Habitual Tea Consumption Increases the Incidence of Metabolic Syndrome in Middle-Aged and Older Individuals
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Study Variables
2.3. Statistical Analysis
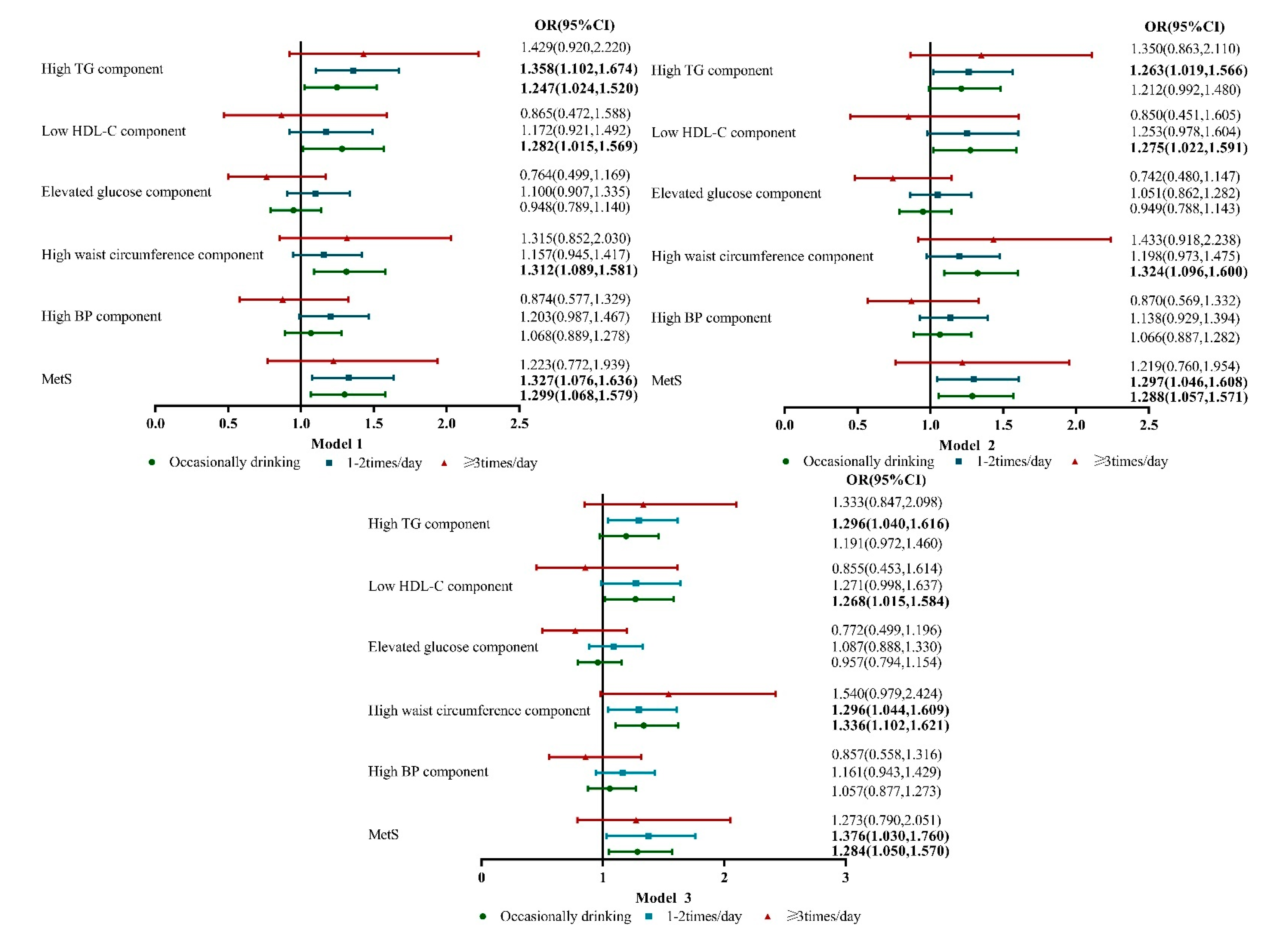
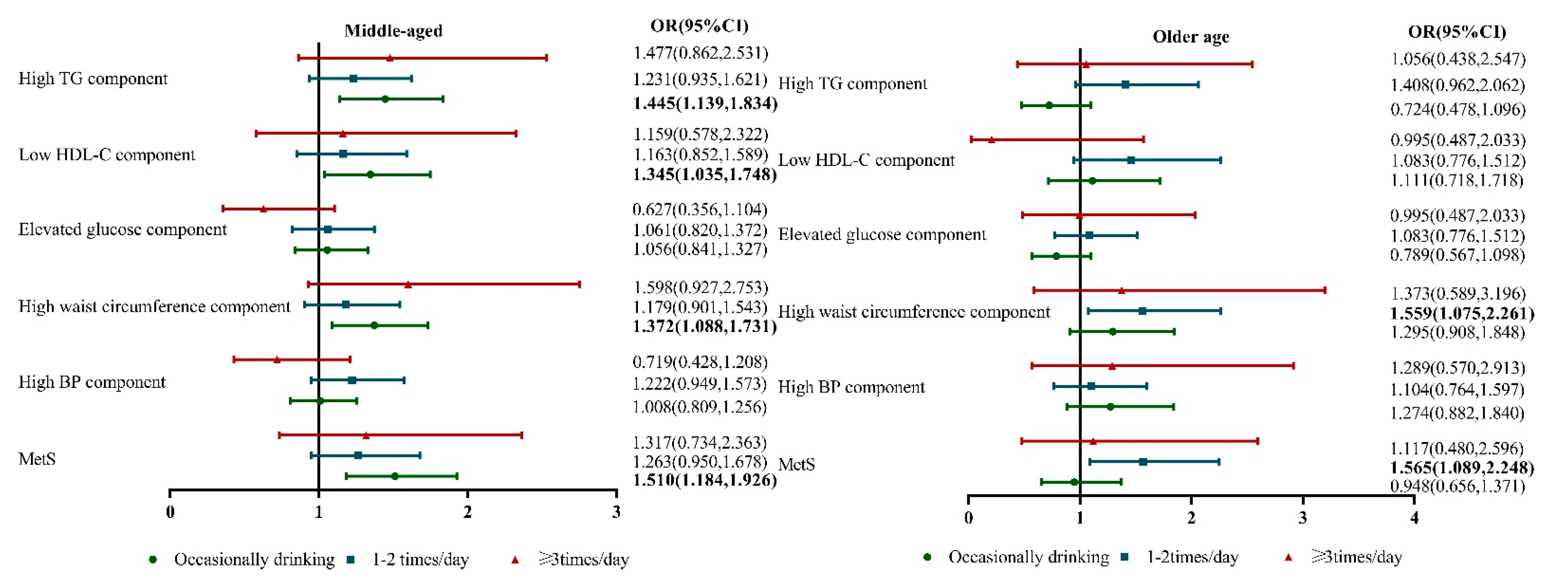
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, F.; Bo, Y.; Zhao, L.; Li, Y.; Ju, L.; Fang, H.; Piao, W.; Yu, D.; Lao, X. Prevalence and Influencing Factors of Metabolic Syndrome among Adults in China from 2015 to 2017. Nutrients 2021, 13, 4475. [Google Scholar] [CrossRef]
- Özmen, M.; Yersal, Ö.; Öztürk, S.; Soysal, D.; Köseeoğlu, M. Prevalence of the metabolic syndrome in rheumatoid arthritis. Eur. J. Rheumatol. 2014, 1, 1–4. [Google Scholar] [CrossRef]
- Gu, D.; Reynolds, K.; Wu, X.; Chen, J.; Duan, X.; Reynolds, R.; Whelton, P.; He, J. Prevalence of the metabolic syndrome and overweight among adults in China. Lancet 2005, 365, 1398–1405. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, L.; Yu, D.; Wang, Z.; Ding, G. Metabolic syndrome prevalence and its risk factors among adults in China: A nationally representative cross-sectional study. PLoS ONE 2018, 13, e0199293. [Google Scholar] [CrossRef]
- Du, Z.; Xing, L.; Liu, S.; Jing, L.; Tian, Y.; Zhang, B.; Yan, H.; Lin, M.; Yu, S.; Sun, Y. Prevalence and determinants of metabolic syndrome based on three definitions in rural northeast China. Public Health Nutr. 2020, 23, 3379–3386. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Yang, S.; Li, S.; Su, M.; Wang, N.; Chen, Y.; Jiang, Q.; Fu, C. Prevalences of metabolic syndrome and its sex-specific association with socioeconomic status in rural China: A cross-sectional study. BMC Public Health 2021, 21, 2033. [Google Scholar] [CrossRef]
- Akimov, A.; Gemueva, K.; Semenova, N. The Seventh Population Census in the PRC: Results and Prospects of the Country’s Demographic Development. Her. Russ. Acad. Sci. 2021, 91, 724–735. [Google Scholar] [CrossRef]
- Tang, G.Y.; Meng, X.; Gan, R.Y.; Zhao, C.N.; Liu, Q.; Feng, Y.B.; Li, S.; Wei, X.L.; Atanasov, A.G.; Corke, H.; et al. Health Functions and Related Molecular Mechanisms of Tea Components: An Update Review. Int. J. Mol. Sci. 2019, 20, 6196. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Zhang, H.; Qi, R.; Tsao, R.; Mine, Y. Recent Advances in the Understanding of the Health Benefits and Molecular Mechanisms Associated with Green Tea Polyphenols. J. Agric. Food Chem. 2019, 67, 1029–1043. [Google Scholar] [CrossRef] [PubMed]
- Hartley, L.; Flowers, N.; Holmes, J.; Clarke, A.; Stranges, S.; Hooper, L.; Rees, K. Green and black tea for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2013, 2013, CD009934. [Google Scholar] [CrossRef]
- Hayat, K.; Iqbal, H.; Malik, U.; Bilal, U.; Mushtaq, S. Tea and its consumption: Benefits and risks. Crit. Rev. Food Sci. Nutr. 2015, 55, 939–954. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Jeong, G.; Yang, J.; Lee, K.; Kronbichler, A.; van der Vliet, H.; Grosso, G.; Galvano, F.; Aune, D.; Kim, J.; et al. Tea Consumption and Risk of Cancer: An Umbrella Review and Meta-Analysis of Observational Studies. Adv. Nutr. 2020, 11, 1437–1452. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi-Roshan, M.; Salari, A.; Ghorbani, Z.; Ashouri, A. The effects of regular consumption of green or black tea beverage on blood pressure in those with elevated blood pressure or hypertension: A systematic review and meta-analysis. Complement. Ther. Med. 2020, 51, 102430. [Google Scholar] [CrossRef]
- Sirotkin, A.; Kolesárová, A. The anti-obesity and health-promoting effects of tea and coffee. Physiol. Res. 2021, 70, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Goto, A.; Noma, H.; Iso, H.; Hayashi, K.; Noda, M. Effects of Coffee and Tea Consumption on Glucose Metabolism: A Systematic Review and Network Meta-Analysis. Nutrients 2018, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Wang, R.; Liu, J.; Ma, Q.; Pan, C. Habitual tea consumption and 5-year incident metabolic syndrome among older adults: A community-based cohort study. BMC Geriatr. 2021, 21, 728. [Google Scholar] [CrossRef]
- Sun, Y.; Mu, J.; Wang, D.; Ouyang, N.; Xing, L.; Guo, X.; Zhao, C.; Ren, G.; Ye, N.; Zhou, Y.; et al. A village doctor-led multifaceted intervention for blood pressure control in rural China: An open, cluster randomised trial. Lancet 2022, 399, 1964–1975. [Google Scholar] [CrossRef]
