Prevalence and Relevance of Vitamin D Deficiency in Newly Diagnosed Breast Cancer Patients: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gröber, U.; Holzhauer, P.; Kisters, K.; Holick, M.F.; Adamietz, I.A. Micronutrients in oncological intervention. Nutrients 2016, 8, 163. [Google Scholar] [CrossRef]
- Gröber, U.; Hübner, J.; Holzhauer, P.; Kleeberg, U.R. Antioxidanzien und andere Mikronährstoffe in der komplementären Onkologie. Onkologe 2010, 16, 73–79. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Hintzpeter, B.; Mensink, G.B.M.; Thierfelder, W.; Müller, M.J.; Scheidt-Nave, C. Vitamin D status and health correlates among German adults. Eur. J. Clin. Nutr. 2008, 62, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Galesanu, C.; Mocanu, V. Vitamin D deficiency and the clinical consequences. Rev. Med. Chir. Soc. Med. Nat. Iasi 2015, 119, 310–318. [Google Scholar]
- Linowiecka, K.; Wolnicka-Głubisz, A.; Brozyna, A. Vitamin D endocrine system in breast cancer. Acta Biochim. Pol. 2021, 68, 489–497. [Google Scholar] [CrossRef]
- De La Puente-Yagüe, M.; Cuadrado-Cenzual, M.A.; Ciudad-Cabañas, M.J.; Hernández-Cabria, M.; Collado-Yurrita, L. Vitamin D: And its role in breast cancer. Kaohsiung J. Med. Sci. 2018, 34, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Shao, T.; Klein, P.; Grossbard, M.L. Vitamin D and Breast Cancer. Oncologist 2012, 17, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Vanhevel, J.; Verlinden, L.; Doms, S.; Wildiers, H.; Verstuyf, A. The role of vitamin D in breast cancer risk and progression. Endocr. Relat. Cancer 2022, 29, R33–R55. [Google Scholar] [CrossRef] [PubMed]
- Filip-Psurska, B.; Zachary, H.; Strzykalska, A.; Wietrzyk, J. Vitamin D, Th17 Lymphocytes, and Breast Cancer. Cancers 2022, 14, 3649. [Google Scholar] [CrossRef] [PubMed]
- Segovia-Mendoza, M.; García-Quiroz, J.; Díaz, L.; García-Becerra, R. Combinations of Calcitriol with Anticancer Treatments for Breast Cancer: An Update. Int. J. Mol. Sci. 2021, 22, 12741. [Google Scholar] [CrossRef] [PubMed]
- Blasiak, J.; Pawlowska, E.; Chojnacki, J.; Szczepanska, J.; Fila, M.; Chojnacki, C. Vitamin D in triple-negative and BRCA1-deficient breast cancer—Implications for pathogenesis and therapy. Int. J. Mol. Sci. 2020, 21, 3670. [Google Scholar] [CrossRef]
