Abstract
Arterial stiffness is often increased in overweight/obese subjects before the development of hypertension. It is also one of the earliest indicators of increased cardiovascular disease risk and can be considered a good predictor of the development of subclinical cardiovascular dysfunction. Arterial stiffness is a significant prognostic factor influencing cardiovascular risk, which dietary habits can modify. Obese patients should use the caloric-restricted diet because it augments aortic distensibility, diminishes pulse wave velocity (PWV), and increases the activity of endothelial nitric oxide synthases. High intake of saturated fatty acids (SFA), trans fats, and cholesterol, typical for the Western diet, impairs endothelial function and raises brachial-ankle PWV. The replacement of SFA with monounsaturated (MUFA) or polyunsaturated fatty acids (PUFA) derived from seafood and plants diminishes the risk of arterial stiffness. The dairy product intake (excluding butter) decreases PWV in the general population. The high-sucrose diet causes toxic hyperglycemia and increases arterial stiffness. Complex carbohydrates with a low glycemic index (including isomaltose) should be recommended to keep vascular health. The high sodium intake (>10 g/day), particularly associated with low potassium consumption, has a deleterious effect on arterial stiffness (↑ baPWV). Since vegetables and fruits are good sources of vitamins and phytochemicals, they should be recommended in patients with high PWV. Thus, the dietary recommendation to prevent arterial stiffness should be similar to the Mediterranean diet, which is rich in dairy products, plant oils, and fish, with a minimal red meat intake and five servings of fruits and vegetables daily.
1. Introduction
Currently, obesity is a serious chronic disease, which constitutes not only a health problem but also a social and economic one. The number of overweight and obese subjects is still increasing. This is why the global nature of the obesity epidemic was recognized by the WHO, in 1997. In 2022, the World Health Organization (WHO) reported that there are about 2 billion adults who are overweight, whilst 650 million are obese. It is expected that 2.7 billion adults will be overweight, and over 1 billion will be obese by 2025 if these rates do not slow down [1,2].
Both being overweight and obese have a huge impact on the development of many diseases such as nonalcoholic fatty liver, type 2 diabetes, cardiovascular disease (CVD), hypertension and stroke, various forms of cancer, musculoskeletal diseases, chronic kidney disease, sleep apnea, as well as mental health problems. Additionally, overweight and obese subjects are also about three times more likely to be hospitalized for COVID-19. They also have an increased risk of intensive care unit admission, invasive mechanical ventilation, and death [1,2,3,4,5].
Additionally, it has been shown that subjects with obesity are likely to have an increase in aortic stiffness, independent of blood pressure level, ethnicity, and age [6]. Moreover, arterial stiffness is often increased in overweight/obese subjects before the development of hypertension [7]. In their study, Kim HL et al. have shown that arterial stiffness may be more strongly associated with abdominal obesity than with overall obesity [8]. It has also been shown that body fat percentage is directly associated with arterial stiffness in long-lived populations, consistent with individuals with lean muscle having more elastic arteries [9]. An increase of two percentage points in the average BMI of society reduces the average life expectancy by one year [10].
Arterial stiffness is an independent risk factor, contributing to the development, progression, and mortality of CVD. It is also one of the earliest indicators of increased CVD risk and can be considered a good predictor of the development of subclinical cardiovascular dysfunction [11,12]. Furthermore, on the one hand, arterial stiffness increases with cardiovascular ageing, but on the other hand, it may be accelerated and occurs earlier in the presence of obesity, insulin resistance, and diabetes [13]. However, increased arterial stiffness is also observed in young people (age 10–24 years) with obesity and obesity-related type 2 diabetes mellitus and is an independent predictor of arterial stiffness even after adjusting for cardiovascular risk factors [14]. Furthermore, the development of vascular stiffness due to obesity is more common in women than in men, and obese and insulin-resistant women lose the protection of the CVD associated with estrogen [15].
Being overweight and obese is related to arterial stiffness and is associated with hypertension. Interestingly, isolated systolic hypertension with high arterial stiffness responds weakly to antihypertensive drugs. As a result, the velocity of the aortic pulse wave (PWV) does not reduce significantly after hypotensive management [16]. A lower response to pharmacological treatment is observed in patients with a high grade of arterial stiffness than in subjects with more distensible arteries. In such cases, non-pharmacological interventions can be beneficial. The study by Dengo et al. has shown that reduced body mass during four months in overweight and obese patients significantly improves aortic and carotid stiffness [17]. Thus, dietary interventions leading to body mass reduction are required to decrease arterial stiffness significantly by improving neuromodulation and blood pressure control [18,19,20].
In this review, we attempt to discuss several aspects related to the association between overweight and obesity and arterial stiffness as well as the influence of body mass reduction and dietary interventions on arterial stiffness on the basis of the most recently published studies.
