Is It Time for a Requiem for Creatine Supplementation-Induced Kidney Failure? A Narrative Review
Abstract
1. Introduction
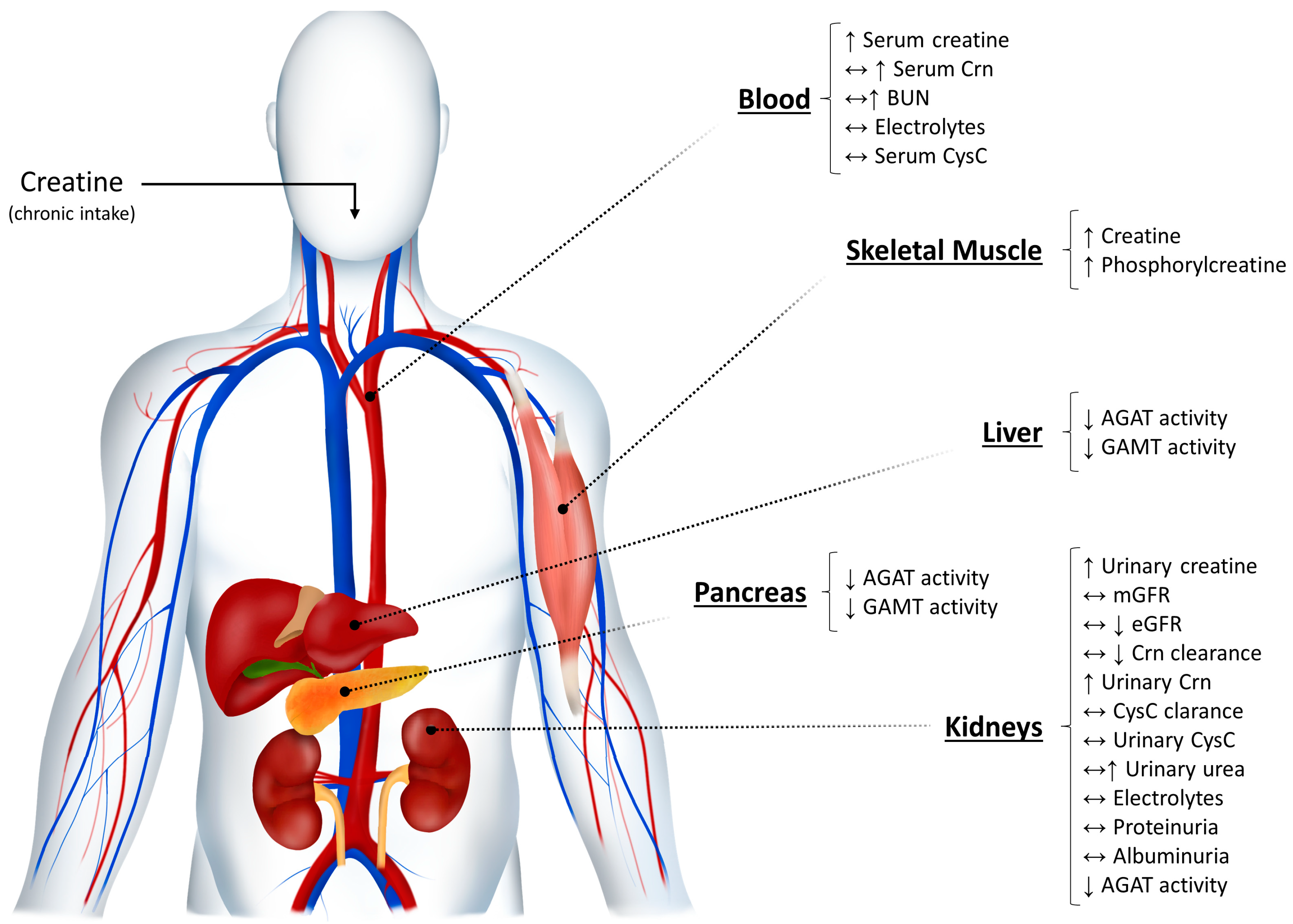
2. The Kidneys and Creatine Metabolism: A Brief Overview
3. Creatine Supplementation and Kidney Function
3.1. Evidence from Animal Models
3.2. Evidence from Case-Studies
3.3. Evidence from Controlled Studies in Humans
4. Gaps and Recommendations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gualano, B.; Roschel, H.; Lancha, A.H., Jr.; Brightbill, C.E.; Rawson, E.S. In sickness and in health: The widespread application of creatine supplementation. Amino Acids 2012, 43, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Wallimann, T.; Wyss, M.; Brdiczka, D.; Nicolay, K.; Eppenberger, H.M. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: The ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem. J. 1992, 281 Pt 1, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.C.; Soderlund, K.; Hultman, E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin. Sci. (Lond.) 1992, 83, 367–374. [Google Scholar] [CrossRef]
- Cooper, R.; Naclerio, F.; Allgrove, J.; Jimenez, A. Creatine supplementation with specific view to exercise/sports performance: An update. J. Int. Soc. Sports Nutr. 2012, 9, 33. [Google Scholar] [CrossRef]
- Alves, C.R.; Santiago, B.M.; Lima, F.R.; Otaduy, M.C.; Calich, A.L.; Tritto, A.C.; de Sa Pinto, A.L.; Roschel, H.; Leite, C.C.; Benatti, F.B.; et al. Creatine supplementation in fibromyalgia: A randomized, double-blind, placebo-controlled trial. Arthritis Care Res. (Hoboken) 2013, 65, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Neves, M., Jr.; Gualano, B.; Roschel, H.; Fuller, R.; Benatti, F.B.; Pinto, A.L.; Lima, F.R.; Pereira, R.M.; Lancha, A.H., Jr.; Bonfa, E. Beneficial effect of creatine supplementation in knee osteoarthritis. Med. Sci. Sports Exerc. 2011, 43, 1538–1543. [Google Scholar] [CrossRef]
- Gualano, B.; Painneli, V.D.S.; Roschel, H.; Artioli, G.G.; Neves, M., Jr.; De Sa Pinto, A.L.; Da Silva, M.E.; Cunha, M.R.; Otaduy, M.C.; Leite Cda, C.; et al. Creatine in type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Med. Sci. Sports Exerc. 2011, 43, 770–778. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A.; Roy, B.D.; MacDonald, J.R. A randomized, controlled trial of creatine monohydrate in patients with mitochondrial cytopathies. Muscle Nerve 1997, 20, 1502–1509. [Google Scholar] [CrossRef]
- Banerjee, B.; Sharma, U.; Balasubramanian, K.; Kalaivani, M.; Kalra, V.; Jagannathan, N.R. Effect of creatine monohydrate in improving cellular energetics and muscle strength in ambulatory Duchenne muscular dystrophy patients: A randomized, placebo-controlled 31P MRS study. Magn. Reson. Imaging 2010, 28, 698–707. [Google Scholar] [CrossRef]
- Kley, R.A.; Tarnopolsky, M.A.; Vorgerd, M. Creatine for treating muscle disorders. Cochrane Database Syst. Rev. 2013, 2013, CD004760. [Google Scholar] [CrossRef]
- Hass, C.J.; Collins, M.A.; Juncos, J.L. Resistance training with creatine monohydrate improves upper-body strength in patients with Parkinson disease: A randomized trial. Neurorehabil. Neural Repair 2007, 21, 107–115. [Google Scholar] [CrossRef]
- Fuld, J.P.; Kilduff, L.P.; Neder, J.A.; Pitsiladis, Y.; Lean, M.E.; Ward, S.A.; Cotton, M.M. Creatine supplementation during pulmonary rehabilitation in chronic obstructive pulmonary disease. Thorax 2005, 60, 531–537. [Google Scholar] [CrossRef]
- Andrews, R.; Greenhaff, P.; Curtis, S.; Perry, A.; Cowley, A.J. The effect of dietary creatine supplementation on skeletal muscle metabolism in congestive heart failure. Eur. Heart J. 1998, 19, 617–622. [Google Scholar] [CrossRef]
- Gordon, A.; Hultman, E.; Kaijser, L.; Kristjansson, S.; Rolf, C.J.; Nyquist, O.; Sylven, C. Creatine supplementation in chronic heart failure increases skeletal muscle creatine phosphate and muscle performance. Cardiovasc. Res. 1995, 30, 413–418. [Google Scholar] [CrossRef]
- Pritchard, N.R.; Kalra, P.A. Renal dysfunction accompanying oral creatine supplements. Lancet 1998, 351, 1252–1253. [Google Scholar] [CrossRef]
- Koshy, K.M.; Griswold, E.; Schneeberger, E.E. Interstitial nephritis in a patient taking creatine. N. Engl. J. Med. 1999, 340, 814–815. [Google Scholar] [PubMed]