- Lope, V.; Castelló, A.; Mena-Bravo, A.; Amiano, P.; Aragonés, N.; Fernández-Villa, T.; Guevara, M.; Dierssen-Sotos, T.; Fernandez-Tardon, G.; Castano-Vinyals, G.; et al. Serum 25-hydroxyvitamin D and breast cancer risk by pathological subtype (MCC-Spain). J. Steroid Biochem. Mol. Biol. 2018, 182, 4–13. [Google Scholar] [CrossRef]
- Min, K.-W.; Kim, D.-H.; Do, S.-I.; Pyo, J.-S.; Chae, S.W.; Sohn, J.H.; Kim, K.; Lee, H.J.; Kim, D.H.; Oh, S.; et al. High Ki67/BCL2 index is associated with worse outcome in early stage breast cancer. Postgrad Med. J. 2016, 92, 707–714. [Google Scholar] [CrossRef]
- Yang, Z.J.; Yu, Y.; Chi, J.R.; Guan, M.; Zhao, Y.; Cao, X.C. The combined pN stage and breast cancer subtypes in breast cancer: A better discriminator of outcome can be used to refine the 8th AJCC staging manual. Breast Cancer 2018, 25, 315–324. [Google Scholar] [CrossRef]
- Bardia, A.; Hurvitz, S. Targeted Therapy for Premenopausal Women with HR+, HER2− Advanced Breast Cancer: Focus on Special Considerations and Latest Advances. Clin. Cancer Res. 2018, 24, 5206–5218. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Connolly, J.L.; Edge, S.B.; Mittendorf, E.A.; Rugo, H.S.; Solin, L.J.; Weaver, D.L.; Winchester, D.J.; Hortobagyi, G.N. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 290–303. [Google Scholar] [CrossRef]
- Osmani, F.; Hajizadeh, E.; Rasekhi, A.; Akbari, M.E. Prognostic factors associated with locoronal relapses, metastatic relapses, and death among women with breast cancer. Population-based cohort study. Breast 2019, 48, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.-X.; Qin, Q.-H.; Yang, W.-P.; Mo, Q.-G.; Wei, C.-Y. Prognostic value of Ki67 expression in HR-negative breast cancer before and after neoadjuvant chemotherapy. Int. J. Clin. Exp. Pathol. 2014, 7, 6862–6870. [Google Scholar]
- Interdisziplinäre S3-Leitlinie für Die Früherkennung, Diagnostik, Therapie und Nachsorge des Mammakarzinoms; AWMF-Registernummer: 032-045OL; AWMF: Berlin, Germany, 2021.
- Li, X.; Yang, J.; Peng, L.; Sahin, A.A.; Huo, L.; Ward, K.C.; O’Regan, R.; Torres, M.A.; Meisel, J.L. Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res. Treat. 2017, 161, 279–287. [Google Scholar] [CrossRef]
