeo, T.; Roncoroni, L.; Lombardo, V.; Tomba, C.; Elli, L.; Sieri, S.; Grioni, S.; Bardella, M.T.; Agostoni, C.; Doneda, L.; et al. Evaluation of a Modified Italian European Prospective Investigation into Cancer and Nutrition Food Frequency Questionnaire for Individuals with Celiac Disease. J. Acad. Nutr. Diet. 2016, 116, 1810–1816. [Google Scholar] [CrossRef]
- Ortega, R.M.; Pérez-Rodrigo, C.; López-Sobaler, A.M. Dietary assessment methods: Dietary records. Nutr. Hosp. 2015, 31 (Suppl. 3), 38–45. [Google Scholar] [CrossRef]
- Dauncey, M.J. Metabolic effects of altering the 24 h energy intake in man, using direct and indirect calorimetry. Br. J. Nutr. 1980, 43, 257–269. [Google Scholar] [CrossRef]
- Jésus, P.; Achamrah, N.; Grigioni, S.; Charles, J.; Rimbert, A.; Folope, V.; Petit, A.; Déchelotte, P.; Coëffier, M. Validity of predictive equations for resting energy expenditure according to the body mass index in a population of 1726 patients followed in a Nutrition Unit. Clin. Nutr. 2015, 34, 529–535. [Google Scholar] [CrossRef]
- Silver, H.J.; Wall, R.; Hollingsworth, E.; Pruitt, A.; Shotwell, M.; Simmons, S. Simple kcal/kg formula is comparable to prediction equations for estimating resting energy expenditure in older cognitively impaired long term care residents. J. Nutr. Health Aging 2013, 17, 39–44. [Google Scholar] [CrossRef]
- Frankenfield, D.; Roth-Yousey, L.; Compher, C. Comparison of predictive equations for resting metabolic rate in healthy nonobese and obese adults: A systematic review. J. Am. Diet. Assoc. 2005, 105, 775–789. [Google Scholar] [CrossRef]
- Lindsey, B.W.; Shookster, D.E.; Martin, J.R.; Cortes, N.N. Predictive Accuracy of the Nelson Equation via BodPod Compared to Commonly Used Equations to Estimate Resting Metabolic Rate in Adults. Int. J. Exerc. Sci. 2021, 14, 1166–1177. [Google Scholar]
- Finkler, E.; Heymsfield, S.B.; St-Onge, M.P. Rate of weight loss can be predicted by patient characteristics and intervention strategies. J. Acad. Nutr. Diet. 2012, 112, 75–80. [Google Scholar] [CrossRef]
- Battley, E.H. The advantages and disadvantages of direct and indirect calorimetry. Thermochim. Acta 1995, 250, 337–352. [Google Scholar] [CrossRef]
- Kenny, G.P.; Notley, S.R.; Gagnon, D. Direct calorimetry: A brief historical review of its use in the study of human metabolism and thermoregulation. Eur. J. Appl. Physiol. 2017, 117, 1765–1785. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.C.; Shin, D.; Leem, C.H.; Joo, S. Development of a Portable Respiratory Gas Analyzer for Measuring Indirect Resting Energy Expenditure (REE). J. Healthc. Eng. 2021, 2021, 8870749. [Google Scholar] [CrossRef] [PubMed]
- Briassoulis, G.; Michaeloudi, E.; Fitrolaki, D.M.; Spanaki, A.M.; Briassouli, E. Influence of different ventilator modes on Vo(2) and Vco(2) measurements using a compact metabolic monitor. Nutrition 2009, 25, 1106–1114. [Google Scholar] [CrossRef]
- McClave, S.A.; Lowen, C.C.; Kleber, M.J.; McConnell, J.W.; Jung, L.Y.; Goldsmith, L.J. Clinical use of the respiratory quotient obtained from indirect calorimetry. JPEN J. Parenter Enteral. Nutr. 2003, 27, 21–26. [Google Scholar] [CrossRef]
- Cardo, A.; Churruca, I.; Lasa, A.; Navarro, V.; Vázquez-Polo, M.; Perez-Junkera, G.; Larretxi, I. Nutritional Imbalances in Adult Celiac Patients Following a Gluten-Free Diet. Nutrients 2021, 13, 2877. [Google Scholar] [CrossRef]
- Drewnowski, A.; Fulgoni, V.L. 3rd. New Nutrient Rich Food Nutrient Density Models That Include Nutrients and MyPlate Food Groups. Front. Nutr. 2020, 7, 107. [Google Scholar] [CrossRef]