- Yu, S.; Guo, X.; Yang, H.; Zheng, L.; Sun, Y. An update on the prevalence of metabolic syndrome and its associated factors in rural northeast China. BMC Public Health 2014, 14, 877. [Google Scholar] [CrossRef]
- Huang, S.; Li, J.; Wu, Y.; Ranjbar, S.; Xing, A.; Zhao, H.; Wang, Y.; Shearer, G.C.; Bao, L.; Lichtenstein, A.H.; et al. Tea Consumption and Longitudinal Change in High-Density Lipoprotein Cholesterol Concentration in Chinese Adults. J. Am. Heart Assoc. 2018, 7, e008814. [Google Scholar] [CrossRef]
- Hernández-Alonso, P.; Salas-Salvadó, J.; Ruiz-Canela, M.; Corella, D.; Estruch, R.; Fitó, M.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; Lapetra, J.; et al. High dietary protein intake is associated with an increased body weight and total death risk. Clin. Nutr. 2016, 35, 496–506. [Google Scholar] [CrossRef]
- Alberti, K.; Eckel, R.; Grundy, S.; Zimmet, P.; Cleeman, J.; Donato, K.; Fruchart, J.; James, W.; Loria, C.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Grosso, G.; Stepaniak, U.; Micek, A.; Topor-Mądry, R.; Pikhart, H.; Szafraniec, K.; Pająk, A. Association of daily coffee and tea consumption and metabolic syndrome: Results from the Polish arm of the HAPIEE study. Eur. J. Nutr. 2015, 54, 1129–1137. [Google Scholar] [CrossRef]
- Chang, C.-S.; Chang, Y.-F.; Liu, P.-Y.; Chen, C.-Y.; Tsai, Y.-S.; Wu, C.-H. Smoking, habitual tea drinking and metabolic syndrome in elderly men living in rural community: The Tianliao old people (TOP) study 02. PLoS ONE 2012, 7, e38874. [Google Scholar] [CrossRef] [PubMed]
- Vernarelli, J.A.; Lambert, J.D. Tea consumption is inversely associated with weight status and other markers for metabolic syndrome in US adults. Eur. J. Nutr. 2013, 52, 1039–1048. [Google Scholar] [CrossRef]
- Hursel, R.; Viechtbauer, W.; Westerterp-Plantenga, M.S. The effects of green tea on weight loss and weight maintenance: A meta-analysis. Int. J. Obes. 2009, 33, 956–961. [Google Scholar] [CrossRef]
- Phung, O.; Baker, W.; Matthews, L.; Lanosa, M.; Thorne, A.; Coleman, C. Effect of green tea catechins with or without caffeine on anthropometric measures: A systematic review and meta-analysis. J. Clin. Nutr. 2010, 91, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.C.; Thi Phuong, T.N.; Minh, L.B.; Minh Thuc, V.T.; Bac, N.D.; Van Tien, N.; Pham, V.H.; Show, P.L.; Tao, Y.; Ngoc, V.T.N.; et al. The effects of green tea on lipid metabolism and its potential applications for obesity and related metabolic disorders—An existing update. Diabetes Metab. Syndr. 2019, 13, 1667–1673. [Google Scholar] [CrossRef]
- Shen, W.; Pan, Y.; Jin, B.; Zhang, Z.; You, T.; Qu, Y.; Han, M.; Yuan, X.; Zhang, Y. Effects of Tea Consumption on Anthropometric Parameters, Metabolic Indexes and Hormone Levels of Women with Polycystic Ovarian Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. 2021, 12, 736867. [Google Scholar] [CrossRef] [PubMed]
- Chieng, D.; Kistler, P. Coffee and tea on cardiovascular disease (CVD) prevention. Trends Cardiovasc. Med. 2021, 32, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Janssens, P.L.; Hursel, R.; Westerterp-Plantenga, M.S. Long-term green tea extract supplementation does not affect fat absorption, resting energy expenditure, and body composition in adults. J. Nutr. 2015, 145, 864–870. [Google Scholar] [CrossRef]
- Peluso, I.; Teichner, A.; Manafikhi, H.; Palmery, M. Camellia sinensis in asymptomatic hyperuricemia: A meta-analysis of tea or tea extract effects on uric acid levels. Crit. Rev. Food Sci. Nutr. 2017, 57, 391–398. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, Y.; Li, X.A.; Li, L.J.; Xie, X.; Huang, Y.Z.; Deng, Y.H.; Zeng, C.; Lei, G.H. Is tea consumption associated with the serum uric acid level, hyperuricemia or the risk of gout? A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2017, 18, 95. [Google Scholar] [CrossRef]
- Sumiyoshi, H.; Ohyama, Y.; Imai, K.; Kurabayashi, M.; Saito, Y.; Nakamura, T. Association of Uric Acid with Incident Metabolic Syndrome in a Japanese General Population. Int. Heart J. 2019, 60, 830–835. [Google Scholar] [CrossRef]