- Zemlin, C.; Stuhlert, C.; Schleicher, J.T.; Wörmann, C.; Altmayer, L.; Lang, M.; Scherer, L.-S.; Thul, I.C.; Muller, C.; Kaiser, E.; et al. Longitudinal assessment of physical activity, fitness, body composition, immunological biomarkers, and psychological parameters during the first year after diagnosis in women with non-metastatic breast cancer: The BEGYN study protocol. Front. Oncol. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- James, D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Elston, C.W.; Ellis, I.O. Pathological prognostic factors in breast cancer, I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology 1991, 19, 403–410. [Google Scholar] [CrossRef]
- Nielsen, T.O.; Leung, S.C.Y.; Rimm, D.L.; Dodson, A.; Acs, B.; Badve, S.; Denkert, C.; Ellis, M.J.; Fineberg, S.; Flowers, M.; et al. Assessment of Ki67 in Breast Cancer: Updated Recommendations from the International Ki67 in Breast Cancer Working Group. JNCI J. Natl. Cancer Inst. 2021, 113, 808–819. [Google Scholar] [CrossRef]
- Welsh, J.E. Vitamin D and breast cancer: Mechanistic update. JBMR Plus 2021, 5, e10582. [Google Scholar] [CrossRef]
- Giovannucci, E. Vitamin D and cancer incidence in the Harvard Cohorts. Ann. Epidemiol. 2009, 19, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, U.; Khan, S.; Azam, I.; Khan, A.H.; Maqbool, A.; Hanif, M.; Gill, T.; Iqbal, R.; Callen, D. A multicenter case control study of association of vitamin D with breast cancer among women in Karachi, Pakistan. PLoS ONE 2020, 15, e0225402. [Google Scholar] [CrossRef]
- Estébanez, N.; Gómez-Acebo, I.; Palazuelos, C.; Llorca, J.; Dierssen-Sotos, T. Vitamin D exposure and Risk of Breast Cancer: A meta-analysis. Sci. Rep. 2018, 8, 9039. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.D.; Aggarwal, A.; Swami, S.; Krishnan, A.V.; Ji, L.; Albertelli, M.A.; Feldman, B.J. Tumor autonomous effects of vitamin D deficiency promote breast cancer metastasis. Endocrinology 2016, 157, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Al-Azhri, J.; Zhang, Y.; Bshara, W.; Zirpoli, G.; McCann, S.E.; Khoury, T.; Morisson, C.D.; Edge, S.B.; Ambrosone, C.B.; Yao, S. Tumor Expression of Vitamin D Receptor and Breast Cancer Histopathological Characteristics and Prognosis. Clin. Cancer Res. 2017, 23, 97–103. [Google Scholar] [CrossRef]