- Borneo, R.; León, A.E. Whole grain cereals: Functional components and health benefits. Food Funct. 2012, 3, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Taetzsch, A.; Das, S.K.; Brown, C.; Krauss, A.; Silver, R.E.; Roberts, S.B. Are Gluten-Free Diets More Nutritious? An Evaluation of Self-Selected and Recommended Gluten-Free and Gluten-Containing Dietary Patterns. Nutrients 2018, 10, 1881. [Google Scholar] [CrossRef] [PubMed]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten free diet and nutrient deficiencies: A review. Clin. Nutr. 2016, 35, 1236–1241. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F. Gluten-Free Diet: Gaps and Needs for a Healthier Diet. Nutrients 2019, 11, 170. [Google Scholar] [CrossRef]
- Wu, J.H.; Neal, B.; Trevena, H.; Crino, M.; Stuart-Smith, W.; Faulkner-Hogg, K.; Louie, J.C.Y.; Dunford, E. Are gluten-free foods healthier than non-gluten-free foods? An evaluation of supermarket products in Australia. Br. J. Nutr. 2015, 114, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Wolters, M.; Ahrens, J.; Romaní-Pérez, M.; Watkins, C.; Sanz, Y.; Benítez-Páez, A.; Stanton, C.; Günther, K. Dietary fat, the gut microbiota, and metabolic health–A systematic review conducted within the MyNewGut project. Clin. Nutr. 2019, 38, 2504–2520. [Google Scholar] [CrossRef] [PubMed]
- Hopman, E.G.; le Cessie, S.; von Blomberg, B.M.; Mearin, M.L. Nutritional management of the gluten-free diet in young people with celiac disease in The Netherlands. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 102–108. [Google Scholar] [CrossRef]
- Thompson, T.; Dennis, M.; Higgins, L.A.; Lee, A.R.; Sharrett, M.K. Gluten-free diet survey: Are Americans with coeliac disease consuming recommended amounts of fibre, iron, calcium and grain foods? J. Hum. Nutr. Diet. 2005, 18, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Zuccotti, G.; Fabiano, V.; Dilillo, D.; Picca, M.; Cravidi, C.; Brambilla, P. Intakes of nutrients in Italian children with celiac disease and the role of commercially available gluten-free products. J. Hum. Nutr. Diet. 2013, 26, 436–444. [Google Scholar] [CrossRef]
- Kitts, D.D.; Jones, P.J.H. Dietary fats: Discriminative partitioning for energy and synthesis of triacylglycerides. Food Res. Int. 1996, 29, 57–69. [Google Scholar] [CrossRef]
- Niland, B.; Cash, B.D. Health Benefits and Adverse Effects of a Gluten-Free Diet in Non-Celiac Disease Patients. Gastroenterol. Hepatol. 2018, 14, 82–91. [Google Scholar]
- Penagini, F.; Dilillo, D.; Meneghin, F.; Mameli, C.; Fabiano, V.; Zuccotti, G.V. Gluten-free diet in children: An approach to a nutritionally adequate and balanced diet. Nutrients 2013, 5, 4553–4565. [Google Scholar] [CrossRef]
- Larretxi, I.; Txurruka, I.; Navarro, V.; Lasa, A.; Bustamante, M.Á.; Fernández-Gil, M.d.P.; Simón, E.; Miranda, J. Micronutrient Analysis of Gluten-Free Products: Their Low Content Is Not Involved in Gluten-Free Diet Imbalance in a Cohort of Celiac Children and Adolescent. Foods. 2019, 8, 321. [Google Scholar] [CrossRef]
- Paddon-Jones, D.; Westman, E.; Mattes, R.D.; Wolfe, R.R.; Astrup, A.; Westerterp-Plantenga, M. Protein, weight management, and satiety. Am. J. Clin. Nutr. 2008, 87, 1558S–1561S. [Google Scholar] [CrossRef] [PubMed]
- Martín-Masot, R.; Nestares, M.T.; Diaz-Castro, J.; López-Aliaga, I.; Alférez, M.J.M.; Moreno-Fernandez, J.; Maldonado, J. Multifactorial Etiology of Anemia in Celiac Disease and Effect of Gluten-Free Diet: A Comprehensive Review. Nutrients 2019, 11, 2557. [Google Scholar] [CrossRef] [PubMed]
- Jivraj, A.; Hutchinson, J.M.; Ching, E.; Marwaha, A.; Verdu, E.F.; Armstrong, D.; Pinto-Sanchez, M.I. Micronutrient deficiencies are frequent in adult patients with and without celiac disease on a gluten-free diet, regardless of duration and adherence to the diet. Nutrition 2022, 103–104, 111809. [Google Scholar] [CrossRef]
- Marciniak, M.; Szymczak-Tomczak, A.; Mahadea, D.; Eder, P.; Dobrowolska, A.; Krela-Kaźmierczak, I. Multidimensional Disadvantages of a Gluten-Free Diet in Celiac Disease: A Narrative Review. Nutrients 2021, 13, 643. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Brar, P.S.; Lee, A.R.; Green, P.H.R. Body mass index in celiac disease: Beneficial effect of a gluten-free diet. J. Clin. Gastroenterol. 2010, 44, 267–271. [Google Scholar] [CrossRef]
