Evaluation and Management of Nutritional Consequences of Chronic Liver Diseases
Abstract
:1. Introduction
2. Consequences of Liver Disease for Nutritional Status
2.1. Impaired Dietary Intake
2.2. Altered Macro- and Micronutrient Metabolism
2.2.1. Macronutrients Metabolism
2.2.2. Micronutrients Metabolism
2.3. Energy Metabolism Disturbances
2.4. Increase in Energy Expenditure
2.5. Nutrient Malabsorption
2.6. Sarcopenia and Muscle Function
2.7. Metabolic Osteopathy
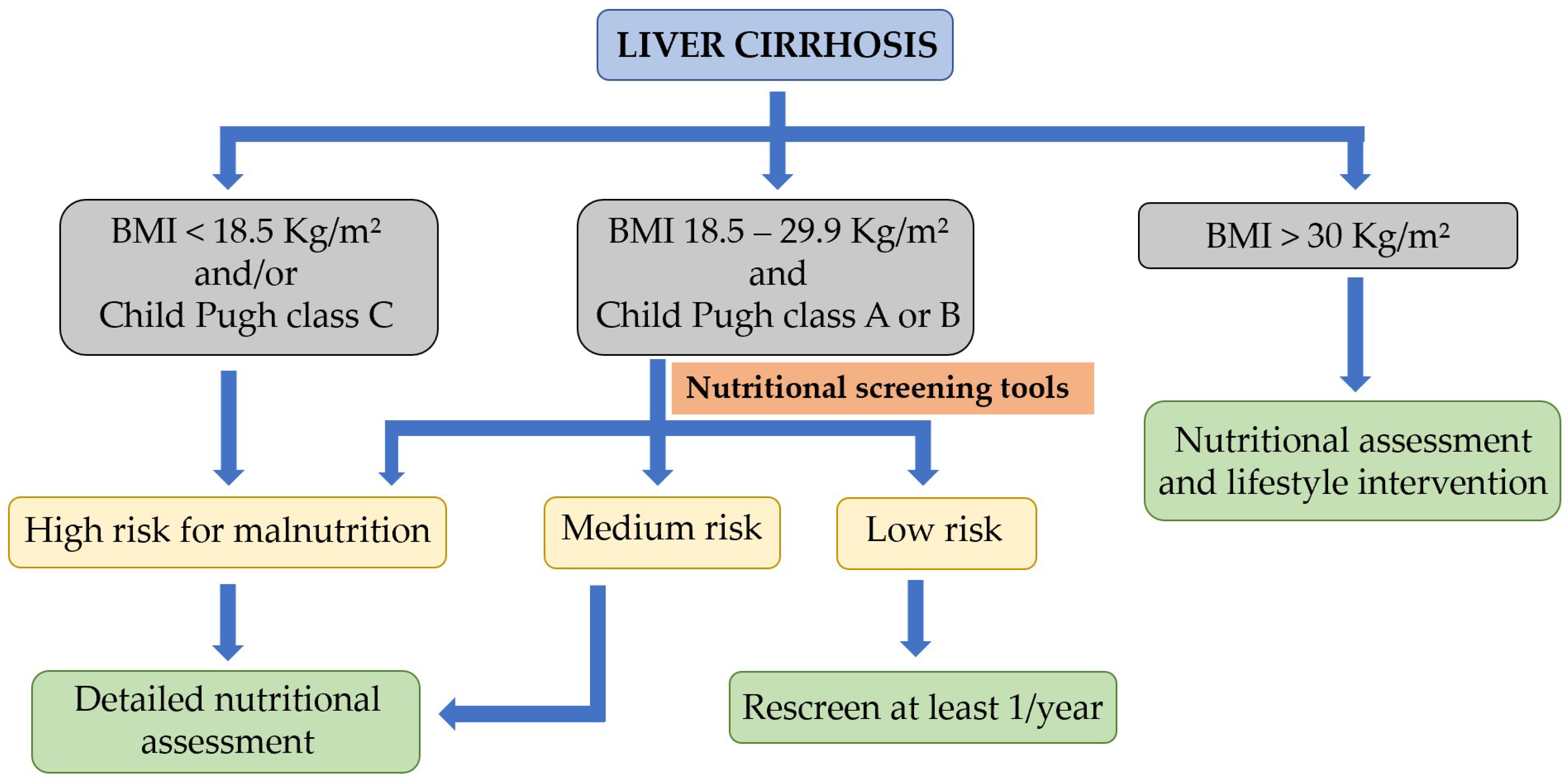
3. Nutritional Screening and Assessment of Patients with Chronic Liver Disease
3.1. Nutritional Screening and Risk of Malnutrition
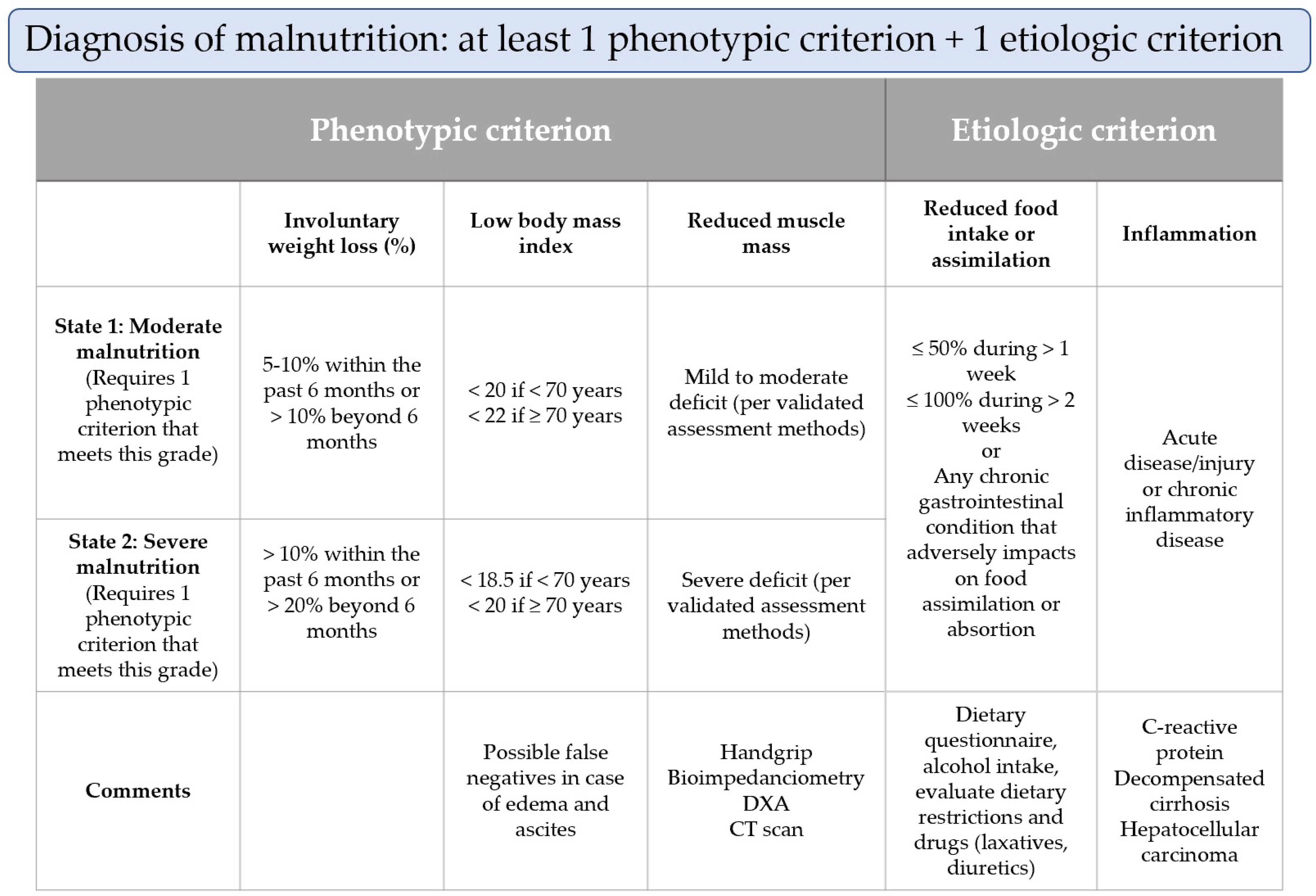
3.2. Nutritional Assessment of Malnutrition
3.2.1. Assessment of Reduced Intake
3.2.2. Weight Loss and Body Mass Index
3.2.3. Muscle Mass and Body Composition
3.2.4. Disease Burden or Inflammation
4. Nutritional Intervention in Liver Disease
4.1. Nutritional Intervention in Patients with Hepatic Cirrhosis
4.1.1. Energy Intake: General Recommendations, Enteral Nutrition, and Parenteral Nutrition
4.1.2. Protein Intake
4.1.3. Vitamins and Minerals
4.1.4. Sodium
| Intake/Uptake | Physical Activity [1,90,92,95,96,97,98,99,100,101,102,103] |
|---|---|
Weight-based equations (using ideal body weight) [10,83,84,85]:
| Personalized activity prescription:
|
Protein intake [1,10]:
| — |
| Frequent, small meals and minimized fasting: daily intake divided into six meals (late evening snack) [80,81,82] | |
| Micronutrient and vitamin administration if it is suspected or demonstrated [10,122,123,124,125] | |
| A moderate restriction of sodium intake (80–120 mmol/day) is recommended in patients with moderate, uncomplicated ascites [128] | |
| Non-osmotic fluid restriction (1000 mL/day) can be useful if serum sodium concentration < 125 mmol/L [128] | |
| Address barriers to intake (e.g., liberalize sodium restrictions as needed) |
4.2. Nutritional Intervention in Patients with MAFLD
4.2.1. Lifestyle Modifications in MAFLD
4.2.2. Roles of Different Micro- and Macronutrients
4.2.3. Dietary Patterns
4.3. Nutritional Interventions in Patients with Alcohol-Associated Liver Disease
4.3.1. General Recommendations
4.3.2. Alcoholic Hepatitis
4.3.3. Alcohol-Associated Cirrhosis
4.4. Nutrition-Related Aspects in Liver Transplantation
4.5. Nutrition-Related Aspects in Other Etiologies
4.5.1. Nutritional Intervention in Patients with Hereditary Hemochromatosis
4.5.2. Nutritional Intervention in Patients with Wilson’s disease
4.5.3. Nutritional Intervention in Patients with Cholestatic Liver Disease
4.6. Probiotics as Emerging Treatments for Liver Disease
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lai, J.C.; Tandon, P.; Bernal, W.; Tapper, E.B.; Ekong, U.; Dasarathy, S.; Carey, E.J. Malnutrition, Frailty, and Sarcopenia in Patients with Cirrhosis: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021, 74, 1611–1644. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN Guidelines on Definitions and Terminology of Clinical Nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Plank, L.D.; McCall, J.L.; Gillanders, L.K.; McIlroy, K.; Gane, E.J. Body Composition, Muscle Function, and Energy Expenditure in Patients with Liver Cirrhosis: A Comprehensive Study. Am. J. Clin. Nutr. 2007, 85, 1257–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moctezuma-Velázquez, C.; García-Juárez, I.; Soto-Solís, R.; Hernández-Cortés, J.; Torre, A. Nutritional Assessment and Treatment of Patients with Liver Cirrhosis. Nutrition 2013, 29, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Cañamares-Orbis, P.; Bernal-Monterde, V.; Sierra-Gabarda, O.; Casas-Deza, D.; Garcia-Rayado, G.; Cortes, L.; Lué, A. Impact of Liver and Pancreas Diseases on Nutritional Status. Nutrients 2021, 13, 1650. [Google Scholar] [CrossRef]
- Kim, G.; Kang, S.H.; Kim, M.Y.; Baik, S.K. Prognostic Value of Sarcopenia in Patients with Liver Cirrhosis: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0186990. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Yu, Q.; Peng, H.; Zhen, Z. Alterations of gut microbiome and effects of probiotic therapy in patients with liver cirrhosis: A systematic review and meta-analysis. Medicine 2022, 101, e32335. [Google Scholar] [CrossRef]
- Ali, S.H.; Abu Sneineh, A.; Hasweh, R. Nutritional Assessment in Patients with Liver Cirrhosis. World J. Hepatol. 2022, 14, 1694–1703. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on Nutrition in Chronic Liver Disease. J. Hepatol. 2019, 70, 172–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bischoff, S.C.; Ockenga, J.; Eshraghian, A.; Barazzoni, R.; Busetto, L.; Campmans-Kuijpers, M.; Cardinale, V.; Chermesh, I.; Kani, H.T.; Khannoussi, W.; et al. Practical Guideline on Obesity Care in Patients with Gastrointestinal and Liver Diseases—Joint ESPEN/UEG Guideline. Clin. Nutr. 2023, 42, 987–1024. [Google Scholar] [CrossRef]
- Plauth, M.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Bischoff, S.C. ESPEN Guideline on Clinical Nutrition in Liver Disease. Clin. Nutr. 2019, 38, 485–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Losera, C.; Aschl, G.; He, X.; Mathus-Vliegen, E.M.H.; Muscaritoli, M.; Niv, Y.; Rollins, H.; Singer, P.; Skelly, R.H. Consensus Statement; ESPEN Guidelines on Artificial Enteral Nutrition—Percutaneous Endoscopic Gastrostomy (PEG). Clin. Nutr. 2005, 24, 848–861. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barazzoni, R.; Busetto, L.; Campmans-Kuijpers, M.; Cardinale, V.; Chermesh, I.; Eshraghian, A.; Kani, H.T.; Khannoussi, W.; Lacaze, L.; et al. European Guideline on Obesity Care in Patients with Gastrointestinal and Liver Diseases—Joint ESPEN/UEG Guideline. Clin. Nutr. 2022, 41, 2364–2405. [Google Scholar] [CrossRef] [PubMed]
- Tsochatzis, E.A.; Bosch, J.; Burroughs, A.K. Liver Cirrhosis. Lancet 2014, 383, 1749–1761. [Google Scholar] [CrossRef]
- Meyer, F.; Bannert, K.; Wiese, M.; Esau, S.; Sautter, L.F.; Ehlers, L.; Aghdassi, A.A.; Metges, C.C.; Garbe, L.-A.; Jaster, R.; et al. Molecular Mechanism Contributing to Malnutrition and Sarcopenia in Patients with Liver Cirrhosis. Int. J. Mol. Sci. 2020, 21, 5357. [Google Scholar] [CrossRef] [PubMed]
- Aqel, B.A.; Scolapio, J.S.; Dickson, R.C.; Burton, D.D.; Bouras, E.P. Contribution of Ascites to Impaired Gastric Function and Nutritional Intake in Patients with Cirrhosis and Ascites. Clin. Gastroenterol. Hepatol. 2005, 3, 1095–1100. [Google Scholar] [CrossRef]
- Clarembeau, F.; Bale, G.; Lanthier, N. Cirrhosis and Insulin Resistance: Current Knowledge, Pathophysiological Mechanisms, Complications and Potential Treatments. Clin. Sci. 2020, 134, 2117–2135. [Google Scholar] [CrossRef]
- Kumar, R.; García-Compeán, D.; Maji, T. Hepatogenous Diabetes: Knowledge, Evidence, and Skepticism. World J. Hepatol. 2022, 14, 1291–1306. [Google Scholar] [CrossRef]
- Dasarathy, S.; Merli, M. Sarcopenia from Mechanism to Diagnosis and Treatment in Liver Disease. J. Hepatol. 2016, 65, 1232–1244. [Google Scholar] [CrossRef] [Green Version]
- Espina, S.; Sanz-Paris, A.; Bernal-Monterde, V.; Casas-Deza, D.; Arbonés-Mainar, J.M. Role of Branched-Chain Amino Acids and Their Derivative β-Hydroxy-β-Methylbutyrate in Liver Cirrhosis. J. Clin. Med. 2022, 11, 7337. [Google Scholar] [CrossRef]
- Espina, S.; Sanz-Paris, A.; Gonzalez-Irazabal, Y.; Pérez-Matute, P.; Andrade, F.; Garcia-Rodriguez, B.; Carpéné, C.; Zakaroff, A.; Bernal-Monterde, V.; Fuentes-Olmo, J.; et al. Randomized Clinical Trial: Effects of β-Hydroxy-β-Methylbutyrate (HMB)-Enriched vs. HMB-Free Oral Nutritional Supplementation in Malnourished Cirrhotic Patients. Nutrients 2022, 14, 2344. [Google Scholar] [CrossRef] [PubMed]