2. The relationship between Obesity, Oxidative Stress, and Inflammation
Obesity is a pathological condition, which is connected with excess calorie intake, and consequent accumulation of free fatty acids (FFA) and carbohydrates. Consequently, the increased oxidative stress markers production occurs via different mechanisms such as activation of protein kinase C (PKC), polyol and hexosamine pathways, and synthesis of superoxide anion [21]. Among oxidative stress markers we distinguish reactive oxygen species (ROS) such as superoxide (O2−), hydrogen peroxide (H2O2), and hydroxyl radical (OH−)]. In physiological conditions, oxidative stress markers are neutralized by antioxidants such as superoxide dismutase (SOD) and glutathione peroxidase (GPX) [22]. Among obese subjects, there is an imbalance between antioxidants and oxidative stress markers, which is not only the result of enhanced ROS concentration but also the result of lowered SOD activity [23]. The accumulation of ROS contributes to cell structure damage such as proteins, DNA, and membranes, and to consequent endothelial dysfunction [24]. Moreover, O2− reacts with nitric oxide (NO) and consequently decreases its bioavailability. In addition, the product of mentioned reaction (ONOO−) promotes the dysfunction of endothelial nitric oxide synthase (eNOS), which causes a further reduction in NO concentration [24]. Increased concentration of FFA contributes to enhanced β-oxidation in mitochondria with a consequent increase in the NADH/NAD+ ratio and increased concentration in advanced glycosylation end products (AGE), and increased activation of PKC, which is responsible for inhibition of eNOS and decreased NO [25]. Increased inflammatory state and oxidative stress are strongly linked with each other and with obesity. Excessive levels of triglycerides and lipids stored in adipocytes among obese patients contribute to hyperplasia and hypertrophy of adipocytes [26]. Enhanced adipocytes induce the elevation of IL-6, IL-8, and leptin and decrease the level of adiponectin, which leads to the consequent accumulation of inflammatory factors in adipose tissue [27]. In addition, macrophages, which are the most numerous immune cells in adipose tissue, consist of two subtypes: M1, with an inflammatory profile, and M2, with an immunosuppressive feature [28]. The pro-inflammatory markers, such as tumor necrosis factor-alpha (TNF-a), and interleukin (IL)-6, secreted from M1 macrophages, induce increased ROS production in adipose tissue of obese patients [29].
3. Arterial Stiffness among Overweight and Obese Subjects
It is known that arterial stiffness increases among obese and overweight individuals, especially with excess abdominal fat [7,21,22] (Figure 1). Increased arterial stiffness contributes to the development of hypertension, due to structural modifications in the vessels and changes in their elasticity, capacity, and resistance, with a consequent loss of vascular compliance [14].
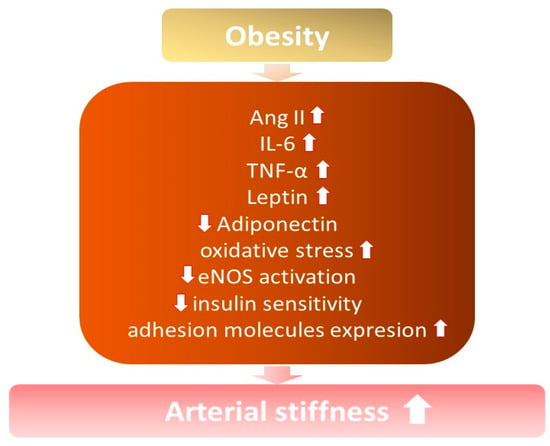
Figure 1.
The influence of obesity on the development of increased arterial stiffness. ↓—decrease; ↑ —increase; Ang II—angiotensin II; IL-6—interleukin 6; TNF-α—tumor necrosis factor; eNOS—endothelial nitric oxide synthase.
3.1. Inflammatory Mediators
Interleukin 6 (IL-6) and tumor necrosis factor α (TNF-α) are the main inflammatory factors, whose level is elevated among obese patients. IL-6 is produced by different cells such as adipocytes and macrophages, which infiltrate adipose tissue and endothelial cells, while TNF—α is primarily a product of macrophages [23,24]. Both contribute to insulin resistance and impaired insulin metabolic signaling, which is crucial for normal endothelial functioning, which is explained below [25].
IL-6 is involved in the regulation of endothelial function by improving the upregulation of vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule 1 (ICAM-1), which have pro-atherogenic facilities [24]. Moreover, IL-6 turned out to be a stimulant of production matrix metalloproteinases, which cause plaque ruptures and changes in arterial vulnerability [26]. Monoclonal antibodies are becoming more and more popular for their therapeutic purposes, especially the IL-6 antagonist, which was widely used during the COVID-19 pandemic [27]. The treatment of atherosclerosis by IL-6 antagonist—tocilizumab—is controversial, while on the one hand, the endothelial function is improved, and arterial stiffness is reduced [28]. On the other hand, the use of tocilizumab in lymphoproliferative disorders causes weight gain and dyslipidemia, which are commonly known as the main risk factors for atherosclerosis [29]. TNF-α, similarly to IL-6, is responsible for the stimulation of VCAM-, ICAM-1, and monocyte chemoattractant protein-1 (MCP-1) production and consequent endothelial dysfunction induced by the synthesis of ROS and inflammatory factors [30]. Treatment with TNF- α antagonist—infliximab—on rodents resulted in a reduction in inflammatory state and protection against insulin resistance and diet-induced obesity [31]. In contrast, studies conducted among participants with insulin resistance did not confirm a positive influence on insulin sensitivity and endothelial function; however, the inflammatory state was reduced [32]. However, obesity is connected with a chronic low-grade inflammatory state, which is confirmed by an increased level of inflammatory markers, especially C-reactive protein, and IL-6, which are significantly higher among obese nonmorbid patients and positively correlate with BMI [33,34].