- Barone, M.; Iannone, A.; Cristofori, F.; Dargenio, V.N.; Indrio, F.; Verduci, E.; Di Leo, A.; Francavilla, R. Risk of obesity during a gluten-free diet in pediatric and adult patients with celiac disease: A systematic review with meta-analysis. Nutr. Rev. 2022, 81, nuac052. [Google Scholar] [CrossRef]
- Dioneda, B.; Healy, M.; Paul, M.; Sheridan, C.; Mohr, A.E.; Arciero, P.J. A Gluten-Free Meal Produces a Lower Postprandial Thermogenic Response Compared to an Iso-Energetic/Macronutrient Whole Food or Processed Food Meal in Young Women: A Single-Blind Randomized Cross-Over Trial. Nutrients 2020, 12, 2035. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; McCarty, T.R.; Lange, A.; Ngu, J.N.; Njei, B. Impact of bariatric surgery on outcomes of patients with celiac disease: A nationwide inpatient sample analysis, 2004–2014. Ann. Gastroenterol. 2019, 32, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Sánchez, M.I.; Causada-Calo, N.; Bercik, P.; Ford, A.; Murray, J.A.; Armstrong, D.; Semrad, C.; Kupfer, S.S.; Alaedini, A.; Moayyedi, P.; et al. Safety of Adding Oats to a Gluten-Free Diet for Patients With Celiac Disease: Systematic Review and Meta-analysis of Clinical and Observational Studies. Gastroenterology 2017, 153, 395–409.e3. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Fabiani, E.; Iacono, G.; D'Agate, C.; Francavilla, R.; Biagi, F.; Volta, U.; Accomando, S.; Picarelli, A.; De Vitis, I.; et al. A prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. Am. J. Clin. Nutr. 2007, 85, 160–166. [Google Scholar] [CrossRef]
- Health Canada’s Position on Gluten-Free Claims. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/food-safety/food-allergies-intolerances/celiac-disease/health-canada-position-gluten-free-claims.html (accessed on 5 October 2022).
- Rubio-Tapia, A.; Rahim, M.W.; See, J.A.; Lahr, B.D.; Wu, T.T.; Murray, J.A. Mucosal recovery and mortality in adults with celiac disease after treatment with a gluten-free diet. Am. J. Gastroenterol. 2010, 105, 1412–1420. [Google Scholar] [CrossRef]
- Penny, H.A.; Baggus, E.M.R.; Rej, A.; Snowden, J.A.; Sanders, D.S. Non-Responsive Coeliac Disease: A Comprehensive Review from the NHS England National Centre for Refractory Coeliac Disease. Nutrients 2020, 12, 216. [Google Scholar] [CrossRef]
- Lionetti, E.; Antonucci, N.; Marinelli, M.; Bartolomei, B.; Franceschini, E.; Gatti, S.; Catassi, G.N.; Verma, A.K.; Monachesi, C.; Catassi, C. Nutritional Status, Dietary Intake, and Adherence to the Mediterranean Diet of Children with Celiac Disease on a Gluten-Free Diet: A Case-Control Prospective Study. Nutrients 2020, 12, 143. [Google Scholar] [CrossRef] [PubMed]
- Mearin, M.L.; Agardh, D.; Antunes, H.; Al-Toma, A.; Auricchio, R.; Castillejo, G.; Carlo, C.; Carolina, C.; Valentina, D.; Jernej, D.; et al. ESPGHAN Position paper on management and follow-up of children and adolescents with celiac disease. J. Pediatr. Gastroenterol. 2022, 75, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Green, P.H.R.; Paski, S.; Ko, C.W.; Rubio-Tapia, A. AGA Clinical Practice Update on Management of Refractory Celiac Disease: Expert Review. Gastroenterology 2022, 163, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Pash, E. Enteral Nutrition: Options for Short-Term Access. Nutr. Clin. Pract. 2018, 33, 170–176. [Google Scholar] [CrossRef]
- García-Manzanares, A.; Lucendo, A.J. Nutritional and dietary aspects of celiac disease. Nutr. Clin. Pract. 2011, 26, 163–173. [Google Scholar] [CrossRef]
- Lappas, B.M.; Patel, D.; Kumpf, V.; Adams, D.W.; Seidner, D.L. Parenteral Nutrition: Indications, Access, and Complications. Gastroenterol. Clin. N. Am. 2018, 47, 39–59. [Google Scholar] [CrossRef]
- Rubio-Tapia, A.; Murray, J.A. Classification and management of refractory coeliac disease. Gut 2010, 59, 547–557. [Google Scholar] [CrossRef]