- Campollo, O.; Sprengers, D.; McIntyre, N. The BCAA/AAA Ratio of Plasma Amino Acids in Three Different Groups of Cirrhotics. Rev. Investig. Clin. 1992, 44, 513–518. [Google Scholar]
- Espina, S.; Gonzalez-Irazabal, Y.; Sanz-Paris, A.; Lopez-Yus, M.; Garcia-Sobreviela, M.P.; del Moral-Bergos, R.; Garcia-Rodriguez, B.; Fuentes-Olmo, J.; Bernal-Monterde, V.; Arbones-Mainar, J.M. Amino Acid Profile in Malnourished Patients with Liver Cirrhosis and Its Modification with Oral Nutritional Supplements: Implications on Minimal Hepatic Encephalopathy. Nutrients 2021, 13, 3764. [Google Scholar] [CrossRef]
- Zhao, V.M.; Ziegler, T.R. Nutrition Support in End-Stage Liver Disease. Crit. Care Nurs. Clin. N. Am. 2010, 22, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Meng, Q.-H. Current Understanding of the Metabolism of Micronutrients in Chronic Alcoholic Liver Disease. World J. Gastroenterol. 2020, 26, 4567–4578. [Google Scholar] [CrossRef] [PubMed]
- Send, S.R. Nutritional Management of Cholestasis. Clin. Liver Dis. 2020, 15, 9–12. [Google Scholar] [CrossRef] [Green Version]
- Stirnimann, J.; Stirnimann, G. Nutritional Challenges in Patients with Advanced Liver Cirrhosis. J. Clin. Med. 2019, 8, 1926. [Google Scholar] [CrossRef] [Green Version]
- Licata, A.; Zerbo, M.; Como, S.; Cammilleri, M.; Soresi, M.; Montalto, G.; Giannitrapani, L. The Role of Vitamin Deficiency in Liver Disease: To Supplement or Not Supplement? Nutrients 2021, 13, 4014. [Google Scholar] [CrossRef]
- Zhu, K.; Hunter, M.; Hui, J.; Murray, K.; James, A.; Lim, E.M.; Cooke, B.R.; Walsh, J.P. Longitudinal Stability of Vitamin D Status and Its Association with Bone Mineral Density in Middle-aged Australians. J. Endocr. Soc. 2022, 7, bvac187. [Google Scholar] [CrossRef]
- Trépo, E.; Ouziel, R.; Pradat, P.; Momozawa, Y.; Quertinmont, E.; Gervy, C.; Gustot, T.; Degré, D.; Vercruysse, V.; Deltenre, P.; et al. Marked 25-Hydroxyvitamin D Deficiency Is Associated with Poor Prognosis in Patients with Alcoholic Liver Disease. J. Hepatol. 2013, 59, 344–350. [Google Scholar] [CrossRef]
- Nishikawa, H.; Asai, A.; Fukunishi, S. The Significance of Zinc in Patients with Chronic Liver Disease. Nutrients 2022, 14, 4855. [Google Scholar] [CrossRef]
- Liu, M.; Yang, H.; Mao, Y. Magnesium and Liver Disease. Ann. Transl. Med. 2019, 7, 578. [Google Scholar] [CrossRef]
- Mehkari, Z.; Mohammed, L.; Javed, M.; Althwanay, A.; Ahsan, F.; Oliveri, F.; Goud, H.K.; Rutkofsky, I.H. Manganese, a Likely Cause of ‘Parkinson’s in Cirrhosis’, a Unique Clinical Entity of Acquired Hepatocerebral Degeneration. Cureus 2020, 12, e10448. [Google Scholar] [CrossRef]
- Kamran, U.; Towey, J.; Khanna, A.; Chauhan, A.; Rajoriya, N.; Holt, A. Nutrition in Alcohol-Related Liver Disease: Physiopathology and Management. World J. Gastroenterol. 2020, 26, 2916–2930. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; He, F.; Lian, S.; Xie, B.; Liu, T.; He, J.; Liu, C. Selenium Status in Patients with Chronic Liver Disease: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 952. [Google Scholar] [CrossRef] [PubMed]
- Ii, E.R.W.; Satapathy, S.K. Sarcopenia in the Cirrhotic Patient: Current Knowledge and Future Directions. J. Clin. Exp. Hepatol. 2023, 13, 162–177. [Google Scholar] [CrossRef]
- Qiu, J.; Tsien, C.; Thapalaya, S.; Narayanan, A.; Weihl, C.C.; Ching, J.K.; Eghtesad, B.; Singh, K.; Fu, X.; Dubyak, G.; et al. Hyperammonemia-Mediated Autophagy in Skeletal Muscle Contributes to Sarcopenia of Cirrhosis. Am. J. Physiol. Metab. 2012, 303, E983–E993. [Google Scholar] [CrossRef] [Green Version]
- Vasques, J.; Guerreiro, C.S.; Sousa, J.; Pinto, M.; Cortez-Pinto, H. Nutritional Support in Cirrhotic Patients with Sarcopenia. Clin. Nutr. ESPEN 2019, 33, 12–17. [Google Scholar] [CrossRef]
- Ferreira, S.; Marroni, C.A.; Stein, J.T.; Rayn, R.; Henz, A.C.; Schmidt, N.P.; Carteri, R.B.; Fernandes, S.A. Assessment of Resting Energy Expenditure in Patients with Cirrhosis. World J. Hepatol. 2022, 14, 802–811. [Google Scholar] [CrossRef]
- Anand, A.C. Nutrition and Muscle in Cirrhosis. J. Clin. Exp. Hepatol. 2017, 7, 340–357. [Google Scholar] [CrossRef]
- Chapman, B.; Sinclair, M.; Gow, P.J.; Testro, A.G. Malnutrition in Cirrhosis: More Food for Thought. World J. Hepatol. 2020, 12, 883–896. [Google Scholar] [CrossRef]
- Dolz, C.; Raurich, J.M.; Ibanez, J.; Obrador, A.; Marse, P.; Gaya, J. Ascites Increases the Resting Energy Expenditure in Liver Cirrhosis. Gastroenterology 1991, 100, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Traub, J.; Reiss, L.; Aliwa, B.; Stadlbauer, V. Malnutrition in Patients with Liver Cirrhosis. Nutrients 2021, 13, 540. [Google Scholar] [CrossRef] [PubMed]
- Augustyn, M.; Grys, I.; Kukla, M. Small Intestinal Bacterial Overgrowth and Nonalcoholic Fatty Liver Disease. Clin. Exp. Hepatol. 2019, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gudan, A.; Jamioł-Milc, D.; Hawryłkowicz, V.; Skonieczna-Żydecka, K.; Stachowska, E. The Prevalence of Small Intestinal Bacterial Overgrowth in Patients with Non-Alcoholic Liver Diseases: NAFLD, NASH, Fibrosis, Cirrhosis—A Systematic Review, Meta-Analysis and Meta-Regression. Nutrients 2022, 14, 5261. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Fukunishi, S.; Asai, A.; Nishiguchi, S.; Higuchi, K. Sarcopenia and Frailty in Liver Cirrhosis. Life 2021, 11, 399. [Google Scholar] [CrossRef]
- Nutritional Status in Cirrhosis. Italian Multicentre Cooperative Project on Nutrition in Liver Cirrhosis. J. Hepatol. 1994, 21, 317–325. [Google Scholar]
- Dhaliwal, A.; Armstrong, M.J. Sarcopenia in Cirrhosis: A Practical Overview. Clin. Med. 2020, 20, 489–492. [Google Scholar] [CrossRef]
- Lockwood, A.H.; McDonald, J.M.; Reiman, R.E.; Gelbard, A.S.; Laughlin, J.S.; Duffy, T.E.; Plum, F. The Dynamics of Ammonia Metabolism in Man. Effects of Liver Disease and Hyperammonemia. J. Clin. Investig. 1979, 63, 449–460. [Google Scholar] [CrossRef] [Green Version]
- Henin, G.; Lanthier, N.; Dahlqvist, G. Pathophysiological Changes of the Liver-Muscle Axis in End-Stage Liver Disease: What Is the Right Target? Acta Gastroenterol. Belg. 2022, 85, 611–624. [Google Scholar] [CrossRef]
- Guañabens, N.; Parés, A. Osteoporosis in Chronic Liver Disease. Liver Int. 2018, 38, 776–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis. Report of a WHO Study Group. World Health Organ. Tech. Rep. Ser. 1994, 843, 1–129. [Google Scholar]
- Jadzic, J.; Djonic, D. Bone Loss in Chronic Liver Diseases: Could Healthy Liver Be a Requirement for Good Bone Health? World J. Gastroenterol. 2023, 29, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Menon, K.; Angulo, P.; Weston, S.; Dickson, E.; Lindor, K.D. Bone Disease in Primary Biliary Cirrhosis: Independent Indicators and Rate of Progression. J. Hepatol. 2001, 35, 316–323. [Google Scholar] [CrossRef]
- Ionele, C.M.; Turcu-Stiolica, A.; Subtirelu, M.S.; Ungureanu, B.S.; Sas, T.N.; Rogoveanu, I. Osteoporosis Assessment among Adults with Liver Cirrhosis. J. Clin. Med. 2023, 12, 153. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Plauth, M. ESPEN Practical Guideline: Clinical Nutrition in Liver Disease. Clin. Nutr. 2020, 39, 3533–3562. [Google Scholar] [CrossRef]