3.2. Hyperinsulinemia
It is known that obesity, or even overweight, causes impaired insulin release, with hyperinsulinemia, in the beginning, causing the gradual worsening function of β cells of islets of Langerhans. Normal insulin metabolism plays an essential role in maintaining proper endothelial function [18]. Hyperinsulinemia, which is the result of insulin resistance, causes decreased activation of eNOS through an insulin receptor substrate (IRS)-1/phosphatidylinositol 3-kinase (PI3K) signaling/protein kinase B (Akt)-mediated pathway, a decrease in NO bioavailability, and a consequent increase in arterial stiffness [35]. Moreover, there is a correlation between hyperinsulinemia and impaired tissue transglutaminase (TG2) functioning in developing arterial stiffness. Increased TG2 activation induces remodeling and increased resistance in arteries. Additionally, it decreases the bioavailability of NO as a result of impaired S-nitrosylation of TG2 and increased excretion to the cell surface [35].
3.3. Renin-Angiotensin-Aldosterone System Activation
Obesity, mainly caused by excess energy intake, especially due to a diet that is not rich in carbohydrates and a high-fructose diet, is associated with increased angiotensin II (ANG II) production by different types of adipose tissue, visceral as well as perivascular adipose tissue (PVAT) [36]. Ang II induces vasoconstriction of vessels and, in increased concentrations, is responsible for the activation of immune cells and the consequent production of cytokines and inflammatory factors [37]. Moreover, increased ANG II causes disturbances in phosphorylation and activation of eNOS through IRS-1/2/PI3K/AKT signaling, which causes decreased production and bioavailability of NO [38]. In addition, Ang II induces enhanced production and release of endothelium-dependent vasoconstrictor, especially endothelin-1 by increased preproendothelin-1, which is consequently changed into endothelin-1, and COX-1-prostanoid by endothelial cells [39,40]. In addition, the role of angiotensin-II type 1 receptors (AT1R) and angiotensin-II type 2 receptors (AT2R) in maintaining proper endothelial function is important. Both receptors are expressed in vascular endothelial cells, while activation of AT1R induces vasoconstriction, and activation of AT2R promotes vasodilation [38]. Persistent activation of AT1R by ANG II induces a decrease in eNOS expression and a consequent decrease in NO concentration. Increased ANG II induces impaired insulin-mediated NO synthesis via up activation of AT1R [38]. Moreover, Ang II, such as aldosterone, increases arterial stiffness by promoting the proliferation of vascular smooth muscle cells (VSMCs), fibrosis, and increased collagen deposition [41].
3.4. Adipocyte-Derived Factors
The synthesis and release of factors derived from adipocytes by visceral as well as PVAT are impaired among obese patients. In addition to the factors mentioned above, there is an important change in the production and release of adiponectin and leptin [42,43].
Almabrouk et al. presented their study with mice fed a high-fat diet, and the level of adiponectin was reduced by 70% [44]. There are some data on humans which confirm the point of view that obesity decreases the level of adiponectin, and the relationship between the level of adiponectin and arterial stiffness is inversely proportional [42,43,45]. Furthermore, among obese patients, the peroxisome proliferator-activated receptor gamma (PPAR-γ), which is responsible for the differentiation of adipocytes, is down-regulated, causing a decrease in adiponectin [46]. As a consequence, the anticontractile effect of adiponectin, caused by the activation of adenosine monophosphate-activated protein kinase (AMPK) and consequent phosphorylation of eNOS, is weakened.
The level of leptin among obese patients is enhanced, while the synthesis of leptin by adipocytes is directly proportional to its size [43]. Leptin has anticontractile properties by increasing NO synthesis; however, prolonged exposure of the endothelium to leptin causes a decrease in NO bioavailability and a consequent inverse effect on vascular tone [47]. In addition, leptin provokes platelet aggregation and atherothrombosis, stimulates ROS synthesis, and causes consequent endothelial dysfunction, resulting in increased arterial stiffness [48,49].
4. Influence of Body Mass Reduction on Arterial Stiffness in Overweight and Obese Subjects
The randomized, controlled study conducted on overweight and obese middle-aged and older adults showed that intentional weight loss (hypocaloric diet alone without increases in physical activity) reduces large artery stiffness. This effect is independent of a reduction in abdominal adiposity and is associated with accelerated arterial stiffening and an increased risk of adverse cardiovascular events [17,50,51,52,53]. Thus, body mass fat loss is an independent predictor of arterial stiffness [17]. This effect is observed with modest weight losses of 5% to <10%, associated with a significant decrease in cardiovascular risk in one year. The more considerable weight loss has more significant benefits [50]. For example, modest weight loss markedly improves glycemic control [54], blood pressure [51], and triglycerides [52], and increases HDL cholesterol [53]. The effects of body mass reduction on CVD risk factors are initially the biggest, even if weight losses are maintained for a long time [55].