- Voutsadakis, I.A. Vitamin D receptor (VDR) and metabolizing enzymes CYP27B1 and CYP24A1 in breast cancer. Mol. Biol. Rep. 2020, 47, 9821–9830. [Google Scholar] [CrossRef]
- Blasiak, J.; Chojnacki, J.; Pawlowska, E.; Jablkowska, A.; Chojnacki, C. Vitamin D May Protect against Breast Cancer through the Regulation of Long Noncoding RNAs by VDR Signaling. Int. J. Mol. Sci. 2022, 23, 3189. [Google Scholar] [CrossRef] [PubMed]
- Harvie, M. Nutritional supplements and cancer: Potential benefits and proven harms. Am. Soc. Clin. Oncol. Educ. Book 2014, 29, e478-86. [Google Scholar] [CrossRef]
- Rosso, C.; Fera, N.; Murugan, N.J.; Voutsadakis, I.A. Vitamin D Levels in Newly Diagnosed Breast Cancer Patients according to Tumor Sub-Types. J. Diet Suppl. 2022, 1–13. [Google Scholar] [CrossRef]
- Pérez-Bilbao, T.; Alonso-Dueñas, M.; Peinado, A.B.; San Juan, A.F. Effects of Combined Interventions of Exercise and Diet or Exercise and Supplementation on Breast Cancer Patients: A Systematic Review. Nutrients 2023, 15, 1013. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Beydoun, M.A.; Beydoun, H.A.; Chen, X.; Zonderman, A.B.; Wood, R.J. Vitamin D and breast cancer: A systematic review and meta-analysis of observational studies. Clin. Nutr. ESPEN 2019, 30, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Merker, M.; Amsler, A.; Pereira, R.; Bolliger, R.; Tribolet, P.; Braun, N.; Hoess, C.; Pavlicek, V.; Bilz, S.; Sigrist, S.; et al. Vitamin D deficiency is highly prevalent in malnourished inpatients and associated with higher mortality. Medicine 2019, 98, e18113. [Google Scholar] [CrossRef]
- Gaudio, A.; Xourafa, A.; Rapisarda, R.; Castellino, P. Therapeutic Options for the Management of Aromatase Inhibitor- Associated Bone Loss. Endocr. Metab. Immune Disord. Drug Targets 2022, 22, 259–273. [Google Scholar] [CrossRef]
- Jennaro, T.S.; Fang, F.; Kidwell, K.M.; Smith, E.M.L.; Vangipuram, K.; Burness, M.L.; Griggs, J.J.; Van Poznak, C.; Hayes, D.F.; Henry, N.L.; et al. Vitamin D deficiency increases severity of paclitaxel-induced peripheral neuropathy. Breast Cancer Res. Treat. 2020, 180, 707–714. [Google Scholar] [CrossRef]