- Morgan, M.Y.; Madden, A.M.; Soulsby, C.T.; Morris, R.W. Derivation and Validation of a New Global Method for Assessing Nutritional Status in Patients with Cirrhosis. Hepatology 2006, 44, 823–835. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, Y.; Feng, Y.; Wang, R.; Yao, N.; Zhang, M.; Liu, X.; Liu, H.; Shi, L.; Zhu, L.; et al. Royal Free Hospital-Nutritional Prioritizing Tool Improves the Prediction of Malnutrition Risk Outcomes in Liver Cirrhosis Patients Compared with Nutritional Risk Screening 2002. Br. J. Nutr. 2020, 124, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Borhofen, S.M.; Gerner, C.; Lehmann, J.; Fimmers, R.; Görtzen, J.; Hey, B.; Geiser, F.; Strassburg, C.P.; Trebicka, J. The Royal Free Hospital-Nutritional Prioritizing Tool Is an Independent Predictor of Deterioration of Liver Function and Survival in Cirrhosis. Dig. Dis. Sci. 2016, 61, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Booi, A.N.; Menendez, J.; Norton, H.J.; Anderson, W.E.; Ellis, A.C. Validation of a Screening Tool to Identify Undernutrition in Ambulatory Patients with Liver Cirrhosis. Nutr. Clin. Pract. 2015, 30, 683–689. [Google Scholar] [CrossRef]
- White, J.V.; Guenter, P.; Jensen, G.; Malone, A.; Schofield, M. Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: Characteristics Recommended for the Identification and Documentation of Adult Malnutrition (Undernutrition). JPEN J. Parenter. Enter. Nutr. 2012, 36, 275–283. [Google Scholar] [CrossRef] [Green Version]
- Deza, D.C.; Msc, M.E.B.G.; Sanz-París, A.; Msc, M.L.B.; Bonilla, E.M.F.; Monterde, V.B.; Mainar, J.M.A.; Olmo, J.F. Mini Nutritional Assessment—Short Form Is a Useful Malnutrition Screening Tool in Patients with Liver Cirrhosis, Using the Global Leadership Initiative for Malnutrition Criteria as the Gold Standard. Nutr. Clin. Pract. 2021, 36, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Harimoto, N.; Yoshizumi, T.; Inokuchi, S.; Itoh, S.; Adachi, E.; Ikeda, Y.; Uchiyama, H.; Utsunomiya, T.; Kajiyama, K.; Kimura, K.; et al. Prognostic Significance of Preoperative Controlling Nutritional Status (CONUT) Score in Patients Undergoing Hepatic Resection for Hepatocellular Carcinoma: A Multi-Institutional Study. Ann. Surg. Oncol. 2018, 25, 3316–3323. [Google Scholar] [CrossRef] [PubMed]
- Serón-Arbeloa, C.; Labarta-Monzón, L.; Puzo-Foncillas, J.; Mallor-Bonet, T.; Lafita-López, A.; Bueno-Vidales, N.; Montoro-Huguet, M. Malnutrition Screening and Assessment. Nutrients 2022, 14, 2392. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.; et al. GLIM Criteria for the Diagnosis of Malnutrition—A Consensus Report from the Global Clinical Nutrition Community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmese, F.; Bolondi, I.; Giannone, F.A.; Zaccherini, G.; Tufoni, M.; Baldassarre, M.; Caraceni, P. The Analysis of Food Intake in Patients with Cirrhosis Waiting for Liver Transplantation: A Neglected Step in the Nutritional Assessment. Nutrients 2019, 11, 2462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega, R.M.; Perez-Rodrigo, C.; Lopez-Sobaler, A.M. Dietary Assessment Methods: Dietary Records. Nutr. Hosp. 2015, 31 (Suppl. S3), 38–45. [Google Scholar] [CrossRef]
- Gabrielson, D.K.; Scaffidi, D.; Leung, E.; Stoyanoff, L.; Robinson, J.; Nisenbaum, R.; Brezden-Masley, C.; Darling, P.B. Use of an Abridged Scored Patient-Generated Subjective Global Assessment (abPG-SGA) as a Nutritional Screening Tool for Cancer Patients in an Outpatient Setting. Nutr. Cancer 2013, 65, 234–239. [Google Scholar] [CrossRef]
- Tandon, P.; Ney, M.; Irwin, I.; Ma, M.M.; Gramlich, L.; Bain, V.G.; Esfandiari, N.; Baracos, V.; Montano-Loza, A.J.; Myers, R.P. Severe Muscle Depletion in Patients on the Liver Transplant Wait List: Its Prevalence and Independent Prognostic Value. Liver Transplant. 2012, 18, 1209–1216. [Google Scholar] [CrossRef]
- Kouassi, K.; Bagny, A.; Kaaga, L.; Bouglouga, O.; Anani-Soh, L.L.; Lamboni, C.; Redah, D. Prevalence of Protein-Energy Undernutrition Evaluated by the Measurement of Triceps Skinfold Thickness and Mid-Arm Muscle Circumference of 103 Adults with Cirrhosis of the Liver Hospitalized in the Department of Hepatology and Gastroenterology of the Lomé Campus University Hospital (Togo). Méd. Santé Trop. 2014, 24, 208–213. [Google Scholar] [CrossRef]
- Topan, M.-M.; Sporea, I.; Dănilă, M.; Popescu, A.; Ghiuchici, A.-M.; Lupușoru, R.; Șirli, R. Comparison of Different Nutritional Assessment Tools in Detecting Malnutrition and Sarcopenia among Cirrhotic Patients. Diagnostics 2022, 12, 893. [Google Scholar] [CrossRef]
- Bunchorntavakul, C.; Reddy, K.R. Review Article: Malnutrition/Sarcopenia and Frailty in Patients with Cirrhosis. Aliment. Pharmacol. Ther. 2020, 51, 64–77. [Google Scholar] [CrossRef] [Green Version]
- Carey, E.J.; Lai, J.C.; Wang, C.W.; Dasarathy, S.; Lobach, I.; Montano-Loza, A.J.; Dunn, M.A. A Multicenter Study to Define Sarcopenia in Patients with End-Stage Liver Disease. Liver Transplant. 2017, 23, 625–633. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, M.; Chapman, B.; Hoermann, R.; Angus, P.W.; Testro, A.; Scodellaro, T.; Gow, P.J. Handgrip Strength Adds More Prognostic Value to the Model for End-Stage Liver Disease Score Than Imaging-Based Measures of Muscle Mass in Men with Cirrhosis. Liver Transplant. 2019, 25, 1480–1487. [Google Scholar] [CrossRef]
- Mori, N.; Maeda, K.; Fujimoto, Y.; Nonogaki, T.; Ishida, Y.; Ohta, R.; Shimizu, A.; Ueshima, J.; Nagano, A.; Fukushima, R. Prognostic Implications of the Global Leadership Initiative on Malnutrition Criteria as a Routine Assessment Modality for Malnutrition in Hospitalized Patients at a University Hospital. Clin. Nutr. 2023, 42, 166–172. [Google Scholar] [CrossRef]
- Santos, B.C.; Fonseca, A.L.F.; Ferreira, L.G.; Ribeiro, H.S.; Correia, M.I.T.D.; Lima, A.S.; e Penna, F.G.C.; Anastácio, L.R. Different Combinations of the GLIM Criteria for Patients Awaiting a Liver Transplant: Poor Performance for Malnutrition Diagnosis but a Potentially Useful Prognostic Tool. Clin. Nutr. 2022, 41, 97–104. [Google Scholar] [CrossRef]
- Prijatmoko, D.; Strauss, B.J.; Lambert, J.R.; Sievert, W.; Stroud, D.B.; Wahlqvist, M.L.; Katz, B.; Colman, J.; Jones, P.; Korman, M.G. Early Detection of Protein Depletion in Alcoholic Cirrhosis: Role of Body Composition Analysis. Gastroenterology 1993, 105, 1839–1845. [Google Scholar] [CrossRef]
- Nielsen, K.; Kondrup, J.; Martinsen, L.; Døssing, H.; Larsson, B.; Stilling, B.; Jensen, M.G. Long-Term Oral Refeeding of Patients with Cirrhosis of the Liver. Br. J. Nutr. 1995, 74, 557–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwasa, M.; Iwata, K.; Hara, N.; Hattori, A.; Ishidome, M.; Sekoguchi-Fujikawa, N.; Mifuji-Moroka, R.; Sugimoto, R.; Fujita, N.; Kobayashi, Y.; et al. Nutrition Therapy Using a Multidisciplinary Team Improves Survival Rates in Patients with Liver Cirrhosis. Nutrition 2013, 29, 1418–1421. [Google Scholar] [CrossRef] [PubMed]
- Tsien, C.D.; McCullough, A.J.; Dasarathy, S. Late Evening Snack: Exploiting a Period of Anabolic Opportunity in Cirrhosis. J. Gastroenterol. Hepatol. 2012, 27, 430–441. [Google Scholar] [CrossRef] [PubMed]
- de Venne, W.P.V.-V.; Westerterp, K.R.; van Hoek, B.; Swart, G.R. Energy Expenditure and Substrate Metabolism in Patients with Cirrhosis of the Liver: Effects of the Pattern of Food Intake. Gut 1995, 36, 110–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zillikens, M.; Berg, J.v.D.; Wattimena, J.; Rietveld, T.; Swart, G. Nocturnal Oral Glucose Supplementation: The Effects on Protein Metabolism in Cirrhotic Patients and in Healthy Controls. J. Hepatol. 1993, 17, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Hipskind, P.; Glass, C.; Charlton, D.; Nowak, D.; Dasarathy, S. Do Handheld Calorimeters Have a Role in Assessment of Nutrition Needs in Hospitalized Patients? Nutr. Clin. Pract. 2011, 26, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Kearns, P.J.; Young, H.; Garcia, G.; Blaschke, T.; O’Hanlon, G.; Rinki, M.; Sucher, K.; Gregory, P. Accelerated Improvement of Alcoholic Liver Disease with Enteral Nutrition. Gastroenterology 1992, 102, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Kondrup, J.; Müller, M.J. Energy and Protein Requirements of Patients with Chronic Liver Disease. J. Hepatol. 1997, 27, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Román, E.; Torrades, M.T.; Nadal, M.J.; Cárdenas, G.; Nieto, J.C.; Vidal, S.; Bascuñana, H.; Juárez, C.; Guarner, C.; Córdoba, J.; et al. Randomized Pilot Study: Effects of an Exercise Programme and Leucine Supplementation in Patients with Cirrhosis. Dig. Dis. Sci. 2014, 59, 1966–1975. [Google Scholar] [CrossRef]