5. The Impact of Diet on Arterial Stiffness
Since arterial stiffness influences vascular ageing and is an independent predictor of CVD and related mortality, the analyses of factors that may decrease its value are a reason for scientific curiosity [56,57]. An increase in vascular stiffness mediates the adverse effects of major cardiovascular risks, including atherosclerosis, hypertension, and diabetes. Thus, preventing arterial stiffness seems reasonable in improving cardiovascular health. In the literature, various nutritional interventions regarding arterial stiffness have recently been widely discussed [58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133]. The most important include caloric restriction (CR), diets with reduced saturated fat, simple carbohydrates, and sodium intake, and dietary intervention with vitamin supplementation or antioxidative nutrients such as polyphenols. On the contrary, a high-energy and unbalanced diet results in hyperglycemia, elevated non-sterified fatty acid levels, and insulin resistance leading to oxidative stress and inflammation and, consequently, contributing to endothelial dysfunction and the progression of arterial stiffness progression [58].
5.1. Caloric Restriction
Arterial stiffness is a significant prognostic factor of CVD, influenced by dietary habits. During the planning of diets, it is crucial to have adequate caloric intake, which improves multiple metabolic parameters and vascular health [59,60,61,62,63,64,65,66]. Ahmet 2011, For example, CR reduces the risk of obesity, diabetes, and CVD and thus increases the lifespan in human and animal models [66]. Food restriction prolongs life in rodents by revealing antiaging effects on various physiologic and pathologic processes [59,60]. CR delays age-related decline in cardiac function, fibrosis, and arterial stiffness [59]. Decreased energy intake prevents increases in arterial stiffness associated with ageing through the inhibition of unbeneficial changes in collagen and elastin [61]. Furthermore, a caloric-restricted diet might influence the ageing process of the arterial wall through various structural alterations in cells and matrix. For example, it delays age-related degenerative features by decreasing the collagenases, increasing the elastin amount, and preserving the vascular smooth muscle in the aorta [60]. Consequently, a larger increase in aortic distensibility and a decrease in PWV are observed [59,60]. Another mechanism present during CR is the possibility of increased activity of endothelial nitric oxide synthases (eNOS). This enzyme increases nitric oxide bioavailability and protects against oxidative stress. As a result, basal NO production increases the distensibility of the large arteries. This mechanism underlines the endothelial role in developing arterial stiffness [62].
Weight loss in overweight and obese individuals is associated with reduced arterial stiffness [63]. Modest weight loss (mean 8% of initial body weight) achieved with diet and lifestyle interventions improves PWV. If weight loss exceeds 10% of initial body weight, it improves the carotid-femoral PWV (cfPWV) of 0.8 m/s [64]. A similar effect of body mass reduction is observed after menopause. The analysis of postmenopausal women obese women has shown that a hypocaloric diet decreases brachial-ankle PWV (baPWV) mainly by reducing femoral-ankle PWV, and this reduction is related to the loss of truncal fat in obese postmenopausal women [65].
Many data showed that caloric CR is strictly related to reduced risk of obesity and decreased arterial stiffness. However, scientific curiosity arises with the question if there is an association between specific dietary components, including macroelements (fat, protein, carbohydrate), microelements, vitamins, and some nutrients, such as polyphenols and carotenoids, on arterial stiffness. All of these nutrients may influence arterial stiffness and change endothelial function. Thus, in this article, the analysis of what kinds of dietary interventions can decrease arterial stiffness and, therefore, improve vascular health and overall cardiovascular risk is analyzed.
5.2. Fat and Fatty Acids Influence Arterial Stiffness
Several studies underline that both the quantity and quality of fat intake influence the CVD risk [67,68,69] and affect arterial stiffness [69,70,71]. The recommendations of the WHO guideline underline the need to limit fat intake, mainly saturated fatty acids (SFA) and cholesterol, to delay or prevent CVD (Table 1). Simultaneously, increased consumption of unsaturated fatty acids should be considered during diet planning (both polyunsaturated—PUFA and/or monounsaturated fatty acids—MUFA) [72]. Recent data meticulously analyzed the impact of dietary fat quantity and quality on arterial stiffness [69,70,71]. Animal studies showed that high-fat and high-sucrose diets cause the of obesity before or alongside arterial stiffness. In contrast, a normal caloric diet induces weight loss in obese animals and decreases arterial stiffness [63].
One of the leading factors influencing cardiovascular risk is a Western diet, rich in saturated and refined fats, and high-sucrose products [73]. SFA are the most deleterious for vascular health, leading to arterial stiffness. The intake of SFA and trans-fat is associated with a higher risk of CVD [67]. On the contrary, low consumption of SFA for at least two years is associated with a 17% reduction in coronary heart disease events (CHD) [68] and decreases mortality from atherosclerotic vascular diseases in elderly Australian women (n = 1469) [69]. The impairment of endothelial function is observed after high-fat meals reach SFA, compared to low-fat meals [71]. A diet containing saturated fat has an unbeneficial influence on vascular health. Such a diet raises brachial-ankle pulse wave velocity (baPWV) in Japanese type 2 diabetic outpatients (n = 733) without a history of CVD. On the contrary, lower intake of SFA is correlated with persistently higher baPWV (measured at baseline at 2 and 5 years) [74]. Moreover, decreased amounts of dietary saturated fats by replacing them with polyunsaturated vegetable oil reduced CVD by 30%, and this outcome is similar to the statin-induced reduction [76]. A similar effect is observed if SFA is substituted by unsaturated fatty acids (derived from fish or plants), and such dietary modifications positively influence arterial stiffness [75].