- Fu, Y.Q.; Yang, H.; Zheng, J.S.; Zeng, X.Y.; Zeng, W.; Fan, Z.F.; Chen, M.; Wang, L.; Li, D. Positive association between metabolic syndrome and serum uric acid in Wuhan. Asia Pac. J. Clin. Nutr. 2017, 26, 343–350. [Google Scholar]
- Zurlo, A.; Veronese, N.; Giantin, V.; Maselli, M.; Zambon, S.; Maggi, S.; Musacchio, E.; Toffanello, E.D.; Sartori, L.; Perissinotto, E.; et al. High serum uric acid levels increase the risk of metabolic syndrome in elderly women: The PRO.V.A study. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 27–35. [Google Scholar] [CrossRef]
- King, C.; Lanaspa, M.A.; Jensen, T.; Tolan, D.R.; Sánchez-Lozada, L.G.; Johnson, R.J. Uric Acid as a Cause of the Metabolic Syndrome. Contrib. Nephrol. 2018, 192, 88–102. [Google Scholar]
- Mazzali, M.; Hughes, J.; Kim, Y.G.; Jefferson, J.A.; Kang, D.H.; Gordon, K.L.; Lan, H.Y.; Kivlighn, S.; Johnson, R.J. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 2001, 38, 1101–1106. [Google Scholar] [CrossRef]
- Sánchez-Lozada, L.; Soto, V.; Tapia, E.; Avila-Casado, C.; Sautin, Y.; Nakagawa, T.; Franco, M.; Rodríguez-Iturbe, B.; Johnson, R. Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Am. J. Physiol. Ren. Physiol. 2008, 295, F1134–F1141. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Lozada, L.; Tapia, E.; López-Molina, R.; Nepomuceno, T.; Soto, V.; Avila-Casado, C.; Nakagawa, T.; Johnson, R.; Herrera-Acosta, J.; Franco, M. Effects of acute and chronic L-arginine treatment in experimental hyperuricemia. Am. J. Physiol. Renal. Physiol. 2007, 292, F1238–F1244. [Google Scholar] [CrossRef] [PubMed]
- Gasińska, A.; Gajewska, D. Tea and coffee as the main sources of oxalate in diets of patients with kidney oxalate stones. Rocz. Panstw. Zakl. Hig. 2007, 58, 61–67. [Google Scholar] [PubMed]
- Wu, A.; Spicer, D.; Stanczyk, F.; Tseng, C.-C.; Yang, C.; Pike, M. Effect of 2-month controlled green tea intervention on lipoprotein cholesterol, glucose, and hormone levels in healthy postmenopausal women. Cancer Prev. Res. 2012, 5, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Suliburska, J.; Bogdanski, P.; Szulinska, M.; Stepien, M.; Pupek-Musialik, D.; Jablecka, A. Effects of green tea supplementation on elements, total antioxidants, lipids, and glucose values in the serum of obese patients. Biol. Trace Elem. Res. 2012, 149, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wen, Y.; Du, Y.; Yan, X.; Guo, H.; Rycroft, J.A.; Boon, N.; Kovacs, E.M.; Mela, D.J. Effects of catechin enriched green tea on body composition. Obesity (Silver Spring Md.) 2010, 18, 773–779. [Google Scholar] [CrossRef]
- Mielgo-Ayuso, J.; Barrenechea, L.; Alcorta, P.; Larrarte, E.; Margareto, J.; Labayen, I. Effects of dietary supplementation with epigallocatechin-3-gallate on weight loss, energy homeostasis, cardiometabolic risk factors and liver function in obese women: Randomised, double-blind, placebo-controlled clinical trial. Br. J. Nutr. 2014, 111, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Golozar, A.; Khademi, H.; Kamangar, F.; Poutschi, H.; Islami, F.; Abnet, C.; Freedman, N.; Taylor, P.; Pharoah, P.; Boffetta, P.; et al. Diabetes mellitus and its correlates in an Iranian adult population. PLoS ONE 2011, 6, e26725. [Google Scholar] [CrossRef]
- Hayashino, Y.; Fukuhara, S.; Okamura, T.; Tanaka, T.; Ueshima, H.; HIPOP-OHP Research Group. High oolong tea consumption predicts future risk of diabetes among Japanese male workers: A prospective cohort study. Diabet. Med. 2011, 28, 805–810. [Google Scholar] [CrossRef]
- Yang, J.; Mao, Q.X.; Xu, H.X.; Ma, X.; Zeng, C.Y. Tea consumption and risk of type 2 diabetes mellitus: A systematic review and meta-analysis update. BMJ Open 2014, 4, e005632. [Google Scholar] [CrossRef]
- Evangelou, E.; Ntritsos, G.; Chondrogiorgi, M.; Kavvoura, F.; Hernández, A.; Ntzani, E.; Tzoulaki, I. Exposure to pesticides and diabetes: A systematic review and meta-analysis. Environ. Int. 2016, 91, 60–68. [Google Scholar] [CrossRef]
- Ngwa, E.N.; Kengne, A.P.; Tiedeu-Atogho, B.; Mofo-Mato, E.P.; Sobngwi, E. Persistent organic pollutants as risk factors for type 2 diabetes. Diabetol. Metab. Syndr. 2015, 7, 41. [Google Scholar] [CrossRef]
- Lee, S.-A.; Dai, Q.; Zheng, W.; Gao, Y.-T.; Blair, A.; Tessari, J.; Ji, B.T.; Shu, X.-O. Association of serum concentration of organochlorine pesticides with dietary intake and other lifestyle factors among urban Chinese women. Environ. Int. 2007, 33, 157–163. [Google Scholar] [CrossRef]