- Román, E.; García-Galcerán, C.; Torrades, T.; Herrera, S.; Marín, A.; Doñate, M.; Alvarado-Tapias, E.; Malouf, J.; Nácher, L.; Serra-Grima, R.; et al. Effects of an Exercise Programme on Functional Capacity, Body Composition and Risk of Falls in Patients with Cirrhosis: A Randomized Clinical Trial. PLoS ONE 2016, 11, e0151652. [Google Scholar] [CrossRef]
- Zenith, L.; Meena, N.; Ramadi, A.; Yavari, M.; Harvey, A.; Carbonneau, M.; Ma, M.; Abraldes, J.G.; Paterson, I.; Haykowsky, M.J.; et al. Eight Weeks of Exercise Training Increases Aerobic Capacity and Muscle Mass and Reduces Fatigue in Patients with Cirrhosis. Clin. Gastroenterol. Hepatol. 2014, 12, 1920–1926.e2. [Google Scholar] [CrossRef]
- Kruger, C.; McNeely, M.L.; Bailey, R.J.; Yavari, M.; Abraldes, J.G.; Carbonneau, M.; Newnham, K.; DenHeyer, V.; Ma, M.; Thompson, R.; et al. Home Exercise Training Improves Exercise Capacity in Cirrhosis Patients: Role of Exercise Adherence. Sci. Rep. 2018, 8, 99. [Google Scholar] [CrossRef] [Green Version]
- Berzigotti, A.; Albillos, A.; Villanueva, C.; Genescá, J.; Ardevol, A.; Augustín, S.; Calleja, J.L.; Bañares, R.; García-Pagán, J.C.; Mesonero, F.; et al. Effects of an Intensive Lifestyle Intervention Program on Portal Hypertension in Patients with Cirrhosis and Obesity: The SportDiet Study. Hepatology 2017, 65, 1293–1305. [Google Scholar] [CrossRef]
- Macías-Rodríguez, R.U.; Ilarraza-Lomelí, H.; Ruiz-Margáin, A.; Ponce-De-León-Rosales, S.; Vargas-Vorácková, F.; García-Flores, O.; Torre, A.; Duarte-Rojo, A. Changes in Hepatic Venous Pressure Gradient Induced by Physical Exercise in Cirrhosis: Results of a Pilot Randomized Open Clinical Trial. Clin. Transl. Gastroenterol. 2016, 7, e180. [Google Scholar] [CrossRef]
- Aamann, L.; Dam, G.; Borre, M.; Drljevic-Nielsen, A.; Overgaard, K.; Andersen, H.; Vilstrup, H.; Aagaard, N.K. Resistance Training Increases Muscle Strength and Muscle Size in Patients with Liver Cirrhosis. Clin. Gastroenterol. Hepatol. 2020, 18, 1179–1187.e6. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.W.; Ferrando, A.; White, M.G.; Dennis, R.A.; Xie, J.; Pauly, M.; Park, S.; Bartter, T.; Dunn, M.A.; Ruiz-Margain, A.; et al. Home-Based Physical Activity and Diet Intervention to Improve Physical Function in Advanced Liver Disease: A Randomized Pilot Trial. Dig. Dis. Sci. 2020, 65, 3350–3359. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.C.; Dodge, J.L.; Kappus, M.R.; Wong, R.; Mohamad, Y.; Segev, D.L.; McAdams-DeMarco, M. A Multicenter Pilot Randomized Clinical Trial of a Home-Based Exercise Program for Patients with Cirrhosis: The Strength Training Intervention (STRIVE). Am. J. Gastroenterol. 2021, 116, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Bandi, J.-C.; García-Pagán, J.C.; Escorsell, A.; François, E.; Moitinho, E.; Rodés, J.; Bosch, J. Effects of Propranolol on the Hepatic Hemodynamic Response to Physical Exercise in Patients with Cirrhosis. Hepatology 1998, 28, 677–682. [Google Scholar] [CrossRef]
- Lin, F.; Ferrando, A.A.; Dennis, R.A.; Dunn, M.A.; Kim, W.R.; Duarte-Rojo, A. Exercise-Induced Hyperammonemia Does Not Precipitate Overt Hepatic Encephalopathy. Hepatology 2020, 72, 778–780. [Google Scholar] [CrossRef]
- Tandon, P.; Ismond, K.P.; Riess, K.; Duarte-Rojo, A.; Al-Judaibi, B.; Dunn, M.A.; Holman, J.; Howes, N.; Haykowsky, M.J.F.; Josbeno, D.A.; et al. Exercise in Cirrhosis: Translating Evidence and Experience to Practice. J. Hepatol. 2018, 69, 1164–1177. [Google Scholar] [CrossRef] [Green Version]
- Lai, J.C.; Sonnenday, C.J.; Tapper, E.B.; Duarte-Rojo, A.; Dunn, M.A.; Bernal, W.; Carey, E.J.; Dasarathy, S.; Kamath, B.M.; Kappus, M.R.; et al. Frailty in Liver Transplantation: An Expert Opinion Statement from the American Society of Transplantation Liver and Intestinal Community of Practice. Am. J. Transplant. 2019, 19, 1896–1906. [Google Scholar] [CrossRef]
- Berzigotti, A.; Garcia-Tsao, G.; Bosch, J.; Grace, N.D.; Burroughs, A.K.; Morillas, R.; Escorsell, A.; Garcia-Pagan, J.C.; Patch, D.; Matloff, D.S.; et al. Obesity Is an Independent Risk Factor for Clinical Decompensation in Patients with Cirrhosis. Hepatology 2011, 54, 555–561. [Google Scholar] [CrossRef] [Green Version]
- Everhart, J.E.; Lok, A.S.; Kim, H.; Morgan, T.R.; Lindsay, K.L.; Chung, R.T.; Bonkovsky, H.L.; Ghany, M.G. Weight-Related Effects on Disease Progression in the Hepatitis C Antiviral Long-Term Treatment Against Cirrhosis Trial. Gastroenterology 2009, 137, 549–557. [Google Scholar] [CrossRef] [Green Version]
- Cabre, E.; Gonzalez-Huix, F.; Abad-Lacruz, A.; Esteve, M.; Acero, D.; Fernandez-Bañares, F.; Xiol, X.; Gassull, M. Effect of Total Enteral Nutrition on the Short-Term Outcome of Severely Malnourished Cirrhotics. Gastroenterology 1990, 98, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Borderie, C.; Ripault, M.-P.; Silvain, C.; De Ledinghen, V.; Beau, P.; Mannant, P.-R.; Beauchant, M. Early Feeding or Enteral Nutrition in Patients with Cirrhosis after Bleeding from Esophageal Varices? (A Randomized Controlled Study). Dig. Dis. Sci. 1997, 42, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Calvey, H.; Davis, M.; Williams, R. Prospective Study of Nasogastric Feeding via East Grinstead® or Viomedex® Tubes Compared with Oral Dietary Supplementation in Patients with Cirrhosis. Clin. Nutr. 1984, 3, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Baltz, J.G.; Argo, C.K.; Al-Osaimi, A.M.; Northup, P.G. Mortality after Percutaneous Endoscopic Gastrostomy in Patients with Cirrhosis: A Case Series. Gastrointest. Endosc. 2010, 72, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, M.; Gkolfakis, P.; Despott, E.J.; Ballarin, A.; Beyna, T.; Boeykens, K.; Elbe, P.; Gisbertz, I.; Hoyois, A.; Mosteanu, O.; et al. Endoscopic management of enteral tubes in adult patients—Part 1: Definitions and indications. European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2021, 53, 81–92. [Google Scholar] [CrossRef]
- Plauth, M.; Cabré, E.; Campillo, B.; Kondrup, J.; Marchesini, G.; Schütz, T.; Shenkin, A.; Wendon, J. ESPEN Guidelines on Parenteral Nutrition: Hepatology. Clin. Nutr. 2009, 28, 436–444. [Google Scholar] [CrossRef]
- Vilstrup, H.; Gluud, C.; Hardt, F.; Kristensen, M.; Køhler, O.; Melgaard, B.; Dejgaard, A.; Hansen, B.A.; Krintel, J.J.; Schütten, H.J.; et al. Branched Chain Enriched Amino Acid versus Glucose Treatment of Hepatic Encephalopathy: A Double-Blind Study of 65 Patients with Cirrhosis. J. Hepatol. 1990, 10, 291–296. [Google Scholar] [CrossRef]
- Wahren, J.; Denis, J.; Desurmont, P.; Eriksson, L.S.; Escoffier, J.-M.; Gauthier, A.P.; Hagenfeldt, L.; Michel, H.; Opolon, P.; Paris, J.-C.; et al. Is Intravenous Administration of Branched Chain Amino Acids Effective in the Treatment of Hepatic Encephalopathy? A Multicenter Study. Hepatology 1983, 3, 475–780. [Google Scholar] [CrossRef]
- Als-Nielsen, B.; Koretz, R.L.; Gluud, L.L.; Gluud, C. Branched-Chain Amino Acids for Hepatic Encephalopathy. Cochrane Database Syst. Rev. 2003, 2, CD001939. [Google Scholar] [CrossRef]