- Robinson, S.J. Acute quadriceps compartment syndrome and rhabdomyolysis in a weight lifter using high-dose creatine supplementation. J. Am. Board Fam. Pract. 2000, 13, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Barisic, N.; Bernert, G.; Ipsiroglu, O.; Stromberger, C.; Muller, T.; Gruber, S.; Prayer, D.; Moser, E.; Bittner, R.E.; Stockler-Ipsiroglu, S. Effects of oral creatine supplementation in a patient with MELAS phenotype and associated nephropathy. Neuropediatrics 2002, 33, 157–161. [Google Scholar] [CrossRef]
- Revai, T.; Sapi, Z.; Benedek, S.; Kovacs, A.; Kaszas, I.; Viranyi, M.; Winkler, G. Severe nephrotic syndrome in a young man taking anabolic steroid and creatine long term. Orv. Hetil. 2003, 144, 2425–2427. [Google Scholar] [PubMed]
- Thorsteinsdottir, B.; Grande, J.P.; Garovic, V.D. Acute renal failure in a young weight lifter taking multiple food supplements, including creatine monohydrate. J. Ren. Nutr. 2006, 16, 341–345. [Google Scholar] [CrossRef]
- Taner, B.; Aysim, O.; Abdulkadir, U. The effects of the recommended dose of creatine monohydrate on kidney function. NDT Plus 2011, 4, 23–24. [Google Scholar] [CrossRef]
- Edmunds, J.W.; Jayapalan, S.; DiMarco, N.M.; Saboorian, M.H.; Aukema, H.M. Creatine supplementation increases renal disease progression in Han:SPRD-cy rats. Am. J. Kidney Dis. 2001, 37, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.G.; De Toledo Bergamaschi, C.; Lazaretti-Castro, M.; Heilberg, I.P. Effects of creatine supplementation on body composition and renal function in rats. Med. Sci. Sports Exerc. 2005, 37, 1525–1529. [Google Scholar] [CrossRef]
- Souza, R.A.; Miranda, H.; Xavier, M.; Lazo-Osorio, R.A.; Gouvea, H.A.; Cogo, J.C.; Vieira, R.P.; Ribeiro, W. Effects of high-dose creatine supplementation on kidney and liver responses in sedentary and exercised rats. J. Sports Sci. Med. 2009, 8, 672–681. [Google Scholar] [PubMed]
- Silverthorn, D.U. Human Physiology; Pearson Education Inc.: San Francisco, CA, USA, 2018. [Google Scholar]
- Boron, W.F.; Boulpaep, E.L. Medical Physiology; Saunders, Elsevier Inc.: Philadelphia, PA, USA, 2016. [Google Scholar]
- Ross, C.; Holohan, P.D. Transport of organic anions and cations in isolated renal plasma membranes. Annu. Rev. Pharmacol. Toxicol. 1983, 23, 65–85. [Google Scholar] [CrossRef] [PubMed]
- Weiner, I.M.; Mudge, G.H. Renal Tubular Mechanisms for Excretion of Organic Acids and Bases. Am. J. Med. 1964, 36, 743–762. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Rabelink, T. Glomerular proteinuria: A complex interplay between unique players. Adv. Chronic Kidney Dis. 2011, 18, 233–242. [Google Scholar] [CrossRef]
- Tryggvason, K.; Pettersson, E. Causes and consequences of proteinuria: The kidney filtration barrier and progressive renal failure. J. Intern. Med. 2003, 254, 216–224. [Google Scholar] [CrossRef]
- Hayslett, J.P. Functional adaptation to reduction in renal mass. Physiol. Rev. 1979, 59, 137–164. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J.; Tighiouart, H.; Greene, T.; Inker, L.A. Measured and estimated glomerular filtration rate: Current status and future directions. Nat. Rev. Nephrol. 2020, 16, 51–64. [Google Scholar] [CrossRef]
- Shlipak, M.G.; Matsushita, K.; Arnlov, J.; Inker, L.A.; Katz, R.; Polkinghorne, K.R.; Rothenbacher, D.; Sarnak, M.J.; Astor, B.C.; Coresh, J.; et al. Cystatin C versus creatinine in determining risk based on kidney function. N. Engl. J. Med. 2013, 369, 932–943. [Google Scholar] [CrossRef]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.A.; Coresh, J.; Greene, T.; Levey, A.S. Assessing kidney function--measured and estimated glomerular filtration rate. N. Engl. J. Med. 2006, 354, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef] [PubMed]
- Delanghe, J.; De Slypere, J.P.; De Buyzere, M.; Robbrecht, J.; Wieme, R.; Vermeulen, A. Normal reference values for creatine, creatinine, and carnitine are lower in vegetarians. Clin. Chem. 1989, 35, 1802–1803. [Google Scholar] [CrossRef] [PubMed]
- Derave, W.; Marescau, B.; Vanden Eede, E.; Eijnde, B.O.; De Deyn, P.P.; Hespel, P. Plasma guanidino compounds are altered by oral creatine supplementation in healthy humans. J. Appl. Physiol. (1985) 2004, 97, 852–857. [Google Scholar] [CrossRef]