- Ullah, N.; Rafique, N.; Nazir, A.; Anwar, S.; Altaf, N.; Ahmed, G. Effect of decoction of Camellia sinensis on blood pressure and heart rate. Med. Forum Mon. 2011, 22, 46–48. [Google Scholar]
- Son, J.T.; Lee, E. Effects of green tea ingestion on postprandial drops in blood pressure in older adults. J. Gerontol. Nurs. 2012, 38, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, W.; Xiong, Y.; Cooper, R.S.; Du Raza-Arvizu, R.; Cao, G.; Wang, Y.; Ji, P.; Bian, R.; Xu, J. The Association Between Tea Consumption and Hyperhomocysteine in Chinese Hypertensive Patients. Am. J. Hypertens. 2019, 32, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, X.; Chen, S.; Zheng, L.; Yang, H.; Sun, G.; Yu, S.; Li, W.; Zhou, L.; Wang, J.; et al. Hyperhomocysteinemia independently associated with the risk of hypertension: A cross-sectional study from rural China. J. Hum. Hypertens. 2016, 30, 508–512. [Google Scholar] [CrossRef]
- Atif, A.; Rizvi, M.A.; Tauheed, S.; Aamir, I.; Majeed, F.; Siddiqui, K.; Khan, S. Serum homocysteine concentrations in patients with hypertension. Pak. J. Physiol. 2008, 4, 21–22. [Google Scholar]
- Lee, H.S.; In, S.; Park, T. The Homocysteine and Metabolic Syndrome: A Mendelian Randomization Study. Nutrients 2021, 13, 2440. [Google Scholar] [CrossRef]
- Obeid, R.; Herrmann, W. Homocysteine and lipids: S-adenosyl methionine as a key intermediate. FEBS Lett. 2009, 583, 1215–1225. [Google Scholar] [CrossRef]
- Yoshitomi, R.; Nakayama, K.; Yamashita, S.; Kumazoe, M.; Lin, T.-A.; Mei, C.-Y.; Marugame, Y.; Fujimura, Y.; Maeda-Yamamoto, M.; Kuriyama, S.; et al. Plasma Homocysteine Concentration is Associated with the Expression Level of Folate Receptor 3. Sci. Rep. 2020, 10, 10283. [Google Scholar] [CrossRef]
- Wang, X.-J.; Chen, W.; Fu, X.-T.; Ma, J.-K.; Wang, M.-H.; Hou, Y.-J.; Tian, D.-C.; Fu, X.-Y.; Fan, C.-D. Reversal of homocysteine-induced neurotoxicity in rat hippocampal neurons by astaxanthin: Evidence for mitochondrial dysfunction and signaling crosstalk. Cell Death Discov. 2018, 4, 50. [Google Scholar] [CrossRef]
- Endres, M.; Ahmadi, M.; Kruman, I.; Biniszkiewicz, D.; Meisel, A.; Gertz, K. Folate deficiency increases postischemic brain injury. Stroke 2005, 36, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-M.; Jin, H.-Z. Homocysteine: A Potential Common Route for Cardiovascular Risk and DNA Methylation in Psoriasis. Chin. Med. J. 2017, 130, 1980–1986. [Google Scholar] [CrossRef] [PubMed]
- Bednarska-Makaruk, M.; Graban, A.; Sobczy ńska-Malefora, A.; Harrington, D.J.; Mitchell, M.; Voong, K.; Dai, L.; Łojkowska, W.; Bochy ńska, A.; Ryglewicz, D.; et al. Homocysteine metabolism and the associations of global DNA methylation with selected gene polymorphisms and nutritional factors in patients with dementia. Exp. Gerontol. 2016, 81, 83–91. [Google Scholar] [CrossRef] [PubMed]
- McCully, K.S. Chemical pathology of homocysteine. IV. Excitotoxicity, oxidative stress, endothelial dysfunction, and inflammation. Ann. Clin. Lab. Sci. 2009, 39, 219–232. [Google Scholar] [PubMed]
- Li, X.H.; Yu, F.F.; Zhou, Y.H.; He, J. Association between alcohol consumption and the risk of incident type 2 diabetes: A systematic review and dose-response meta-analysis. Am. J. Clin. Nutr. 2016, 103, 818–829. [Google Scholar] [CrossRef]
- Pan, A.; Wang, Y.; Talaei, M.; Hu, F.B.; Wu, T. Relation of active, passive, and quitting smoking with incident type 2 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015, 3, 958–967. [Google Scholar] [CrossRef]
- InterAct Consortium; Ekelund, U.; Palla, L.; Brage, S.; Franks, P.W.; Peters, T.; Balkau, B.; Diaz, M.J.T.; Huerta, J.M.; Agnoli, C.; et al. Physical activity reduces the risk of incident type 2 diabetes in general and in abdominally lean and obese men and women: The EPIC-InterAct study. Diabetologia 2012, 55, 1944–1952. [Google Scholar] [CrossRef]