- Naylor, C.; O’Rourke, K.; Detsky, A.S.; Baker, J.P. Parenteral Nutrition with Branched-Chain Amino Acids in Hepatic Encephalopathy. Gastroenterology 1989, 97, 1033–1042. [Google Scholar] [CrossRef]
- Córdoba, J.; López-Hellín, J.; Planas, M.; Sabín, P.; Sanpedro, F.; Castro, F.; Esteban, R.; Guardia, J. Normal Protein Diet for Episodic Hepatic Encephalopathy: Results of a Randomized Study. J. Hepatol. 2004, 41, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.C.; Dodge, J.L.; Kappus, M.R.; Dunn, M.A.; Volk, M.L.; Duarte-Rojo, A.; Ganger, D.R.; Rahimi, R.S.; McCulloch, C.E.; Haugen, C.E.; et al. Changes in Frailty Are Associated with Waitlist Mortality in Patients with Cirrhosis. J. Hepatol. 2020, 73, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Merli, M.; Iebba, V.; Giusto, M. What Is New about Diet in Hepatic Encephalopathy. Metab. Brain Dis. 2016, 31, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Maharshi, S.; Sharma, B.C.; Sachdeva, S.; Srivastava, S.; Sharma, P. Efficacy of Nutritional Therapy for Patients with Cirrhosis and Minimal Hepatic Encephalopathy in a Randomized Trial. Clin. Gastroenterol. Hepatol. 2016, 14, 454–460.e3. [Google Scholar] [CrossRef] [Green Version]
- Moctezuma-Velázquez, C.; Low, G.; Mourtzakis, M.; Ma, M.; Burak, K.W.; Tandon, P.; Montano-Loza, A.J. Association between Low Testosterone Levels and Sarcopenia in Cirrhosis: A Cross-Sectional Study. Ann. Hepatol. 2018, 17, 615–623. [Google Scholar] [CrossRef]
- Sinclair, M.; Grossmann, M.; Hoermann, R.; Angus, P.W.; Gow, P.J. Testosterone Therapy Increases Muscle Mass in Men with Cirrhosis and Low Testosterone: A Randomised Controlled Trial. J. Hepatol. 2016, 65, 906–913. [Google Scholar] [CrossRef]
- Marchesini, G.; Bianchi, G.; Merli, M.; Amodio, P.; Panella, C.; Loguercio, C.; Fanelli, F.R.; Abbiati, R. Nutritional Supplementation with Branched-Chain Amino Acids in Advanced Cirrhosis: A Double-Blind, Randomized Trial. Gastroenterology 2003, 124, 1792–1801. [Google Scholar] [CrossRef]
- Muto, Y.; Sato, S.; Watanabe, A.; Moriwaki, H.; Suzuki, K.; Kato, A.; Kato, M.; Nakamura, T.; Higuchi, K.; Nishiguchi, S.; et al. Effects of Oral Branched-Chain Amino Acid Granules on Event-Free Survival in Patients with Liver Cirrhosis. Clin. Gastroenterol. Hepatol. 2005, 3, 705–713. [Google Scholar] [CrossRef]
- Gluud, L.L.; Dam, G.; Les, I.; Córdoba, J.; Marchesini, G.; Borre, M.; Aagaard, N.K.; Vilstrup, H. Branched-Chain Amino Acids for People with Hepatic Encephalopathy. Cochrane Database Syst Rev. 2015, 2, CD001939. [Google Scholar] [CrossRef]
- Ericksen, R.E.; Lim, S.L.; McDonnell, E.; Shuen, W.H.; Vadiveloo, M.; White, P.J.; Ding, Z.; Kwok, R.; Lee, P.; Radda, G.K.; et al. Loss of BCAA Catabolism during Carcinogenesis Enhances mTORC1 Activity and Promotes Tumor Development and Progression. Cell Metab. 2019, 29, 1151–1165.e6. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Margáin, A.; Román-Calleja, B.M.; Moreno-Guillén, P.; González-Regueiro, J.A.; Kúsulas-Delint, D.; Campos-Murguía, A.; Flores-García, N.C.; Macías-Rodríguez, R.U. Nutritional Therapy for Hepatocellular Carcinoma. World J. Gastrointest. Oncol. 2021, 13, 1440–1452. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.; Lee, S.S.; Raman, M. Prevalence and Mechanisms of Malnutrition in Patients with Advanced Liver Disease, and Nutrition Management Strategies. Clin. Gastroenterol. Hepatol. 2012, 10, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Himoto, T.; Masaki, T. Current Trends of Essential Trace Elements in Patients with Chronic Liver Diseases. Nutrients 2020, 12, 2084. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.E.; Conte, D.; Massironi, S. Diagnosis and Treatment of Nutritional Deficiencies in Alcoholic Liver Disease: Overview of Available Evidence and Open Issues. Dig. Liver Dis. 2015, 47, 819–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paternostro, R.; Wagner, D.; Reiberger, T.; Mandorfer, M.; Schwarzer, R.; Ferlitsch, M.; Trauner, M.; Peck-Radosavljevic, M.; Ferlitsch, A. Low 25-OH-Vitamin D Levels Reflect Hepatic Dysfunction and Are Associated with Mortality in Patients with Liver Cirrhosis. Wien. Klin. Wochenschr. 2017, 129, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Bjelakovic, M.; Nikolova, D.; Bjelakovic, G.; Gluud, C. Vitamin D Supplementation for Chronic Liver Diseases in Adults. Cochrane Database Syst. Rev. 2021, 8, CD011564. [Google Scholar] [CrossRef]
- Savić, Ž; Vračarić, V.; Milić, N.; Nićiforović, D.; Damjanov, D.; Pellicano, R.; Medić-Stojanoska, M.; Abenavoli, L. Vitamin D Supplementation in Patients with Alcoholic Liver Cirrhosis: A Prospective Study. Miner. Med. 2018, 109, 352–357. [Google Scholar] [CrossRef]
- Okubo, T.; Atsukawa, M.; Tsubota, A.; Ono, H.; Kawano, T.; Yoshida, Y.; Arai, T.; Hayama, K.; Itokawa, N.; Kondo, C.; et al. Effect of Vitamin D Supplementation on Skeletal Muscle Volume and Strength in Patients with Decompensated Liver Cirrhosis Undergoing Branched Chain Amino Acids Supplementation: A Prospective, Randomized, Controlled Pilot Trial. Nutrients 2021, 13, 1874. [Google Scholar] [CrossRef]
- Grover, I.; Gunjan, D.; Singh, N.; Benjamin, J.; Ramakrishnan, L.; Pandey, R.M.; Sati, H.C.; Saraya, A. Effect of Vitamin D Supplementation on Vitamin D Level and Bone Mineral Density in Patients with Cirrhosis: A Randomized Clinical Trial. Am. J. Gastroenterol. 2021, 116, 2098–2104. [Google Scholar] [CrossRef]
- Garrett-Laster, M.; Russell, R.M.; Jacques, P.F. Impairment of Taste and Olfaction in Patients with Cirrhosis: The Role of Vitamin A. Hum. Nutr. Clin. Nutr. 1984, 38, 203–214. [Google Scholar]
- Bresci, G.; Parisi, G.; Banti, S. Management of Hepatic Encephalopathy with Oral Zinc Supplementation: A Long-Term Treatment. Eur. J. Med. 1993, 2, 414–416. [Google Scholar]
- Angeli, P.; Bernardi, M.; Villanueva, C.; Francoz, C.; Mookerjee, R.P.; Trebicka, J.; Krag, A.; Laleman, W.; Gines, P. EASL Clinical Practice Guidelines for the Management of Patients with Decompensated Cirrhosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohara, M.; Ogawa, K.; Suda, G.; Kimura, M.; Maehara, O.; Shimazaki, T.; Suzuki, K.; Nakamura, A.; Umemura, M.; Izumi, T.; et al. L-Carnitine Suppresses Loss of Skeletal Muscle Mass in Patients with Liver Cirrhosis. Hepatol. Commun. 2018, 2, 910–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malaguarnera, M.; Vacante, M.; Giordano, M.; Pennisi, G.; Bella, R.; Rampello, L.; Malaguarnera, M.; Volti, G.L.; Galvano, F. Oral Acetyl-l-Carnitine Therapy Reduces Fatigue in Overt Hepatic Encephalopathy: A Randomized, Double-Blind, Placebo-Controlled Study. Am. J. Clin. Nutr. 2011, 93, 799–808. [Google Scholar] [CrossRef] [Green Version]
- Vernon, G.; Baranova, A.; Younossi, Z.M. Systematic review: The Epidemiology and Natural History of Non-Alcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis in Adults. Aliment. Pharmacol. Ther. 2011, 34, 274–285. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Blissett, D.; Blissett, R.; Henry, L.; Stepanova, M.; Younossi, Y.; Racila, A.; Hunt, S.; Beckerman, R. The Economic and Clinical Burden of Nonalcoholic Fatty Liver Disease in the United States and Europe. Hepatology 2016, 64, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S.; Lotan, R.; Shlomai, A.; Webb, M.; Harrari, G.; Buch, A.; Kaluski, D.N.; Halpern, Z.; Oren, R. Predictors for Incidence and Remission of NAFLD in the General Population during a Seven-Year Prospective Follow-Up. J. Hepatol. 2012, 56, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Lazo, M.; Solga, S.F.; Horska, A.; Bonekamp, S.; Diehl, A.M.; Brancati, F.L.; Wagenknecht, L.E.; Pi-Sunyer, F.X.; Kahn, S.E.; Clark, J.M.; et al. Effect of a 12-Month Intensive Lifestyle Intervention on Hepatic Steatosis in Adults with Type 2 Diabetes. Diabetes Care 2010, 33, 2156–2163. [Google Scholar] [CrossRef] [Green Version]