- Natelson, S.; Sherwin, J.E. Proposed mechanism for urea nitrogen re-utilization: Relationship between urea and proposed guanidine cycles. Clin. Chem. 1979, 25, 1343–1344. [Google Scholar] [CrossRef] [PubMed]
- Earnest, C.P.; Almada, A.L.; Mitchell, T.L. High-performance capillary electrophoresis-pure creatine monohydrate reduces blood lipids in men and women. Clin. Sci. (Lond.) 1996, 91, 113–118. [Google Scholar] [CrossRef]
- Poortmans, J.R.; Auquier, H.; Renaut, V.; Durussel, A.; Saugy, M.; Brisson, G.R. Effect of short-term creatine supplementation on renal responses in men. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 76, 566–567. [Google Scholar] [CrossRef]
- Poortmans, J.; Francaux, M.J.T.L. Renal dysfunction accompanying oral creatine supplements. Lancet 1998, 352, 234. [Google Scholar] [CrossRef]
- Poortmans, J.R.; Francaux, M. Long-term oral creatine supplementation does not impair renal function in healthy athletes. Med. Sci. Sports Exerc. 1999, 31, 1108–1110. [Google Scholar] [CrossRef]
- Mihic, S.; MacDonald, J.R.; McKenzie, S.; Tarnopolsky, M. Acute creatine loading increases fat-free mass, but does not affect blood pressure, plasma creatinine, or CK activity in men and women. Med. Sci. Sports Exerc. 2000, 32, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.M.; Sewell, D.A.; Casey, A.; Steenge, G.; Greenhaff, P.L. Dietary creatine supplementation does not affect some haematological indices, or indices of muscle damage and hepatic and renal function. Br. J. Sports Med. 2000, 34, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, D.L.; Mayhew, J.L.; Ware, J.S. Effects of long-term creatine supplementation on liver and kidney functions in American college football players. Int. J. Sport Nutr. Exerc. Metab. 2002, 12, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Brose, A.; Parise, G.; Tarnopolsky, M.A. Creatine supplementation enhances isometric strength and body composition improvements following strength exercise training in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, 11–19. [Google Scholar] [CrossRef]
- Eijnde, B.O.; Van Leemputte, M.; Goris, M.; Labarque, V.; Taes, Y.; Verbessem, P.; Vanhees, L.; Ramaekers, M.; Vanden Eynde, B.; Van Schuylenbergh, R.; et al. Effects of creatine supplementation and exercise training on fitness in men 55–75 yr old. J. Appl. Physiol. (1985) 2003, 95, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B.; Melton, C.; Rasmussen, C.J.; Greenwood, M.; Lancaster, S.; Cantler, E.C.; Milnor, P.; Almada, A.L. Long-term creatine supplementation does not significantly affect clinical markers of health in athletes. Mol. Cell. Biochem. 2003, 244, 95–104. [Google Scholar] [CrossRef]
- Groeneveld, G.J.; Beijer, C.; Veldink, J.H.; Kalmijn, S.; Wokke, J.H.; van den Berg, L.H. Few adverse effects of long-term creatine supplementation in a placebo-controlled trial. Int. J. Sports Med. 2005, 26, 307–313. [Google Scholar] [CrossRef]
- Poortmans, J.R.; Kumps, A.; Duez, P.; Fofonka, A.; Carpentier, A.; Francaux, M. Effect of oral creatine supplementation on urinary methylamine, formaldehyde, and formate. Med. Sci. Sports Exerc. 2005, 37, 1717–1720. [Google Scholar] [CrossRef]
- Cancela, P.; Ohanian, C.; Cuitiño, E.; Hackney, A.C. Creatine supplementation does not affect clinical health markers in football players. Br. J. Sports Med. 2008, 42, 731–735. [Google Scholar] [CrossRef]
- Bender, A.; Samtleben, W.; Elstner, M.; Klopstock, T. Long-term creatine supplementation is safe in aged patients with Parkinson disease. Nutr. Res. 2008, 28, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Gualano, B.; Ugrinowitsch, C.; Novaes, R.B.; Artioli, G.G.; Shimizu, M.H.; Seguro, A.C.; Harris, R.C.; Lancha, A.H., Jr. Effects of creatine supplementation on renal function: A randomized, double-blind, placebo-controlled clinical trial. Eur. J. Appl. Physiol. 2008, 103, 33–40. [Google Scholar] [CrossRef]
- Spillane, M.; Schoch, R.; Cooke, M.; Harvey, T.; Greenwood, M.; Kreider, R.; Willoughby, D.S. The effects of creatine ethyl ester supplementation combined with heavy resistance training on body composition, muscle performance, and serum and muscle creatine levels. J. Int. Soc. Sports Nutr. 2009, 6, 6. [Google Scholar] [CrossRef]