- Poskitt, E.M.E.; Cole, T.J.; Lawson, D.E.M. Diet, sunlight, and 25-hydroxy vitamin D in healthy children and adults. Br. Med. J. 1979, 1, 221–223. [Google Scholar] [CrossRef]
- Heidari, B.; Mirghassemi, M.B.H. Seasonal variations in serum vitamin D according to age and sex. Caspian J. Intern. Med. 2012, 3, 535–540. [Google Scholar]
- Shakeri, H.; Pournaghi, S.J.; Hashemi, J.; Mohammad-Zadeh, M.; Akaberi, A. Do sufficient vitamin D levels at the end of summer in children and adolescents provide an assurance of vitamin D sufficiency at the end of winter? A cohort study. J. Pediatr. Endocrinol. Metab. 2017, 30, 1041–1046. [Google Scholar] [CrossRef]
- Park, S.-H.; Hoang, T.; Kim, J. Dietary Factors and Breast Cancer Prognosis among Breast Cancer Survivors: A Systematic Review and Meta-Analysis of Cohort Studies. Cancers 2021, 13, 5329. [Google Scholar] [CrossRef] [PubMed]
- Buono, G.; Giuliano, M.; De Angelis, C.; Lauria, R.; Forestieri, V.; Pensabene, M.; Bruzzese, D.; De Placido, S.; Arpino, G. Pretreatment Serum Concentration of Vitamin D and Breast Cancer Characteristics: A Prospective Observational Mediterranean Study. Clin. Breast Cancer 2017, 17, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Mego, M.; Vlkova, B.; Minarik, G.; Cierna, Z.; Karaba, M.; Benca, J.; Sedlackova, T.; Cholujova, D.; Gronesova, P.; Kalavska, K.; et al. Vitamin D and circulating tumor cells in primary breast cancer. Front. Oncol. 2022, 12. [Google Scholar] [CrossRef]
- Tommie, J.L.; Pinney, S.M.; Nommsen-Rivers, L.A. Serum Vitamin D Status and Breast Cancer Risk by Receptor Status: A Systematic Review. Nutr. Cancer 2018, 70, 804–820. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Kwan, M.L.; Ergas, I.J.; Roh, J.M.; Cheng, T.-Y.D.; Hong, C.-C.; McCann, S.E.; Tang, L.; Davis, W.; Liu, S.; et al. Association of Serum Level of Vitamin D at Diagnosis With Breast Cancer Survival. JAMA Oncol. 2017, 3, 351. [Google Scholar] [CrossRef]