- Jamma, S.; Rubio-Tapia, A.; Kelly, C.P.; Murray, J.; Najarian, R.; Sheth, S.; Schuppan, D.; Dennis, M.; Leffler, D.A. Celiac crisis is a rare but serious complication of celiac disease in adults. Clin. Gastroenterol. Hepatol. 2010, 8, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Braunschweig, C.L.; Levy, P.; Sheean, P.M.; Wang, X. Enteral compared with parenteral nutrition: A meta-analysis. Am. J. Clin. Nutr. 2001, 74, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Hall, N.J.; Rubin, G.; Charnock, A. Systematic review: Adherence to a gluten-free diet in adult patients with coeliac disease. Aliment. Pharmacol. Ther. 2009, 30, 315–330. [Google Scholar] [CrossRef]
- Pinto-Sanchez, M.I.; Bai, J.C. Toward New Paradigms in the Follow Up of Adult Patients With Celiac Disease on a Gluten-Free Diet. Front. Nutr. 2019, 6, 153. [Google Scholar] [CrossRef]
- Katarzyna, G.; Dardzińska, J.; Guzek, M.; Adrych, K.; Kochan, Z.; Małgorzewicz, S. Expanded Role of a Dietitian in Monitoring a Gluten-Free Diet in Patients with Celiac Disease: Implications for Clinical Practice. Nutrients 2021, 13, 1859. [Google Scholar] [CrossRef]
- Coto, L.; Mendia, I.; Sousa, C.; Bai, J.C.; Cebolla, A. Determination of gluten immunogenic peptides for the management of the treatment adherence of celiac disease: A systematic review. World J. Gastroenterol. 2021, 27, 6306–6321. [Google Scholar] [CrossRef] [PubMed]
| Tools | Components | Scoring | Advantages | Disadvantages |
|---|---|---|---|---|
| SCREENING | ||||
| Nutritional Risk Screening 2002 (NRS-2002) [20,21] | Used in an inpatient setting. Four questions in pre-screening. | If the response is positive, subsequent assessment evaluates the nutritional status and disease severity. Score ≥ 3 indicates malnutrition/malnutrition risk | Simple, well-validated tool Very reliable Can be completed within 3–5 min | Requires trained staff |
| Malnutrition Universal Screening Tool (MUST) [20,24] | Five-step screening tool Combines BMI score, weight loss score, and acute disease effect score to obtain malnutrition risk score. | Scores: 0 (low risk), 1 (medium risk), and ≥2 (high risk). | Malnutrition can be detected across a variety of community care settings Useful for determining the grade of malnutrition risk | Does not include low dietary intake Requires BMI and percent weight loss calculations, which can be time-consuming |
| ASSESSMENT | ||||
| Mini Nutritional Assessment (MNA) [20,22] | Combines nutrition screening and assessment. Covers 4 domains (nutrient intake, anthropometric measurement, global assessment, and subjective assessment). | Scores: 0–7 (malnourished), 8–11 (malnutrition risk), and 12–14 (normal nutritional status). Score ≤ 11 indicates the need for further assessment. | Quick evaluation tool No biochemical tests required Non-invasive | Useful only in limited patient populations Relies on patient self-assessment |
| Mini Nutritional Assessment short-form (MNA-SF) [20,23] | A short version of MNA. Covers six items (food intake, weight loss, mobility, psychological stress, neuropsychological symptoms, and BMI). | Score ≤ 11 indicates malnutrition/malnutrition risk, subsequently requiring full MNA. | Faster than complete MNA Considered as effective | Requires MNA when the patient has malnutrition risk |
| Subjective Global Assessment (SGA) [18,19,20] | Assessment by a healthcare (HC) provider. Seven domains (nutrient intake, weight change, symptoms, functional capacity, metabolic requirement, physical examination, and contributing factor). | Rating: SGA A (well nourished, no risk of malnutrition), SGA B (mild/moderate risk), and SGA C (severe risk). | A non-invasive and inexpensive tool Requires basic training Simple to incorporate in routine follow-ups | Only studied in some populations Does not include biochemical data Allows for subjective determination Need for physical examination |