- Gualano, B.; de Salles Painelli, V.; Roschel, H.; Lugaresi, R.; Dorea, E.; Artioli, G.G.; Lima, F.R.; da Silva, M.E.; Cunha, M.R.; Seguro, A.C.; et al. Creatine supplementation does not impair kidney function in type 2 diabetic patients: A randomized, double-blind, placebo-controlled, clinical trial. Eur. J. Appl. Physiol. 2011, 111, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Neves, M., Jr.; Gualano, B.; Roschel, H.; Lima, F.R.; Lucia de Sa-Pinto, A.; Seguro, A.C.; Shimizu, M.H.; Sapienza, M.T.; Fuller, R.; Lancha, A.H., Jr.; et al. Effect of creatine supplementation on measured glomerular filtration rate in postmenopausal women. Appl. Physiol. Nutr. Metab. 2011, 36, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.P.P.F.; Molina, G.E.; Fontana, K.E. Creatine supplementation associated with resistance training does not alter renal and hepatic functions. Rev. Bras. Med. Esporte 2011, 17, 237–241. [Google Scholar] [CrossRef]
- Lugaresi, R.; Leme, M.; de Salles Painelli, V.; Murai, I.H.; Roschel, H.; Sapienza, M.T.; Lancha Junior, A.H.; Gualano, B. Does long-term creatine supplementation impair kidney function in resistance-trained individuals consuming a high-protein diet? J. Int. Soc. Sports Nutr. 2013, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.P.; Solis, M.Y.; Sapienza, M.T.; Otaduy, M.C.; de Sa Pinto, A.L.; Silva, C.A.; Sallum, A.M.; Pereira, R.M.; Gualano, B. Efficacy and safety of creatine supplementation in childhood-onset systemic lupus erythematosus: A randomized, double-blind, placebo-controlled, crossover trial. Lupus 2014, 23, 1500–1511. [Google Scholar] [CrossRef]
- Chilibeck, P.D.; Candow, D.G.; Landeryou, T.; Kaviani, M.; Paus-Jenssen, L. Effects of Creatine and Resistance Training on Bone Health in Postmenopausal Women. Med. Sci. Sports Exerc. 2015, 47, 1587–1595. [Google Scholar] [CrossRef]
- Writing Group for the NINDS Exploratory Trials in Parkinson Disease (NET-PD) Investigators; Kieburtz, K.; Tilley, B.C.; Elm, J.J.; Babcock, D.; Hauser, R.; Ross, G.W.; Augustine, A.H.; Augustine, E.U.; Aminoff, M.J.; et al. Effect of creatine monohydrate on clinical progression in patients with Parkinson disease: A randomized clinical trial. JAMA 2015, 313, 584–593. [Google Scholar] [CrossRef]
- Lobo, D.M.; Tritto, A.C.; da Silva, L.R.; de Oliveira, P.B.; Benatti, F.B.; Roschel, H.; Niess, B.; Gualano, B.; Pereira, R.M. Effects of long-term low-dose dietary creatine supplementation in older women. Exp. Gerontol. 2015, 70, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.T.; Dorr, F.A.; Pinto, E.; Solis, M.Y.; Artioli, G.G.; Fernandes, A.L.; Murai, I.H.; Dantas, W.S.; Seguro, A.C.; Santinho, M.A.; et al. Can creatine supplementation form carcinogenic heterocyclic amines in humans? J. Physiol. 2015, 593, 3959–3971. [Google Scholar] [CrossRef] [PubMed]
- Blancquaert, L.; Baguet, A.; Bex, T.; Volkaert, A.; Everaert, I.; Delanghe, J.; Petrovic, M.; Vervaet, C.; De Henauw, S.; Constantin-Teodosiu, D.; et al. Changing to a vegetarian diet reduces the body creatine pool in omnivorous women, but appears not to affect carnitine and carnosine homeostasis: A randomised trial. Br. J. Nutr. 2018, 119, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Domingues, W.J.R.; Ritti-Dias, R.M.; Cucato, G.G.; Wolosker, N.; Zerati, A.E.; Puech-Leao, P.; Nunhes, P.M.; Moliterno, A.A.; Avelar, A. Does Creatine Supplementation Affect Renal Function in Patients with Peripheral Artery Disease? A Randomized, Double Blind, Placebo-controlled, Clinical Trial. Ann. Vasc. Surg. 2020, 63, 45–52. [Google Scholar] [CrossRef]
- Sales, L.P.; Pinto, A.J.; Rodrigues, S.F.; Alvarenga, J.C.; Goncalves, N.; Sampaio-Barros, M.M.; Benatti, F.B.; Gualano, B.; Rodrigues Pereira, R.M. Creatine Supplementation (3 g/d) and Bone Health in Older Women: A 2-Year, Randomized, Placebo-Controlled Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 931–938. [Google Scholar] [CrossRef]
- Roschel, H.; Hayashi, A.P.; Fernandes, A.L.; Jambassi-Filho, J.C.; Hevia-Larrain, V.; de Capitani, M.; Santana, D.A.; Goncalves, L.S.; de Sa-Pinto, A.L.; Lima, F.R.; et al. Supplement-based nutritional strategies to tackle frailty: A multifactorial, double-blind, randomized placebo-controlled trial. Clin. Nutr. 2021, 40, 4849–4858. [Google Scholar] [CrossRef]