- Kim, H.J.; Koh, B.S.; Yu, J.H.; Lee, J.W.; Son, B.H.; Kim, S.B.; Ahn, S.H. Changes in serum hydroxyvitamin D levels of breast cancer patients during tamoxifen treatment or chemotherapy in premenopausal breast cancer patients. Eur. J. Cancer 2014, 50, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Kok, D.E.; van den Berg, M.M.G.A.; Posthuma, L.; van ’t Erve, I.; van Duijnhoven, F.J.B.; de Roos, W.K.; Grosfeld, S.; Los, M.; Sommeijer, D.W.; van Laarhoven, H.W.M.; et al. Changes in Circulating Levels of 25-hydroxyvitamin D3 in Breast Cancer Patients Receiving Chemotherapy. Nutr. Cancer 2019, 71, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Haule, C.C.; Kim, J.H.; Lim, S.M.; Yoon, K.H.; Kim, J.Y.; Park, H.S.; Park, S.; Kim, S.I.; Cho, Y.U.; et al. Association between Changes in Serum 25-Hydroxyvitamin D Levels and Survival in Patients with Breast Cancer Receiving Neoadjuvant Chemotherapy. J. Breast Cancer 2018, 21, 134. [Google Scholar] [CrossRef]
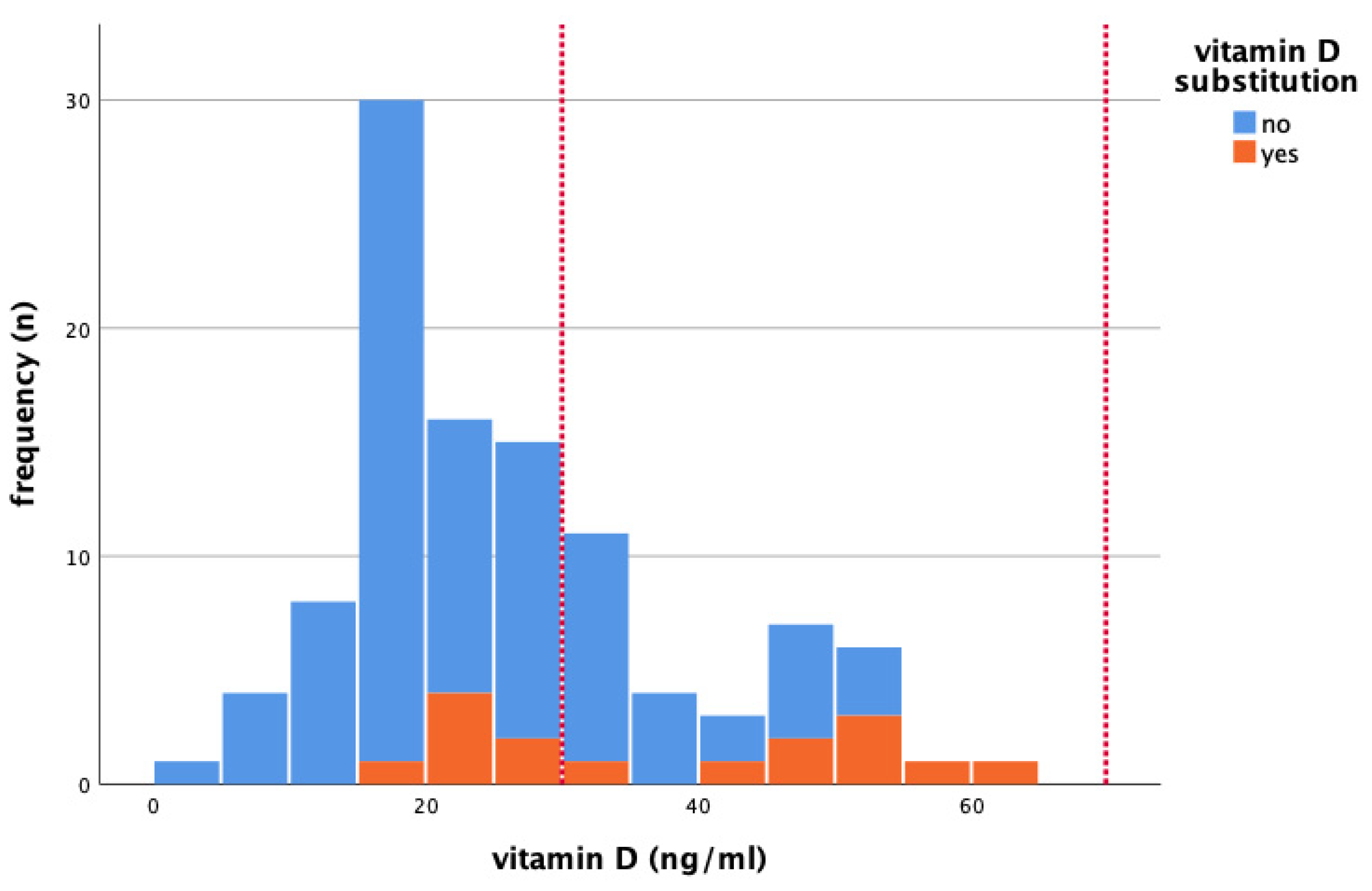
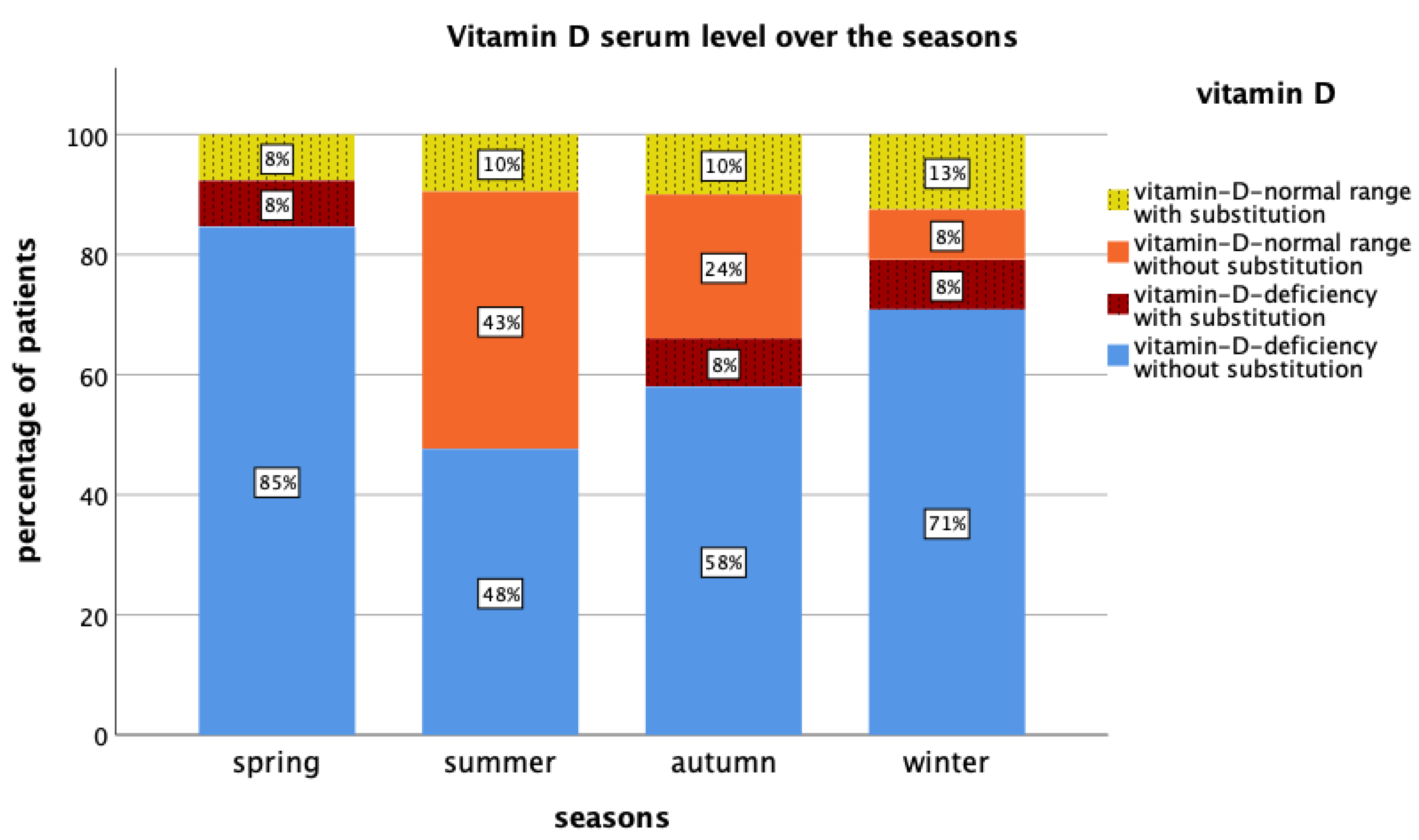
| Tumor Biology | Frequency (n) | Percentage (%) | 25(OH)D (Median (Min–Max)) |
|---|---|---|---|
| Luminal A (ER and/or PR positive, Ki67 < 25) | 48 | 43.6 | 23 ng/mL (5–65 ng/mL) |
| Luminal B (ER and/or PR positive, Ki67 ≥ 25) | 24 | 21.9 | 19 ng/mL (10–63 ng/mL) |
| Her2 positive (ER/PR positive or negative) | 27 | 24.5 | 24 ng/mL (12–57 ng/mL) |
| Triple negative (ER, PR and Her2 negative) | 11 | 10.0 | 30 ng/mL (20–54 ng/mL) |
| Total | 110 | 100 | 24 ng/mL (5–65 ng/mL) |
| Tumor Entity | Frequency (n) | Percentage (%) | 25(OH)D (Median (Min–Max)) |
|---|---|---|---|
| NST | 91 | 82.7 | 24 ng/mL (5–65 ng/mL) |
| Invasive lobular | 12 | 10.9 | 29 ng/mL (7–53 ng/mL) |
| Inflammatory | 2 | 1.8 | 32 ng/mL (11–53 ng/mL) |
| Mucinous | 1 | 0.9 | 15 ng/mL |
| Tubular | 2 | 1.8 | 16 ng/mL |
| Metaplastic | 1 | 0.9 | 12 ng/mL |
| Mixed (NST, tubular) | 1 | 0.9 | 47 ng/mL |
| Total | 110 | 100 | 24 ng/mL (5–65 ng/mL) |
| cT | Frequency (n) | Percentage (%) | 25(OH)D (Median (Min–Max)) |
| cT0 * | 3 | 2.7 | 31 ng/mL (27–57 ng/mL) |
| cT1 | 71 | 64.5 | 22 ng/mL (5–65 ng/mL) |
| cT2 | 31 | 28.2 | 24 ng/mL (6–48 ng/mL) |
| cT3 | 1 | 0.9 | 30 ng/mL |
| cT4 | 4 | 3.6 | 38 ng/mL (11–53 ng/mL) |
| cN | Frequency (n) | Percentage (%) | |
| cN0 | 83 | 75.5 | 25 ng/mL (5–65 ng/mL) |
| cN+ | 27 | 24.5 | 23 ng/mL (6–57 ng/mL) |
| M | Frequency (n) | Percentage (%) | |
| M0 | 110 | 100 | 24 ng/mL (5–65 ng/mL) |