| Patient-Generated Subjective Global Assessment (PG-SGA) [25] | Self-assessment by patient and assessment by HC provider. Patient-generated components (weight history, food intake, symptoms, activities, and function). HC provider component (weight loss, disease/nutritional requirements, metabolic demand, and physical exam). | The score is based on:
| Autonomy for patient Improved patient-clinician interaction Dynamic evaluation of the nutritional status | Patients may misinterpret the question Can be difficult to answer honestly The duration of recall can be long for patients |
| Global Leadership Initiative on Malnutrition (GLIM) criteria [26] | Framework for diagnosing malnutrition based on combinations of phenotypic (non-voluntary weight loss, low BMI, and reduced muscle mass) and etiologic (reduced food intake, disease burden/inflammatory condition) criteria. | Malnutrition is assessed based on (1) phenotypic (weight loss, low BMI, and reduced muscle mass) and (2) etiologic criteria (reduced food intake, malabsorption, and disease burden/inflammatory condition). One phenotypic and one etiologic criterion are required to define malnutrition. | High sensitivity Good performance as a screening tool | Low performance compared with SGA Low specificity False positive risk is high |
| Food Group | Gluten-Free | Gluten Containing |
|---|---|---|
| Grains | Rice, corn, corn, tapioca, millet, sorghum, teff, buckwheat, pure gluten-free oats and quinoa | Barley, bulgar, couscous, and durum wheat and other types of wheat (einkorn, emmer, farro, kamut, and spelt (dinkel) Derived from barley (malt extract, flavoring, syrup, and vinegar) Non-pure gluten-free oats Rye, semolina, and triticale |
| Sugar | Sugar, honey, and sweeteners | |
| Meats | Fresh and frozen plain meats, offal, jerky, cured ham, and cooked ham (no flavorings), fresh and frozen fish and seafood without breading, canned, or in oil | Processed meat may contain gluten Breaded chicken, fish, or meat |
| Fruits and vegetables | Fresh, in-syrup, and most dried fruits (except dried figs, which may contain gluten), and vegetables | Processed fruits, jams, or vegetables flavored may contain gluten |
| Nuts | Raw nuts (roasted nuts may contain gluten), shelled and unshelled | Flavored nuts or mixed nuts may contain gluten |
| Condiments | Oil and traditional butter, vinegar | Flavored oils may contain gluten Soy sauce often contains gluten |
| Eggs | Eggs | Processed, scrambled, omelets may contain gluten |
| Hot and soft drinks | Coffee beans or ground coffee, unprocessed herbal teas, soft drinks (orange, lemon, cola, etc.), and sodas | Flavored coffees and shakes may contain gluten |
| Milk and dairy products | Cheeses, cottage cheese, cream, natural yogurts, and fresh curd | Processed, flavored, or mixed dairy may contain gluten |
| Legumes | Dried and cooked legumes in natural preserves Careful with lentils—check and remove any foreign grain if found | Processed legumes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdi, F.; Zuberi, S.; Blom, J.-J.; Armstrong, D.; Pinto-Sanchez, M.I. Nutritional Considerations in Celiac Disease and Non-Celiac Gluten/Wheat Sensitivity. Nutrients 2023, 15, 1475. https://doi.org/10.3390/nu15061475
Abdi F, Zuberi S, Blom J-J, Armstrong D, Pinto-Sanchez MI. Nutritional Considerations in Celiac Disease and Non-Celiac Gluten/Wheat Sensitivity. Nutrients. 2023; 15(6):1475. https://doi.org/10.3390/nu15061475
Chicago/Turabian StyleAbdi, Fardowsa, Saania Zuberi, Jedid-Jah Blom, David Armstrong, and Maria Ines Pinto-Sanchez. 2023. "Nutritional Considerations in Celiac Disease and Non-Celiac Gluten/Wheat Sensitivity" Nutrients 15, no. 6: 1475. https://doi.org/10.3390/nu15061475
APA StyleAbdi, F., Zuberi, S., Blom, J.-J., Armstrong, D., & Pinto-Sanchez, M. I. (2023). Nutritional Considerations in Celiac Disease and Non-Celiac Gluten/Wheat Sensitivity. Nutrients, 15(6), 1475. https://doi.org/10.3390/nu15061475