Conversely to SFA, unsaturated fatty acids have a beneficial influence on vascular health. One of the unsaturated fatty acids is MUFA (mainly oleic acid), which is present in nuts, avocados, unrefined (virgin) olive oil, safflower, and canola oil [76]. The replacement of trans fat with MUFAs is inversely associated with CVD [67]. A beneficial effect is also observed if MUFA substitutes for SFA, and this modification causes a lower arterial pulse pressure in diabetic patients (The National Health and Nutrition Examination Survey (NHANES), 2007–2008) [70]. Nevertheless, the exact role of MUFA is not fully defined because some data shows that the replacement of SFAs with MUFA did not cause cardiovascular risk reduction [68].
In addition to MUFA, PUFA intake is also considered beneficial for vascular health. Among PUFA, both n-6 fractions (linoleic acid in vegetable oils) and n-3 fractions (seafood and plant sources) reduce CHD risk [77,78]. The study of Guasch-Ferre et al. showed that the intake of MUFAs and PUFAs in general populations is related to a lower risk of CVD and death [67]. Furthermore, replacing SFA with PUFA results in a 27% reduction in the risk of CHD prevalence [68]. In contrast, the study by Vafeiadou et al. has shown that the substitution of 9.5–9.6% of total energy dietary SFAs with MUFA or n-6 PUFA did not significantly affect the percentage of flow-mediated dilatation. However, such a diet has beneficial effects on biomarkers affecting arterial stiffness, such as E-selectin as serum lipids (fasting serum total cholesterol LDL and CH to HDL ratio) [79].
Many studies have shown that fish consumption has a beneficial influence on myocardial infarction [80] and hypertension [81] and, thus, lower CHD incidence and mortality [82]. The analysis of the Japanese population (n = 41,578, aged 40 to 59) who were free of prior diagnosis of CVD showed that high frequency or a large amount of fish intake (eight times per week or 180 g/day) versus lowest (23 g/day) reduced risk of nonfatal coronary events [83]. Fish and fish oils include PUFA, which reveals a beneficial influence on cardiovascular risk. PUFA, mainly linoleic acid (18: 2n − 6), and n-3 PUFA have a beneficial effect on vascular function. Among PUFA, the essential fatty acids eicosapentaenoic EPA (20: 5n − 3) and docosahexaenoic DHA (22: 6n − 3) reduce blood pressure and increase endothelium-dependent vasodilation in diabetic and hypertensive patients [71]. Observational studies report decreased arterial stiffness (measured by PWV of the aorta, intima-media thickness of the carotid artery, and atherosclerotic plaques in individuals who habitually eat larger amounts of fish [84]. Some studies suggest that the effect of omega-3 fatty acids may be related to age. For example, 12 weeks of daily omega-3 fatty acids supplementation decreases carotid-femoral PWV in older (66 ± 2-year-old) but not young (25 ± 1-year-old) healthy adults [85]. Moreover, in obesity, the 25% energy deficit diet, including 4 g/d of essential fatty acids—EFA (46% EPA and 38% DHA)—supplementation, improves metabolic parameters and arterial stiffness in obese participants much more than the caloric-restriction diet alone. The changes in systolic blood pressure (SBP), heart rate, plasma TGs, and large and small artery elasticity were significantly greater in the CR with EFA intake. This study shows that supplementation with n-3 EFA improves the elasticity of large and small arteries independently of weight loss in obese adults [86].
One of the most well-analyzed nutritional diets is the Mediterranean one. This diet has received particular scientific interest in recent years as it protects against CVD, particularly compared to a Western diet. The health benefits are related to the balanced composition of macronutrients (carbohydrates, proteins, and fats) [87]. The Mediterranean diet is characterized by a moderate consumption of lean meat and fish with a minimal intake of red or processed meat, minimalizing saturated fat intake [88] This diet habit is associated with a reduction in all-cause and cardiovascular mortality risk caused by decreased inflammation, improvement of endothelial function, and flow-mediated dilatation [89]. Thus, this diet should be recommended in patients with arterial stiffness.

Table 1.
Dietary effect of various nutrients on arterial stiffness and cardiovascular risk.
Table 1.