- Cui, X.; Wang, B.; Wu, Y.; Xie, L.; Xun, P.; Tang, Q.; Cai, W.; Shen, X. Vegetarians have a lower fasting insulin level and higher insulin sensitivity than matched omnivores: A cross-sectional study. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.A.; Kim, J.; Cho, E.R.; Shin, A. Dietary patterns and the prevalence of metabolic syndrome in Korean women. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Kelemen, L.E.; Kushi, L.H.; Jacobs DRJr Cerhan, J.R. Associations of dietary protein with disease and mortality in a prospective study of postmenopausal women. Am. J. Epidemiol. 2005, 161, 239–249. [Google Scholar] [CrossRef]
- Alonso, A.; Beunza, J.J.; Bes-Rastrollo, M.; Pajares, R.M.; Martínez-González, M.A. Vegetable protein and fiber from cereal are inversely associated with the risk of hypertension in a Spanish cohort. Arch. Med. Res. 2006, 37, 778–786. [Google Scholar] [CrossRef]
- Stamler, J.; Brown, I.J.; Daviglus, M.L.; Chan, Q.; Miura, K.; Okuda, N.; Ueshima, H.; Zhao, L.; Elliott, P. Dietary glycine and blood pressure: The International Study on Macro/Micronutrients and Blood Pressure. Am. J. Clin. Nutr. 2013, 98, 136–145. [Google Scholar] [CrossRef]
- Stamler, J.; Brown, I.J.; Daviglus, M.L.; Chan, Q.; Kesteloot, H.; Ueshima, H.; Zhao, L.; Elliott, P.; INTERMAP Research Group. Glutamic acid, the main dietary amino acid, and blood pressure: The INTERMAP Study (International Collaborative Study of Macronutrients, Micronutrients and Blood Pressure). Circulation 2009, 120, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Wittenbecher, C.; Mühlenbruch, K.; Kröger, J.; Jacobs, S.; Kuxhaus, O.; Floegel, A.; Fritsche, A.; Pischon, T.; Prehn, C.; Adamski, J.; et al. Amino acids, lipid metabolites, and ferritin as potential mediators linking red meat consumption to type 2 diabetes. Am. J. Clin. Nutr. 2015, 101, 1241–1250. [Google Scholar] [CrossRef]
- Bibbins-Domingo, K.; Chertow, G.M.; Coxson, P.G.; Moran, A.; Lightwood, J.M.; Pletcher, M.J.; Goldman, L. Projected effect of dietary salt reductions on future cardiovascular disease. N. Engl. J. Med. 2010, 362, 590–599. [Google Scholar] [CrossRef]
- Smith-Spangler, C.M.; Juusola, J.L.; Enns, E.A.; Owens, D.K.; Garber, A.M. Population strategies to decrease sodium intake and the burden of cardiovascular disease: A cost-effectiveness analysis. Ann. Intern. Med. 2010, 152, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Männistö, S.; Kontto, J.; Kataja-Tuomola, M.; Albanes, D.; Virtamo, J. High processed meat consumption is a risk factor of type 2 diabetes in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention study. Br. J. Nutr. 2010, 103, 1817–1822. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Neusner, A.; Longato, L.; Lawton, M.; Wands, J.R.; de la Monte, S.M. Nitrosamine exposure causes insulin resistance diseases: Relevance to type 2 diabetes mellitus, non-alcoholic steatohepatitis, and Alzheimer’s disease. J. Alzheimers Dis. 2009, 17, 827–844. [Google Scholar] [PubMed]
- Kleinbongard, P.; Dejam, A.; Lauer, T.; Jax, T.; Kerber, S.; Gharini, P.; Balzer, J.; Zotz, R.B.; Scharf, R.E.; Willers, R.; et al. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic. Biol. Med. 2006, 40, 295–302. [Google Scholar] [CrossRef]
- Pereira, E.C.; Ferderbar, S.; Bertolami, M.C.; Faludi, A.A.; Monte, O.; Xavier, H.T.; Pereira, T.V.; Abdalla, D.S. Biomarkers of oxidative stress and endothelial dysfunction in glucose intolerance and diabetes mellitus. Clin. Biochem. 2008, 41, 1454–1460. [Google Scholar] [CrossRef]
- Peppa, M.; Goldberg, T.; Cai, W.; Rayfield, E.; Vlassara, H. Glycotoxins: A missing link in the “relationship of dietary fat and meat intake in relation to risk of type 2 diabetes in men”. Diabetes Care 2002, 25, 1898–1899. [Google Scholar] [CrossRef]
- Azadbakht, L.; Esmaillzadeh, A. Red meat intake is associated with metabolic syndrome and the plasma C-reactive protein concentration in women. J. Nutr. 2009, 139, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Steffen, L.M.; Jacobs, D.R., Jr. Association between serum gamma-glutamyltransferase and dietary factors: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am. J. Clin. Nutr. 2004, 79, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Baranczewski, P.; Gustafsson, J.A.; Moller, L. DNA adduct formation of 14 heterocyclic aromatic amines in mouse tissue after oral administration and characterization of the DNA adduct formed by 2-amino-9H-pyrido[2,3-b]indole (AalphaC), analysed by 32P_HPLC. Biomarkers 2004, 9, 243–257. [Google Scholar] [CrossRef]