- Taes, Y.E.; Delanghe, J.R.; Wuyts, B.; van de Voorde, J.; Lameire, N.H. Creatine supplementation does not affect kidney function in an animal model with pre-existing renal failure. Nephrol. Dial. Transplant. 2003, 18, 258–264. [Google Scholar] [CrossRef]
- Sewell, D.; Harris, R.C. Effects of creatine supplementation in the Thoroughbred horse. Equine Vet. J. 1995, 27, 239–242. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A.; Bourgeois, J.M.; Snow, R.; Keys, S.; Roy, B.D.; Kwiecien, J.M.; Turnbull, J. Histological assessment of intermediate- and long-term creatine monohydrate supplementation in mice and rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R762–R769. [Google Scholar] [CrossRef]
- Gualano, B.; Ferreira, D.C.; Sapienza, M.T.; Seguro, A.C.; Lancha, A.H., Jr. Effect of short-term high-dose creatine supplementation on measured GFR in a young man with a single kidney. Am. J. Kidney Dis. 2010, 55, e7–e9. [Google Scholar] [CrossRef]
- Burdmann, E.A.; Andoh, T.F.; Yu, L.; Bennett, W.M. Cyclosporine nephrotoxicity. Semin. Nephrol. 2003, 23, 465–476. [Google Scholar] [CrossRef]
- Poortmans, J.R.; Vanderstraeten, J. Kidney function during exercise in healthy and diseased humans. An update. Sports Med. 1994, 18, 419–437. [Google Scholar] [CrossRef] [PubMed]
- Parente Filho, S.L.A.; Gomes, P.; Forte, G.A.; Lima, L.L.L.; Silva Junior, G.B.D.; Meneses, G.C.; Martins, A.M.C.; Daher, E.F. Kidney disease associated with androgenic-anabolic steroids and vitamin supplements abuse: Be aware! Nefrologia 2020, 40, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Tucker, J.; Fischer, T.; Upjohn, L.; Mazzera, D.; Kumar, M. Unapproved Pharmaceutical Ingredients Included in Dietary Supplements Associated With US Food and Drug Administration Warnings. JAMA Netw. Open 2018, 1, e183337. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC consensus statement: Dietary supplements and the high-performance athlete. Br. J. Sports Med. 2018, 52, 439–455. [Google Scholar] [CrossRef]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- de Souza, E.S.A.; Pertille, A.; Reis Barbosa, C.G.; Aparecida de Oliveira Silva, J.; de Jesus, D.V.; Ribeiro, A.; Baganha, R.J.; de Oliveira, J.J. Effects of Creatine Supplementation on Renal Function: A Systematic Review and Meta-Analysis. J. Ren. Nutr. 2019, 29, 480–489. [Google Scholar] [CrossRef]
| Study | Sample Characteristics | Experimental Groups | Creatine Supplementation Protocol | Comparison | Main Findings |
|---|---|---|---|---|---|
| Edmunds et al. (2001) [22] | Han:SPRD-Cy Rats * | (i) Creatine diet (ii) Control diet | Creatine/glutamine (5:1 w/w) L: 2.4 g∙kg−1∙day−1 of diet for 7 days M: 0.48 g∙kg−1∙day−1 of diet for 35 days | Purified diet | ↑ Serum Crn # ↓ Crn clearance ↑ BUN ↑ Cyst scores |
| Taes et al. (2003) [69] | Male Wistar rats | (i) Sham-operated/control diet (ii) Nephrectomized/control diet (iii) Sham-operated/creatine diet (iv) Nephrectomized/creatine diet | Creatine monohydrate (2% of diet∙day−1) for 4 weeks | Soy-based chow (14% protein) | ↔ mGFR † ↔ Serum Crn † ↔ Crn clearance † ↔ BUN † ↔ Urea clearance † ↔ Serum CysC † ↔ Proteinuria † ↔ Albuminuria † |
| Ferreira et al. (2005) [23] | Male Wistar rats | (i) Sedentary/control diet (ii) AE/control diet (iii) Sedentary/creatine diet (iv) AE/creatine diet | Creatine monohydrate 2 g∙kg−1∙day−1 of diet for 10 weeks | Standard chow | ↓ mGFR & ↓ RPF & ↓ Filtration fraction & ↔ Proteinuria ↔ UFR |
| Souza et al. (2009) [24] | Male Wistar rats | (i) Sedentary/control diet (ii) AE/control diet (iii) Sedentary/creatine diet (iv) AE/creatine diet | Creatine monohydrate L: 5 g∙kg−1∙day−1 of diet for 1 week M: 1 g∙kg−1∙day−1 of diet for 4–8 weeks | Not specified | ↑ Serum Crn & ↑ BUN & ↑ RHA & |
| Study | Study Design | Patient Characteristics | Creatine Supplementation Protocol | Concomitant Use of Other Substances | Main Findings |
|---|---|---|---|---|---|
| Pritchard & Kalra (1998) [15] | Retrospective | 25-year-old man with FSG and frequently relapsing NS undergoing pre-season soccer training regime | L: 15 g∙day−1 for 1 week M: 2 g∙day−1 for 7 weeks | Cyclosporine | ↓ mGFR ↑ Serum Crn ↓ Crn clearance |
| Koshy et al. (1999) [16] | Retrospective | 20-year-old healthy man | Creatine monohydrate 20 g∙day−1 for 4 weeks | None | ↑ Serum Crn ↑ Proteinuria ↑ Hematuria |