| Grading | Frequency (n) | Percentage (%) | |
| G1 | 10 | 9.2 | 30 ng/mL (13–47 ng/mL) |
| G2 | 56 | 51.4 | 22 ng/mL (5–65 ng/mL) |
| G3 | 43 | 39.4 | 26 ng/mL (10–63 ng/mL) |
| Total | 110 | 100 | 24 ng/mL (5–65 ng/mL) |
| Spring | Frequency (n) | Percentage (%) |
| <10 min | 25 | 27.5 |
| 10–20 min | 26 | 28.6 |
| 15–25 min | 12 | 13.2 |
| 25–50 min | 19 | 20.9 |
| >50 min | 9 | 9.9 |
| Summer | Frequency (n) | Percentage (%) |
| <5 min | 16 | 17.6 |
| 5–10 min | 17 | 18.7 |
| 10–15 min | 15 | 16.5 |
| 15–30 min | 17 | 18.7 |
| >30 min | 26 | 28.6 |
| Autumn | Frequency (n) | Percentage (%) |
| <10 min | 21 | 23.1 |
| 10–20 min | 22 | 24.2 |
| 15–25 min | 12 | 13.2 |
| 25–50 min | 25 | 27.5 |
| >50 min | 11 | 12.1 |
| Winter | Frequency (n) | Percentage (%) |
| <10 min | 35 | 38.5 |
| 10–20 min | 17 | 18.7 |
| 15–25 min | 15 | 16.5 |
| 25–50 min | 16 | 17.6 |
| >50 min | 8 | 8.8 |
| Spring | Frequency (n) | Percentage (%) |
| Never | 50 | 54.9 |
| 1–3 days/week | 21 | 23.1 |
| 3–6 days/week | 6 | 6.6 |
| Every day | 14 | 15.4 |
| Summer | Frequency (n) | Percentage (%) |
| Never | 6 | 6.6 |
| 1–3 days/week | 32 | 35.2 |
| 3–6 days/week | 22 | 24.2 |
| Every day | 31 | 34.1 |
| Autumn | Frequency (n) | Percentage (%) |
| Never | 49 | 53.8 |
| 1–3 days/week | 25 | 27.5 |
| 3–6 days/week | 8 | 8.8 |
| Every day | 9 | 9.9 |
| Winter | Frequency (n) | Percentage (%) |
| Never | 69 | 75.8 |
| 1–3 days/week | 11 | 12.1 |
| 3–6 days/week | 4 | 4.4 |
| Every day | 7 | 7.7 |
| Spring | Frequency (n) | Percentage (%) |
| Never | 52 | 57.1 |
| 1–3 days/week | 11 | 12.1 |
| 3–6 days/week | 6 | 6.6 |
| Every day | 22 | 24.2 |
| Summer | Frequency (n) | Percentage (%) |
| Never | 15 | 16.5 |
| 1–3 days/week | 23 | 25.3 |
| 3–6 days/week | 19 | 20.9 |
| Every day | 34 | 37.4 |
| Autumn | Frequency (n) | Percentage (%) |
| Never | 52 | 57.1 |
| 1–3 days/week | 15 | 16.5 |
| 3–6 days/week | 4 | 4.4 |
| Every day | 20 | 22.0 |
| Winter | Frequency (n) | Percentage (%) |
| Never | 62 | 68.1 |
| 1–3 days/week | 7 | 7.7 |
| 3–6 days/week | 6 | 6.6 |
| Every day | 16 | 17.6 |
| Lifestyle factors | Age, BMI, alcohol, smoking, skin type, prior cancer history, season of blood sampling (spring, summer, autumn, winter), daily sun exposure (spring, summer, autumn, winter), 25(OH)D substitution |