- Gertig, D.M.; Hankinson, S.E.; Hough, H.; Spiegelman, D.; Colditz, G.A.; Willett, W.C.; Kelsey, K.T.; Hunter, D.J. N-acetyl transferase 2 genotypes, meat intake and breast cancer risk. Int. J. Cancer 1999, 80, 13–17. [Google Scholar] [CrossRef]
- Hansen, E.S. International Commission for Protection Against Environmental Mutagens and Carcinogens. ICPEMC Working Paper 7/1/2. Shared risk factors for cancer and atherosclerosis—A review of the epidemiological evidence. Mutat. Res. 1990, 239, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Kanková, K. Diabetic threesome (hyperglycaemia, renal function and nutrition) and advanced glycation end products: Evidence for the multiple-hit agent? Proc. Nutr. Soc. 2008, 67, 60–74. [Google Scholar] [CrossRef]
- Santarelli, R.L.; Pierre, F.; Corpet, D.E. Processed meat and colorectal cancer: A review of epidemiologic and experimental evidence. Nutr. Cancer 2008, 60, 131–144. [Google Scholar] [CrossRef]
- Spiteller, G. Peroxyl radicals are essential reagents in the oxidation steps of the Maillard reaction leading to generation of advanced glycation end products. Ann. N. Y. Acad. Sci. 2008, 1126, 128–133. [Google Scholar] [CrossRef]
- Wakabayashi, K. International Commission for Protection Against Environmental Mutagens and Carcinogens. ICPEMC Working Paper 7/1/3. Animal studies suggesting involvement of mutagen/carcinogen exposure in atherosclerosis. Mutat. Res. 1990, 239, 181–187. [Google Scholar] [CrossRef]
- Wu, K.; Giovannucci, E.; Byrne, C.; Platz, E.A.; Fuchs, C.; Willett, W.C.; Sinha, R. Meat mutagens and risk of distal colon adenoma in a cohort of U.S. men. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1120–1125. [Google Scholar] [CrossRef]
- van der, A.D.L.; Peeters, P.H.; Grobbee, D.E.; Marx, J.J.; van der Schouw, Y.T. Dietary haem iron and coronary heart disease in women. Eur. Heart J. 2005, 26, 257–262. [Google Scholar] [CrossRef]
- Lee, D.H.; Folsom, A.R.; Jacobs, D.R., Jr. Iron, zinc, and alcohol consumption and mortality from cardiovascular diseases: The Iowa Women’s Health Study. Am. J. Clin. Nutr. 2005, 81, 787–791. [Google Scholar] [CrossRef]
- Ascherio, A.; Willett, W.C.; Rimm, E.B.; Giovannucci, E.L.; Stampfer, M.J. Dietary iron intake and risk of coronary disease among men. Circulation 1994, 89, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Klipstein-Grobusch, K.; Grobbee, D.E.; den Breeijen, J.H.; Boeing, H.; Hofman, A.; Witteman, J.C. Dietary iron and risk of myocardial infarction in the Rotterdam Study. Am. J. Epidemiol. 1999, 149, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Tzonou, A.; Lagiou, P.; Trichopoulou, A.; Tsoutsos, V.; Trichopoulos, D. Dietary iron and coronary heart disease risk: A study from Greece. Am. J. Epidemiol. 1998, 147, 161–166. [Google Scholar] [CrossRef]
- Jiang, R.; Ma, J.; Ascherio, A.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Dietary iron intake and blood donations in relation to risk of type 2 diabetes in men: A prospective cohort study. Am. J. Clin. Nutr. 2004, 79, 70–75. [Google Scholar] [CrossRef]
- Rajpathak, S.; Ma, J.; Manson, J.; Willett, W.C.; Hu, F.B. Iron intake and the risk of type 2 diabetes in women: A prospective cohort study. Diabetes Care 2006, 29, 1370–1376. [Google Scholar] [CrossRef]
- Rajpathak, S.N.; Crandall, J.P.; Wylie-Rosett, J.; Kabat, G.C.; Rohan, T.E.; Hu, F.B. The role of iron in type 2 diabetes in humans. Biochim. Biophys. Acta 2009, 1790, 671–681. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M. Oxygen-derived free radicals in postischemic tissue injury. N. Engl. J. Med. 1985, 312, 159–163. [Google Scholar]
- Ambrosio, G.; Zweier, J.L.; Jacobus, W.E.; Weisfeldt, M.L.; Flaherty, J.T. Improvement of postischemic myocardial function and metabolism induced by administration of deferoxamine at the time of reflow: The role of iron in the pathogenesis of reperfusion injury. Circulation 1987, 76, 906–915. [Google Scholar] [CrossRef]
- van der Kraaij, A.M.; Mostert, L.J.; van Eijk, H.G.; Koster, J.F. Iron-load increases the susceptibility of rat hearts to oxygen reperfusion damage. Protection by the antioxidant (þ)-cyanidanol-3 and deferoxamine. Circulation 1988, 78, 442–449. [Google Scholar] [CrossRef]