| Robinson et al. (2000) [17] | Retrospective | 24-year-old healthy man Bodybuilder | 25 g∙day−1 for 12 months | None | ↔ Serum Crn ↔ BUN ↔ Electrolytes ↑ Proteinuria ↑ Hematuria Rhabdomyolysis |
| Barisic et al. (2002) [18] | Prospective | 18-year-old sedentary man with mitochondrial encephalopathy and moderate renal insufficiency | Creatine monohydrate L: 20 g∙day−1 for 12 days M: 5 g∙day−1 for 28 months | Carbamazepine L-thyroxine Lamotrigine Coenzyme Q Riboflavin Vitamin K3 Ascorbic acid L-carnitine | ↑ Serum Crn ↓ Crn clearance ↑ BUN ↑ Proteinuria |
| Révai et al. (2003) [19] | Retrospective | 22-year-old man Supposedly bodybuilder | 200 g∙day−1 continuously | Methandion | MPGN * |
| Thorsteinsdottir et al. (2006) [20] | Retrospective | 24-year-old man Bodybuilder # | Creatine monohydrate 15 g∙day−1 for 6 months | Large amounts of dietary supplements for bodybuilding purposes, including multiple herbs, nonherbal supplements, and vitamins | ↑ Serum Crn ↓ Crn clearance ↑ BUN ↑ Proteinuria ↑ Hematuria |
| Gualano et al. (2010) [72] | Prospective | 20-year-old man with a single kidney and mild renal insufficiency submitted to resistance training and a high-protein diet (2.8 g∙kg−1∙day−1) | Creatine monohydrate L: 20 g∙day−1 for 5 days M: 5 g∙day−1 for 30 days | None | ↔ mGFR ↑ Serum Crn ↓ Crn clearance ↓ BUN ↔ Electrolytes ↔ Proteinuria ↓ Albuminuria |
| Taner et al. (2010) [21] | Retrospective | 18-year-old healthy man Supposedly bodybuilder | Creatine monohydrate L: 20 g∙day−1 for 5 days M: 1 g∙day−1 for 6 weeks | Not reported | ↑ Serum Crn ↑ BUN ↑ Urate ↔ Electrolytes ↑ Proteinuria |
| Study | Sample Characteristics | Experimental Design | Creatine Supplementation Protocol | Main Findings |
|---|---|---|---|---|
| Derave et al. (2004) [38] | Healthy young adults (19 ± 0 years old) | Double-blind randomized controlled trial (i) Creatine (n = 8) (ii) Placebo (n = 8) | Creatine monohydrate L: 20 g∙day−1 for 1 week M: 5 g∙day−1 19 weeks | ↑ Serum Crn ↔ Urinary Crn ↔ BUN |
| Kreider et al. (2003) [49] | Healthy young adults (19 ± 2 years old) College football players | Non-randomized controlled trial (i) Non-creatine control (n = 44) (ii) Creatine 0–6 months (n = 12) (iii) Creatine 7–12 months (n = 25) (iv) Creatine 12–21 months (n = 17) | Creatine monohydrate L: 15.75 g∙day−1 for 5 days M: 5–10 g∙day−1 for 0–21 months | ↔ Serum Crn ↔ BUN ↔ Uric acid ↔ Electrolytes ↔ Plasma protein ↔ Plasma albumin ↔ Plasma globulin |
| Mayhew et al. (2002) [46] | Healthy young adults (20 ± 2 years old) College football players | Non-randomized controlled trial (i) Creatine users (n = 10) (ii) Non-creatine control (n = 13) | Creatine monohydrate 5–20 g∙day−1 for 0.25–5.6 years | ↔ Serum Crn ↔ Crn clearance ↔ BUN |
| Spillane et al. (2009) [55] | Healthy young men (20 ± 2 years old) | Double-blind randomized controlled trial (i) Creatine monohydrate + RT (n = 10) (ii) Creatine ethyl ester + RT (n = 10) (iii) Placebo + RT (n = 10) | Creatine monohydrate or Creatine ethyl ester L: 20 g∙day−1 for 5 days M: 5 g∙day−1 for 43 days | ↔↑ Serum Crn # |
| Cancela et al. (2008) [52] | Healthy young men (20 ± 3 years old) Soccer players | Double-blind randomized controlled trial (i) Creatine (n = 7) (ii) Placebo (n = 7) | Creatine monohydrate L: 15 g∙day−1 for 7 days M: 3 g∙day−1 49 days | ↔ Serum Crn ↔ Crn clearance ↔ BUN ↔ Uric acid ↔ Plasma albumin |
| Poortmans & Francaux (1998) [42] | Healthy young men (21 ± 2 years old) | Non-randomized controlled trial (n = 20) (i) Creatine supplementation (ii) Placebo supplementation | Creatine monohydrate L: 21 g∙day−1 for 5 days M: 3 g∙day−1 for 58 days | ↔ Crn clearance ↔ Urea clearance ↔ Albuminuria |
| Mihic et al. (2000) [44] | Healthy young adults (22 ± 2 years old) Physically active | Randomized controlled trial (i) Creatine (n = 15; 7 men/8 women) (ii) Placebo (n = 15; 8 men/7 women) | Creatine monohydrate 20 g∙day−1 for 5 days | ↔ Serum Crn ↔ Crn clearance |
| Poortmans & Francaux (1999) [43] | Healthy young adults (24 ± 3 years old) (i) Athletes of national and international levels (ii) Physical education and physical therapy students | Non-randomized controlled trial (i) Creatine supplemented (n = 9) (ii) Non-creatine control (n = 85) | Creatine monohydrate 1–80 g∙day−1 for 10–60 months | ↔ Serum Crn ↔ Urinary Crn ↔ Crn clearance ↔ BUN ↔ Urinary urea ↔ Urea clearance ↔ Plasma albumin ↔ Albuminuria ↔ Clearance albumin |