- Patel, N.S.; Doycheva, I.; Peterson, M.R.; Hooker, J.; Kisselva, T.; Schnabl, B.; Seki, E.; Sirlin, C.B.; Loomba, R. Effect of Weight Loss on Magnetic Resonance Imaging Estimation of Liver Fat and Volume in Patients with Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2015, 13, 561–568.e1. [Google Scholar] [CrossRef] [Green Version]
- Promrat, K.; Kleiner, D.E.; Niemeier, H.M.; Jackvony, E.; Kearns, M.; Wands, J.R.; Fava, J.L.; Wing, R.R. Randomized Controlled Trial Testing the Effects of Weight Loss on Nonalcoholic Steatohepatitis. Hepatology 2010, 51, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight Loss through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378.e5. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Li, S.; Li, J.; Wang, J.; Zhang, R.; Zhou, Y.; Yin, Q.; Zheng, Y.; Wang, F.; Xia, Y.; et al. Effects of Omega-3 Fatty Acid in Nonalcoholic Fatty Liver Disease: A Meta-Analysis. Gastroenterol. Res. Pract. 2016, 2016, 1459790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosqvist, F.; Iggman, D.; Kullberg, J.; Cedernaes, J.; Johansson, H.-E.; Larsson, A.; Johansson, L.; Ahlström, H.; Arner, P.; Dahlman, I.; et al. Overfeeding Polyunsaturated and Saturated Fat Causes Distinct Effects on Liver and Visceral Fat Accumulation in Humans. Diabetes 2014, 63, 2356–2368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjermo, H.; Iggman, D.; Kullberg, J.; Dahlman, I.; Johansson, L.; Persson, L.; Berglund, J.; Pulkki, K.; Basu, S.; Uusitupa, M.; et al. Effects of n-6 PUFAs Compared with SFAs on Liver Fat, Lipoproteins, and Inflammation in Abdominal Obesity: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2012, 95, 1003–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortez-Pinto, H.; Jesus, L.; Barros, H.; Lopes, C.; Moura, M.C.; Camilo, M.E. How Different is the Dietary Pattern in Non-Alcoholic Steatohepatitis Patients? Clin. Nutr. 2006, 25, 816–823. [Google Scholar] [CrossRef]
- Vitaglione, P.; Morisco, F.; Mazzone, G.; Amoruso, D.C.; Ribecco, M.T.; Romano, A.; Fogliano, V.; Caporaso, N.; D’Argenio, G. Coffee Reduces Liver Damage in a Rat Model of Steatohepatitis: The Underlying Mechanisms and the Role of Polyphenols and Melanoidins. Hepatology 2010, 52, 1652–1661. [Google Scholar] [CrossRef]
- Molloy, J.W.; Calcagno, C.J.; Williams, C.D.; Jones, F.J.; Torres, D.M.; Harrison, S.A. Association of Coffee and Caffeine Consumption with Fatty Liver Disease, Nonalcoholic Steatohepatitis, and Degree of Hepatic Fibrosis. Hepatology 2012, 55, 429–436. [Google Scholar] [CrossRef]
- Romero-Gómez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with Diet, Physical Activity and Exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Li, C.; Ji, G.; Zhang, L. The Contribution of Dietary Fructose to Non-Alcoholic Fatty Liver Disease. Front. Pharmacol. 2021, 12, 783393. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, D.; Seo, W.; Gao, B.; Yoo, S.; Song, B. Fructose Promotes Leaky Gut, Endotoxemia, and Liver Fibrosis through Ethanol-Inducible Cytochrome P450-2E1–Mediated Oxidative and Nitrative Stress. Hepatology 2021, 73, 2180–2195. [Google Scholar] [CrossRef]
- Choi, J.W.J.; Ford, E.S.; Gao, X.; Choi, H.K. Sugar-Sweetened Soft Drinks, Diet Soft Drinks, and Serum Uric Acid Level: The Third National Health and Nutrition Examination Survey. Arthritis Care Res. 2008, 59, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Fox, C.S.; Jacques, P.F.; Speliotes, E.K.; Hoffmann, U.; Smith, C.E.; Saltzman, E.; McKeown, N.M. Sugar-sweetened beverage, diet soda, and fatty liver disease in the Framingham Heart Study cohorts. J. Hepatol. 2015, 63, 462–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Gómez-gracia, E.; Ruiz-gutiérrez, V.; Fiol, M. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. Z. Gefassmedizin 2013, 10, 28. [Google Scholar]
- Różański, G.; Pheby, D.; Newton, J.L.; Murovska, M.; Zalewski, P.; Słomko, J. Effect of Different Types of Intermittent Fasting on Biochemical and Anthropometric Parameters among Patients with Metabolic-Associated Fatty Liver Disease (MAFLD)—A Systematic Review. Nutrients 2021, 14, 91. [Google Scholar] [CrossRef]
- Crabb, D.W.; Im, G.Y.; Szabo, G.; Mellinger, J.L.; Lucey, M.R. Diagnosis and Treatment of Alcohol-Associated Liver Diseases: 2019 Practice Guidance From the American Association for the Study of Liver Diseases. Hepatology 2020, 71, 306–333. [Google Scholar] [CrossRef]
- Mendenhall, C.L.; Anderson, S.; Weesner, R.E.; Goldberg, S.J.; Crolic, K.A. Protein-Calorie Malnutrition Associated with Alcoholic Hepatitis: Veterans Administration Cooperative Study Group on Alcoholic Hepatitis. Am. J. Med. 1984, 76, 211–222. [Google Scholar] [CrossRef]
- Carvalho, L.; Parise, E.R. Evaluation of Nutritional Status of Nonhospitalized Patients with Liver Cirrhosis. Arq. Gastroenterol. 2006, 43, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Álvares-Da-Silva, M.R.; da Silveira, T.R. Comparison between Handgrip Strength, Subjective Global Assessment, and Prognostic Nutritional Index in Assessing Malnutrition and Predicting Clinical Outcome in Cirrhotic Outpatients. Nutrition 2005, 21, 113–117. [Google Scholar] [CrossRef]
- Styskel, B.; Natarajan, Y.; Kanwal, F. Nutrition in Alcoholic Liver Disease. Clin. Liver Dis. 2019, 23, 99–114. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.-J.; Hylemon, P.B.; Bajaj, J.S. Gut Microbiota, Cirrhosis, and Alcohol Regulate Bile Acid Metabolism in the Gut. Dig. Dis. 2015, 33, 338–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhavsar-Burke, I.; Jansson-Knodell, C.L.; Gilmore, A.C.; Crabb, D.W. Review Article: The Role of Nutrition in Alcohol-Associated Liver Disease. Aliment. Pharmacol. Ther. 2021, 53, 1268–1276. [Google Scholar] [CrossRef]
- Fialla, A.D.; Israelsen, M.; Hamberg, O.; Krag, A.; Gluud, L.L. Nutritional Therapy in Cirrhosis or Alcoholic Hepatitis: A Systematic Review and Meta-Analysis. Liver Int. 2015, 35, 2072–2078. [Google Scholar] [CrossRef] [PubMed]
- Koretz, R.L.; Avenell, A.; Lipman, T.O. Nutritional Support for Liver Disease. Cochrane Database Syst. Rev. 2012, 2012, CD008344. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, C.; Roselle, G.A.; Gartside, P.; Moritz, T.; The Veterans Administration Cooperative Study Groups 119 and 275. Relationship of Protein Calorie Malnutrition to Alcoholic Liver Disease: A Reexamination of Data from Two Veterans Administration Cooperative Studies. Alcohol. Clin. Exp. Res. 1995, 19, 635–641. [Google Scholar] [CrossRef]
- Moreno, C.; Deltenre, P.; Senterre, C.; Louvet, A.; Gustot, T.; Bastens, B.; Hittelet, A.; Piquet, M.-A.; Laleman, W.; Orlent, H.; et al. Intensive Enteral Nutrition Is Ineffective for Patients with Severe Alcoholic Hepatitis Treated with Corticosteroids. Gastroenterology 2016, 150, 903–910.e8. [Google Scholar] [CrossRef] [Green Version]
- Antar, R.; Wong, P.; Ghali, P. A Meta-Analysis of Nutritional Supplementation for Management of Hospitalized Alcoholic Hepatitis. Can. J. Gastroenterol. 2012, 26, 463–467. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. J. Hepatol. 2016, 64, 433–485. [Google Scholar] [CrossRef]
- Stephenson, G.R.; Moretti, E.W.; El-Moalem, H.; Clavien, P.A.; Tuttle-Newhall, J.E. Malnutrition in Liver Transplant Patients. Transplantation 2001, 72, 666–670. [Google Scholar] [CrossRef]