- Fardet, A.; Boirie, Y. Associations between food and beverage groups and major diet-related chronic diseases: An exhaustive review of pooled/meta-analyses and systematic reviews. Nutr. Rev. 2014, 72, 741–762. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Yang, K.; Li, S.; Dai, M.; Chen, G. Effect of green tea consumption on blood lipids: A systematic review and meta-analysis of randomized controlled trials. Nutr. J. 2020, 19, 48. [Google Scholar] [CrossRef]
- Igho-Osagie, E.; Cara, K.; Wang, D.; Yao, Q.; Penkert, L.; Cassidy, A.; Ferruzzi, M.; Jacques, P.; Johnson, E.; Chung, M.; et al. Short-Term Tea Consumption Is Not Associated with a Reduction in Blood Lipids or Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Nutr. 2020, 150, 3269–3279. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, D.; Su, B.; Wei, J.; Găman, M.; Macit, M.S.; Nascimento, I.B.; Guimaraes, N. The effect of green tea supplementation on obesity: A systematic review and dose-response meta-analysis of randomized controlled trials. Phytother. Res. 2020, 34, 2459–2470. [Google Scholar] [CrossRef] [PubMed]
- Zijp, I.M.; Korver, O.; Tijburg, L.B. Effect of tea and other dietary factors on iron absorption. Crit. Rev. Food Sci. Nutr. 2000, 40, 371–398. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, X.; Shen, Y.; Fang, X.; Wang, Y.; Wang, F. Obesity and iron deficiency: A quantitative meta-analysis. Obes. Rev. 2015, 16, 1081–1093. [Google Scholar] [CrossRef]
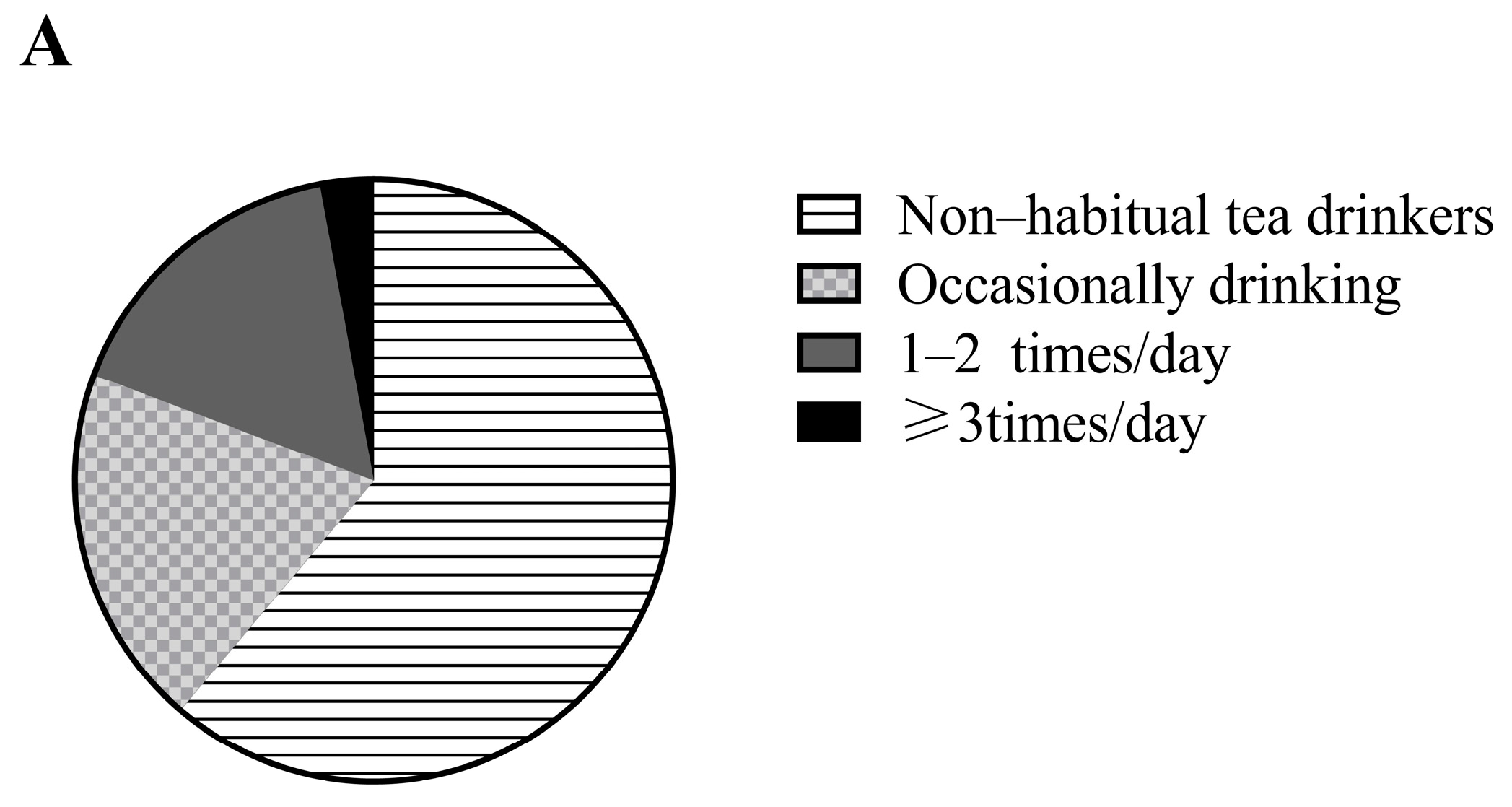

| Baseline Characteristics | Total (n = 3632) | Men (n = 2005) | Women (n = 1627) | p-Value * |
|---|---|---|---|---|
| Age, years | 57.04 ± 8.29 | 58.15 ± 8.57 | 55.67 ± 7.71 | <0.001 |
| Han ethnicity | 3417 (94.1) | 1891 (94.3) | 1526 (93.8) | 0.276 |
| Current smoking (yes) | 1480 (40.7) | 1167 (58.2) | 313 (19.2) | <0.001 |
| Current drinking (yes) | 978 (26.9) | 923 (46.0) | 55 (3.4) | <0.001 |
| Married | 3593 (98.9) | 1972 (98.4) | 1621 (99.6) | <0.001 |
| Primary and lower education | 1966 (54.1) | 948 (47.3) | 1018 (62.6) | <0.001 |
| Sleep duration (h/day) | <0.001 | |||
| ≤7 | 1893 (52.2) | 963 (48.1) | 930 (57.3) | |
| 7–8 | 991 (27.3) | 575 (28.7) | 416 (25.6) | |
| 8–9 | 482 (13.3) | 300 (15.0) | 182 (11.2) | |
| >9 | 261 (7.2) | 165 (8.2) | 96 (5.9) | |
| Annual income (CNY/year) | 0.025 | |||
| ≤5000 | 454 (12.5) | 277 (13.8) | 177 (10.9) | |
| 5000–20,000 | 2111 (58.2) | 1154 (57.6) | 957 (58.9) | |
| >20,000 | 1064 (29.3) | 572 (28.6) | 492 (30.2) | |
| Physical activity | <0.001 | |||
| Low | 1234 (34.3) | 562 (28.3) | 672 (41.6) | |
| Moderate | 647 (18.0) | 350 (17.6) | 297 (18.4) | |
| Severe | 1719 (47.8) | 1073 (54.1) | 646 (40.0) | |
| Diet score (>3) | 1776 (48.9) | 1121 (55.9) | 655 (40.2) | <0.001 |
| SBP, mmHg | 139.93 ± 22.76 | 142.57 ± 22.61 | 136.67 ± 22.53 | <0.001 |
| DBP, mmHg | 80.65 ± 11.16 | 82.34 ± 11.09 | 78.57 ± 10.88 | <0.001 |
| BMI, kg/m2 | 23.43 ± 3.08 | 23.54 ± 2.85 | 23.31 ± 3.34 | 0.026 |
| HDL-C, mmol/L | 1.54 ± 0.39 | 1.52 ± 0.42 | 1.57 ± 0.35 | <0.001 |
| TG, mmol/L | 1.14 ± 0.63 | 1.14 ± 0.67 | 1.14 ± 0.58 | 0.524 |
| FPG, mmol/L | 5.57 ± 1.06 | 5.67 ± 1.17 | 5.43 ± 0.88 | <0.001 |
| eGFR, mL/min per 1.73 m2 | 92.45 ± 13.63 | 92.98 ± 13.06 | 91.79 ± 14.27 | 0.009 |
| Uric acid, μmoI/L | 279.53 ± 77.18 | 313.33 ± 75.16 | 237.87 ± 56.52 | <0.001 |
| AST/ALT | 1.18 (0.95, 1.43) | 1.14 (0.90, 1.41) | 1.22 (1.00, 1.45) | <0.001 |
| Follow-up Characteristics | ||||
| SBP, mmHg | 135.99 ± 21.60 | 139.14 ± 21.34 | 132.10 ± 21.29 | <0.001 |
| DBP, mmHg | 80.01 ± 11.61 | 81.85 ± 11.68 | 77.74 ± 11.12 | <0.001 |
| BMI, kg/m2 | 23.64 ± 3.55 | 24.28 ± 3.28 | 22.86 ± 3.72 | <0.001 |
| HDL-C, mmol/L | 1.46 ± 0.38 | 1.44 ± 0.39 | 1.48 ± 0.37 | 0.005 |
| TG, mmol/L | 1.41 ± 1.10 | 1.39 ± 1.21 | 1.44 ± 0.96 | 0.221 |
| FPG, mmol/L | 5.60 ± 1.28 | 5.75 ± 1.48 | 5.41 ± 0.95 | <0.001 |
| eGFR, mL/min per 1.73 m2 | 91.24 ± 12.24 | 90.55 ± 12.05 | 92.07 ± 12.41 | <0.001 |
| Uric acid, μmoI/L | 284.10 ± 75.39 | 314.60 ± 73.93 | 246.50 ± 58.26 | <0.001 |
| AST/ALT | 1.36 (1.09, 1.68) | 1.33 (1.04, 1.65) | 1.39 (1.14, 1.70) | <0.001 |
| MetS | 1188 (24.0) | 572 (22.2) | 616 (25.8) | 0.203 |
| Non-Habitual Tea Drinkers (n = 2223) | Occasional Drinkers (n = 708) | 1–2 Times/Day (n = 596) | ≥3 Times/Day (n = 105) | p-Value ! | |
|---|---|---|---|---|---|