| Gualano et al. (2008) [54] | Sedentary healthy young men (24 ± 5 years old) * | Double-blind randomized controlled trial (i) Creatine + AT (n = 9) (ii) Placebo + AT (n = 9) | Creatine monohydrate L: 0.3 g∙kg−1∙day−1 for 1 week M: 0.15 g∙kg−1∙day−1 for 11 weeks | ↑ Serum Crn ↓ Serum CysC ↔ Serum electrolytes ↔ Urinary electrolytes |
| Carvalho et al. (2011) [58] | Healthy male adults (24 ± 5 years old) * | Double-blind randomized controlled trial (i) Creatine-absolute + RT (n = 12) (ii) Creatine-relative + RT(n = 11) (iii) Placebo + RT (n = 12) | Creatine monohydrate L: 20 g∙day−1 for 1 week M: 0.03 g∙kg−1 or 5 g∙day−1 for 7 weeks | ↑ Serum Crn † ↔ BUN ↔ Proteinuria ↔ Hematuria |
| Poortmans et al. (2005) [51] | Healthy young men (24 ± 6 years old) | Single group pre-to-post design (i) Creatine supplementation (n = 20) | Creatine monohydrate 21 g∙day−1 for 14 days | ↔ Serum Crn ↔ Urinary Crn ↔ Albuminuria |
| Poortmans et al. (1997) [41] | Healthy young men (25 ± 3 years old) | Non-randomized crossover (n = 5) (i) Creatine supplementation (ii) Placebo supplementation | Creatine monohydrate 20 g∙day−1 for 5 days | ↔ eGFR ↔ Serum Crn ↔ Urinary Crn ↔ Crn clearance ↔ Proteinuria ↔ Albuminuria |
| Robinson et al. (2000) [45] | Healthy young adults (25 ± 5 years old) * Physically active | Randomized placebo-controlled trial (i) Creatine (L; n = 7 men) (ii) Creatine (L; n = 6 men) ‡ (iii) Creatine (M; n = 7 women) (iv) Creatine + RT (M; n = 9 women) (v) Placebo (n = 7 men) (vi) Placebo (n = 6; 3 men/3 women) ‡ (vii) Placebo + RT (n = 6 women) | Creatine monohydrate L: 20 g∙day−1 for 5 days M: 3 g∙day−1 for 8 weeks | ↔ Serum Crn ↔ BUN ↔ Electrolytes ↔ Plasma albumin |
| Lugaresi et al. (2013) [59] | Healthy young men (26 ± 4 years old) * Resistance trained High-protein diet | Double-blind randomized controlled trial (i) Creatine (n = 12) (ii) Placebo (n = 14) | Creatine monohydrate L: 20 g∙day−1 for 1 week M: 5 g∙day−1 for 11 weeks | ↔ mGFR ↔ Serum Crn ↔ BUN ↔ Serum electrolytes ↔ Urinary electrolytes ↔ Proteinuria ↔ Albuminuria |
| Blancquaert et al. (2018) [65] | Healthy young women (26 ± 7 years old) | Randomized controlled trial (i) Omnivorous diet (Control; n = 10) (ii) Vegetarian diet + Placebo (n = 15) (iii) Vegetarian diet + Supplements (n = 15) | Creatine monohydrate 1 g∙day−1 for 3–6 months | ↔ Serum Crn ↔ Urinary Crn |
| Pereira et al. (2015) [64] | Healthy young adults (29 ± 4 years old) | Non-counterbalanced single-blind crossover (i) Creatine (L) (ii) Creatine (M) (iii) Placebo | Creatine monohydrate L: 7 or 20 g∙day−1 for 7 days M: 2 or 5 g∙day−1 for 23 days | ↔ Serum Crn ↔ Urinary Crn |
| Chilibeck et al. (2015) [61] | Postmenopausal women (57 ± 6 years old) | Double-blind randomized controlled trial (i) Creatine + RT (n = 15) (ii) Placebo + RT (n = 18) | Creatine monohydrate 0.1 g∙kg−1∙day−1 for 12 months | ↔ Serum Crn ↔ Crn clearance ↔ BUN ↔ Plasma albumin ↔ Proteinuria ↔ Albuminuria |
| Eijnde et al. (2003) [48] | Physically active healthy older men (63 ± 9 years old) * | Double-blind randomized controlled trial (i) Creatine + CT (n = 15) (ii) Placebo + CT (n = 21) | Creatine monohydrate 5 g∙day−1 for 12 months | ↔ Serum Crn ↔ Urinary Crn ↔ BUN |
| Brose et al. (2003) [47] | Healthy older adults (68 ± 4 years old) * | Double-blind randomized controlled trial (i) Creatine + RT (n = 14; 8 men/6 women) (ii) Placebo + RT (n = 14; 7 men/7 women) | Creatine monohydrate 5 g∙day−1 for 14 weeks | ↑ Serum Crn ↔ Urinary Crn |
| Study | Sample Characteristics | Experimental Design | Creatine Supplementation Protocol | Main Findings |
|---|---|---|---|---|
| Hayashi et al. (2014) [60] | Children with SLE (15 ± 2 years old) | Double-blind randomized placebo-controlled crossover trial (n = 15) (i) Creatine (ii) Placebo | Creatine monohydrate 0.1 g∙kg−1∙day−1 for 12 weeks | ↔ mGFR ↔ Serum Crn ↔ Urinary Crn ↔ BUN ↔ Urinary urea ↔ Serum electrolytes ↔ Proteinuria ↔ Albuminuria |
| Alves et al. (2013) [5] | Middle-aged women with fibromyalgia (49 ± 9 years old) * | Double-blind randomized controlled trial (i) Creatine (n = 15) (ii) Placebo (n = 13) | Creatine monohydrate L: 20 g∙day−1 for 5 days M: 5 g∙day−1 for 15 weeks | ↔ Serum Crn ↔ Urinary Crn ↔ BUN ↔ Urinary urea ↔ Serum electrolytes ↔ Urinary electrolytes ↔ Proteinuria ↔ Albuminuria |