Dietary effect of various nutrients on arterial stiffness and cardiovascular risk.
| Nutrients Influence Arterial Stiffness | |||
|---|---|---|---|
| Component | Dietary Modification | Health Effect/Influence on Arterial Stiffness | References |
| Fat | high-fat and high-sucrose diet (animal studies) | ↑ obesity ↑ arterial stiffness | [64] |
| ↑ SFA in the diet | ↑ arterial stiffness | [67] | |
| high SFA intake | ↑ baPWV at baseline and at 2 and 5 years (diabetic patients) | [74] | |
| substitution of 9.5–9.6% total energy dietary SFAs with either MUFA or n-6 PUFA | no significant effect on the flow-mediated dilatation beneficial effects on biomarkers of arterial stiffness: ↓E-selectin, ↓ fasting serum lipids (CH. LDL, and CH/HDL ratio) | [79] | |
| SFA replacement with MUFA and carbohydrates | ↓ arterial pulse pressure | [70] | |
| fish oils consumption with high EPA and DHA in diabetic hypertensive patients | ↓ blood pressure ↑ endothelium-dependent vasodilation | [70] | |
| a habitual large amount of fish consumption | ↓ arterial stiffness ↓ pulse wave velocity of the aorta ↓ intima-media thickness of the carotid artery ↓ atherosclerotic plaques | [84] | |
| the 25% energy deficit diet, including 4 g/d of EFA (46% EPA and 38% DHA) in obese patients | ↑ large and small artery elasticity (about 20% increase) | [86] | |
| Protein | vegetarian diet vs. omnivore control | ↓ PWV (−8%) in healthy male vegetarians vs. omnivore control sample (7.1 ± 0.8 and 7.7 ± 0.9 m/s, respectively) | [90] |
| vegetarian diet vs. omnivore control | a tendency to ↑ arterial stiffness in omnivores (mainly male) vs. vegetarians (PWV 7.0 ± 1.5 vs. 6.8 ± 1.1 m/s, respectively) limited effect in premenopausal women | [91] | |
| dairy products | inverse correlation with PWV | [92] | |
| milk consumption | ↓SBP (inverse dose-dependent effect) not associated with arterial stiffness | [93] | |
| dairy products (milk, cheese, cream excluding butter) cohort prospective study (22.8 years observation) | high consumption of dairy products ≥ ↓ 1.8% A. no detrimental to arterial stiffness and metabolic markers (insulin, TG, CH) | [93] | |
| casein-derived biologically active tripeptides in dairy products | ↓ angiotensin formation ≥ ↓ HA risk ≥ ↓ arterial stiffness | [94] | |
| dairy products containing ruminant-derived fatty acids (14:0, 15:0, 16:0, 17:0, and 17:1) | no influence on serum lipoproteins, PWV, central blood pressure, and AI. | [95] | |
| Carbohydrate | postprandial glucose in non-diabetic patients | ↑ postprandial glucose concentration ≥ ↑ arterial stiffness with age (measured by CAVIs) postprandial glycemia is an independent predictor of CAVI values in men and women over 50 years old | [96] |
| The high-sucrose diet in diabetic patients with kidney impairments (albuminuria) | postprandial hyperglycemia ≥ ↑ brachial PWV of intermediate-sized arteries (30 min before breakfast and up to 240 min after breakfast) | [94] | |
| low-carbohydrate diets | ↓ serum glucose and lipid level and ↓ aortic stiffness within a short time (four weeks) ≥ ↓ CVD and ↓ diabetes risk | [98,99,100] | |
| dietary carbohydrate restriction (about 645 kcal/day energy deficit) | ↓ body mass, glucose, and lipids ↓ arterial stiffness (↓PWV, significant only in women: from 7.2 ± 03 m/s to 6.3 ± 0.3 m/s, p = 0.028) | [100] | |
| low-GI diet (breakfast) in young, healthy adults compared to high-GI food | ↓ arterial stiffness compared to high-GI food high-GI products ≥ ↑ AI and heart rate (but stratifying data by gender, this interaction remained significant for AI only in males) | [101] | |
| high-GI diet | ↑ arterial stiffness immediately after food intake | [96] | |
| ~600 kcal breakfast including fiber with various GI (fiber amount~4 vs. 20 g and GI~44 vs. 70) | high-fiber diet with low-GI ≥ ↑ FMD four hours after meal ingestion | [102] | |
| A diet containing isomaltulose vs. sucrose glucose | 25 g of isomaltulose consumption ≥ stable baPWV in 30, 60, and 90 min after ingestion compared to the state before ingestion in healthy middle-aged and older adults 25 g sucrose intake ≥ ↑ baPWV 25 g glucose ≥ ↑ baPWV at 30, 60, and 90 min after ingestion and ↑ CAVI at 60 min after glucose intake | [103,104] | |
| Elements | sodium intake: low sodium <6 g, medium 6–10 g, and high >10 g sodium daily intake on arterial stiffness. | high sodium intake (>10 g/day) ≥ ↑ arterial stiffness (baPWV ≥1400 cm/s) | [105] |
| high sodium in normotensive subjects | AIx | [106] | |
| ↓ sodium intake | ↓ blood pressure + ↓ arterial stiffness (independently of antihypertensive effects) | [107] | |
| low-salt diet | was proved (meta-analysis) ≥ a reduction of 89.3 mmol/day in sodium intake in different populations is associated with a 2.84% reduction in PWV | [108] | |
| two weeks of dietary sodium restriction in older adults | ↓ arterial stiffness (rapid improvement of large elastic artery compliance and AIx) ≥ ↓ systolic hypertension | [109] | |