| Prognostic factors | Ki67, grading, triple negative carcinomas, Her2 positive carcinomas, Luminal A carcinomas, Luminal B carcinomas, cT (tumor stage), cN (tumor stage) |
| 25(OH)D Levels | ≤ vs. >10 µg/L | ≤ vs. >20 µg/L | ≤ vs. >30 µg/L |
|---|---|---|---|
| Ki67 | p = 0.33 | p = 0.07 | p = 0.39 |
| Grading | p = 0.10 | p = 0.61 | p = 0.74 |
| Triple negative | p = 1.00 | p = 0.047 | p = 0.74 |
| Her2 positive | p = 0.33 | p = 0.50 | p = 1.00 |
| Luminal A | p = 0.65 | p = 1.00 | p = 0.68 |
| Luminal B | p = 0.29 | p = 0.03 | p = 0.62 |
| cT (tumor stage) | p = 0.95 | p = 0.50 | p = 0.45 |
| cN+ (lymph nodes) | p = 0.60 | p = 1.00 | p = 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zemlin, C.; Altmayer, L.; Stuhlert, C.; Schleicher, J.T.; Wörmann, C.; Lang, M.; Scherer, L.-S.; Thul, I.C.; Spenner, L.S.; Simon, J.A.; et al. Prevalence and Relevance of Vitamin D Deficiency in Newly Diagnosed Breast Cancer Patients: A Pilot Study. Nutrients 2023, 15, 1450. https://doi.org/10.3390/nu15061450
Zemlin C, Altmayer L, Stuhlert C, Schleicher JT, Wörmann C, Lang M, Scherer L-S, Thul IC, Spenner LS, Simon JA, et al. Prevalence and Relevance of Vitamin D Deficiency in Newly Diagnosed Breast Cancer Patients: A Pilot Study. Nutrients. 2023; 15(6):1450. https://doi.org/10.3390/nu15061450
Chicago/Turabian StyleZemlin, Cosima, Laura Altmayer, Caroline Stuhlert, Julia Theresa Schleicher, Carolin Wörmann, Marina Lang, Laura-Sophie Scherer, Ida Clara Thul, Lisanne Sophie Spenner, Jana Alisa Simon, and et al. 2023. "Prevalence and Relevance of Vitamin D Deficiency in Newly Diagnosed Breast Cancer Patients: A Pilot Study" Nutrients 15, no. 6: 1450. https://doi.org/10.3390/nu15061450
APA StyleZemlin, C., Altmayer, L., Stuhlert, C., Schleicher, J. T., Wörmann, C., Lang, M., Scherer, L.-S., Thul, I. C., Spenner, L. S., Simon, J. A., Wind, A., Kaiser, E., Weber, R., Goedicke-Fritz, S., Wagenpfeil, G., Zemlin, M., Solomayer, E.-F., Reichrath, J., & Müller, C. (2023). Prevalence and Relevance of Vitamin D Deficiency in Newly Diagnosed Breast Cancer Patients: A Pilot Study. Nutrients, 15(6), 1450. https://doi.org/10.3390/nu15061450