| Baseline Characteristics | |||||
| Gender (women) | 1216 (54.7) | 232 (32.8) | 165 (27.7) | 14 (13.3) | <0.001 |
| Age, years | 57.12 ± 8.32 | 56.43 ± 8.24 | 57.41 ± 8.27 # | 57.33 ± 7.90 | 0.143 |
| Han ethnicity | 2116 (95.2) | 667 (94.2) | 540 (90.6) | 94 (89.5) | <0.001 |
| Current smoking (yes) | 749 (33.7) | 322 (45.5) | 335 (56.2) | 74 (70.5) | <0.001 |
| Current drinking (yes) | 412 (18.5) | 247 (34.9) | 265 (44.5) | 54 (51.4) | <0.001 |
| Marriage status (yes) | 2204 (99.1) | 705 (99.6) | 581 (97.5) | 103 (98.1) | 0.001 |
| Primary and lower education | 1290 (58.0) | 345 (48.7) | 289 (48.5) | 42 (40.0) | <0.001 |
| Sleep duration (h/day) | <0.001 | ||||
| ≤7 | 1252 (56.4) | 354 (50.2) | 248 (41.6) | 39 (37.1) | |
| 7–8 | 545 (24.5) | 199 (28.2) | 207 (34.7) | 40 (38.1) | |
| 8–9 | 267 (12.0) | 99 (14.0) | 104 (17.4) | 12 (11.4) | |
| >9 | 157 (7.1) | 53 (7.5) | 37 (6.2) | 14 (13.3) | |
| Annual income (CNY/year) | 0.001 | ||||
| ≤5000 | 273 (12.3) | 72 (10.2) | 97 (16.3) | 12 (11.4) | |
| 5000–20,000 | 1279 (57.6) | 414 (58.5) | 363 (60.9) | 55 (52.4) | |
| >20,000 | 668 (30.1) | 222 (31.4) | 136 (22.8) | 38 (36.2) | |
| Physical activity | 0.019 | ||||
| Low | 803 (36.5) | 226 (32.1) | 177 (29.9) | 28 (27.2) | |
| Moderate | 379 (17.2) | 124 (17.6) | 120 (20.3) | 24 (23.3) | |
| Severe | 1021 (46.3) | 353 (50.2) | 294 (49.7) | 51 (49.5) | |
| Diet score (>3) | 1028 (46.2) | 360 (50.8) | 331 (55.5) | 57 (54.3) | <0.001 |
| SBP, mmHg | 137.47 ± 21.96 | 139.54 ± 21.25 * | 148.59 ± 24.84 ^,# | 145.62 ± 24.30 &,$ | <0.001 |
| DBP, mmHg | 79.76 ± 10.84 | 81.15 ± 10.88 * | 82.75 ± 11.87 ^,# | 84.13 ± 12.97 &,$ | <0.001 |
| BMI, kg/m2 | 23.15 ± 3.15 | 23.67 ± 2.94 * | 24.10 ± 2.85 ^,# | 24.04 ± 3.03 &,$ | <0.001 |
| HDL-C, mmol/L | 1.49 ± 0.35 | 1.54 ± 0.38 * | 1.70 ± 0.46 ^,# | 1.65 ± 0.52 &,$ | <0.001 |
| TG, mmol/L | 1.14 ± 0.62 | 1.20 ± 0.73 | 1.07 ± 0.53 ^,# | 1.11 ± 0.56 | 0.006 |
| FPG, mmol/L | 5.56 ± 0.98 | 5.59 ± 1.02 | 5.54 ± 1.15 | 5.61 ± 1.91 | 0.844 |
| eGFR, mL/min per 1.73 m2 | 90.68 ± 13.34 | 93.89 ± 14.58 * | 97.10 ± 12.33 ^,# | 93.81 ± 12.62 &,** | <0.001 |
| Uric acid, μmoI/L | 272.72 ± 74.19 | 292.76 ± 84.63 * | 284.84 ± 75.12 ^ | 304.36 ± 80.14 &,** | <0.001 |
| AST/ALT | 1.15 (0.93, 1.40) | 1.17 (0.92, 1.42) | 1.25 (1.03, 1.50) | 1.31 (1.00, 1.62) | <0.001 |
| Follow-up Characteristics | |||||
| SBP, mmHg | 135.20 ± 21.12 | 135.48 ± 20.73 | 139.26 ± 23.73 ^,# | 137.54 ± 21.60 | 0.001 |
| DBP, mmHg | 79.40 ± 11.32 | 80.26 ± 11.05 | 81.65 ± 12.85 ^,# | 81.77 ± 13.06 & | <0.001 |
| BMI, kg/m2 | 23.34 ± 3.60 | 24.07 ± 3.47 * | 24.15 ± 3.34 ^ | 24.35 ± 3.49 & | <0.001 |
| HDL-C, mmol/L | 1.46 ± 0.37 | 1.45 ± 0.40 | 1.44 ± 0.39 | 1.50 ± 0.42 | 0.437 |
| TG, mmol/L | 1.36 ± 0.94 | 1.48 ± 1.36 * | 1.51 ± 1.33 ^ | 1.48 ± 0.94 | 0.007 |
| FPG, mmol/L | 5.58 ± 1.28 | 5.58 ± 1.19 | 5.69 ± 1.35 ^ | 5.62 ± 1.46 | 0.235 |
| eGFR, mL/min per 1.73 m2 | 91.52 ± 12.33 | 91.65 ± 11.85 | 90.36 ± 11.97 ^ | 87.40 ± 13.47 &,$,** | 0.001 |
| Uric acid, μmoI/L | 274.77 ± 72.80 | 296.00 ± 76.47 * | 299.45 ± 76.41 ^ | 314.16 ± 83.52 &,$ | <0.001 |
| AST/ALT | 1.36 (1.09, 1.69) | 1.31 (1.05, 1.60) | 1.42 (1.12, 1.71) | 1.42 (1.10, 1.73) | 0.001 |
| MetS | 557 (25.1) | 196 (27.7) | 166 (27.9) | 26 (24.8) | 0.359 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.; Wang, B.; Li, G.; Guo, X.; Yang, H.; Sun, Y. Habitual Tea Consumption Increases the Incidence of Metabolic Syndrome in Middle-Aged and Older Individuals. Nutrients 2023, 15, 1448. https://doi.org/10.3390/nu15061448
Yu S, Wang B, Li G, Guo X, Yang H, Sun Y. Habitual Tea Consumption Increases the Incidence of Metabolic Syndrome in Middle-Aged and Older Individuals. Nutrients. 2023; 15(6):1448. https://doi.org/10.3390/nu15061448
Chicago/Turabian StyleYu, Shasha, Bo Wang, Guangxiao Li, Xiaofan Guo, Hongmei Yang, and Yingxian Sun. 2023. "Habitual Tea Consumption Increases the Incidence of Metabolic Syndrome in Middle-Aged and Older Individuals" Nutrients 15, no. 6: 1448. https://doi.org/10.3390/nu15061448
APA StyleYu, S., Wang, B., Li, G., Guo, X., Yang, H., & Sun, Y. (2023). Habitual Tea Consumption Increases the Incidence of Metabolic Syndrome in Middle-Aged and Older Individuals. Nutrients, 15(6), 1448. https://doi.org/10.3390/nu15061448