| Salt restriction for 6 weeks is untreated in patients with mildly raised blood pressure | ↓carotid-femoral PWV ↓ blood pressure ↓urinary albumin and ↓albumin/creatinine ratio | [110] | |
| diet with an additional 20 or 40 mmol K(+)/d from fruit and vegetables in a group with early-stage hypertension | no change in arterial stiffness and endothelial function | [111] | |
| potassium chloride and potassium bicarbonate supplementation | ↑brachial artery flow-mediated dilatation ↓carotid-femoral PWV | [112] | |
| low dietary potassium intake in healthy young adults | ↑ wave reflection and arterial stiffness ↑ potassium excretion ≥ ↓ aortic AIx + ↓ carotid-femoral PWV | [113] | |
| low sodium-to-potassium intake ratio | ↓ aortic AIx + ↓ PWV | [113] | |
| Vitamins | Vitamin D | ↓ central arterial stiffness (about 60.0% m/s) | [114,115,116] |
| not affect arterial stiffness short (2–12 months) vitamin D supplementation (1000 IU/day to 120,000 IU/month of cholecalciferol) ≥ no effect on aortic PWV and AIx | [117,118,119] | ||
| Vitamin C | improves flow-mediated dilation ↓ central blood pressure ↓ ADMA—an endogenous eNOS inhibitor ≥ ↑ vascular stiffness | [120,121] | |
| B vitamins | insufficiency ≥ ↑ homocysteine concentration ≥ endothelial dysfunction ↑ homocysteine + ↑ uric acid levels ≥ ↑ baPWV | [122,123] | |
| phytochemicals | Flavonoids (cranberry juice consumption) | ↓ carotid-femoral PWV (immediate but no chronic vasodilatory effect | [124] |
| Soy isoflavones | ↓ PWV and improving arterial compliance | [125,126,127,128] | |
| no effect on PWV and AIx | [129,130] | ||
| carotenoids (lycopene intake) | ↓ brachial-ankle PWV healthy women ↓ PWV in Korean men | [131,134] | |
| no effect on arterial stiffness in healthy overweight volunteers | [133] | ||
↓—decrease; ↑—increase; ADMA—asymmetric dimethylarginine; AI—augmentation index; baPWV—brachial-ankle pulse wave velocity; CAVI—cardio-ankle vascular index; CH—total cholesterol; CHD—coronary heart disease; CVD—cardiovascular disease; DHA—docosahexaenoic acid; EFA—essential fatty acids; EPA—eicosapentaenoic acid; GI—glycemic index; FMD—flow-mediated dilation; HA—arterial hypertension; HDL—high density lipoproteins; HOMA—homeostasis model assessment; LDL—low density lipoproteins; MUFA—monounsaturated fatty acids; NHANES—National Nutrition and Health Examination Survey; PUFA—polyunsaturated fatty acids; SBP—systolic blood pressure; SFA—saturated fatty acids.
5.3. Protein Intake and Arterial Stiffness
The primary protein sources in the diet are meat, poultry, fish, eggs, and dairy foods. Meat and eggs contain dietary cholesterol and SFA, which deleteriously affect arterial stiffness (discuss below).
The beneficial influence of a low-fat meat diet is seen mainly in studies analyzing the impact of diets typical for omnivores and vegetarians/vegans on arterial stiffness, for example, lower PWV (−8%) in healthy male vegetarians (n = 44) compared to the omnivore control sample (n = 44; 7.1 ± 0.8 and 7.7 ± 0.9 m/s, respectively) [90]. Similarly, the study of Mayra et al. has shown a tendency for higher arterial stiffness in omnivores versus vegetarians (7.0 ± 1.5 and 6.8 ± 1.1 m/s, respectively; p = 0.073), mainly male omnivores (p = 0.006 for the effect of gender and p = 0.294 for the effect of the eating pattern effect), suggesting that the beneficial effect of the vegetarian eating pattern may be limited in premenopausal women [91].
Not every type of dietary protein contained in meat reveals a detrimental effect on arterial stiffness. For example, poultry meat is rich in protein and is characterized by low fat content, a high degree of unsaturation of fatty acids, and low cholesterol levels [134]. Its beneficial effect on human health is related to bioactive substances it contains, such as vitamins and antioxidants, and an anticipated proportion of an n-6-to-n-3 ratio close to the recommended ratio of 4:1 [135].
A good source of protein is dairy products. Many studies underline that dairy consumption may prevent or delay the onset of hypertension and is associated with a lower risk of mortality and major CVD events [136,137]. Since decreased arterial stiffness, similarly to lower blood pressure, can be influenced by dairy food intake, modifying nutritional habits can have a protective effect and increase lifespan. The longitudinal study confirmed the beneficial influence of the inverse relationship between dairy consumption and hypertension risk in the adult cohort without arterial hypertension (n = 2636 subjects). Over seventeen years of observation, the highest intake of total dairy products and low-fat/fat-free dairy foods were inversely correlated with the values of systolic and diastolic blood pressure (DBP). For yogurt, each additional serving was associated with a 6% reduced risk of incident hypertension [137]. A similar association was reported in urban and rural populations (n = 136,384 adults from 21 countries), which were observed over nine years of follow-up. That study proved that dairy consumption was associated with lower mortality risk in CVD events independently of ethnicity [136].