- Montano-Loza, A.J.; Duarte-Rojo, A.; Meza-Junco, J.; Baracos, V.E.; Sawyer, M.B.; Pang, J.X.Q.; Beaumont, C.; Esfandiari, N.; Myers, R.P. Inclusion of Sarcopenia Within MELD (MELD-Sarcopenia) and the Prediction of Mortality in Patients with Cirrhosis. Clin. Transl. Gastroenterol. 2015, 6, e102. [Google Scholar] [CrossRef]
- van Vugt, J.L.A.; Alferink, L.J.M.; Buettner, S.; Gaspersz, M.P.; Bot, D.; Murad, S.D.; Feshtali, S.; van Ooijen, P.M.A.; Polak, W.G.; Porte, R.J.; et al. A model Including Sarcopenia Surpasses the MELD Score in Predicting Waiting List Mortality in Cirrhotic Liver Transplant Candidates: A Competing Risk Analysis in a National Cohort. J. Hepatol. 2018, 68, 707–714. [Google Scholar] [CrossRef] [PubMed]
- DiMartini, A.; Cruz, R.J., Jr.; Dew, M.A.; Myaskovsky, L.; Goodpaster, B.; Fox, K.; Kim, K.H.; Fontes, P. Muscle Mass Predicts Outcomes Following Liver Transplantation. Liver Transplant. 2013, 19, 1172–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merli, M.; Giusto, M.; Gentili, F.; Novelli, G.; Ferretti, G.; Riggio, O.; Corradini, S.G.; Siciliano, M.; Farcomeni, A.; Attili, A.F.; et al. Nutritional Status: Its Influence on the Outcome of Patients Undergoing Liver Transplantation. Liver Int. 2010, 30, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Van Vugt, J.L.A.; Levolger, S.; De Bruin, R.W.F.; van Rosmalen, J.; Metselaar, H.J.; Ijzermans, J.N.M. Systematic Review and Meta-Analysis of the Impact of Computed Tomography-Assessed Skeletal Muscle Mass on Outcome in Patients Awaiting or Undergoing Liver Transplantation. Am. J. Transplant. 2016, 16, 2277–2292. [Google Scholar] [CrossRef] [PubMed]
- Giusto, M.; Lattanzi, B.; Di Gregorio, V.; Giannelli, V.; Lucidi, C.; Merli, M. Changes in Nutritional Status after Liver Transplantation. World J. Gastroenterol. 2014, 20, 10682–10690. [Google Scholar] [CrossRef]
- Wiesner, R.; Edwards, E.; Freeman, R.; Harper, A.; Kim, R.; Kamath, P.; Kremers, W.; Lake, J.; Howard, T.; Merion, R.M.; et al. Model for End-Stage Liver Disease (MELD) and Allocation of Donor Livers. Gastroenterology 2003, 124, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.R.; Mannalithara, A.; Heimbach, J.K.; Kamath, P.S.; Asrani, S.K.; Biggins, S.W.; Wood, N.L.; Gentry, S.E.; Kwong, A.J. MELD 3.0: The Model for End-Stage Liver Disease Updated for the Modern Era. Gastroenterology 2021, 161, 1887–1895.e4. [Google Scholar] [CrossRef]
- Battistella, S.; D’arcangelo, F.; Grasso, M.; Zanetto, A.; Gambato, M.; Germani, G.; Senzolo, M.; Russo, F.P.; Burra, P. Liver Transplantation for Non-Alcoholic Fatty Liver Disease: Indications and Post-Transplant Management. Clin. Mol. Hepatol. 2023, 29, S286–S301. [Google Scholar] [CrossRef]
- Nair, S.; Verma, S.; Thuluvath, P.J. Obesity and Its Effect on Survival in Patients Undergoing Orthotopic Liver Transplantation in the United States. Hepatology 2002, 35, 105–109. [Google Scholar] [CrossRef]
- Martin, P.; DiMartini, A.; Feng, S.; Brown, R., Jr.; Fallon, M. Evaluation for Liver Transplantation in Adults: 2013 Practice Guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology 2014, 59, 1144–1165. [Google Scholar] [CrossRef]
- Merli, M.; Nicolini, G.; Angeloni, S.; Riggio, O. Malnutrition Is a Risk Factor in Cirrhotic Patients Undergoing Surgery. Nutrition 2002, 18, 978–986. [Google Scholar] [CrossRef]
- Parekh, J.; Corley, D.A.; Feng, S. Diabetes, Hypertension and Hyperlipidemia: Prevalence Over Time and Impact on Long-Term Survival after Liver Transplantation. Am. J. Transplant. 2012, 12, 2181–2187. [Google Scholar] [CrossRef] [PubMed]
- Anastácio, L.R.; Correia, M.I.T.D. Nutrition Therapy: Integral Part of Liver Transplant Care. World J. Gastroenterol. 2016, 22, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Le Cornu, K.A.; McKiernan, F.J.; Kapadia, S.A.; Neuberger, J.M. A Prospective Randomized Study of Preoperative Nutritional Supplementation in Patients Awaiting Elective Orthotopic Liver Transplantation1. Transplantation 2000, 69, 1364–1369. [Google Scholar] [CrossRef]
- Zhu, X.-H. Liver-Protecting Effects of Omega-3 Fish Oil Lipid Emulsion in Liver Transplantation. World J. Gastroenterol. 2012, 18, 6141–6147. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Wang, X.; Zheng, H.; Bi, J.; Tan, S.; Li, N. Peri-Operative Immunonutrition in Patients Undergoing Liver Transplantation: A Meta-Analysis of Randomized Controlled Trials. Asia Pac. J. Clin. Nutr. 2015, 24, 583–590. [Google Scholar]
- Doi, J.; Moro, A.; Fujiki, M.; Eghtesad, B.; Quintini, C.; Menon, K.V.N.; Hashimoto, K.; Sasaki, K. Nutrition Support in Liver Transplantation and Postoperative Recovery: The Effects of Vitamin D Level and Vitamin D Supplementation in Liver Transplantation. Nutrients 2020, 12, 3677. [Google Scholar] [CrossRef]
- Mazurak, V.C.; Tandon, P.; Montano-Loza, A.J. Nutrition and the Transplant Candidate. Liver Transplant. 2017, 23, 1451–1464. [Google Scholar] [CrossRef] [Green Version]
- Pietrangelo, A. Hereditary Hemochromatosis—A New Look at an Old Disease. N. Engl. J. Med. 2004, 350, 2383–2397. [Google Scholar] [CrossRef] [Green Version]
- Adams, P.C. Review article: The Modern Diagnosis and Management of Haemochromatosis. Aliment. Pharmacol. Ther. 2006, 23, 1681–1691. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on Haemochromatosis. J. Hepatol. 2022, 77, 479–502. [Google Scholar] [CrossRef] [PubMed]
- Bacon, B.R.; Adams, P.C.; Kowdley, K.V.; Powell, L.W.; Tavill, A.S.; American Association for the Study of Liver Diseases. Diagnosis and management of hemochromatosis: 2011 Practice Guideline by the American Association for the Study of Liver Diseases. Hepatology 2011, 54, 328–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ala, A.; Walker, A.P.; Ashkan, K.; Dooley, J.S.; Schilsky, M.L. Wilson’s Disease. Lancet 2007, 369, 397–408. [Google Scholar] [CrossRef]
- Medici, V.; Trevisan, C.P.; D’Incà, R.; Barollo, M.; Zancan, L.; Fagiuoli, S.; Martines, D.; Irato, P.; Sturniolo, G.C. Diagnosis and Management of Wilson’s Disease. J. Clin. Gastroenterol. 2006, 40, 936–941. [Google Scholar] [CrossRef]
- Weiss, K.H.; Gotthardt, D.N.; Klemm, D.; Merle, U.; Ferenci–Foerster, D.; Schaefer, M.; Ferenci, P.; Stremmel, W. Zinc Monotherapy Is Not as Effective as Chelating Agents in Treatment of Wilson Disease. Gastroenterology 2011, 140, 1189–1198.e1. [Google Scholar] [CrossRef] [PubMed]
- Członkowska, A.; Tarnacka, B.; Litwin, T.; Gajda, J.; Rodo, M. Wilson’s Disease-Cause of Mortality in 164 Patients during 1992-2003 Observation Period. J. Neurol. 2005, 252, 698–703. [Google Scholar] [CrossRef]
- Orlický, J.; Ruscák, M. Some Properties of Mitochondrial and Cell Sap Alanine Aminotransferase of the Rat Heart. Physiol. Bohemoslov. 1976, 25, 223–230. [Google Scholar]
- Yamada, R. Antivitamin B6 Activity of L-Penicillamine in Escherichia coli. Acta Vitaminol. Enzym. 1983, 5, 73–81. [Google Scholar]
- Roberts, E.A.; Schilsky, M.L. A Practice Guideline on Wilson Disease. Hepatology 2003, 37, 1475–1492. [Google Scholar] [CrossRef] [Green Version]
- Kowdley, K.V.; Emond, M.J.; Sadowski, J.A.; Kaplan, M.M. Plasma Vitamin K1 Level Is Decreased in Primary Biliary Cirrhosis. Am. J. Gastroenterol. 1997, 92, 2059–2061. [Google Scholar]
- Floreani, A.; Zappala, F.; Fries, W.; Naccarato, R.; Plebani, M.; D’Angelo, A.; Chiaramonte, M. A 3-Year Pilot Study with 1,25-Dihydroxyvitamin D, Calcium, and Calcitonin for Severe Osteodystrophy in Primary Biliary Cirrhosis. J. Clin. Gastroenterol. 1997, 24, 239–244. [Google Scholar] [CrossRef]