| Earnest et al. (1996) [40] | Middle-aged adults with hypercholesterolemia (51 ± 12 years old) | Double-blind randomized controlled trial (i) Creatine (n = 20; 9 men/11 women) (ii) Placebo (n = 14; 9 men/5 women) | Creatine monohydrate L: 20 g∙day−1 for 5 days M: 10 g∙day−1 for 51 days | ↔ Serum Crn ↔↑ BUN # |
| Gualano et al. (2011) [56] | Sedentary older adults with T2DM (57 ± 6 years old) * | Double-blind randomized controlled trial (i) Creatine + CT (n = 13) (ii) Placebo + CT (n = 12) | Creatine monohydrate 5 g∙day−1 for 12 weeks | ↔ mGFR ↔ Serum Crn ↔ Urinary Crn ↔ Crn clearance ↔ BUN ↔ Urinary urea ↔ Serum electrolytes ↔ Urinary electrolytes ↔ Proteinuria ↔ Albuminuria |
| Neves et al. (2011) [57] | Postmenopausal women with knee osteoarthritis (58 ± 3 years old) | Double-blind randomized controlled trial (i) Creatine (n = 13) (ii) Placebo (n = 11) | Creatine monohydrate L: 20 g∙day−1 for 1 week M: 5 g∙day−1 for 11 weeks | ↔ mGFR ↔ Serum Crn ↔ Urinary Crn ↔ Crn clearance ↔ BUN ↔ Urinary urea ↔ Proteinuria ↔ Albuminuria |
| Lobo et al. (2015) [63] | Postmenopausal women with osteopenia (58 ± 5 years old) * | Double-blind randomized controlled trial (i) Creatine (n = 56) (ii) Placebo (n = 53) | Creatine monohydrate 1 g∙day−1 for 12 months | ↔ Serum Crn ↔ Urinary Crn ↔ Albuminuria |
| Sales et al. (2020) [67] | Postmenopausal women with osteopenia (58 ± 6 years old) | Double-blind randomized controlled trial (i) Creatine (n = 106) (ii) Placebo (n = 94) | Creatine monohydrate 3 g∙day−1 for 24 months | ↔ Serum Crn ↔ Urinary Crn ↔ Albuminuria |
| Groeneveld et al. (2005) [50] | Young to older adults with ALS (58 ± 11 years old) | Double-blind randomized controlled trial (i) Creatine (n = 88; 57 men/31 women) (ii) Placebo (n = 87; 63 men/24 women) | Creatine monohydrate 10 g∙day−1 for 310 days | ↔↑ Serum Crn † ↔ BUN ↔ Albuminuria |
| Bender et al. (2008) [53] | Middle-aged patients with Parkinson (60 ± 10 years old)* | Double-blind randomized controlled trial (i) Creatine (n = 40; 28 men/12 women) (ii) Placebo (n = 20; 15 men/5 women) | Creatine monohydrate L: 20 g∙day−1 for 6 days M: 2–4 g∙day−1 for 6–24 months | ↔↑ Serum Crn ‡ ↔ Urinary Crn ↔ Serum CysC ↔ BUN ↔ Hematuria ↔ Albuminuria |
| Kieburtz et al. (2015) [62] | Middle-aged and older adults with Parkinson (62 ± 10 years old) * | Double-blind randomized controlled trial (i) Creatine (n = 477) (ii) Placebo (n = 478) | Creatine monohydrate 10 g∙day−1 for 5–8 years | ↔↑ Serum Crn ¥ |
| Domingues et al. (2020) [66] | Middle-aged and older adults with peripheral arterial disease (64 ± 9 years old) * | Double-blind randomized controlled trial (i) Creatine (n = 14) (ii) Placebo (n = 15) | Creatine monohydrate L: 20 g∙day−1 for 1 week M: 5 g∙day−1 for 7 weeks | ↔ Serum Crn ↔ Urinary Crn ↔ Crn clearance |
| Roschel et al. (2021) [68] | Pre-frail and frail older adults (72 ± 6 years old) | Double-blind randomized controlled trial (i) Creatine (n = 22) (ii) Creatine + Whey (n = 22) (iii) Whey (n = 22) (iv) Placebo (n = 22) | Creatine monohydrate 6 g∙day−1 for 16 weeks | ↔ mGFR ↔ Serum Crn ↔ Urinary Crn ↔ BUN ↔ Urinary urea ↔ Proteinuria ↔ Albuminuria |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longobardi, I.; Gualano, B.; Seguro, A.C.; Roschel, H. Is It Time for a Requiem for Creatine Supplementation-Induced Kidney Failure? A Narrative Review. Nutrients 2023, 15, 1466. https://doi.org/10.3390/nu15061466
Longobardi I, Gualano B, Seguro AC, Roschel H. Is It Time for a Requiem for Creatine Supplementation-Induced Kidney Failure? A Narrative Review. Nutrients. 2023; 15(6):1466. https://doi.org/10.3390/nu15061466
Chicago/Turabian StyleLongobardi, Igor, Bruno Gualano, Antonio Carlos Seguro, and Hamilton Roschel. 2023. "Is It Time for a Requiem for Creatine Supplementation-Induced Kidney Failure? A Narrative Review" Nutrients 15, no. 6: 1466. https://doi.org/10.3390/nu15061466
APA StyleLongobardi, I., Gualano, B., Seguro, A. C., & Roschel, H. (2023). Is It Time for a Requiem for Creatine Supplementation-Induced Kidney Failure? A Narrative Review. Nutrients, 15(6), 1466. https://doi.org/10.3390/nu15061466