- Maslennikov, R.; Ivashkin, V.; Efremova, I.; Poluektova, E.; Shirokova, E. Probiotics in Hepatology: An update. World J. Hepatol. 2021, 13, 1154–1166. [Google Scholar] [CrossRef]
- Dhiman, R.K.; Thumburu, K.K.; Verma, N.; Chopra, M.; Rathi, S.; Dutta, U.; Singal, A.K.; Taneja, S.; Duseja, A.; Singh, M. Comparative Efficacy of Treatment Options for Minimal Hepatic Encephalopathy: A Systematic Review and Network Meta-Analysis. Clin. Gastroenterol. Hepatol. 2020, 18, 800–812.e25. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Yu, C.-B.; Yang, S.-G.; Cao, H.-C.; Chen, P.; Deng, M.; Li, L.-J. Effect of Probiotic Treatment on Cirrhotic Patients with Minimal Hepatic Encephalopathy: A Meta-Analysis. Hepatobiliary Pancreat. Dis. Int. 2018, 17, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Dalal, R.; McGee, R.G.; Riordan, S.M.; Webster, A.C. Probiotics for People with Hepatic Encephalopathy. Cochrane Database Syst. Rev. 2017, 2, CD008716. [Google Scholar] [CrossRef] [PubMed]
- Román, E.; Nieto, J.C.; Gely, C.; Vidal, S.; Pozuelo, M.; Poca, M.; Juárez, C.; Guarner, C.; Manichanh, C.; Soriano, G. Effect of a Multistrain Probiotic on Cognitive Function and Risk of Falls in Patients with Cirrhosis: A Randomized Trial. Hepatol. Commun. 2019, 3, 632–645. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Heuman, D.M.; Hylemon, P.B.; Sanyal, A.J.; Puri, P.; Sterling, R.K.; Luketic, V.; Stravitz, R.T.; Siddiqui, M.S.; Fuchs, M.; et al. Randomised Clinical Trial: Lactobacillus GG Modulates Gut Microbiome, Metabolome and Endotoxemia in Patients with Cirrhosis. Aliment. Pharmacol. Ther. 2014, 39, 1113–1125. [Google Scholar] [CrossRef] [Green Version]
- Marlicz, W.; Wunsch, E.; Mydlowska, M.; Milkiewicz, M.; Serwin, K.; Mularczyk, M.; Milkiewicz, P.; Raszeja-Wyszomirska, J. The Effect of Short Term Treatment with Probiotic VSL#3 on Various Clinical and Biochemical Parameters in Patients with Liver Cirrhosis. J. Physiol. Pharmacol. 2016, 67, 867–877. [Google Scholar]
- Rincón, D.; Vaquero, J.; Hernando, A.; Galindo, E.; Ripoll, C.; Puerto, M.; Salcedo, M.; Francés, R.; Matilla, A.; Catalina, M.V.; et al. Oral Probiotic VSL#3 Attenuates the Circulatory Disturbances of Patients with Cirrhosis and Ascites. Liver Int. 2014, 34, 1504–1512. [Google Scholar] [CrossRef]


| Nutritional Consequence [Ref.] | Mechanisms in Chronic Liver Disease |
|---|---|
| 1. Impaired dietary intake [8,15,16] | Hyporexia, early satiety, impaired gastric motility, dysgeusia, restrictive diets, and alcohol abuse |
| 2. Altered macro- and micronutrient metabolism [9,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35] | Impaired glucose tolerance with insulin resistance and β-cell dysfunction; higher protein intake secondary to hypermetabolism; increased lipolysis and lipid oxidation. Vitamin deficiencies are secondary to malabsorption and diminished reserves |
| 3. Energy metabolism disturbances [19,36,37,38] | Hypermetabolic state causes a reduced hepatic glycogen synthesis and storage and an increase in gluconeogenesis |
| 4. Increase in energy expenditure [3,39,40,41,42] | Hypermetabolism, malnutrition, chronic inflammation and immunosuppression |
| 5. Nutrient malabsorption [43,44,45,46] | Impaired bile acid metabolism, portal hypertensive enteropathy, gut microbiome dysregulation, and small intestinal bacterial overgrowth |
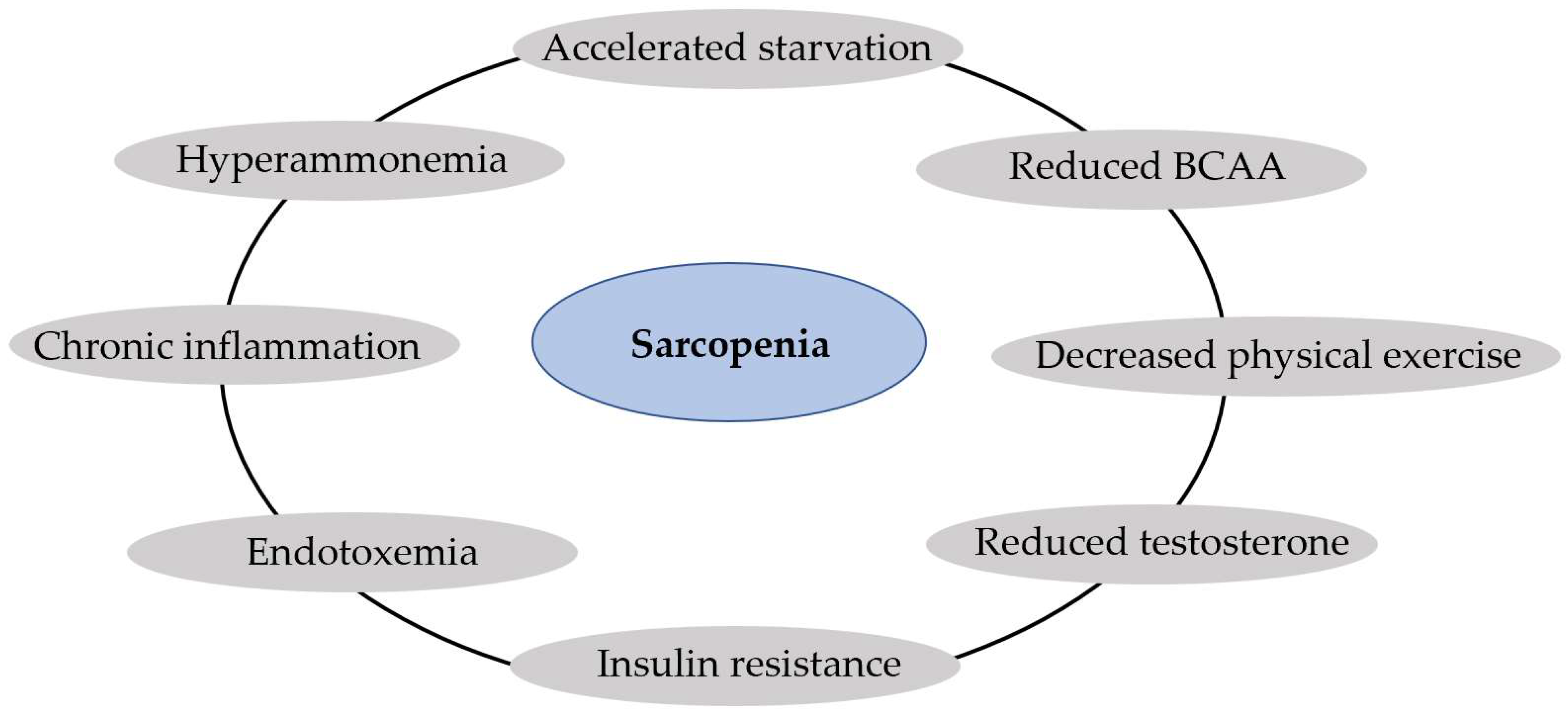
| 6. Sarcopenia and muscle function [19,38,47,48,49,50] | Accelerated starvation, hyperammonemia, endotoxemia, reduced levels of testosterone and branched-chain amino acids, decreased physical exercise |
| 7. Metabolic osteopathy [9,51,52,53,54,55] | Nutritional, hormonal, metabolic, genetic and inflammatory factors |
| Micronutrient | Status in CLD | Imbalance-Associated Liver Disease | Liver- and Muscle-Related Consequences |
|---|---|---|---|
| Fat-soluble vitamins [27,28,30] | |||
| A (retinol) | Deficiency | Cirrhosis, MAFLD | Fibrosis development, progression of MAFLD disease |
| D | Deficiency | Cirrhosis | Liver dysfunction, bone density decreased, progression of frailty, and poor prognosis |
| E | Deficiency | Alcoholic or cholestatic liver disease, cirrhosis | Increased risk of hepatocellular carcinoma |
| K | Insufficiency | Cholestatic liver disease | Liver injury, supplementation involved in hepatocellular carcinoma reduction |
| Water-soluble vitamins [1,25,26,28,34] | |||
| B1 | Deficiency | Alcoholic liver disease | Neurologic dysfunction (Wernicke encephalopathy), high-output heart failure |
| B6 | Deficiency | Cirrhosis | Inadequate antioxidant capabilities of the liver |
| B9 | Deficiency | Alcoholic liver disease | Progression of liver disease, muscle weakness |
| B12 | Increase | Cirrhosis, alcoholic liver disease | No symptoms; association with liver fibrosis and liver failure |
| C | Deficiency | MAFLD | Possible influence on the progression towards MAFLD |
| Minerals [31,32,33,35] | |||
| Zinc (Zn) | Deficiency | Cirrhosis | Hepatic encephalopathy, liver fibrosis, liver carcinogenesis, myopathy |
| Magnesium (Mg) | Deficiency | Cirrhosis | Reduced cognitive performance and reduced muscle strength |
| Manganese (Mn) | Increase | Cirrhosis | Extrapyramidal and neuropsychiatric symptoms |
| Selenium (Se) | Deficiency | Cirrhosis, chronic hepatitis | Development of hepatocellular carcinoma, increased risk of hepatic encephalopathy, muscle pain |
| Iron (Fe) | Increase | Alcoholic liver disease, cirrhosis | Liver fibrosis, increased risk of infections |
| Screening Tool [Ref] | Variables | Strengths | Limitations |
|---|---|---|---|
| NRS-2002 [56] |
| Validated in hospitalized patients | Fluid overload can decrease accuracy Low sensitivity in liver cirrhosis |
| MUST [56] |
| Validated in hospitalized and outpatients Quick and easy | Fluid overload can decrease accuracy Low sensitivity in liver cirrhosis |
| SGA [9,56] |
| Good interobserver reproducibility Good association with various clinical and prognostic variables | Underestimates the prevalence of muscle loss in liver disease patients Based on subjective variables |
| MNA-SF [62] |
| Good sensitivity and specificity to screen malnutrition in cirrhosis | Needs more validation |
| RFH-NPT [8,57,59] |
| Liver disease-specific tool Quick and easy Reduces the impact of fluid retention High sensitivity and specificity to screen malnutrition in cirrhosis Useful predictor of disease progression and outcome | Needs more validation |
| LDUST [60,61] |
| Liver disease-specific tool Reduces the impact of fluid retention | Based on subjective variables Low negative predictive value Needs more validation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espina, S.; Casas-Deza, D.; Bernal-Monterde, V.; Domper-Arnal, M.J.; García-Mateo, S.; Lué, A. Evaluation and Management of Nutritional Consequences of Chronic Liver Diseases. Nutrients 2023, 15, 3487. https://doi.org/10.3390/nu15153487
Espina S, Casas-Deza D, Bernal-Monterde V, Domper-Arnal MJ, García-Mateo S, Lué A. Evaluation and Management of Nutritional Consequences of Chronic Liver Diseases. Nutrients. 2023; 15(15):3487. https://doi.org/10.3390/nu15153487
Chicago/Turabian StyleEspina, Silvia, Diego Casas-Deza, Vanesa Bernal-Monterde, María José Domper-Arnal, Sandra García-Mateo, and Alberto Lué. 2023. "Evaluation and Management of Nutritional Consequences of Chronic Liver Diseases" Nutrients 15, no. 15: 3487. https://doi.org/10.3390/nu15153487
APA StyleEspina, S., Casas-Deza, D., Bernal-Monterde, V., Domper-Arnal, M. J., García-Mateo, S., & Lué, A. (2023). Evaluation and Management of Nutritional Consequences of Chronic Liver Diseases. Nutrients, 15(15), 3487. https://doi.org/10.3390/nu15153487