Dairy products not only decrease the risk of cardiovascular events and hypertension but also have a positive influence on arterial stiffness. An inverse relationship between dairy product intake and arterial stiffness measured by PWV pulse wave velocity was proved in the general population [92]. Similar data analyzing dietary habits in the UK population have shown that the highest milk intake was related to a lower SBP compared to the group without milk consumption. Furthermore, the intake of milk and dairy products was not associated with arterial stiffening [93]. Interestingly, the data of the Swedish cohort study (n = 103,256 adults) analyzed the effect of various groups of dairy products on vascular health. Surprisingly, this study shows a negative association between dairy product intake and the risk of CVD. It proves that intakes of non-fermented milk (≥ 2.5 times/day) are associated with higher all-cause mortality compared with subjects who consumed milk ≤1 time per week. However, fermented milk and cheese intake is associated with lower all-cause mortality [138].
Some disinclinations in the assessment of dairy product intake on arterial stiffening can be associated with the fat content in these foods. However, the analysis of milk, cheese, cream, and butter on aortic PWV, augmentation index (Alx), and blood pressure did not confirm the detrimental effect of dairy products on CVD risk (except butter). This large cohort prospective study (n = 2512 men aged 45 to 59) analyzed cardiovascular health followed up at 5-year intervals for a mean of 22.8 years. The AIx was 1.8% lower in subjects in the highest quartiles of dairy product intake compared with the lowest (p trend = 0.021). Consumption of dairy products, excluding butter, was not detrimental to arterial stiffness and metabolic markers (insulin, triacylglycerol, and total cholesterol) [93]. Additionally, another study established that only butter intake could be positively associated with higher all-cause mortality [138]. Moreover, a systematic review and meta-analysis have shown that dairy product consumption has no detrimental effects on arterial stiffness [92]. Another systematic review has shown that the consumption of various forms of dairy products (including high-fat dairy, milk, and yoghurt) leads to favorable or neutral associations with cardiovascular risk (e.g., stroke, CAD, and hypertension) [139].
The confusing data regarding the influence of dairy products on metabolic parameters and endothelial health can result from the numerous non-fat components in dairy fermented and non-fermented products revealing specific nutritional functions and thus explaining various health benefits. Active molecules present in dairy products are biologically active tripeptides derived from casein. They have antihypertensive properties, which probably result from the ability to reduce angiotensin formation. It has been suggested that casein-derived tripeptides may reduce arterial stiffness and improve endothelial function [94]. It is worth underlining that some dairy products also contain ruminant origin 14:0, 15:0, 16:0, 17:0, and 17:1 fatty acids, which do not influence serum lipoproteins, PWV, central blood pressure, or AIx [95]. Thus, planning a diet containing specific protein products, dairy products should be included in daily nourishment, excluding butter.
5.4. Carbohydrates and Arterial Stiffness
Arterial stiffness is affected by the quantity and quality of ingested carbohydrates, particularly simple sugars, and complex carbohydrates. Moreover, the relationship between arterial stiffness and postprandial blood glucose levels increases with age. This thesis was proved by a large Japanese study (n = 1291, age range 23–85 years), which demonstrated that the 1 h postprandial glucose level is associated with increased arterial stiffness (measured by CAVI—cardio-ankle vascular index) in non-diabetic patients. Furthermore, postprandial glycemia was an independent predictor of CAVI values in men and women over 50 years of age [96].
Not only age but also obesity and diabetes mellitus are related to more advanced arterial stiffness, which increases with carbohydrate consumption. The high-sucrose diet has a deleterious effect on vascular health, causing toxic hyperglycemia and predisposing patients to arterial stiffness development. Such changes are mainly observed in diabetic patients with kidney impairments. The analysis of glucose levels in diabetic subjects with albuminuria led to intermediate-sized arteries stiffness. The measurement of glycemia 30 min before breakfast and up to 240 min after breakfast showed that postprandial hyperglycemia increases brachial PWV [97].
On the contrary, low-carbohydrate diets reduce CVD and diabetes risk not only by decreasing serum glucose and lipid level but also by improving aortic stiffness in a short time (four weeks) [98,99,100]. Dietary carbohydrate restriction is crucial in obese patients with insulin resistance and metabolic syndrome. The decreased carbohydrate intake, which causes an energy deficit of about 645 kcal/day, leads to beneficial metabolic changes (reduced body mass, glucose, and lipids). It also diminishes arterial stiffness (reduction in PWV, significant only in women: from 7.2 ± 0.3 m/s to 6.3 ± 0.3 m/s, p = 0.028) [100].
Complex carbohydrates and fibers (such as cereals and whole meal pasta) are the main components of dietary products with a low glycemic index (GI). Such foods impact metabolic changes and modulate insulin production [140,141]. Moreover, water-soluble fibers lower the absorption of bile acids and, thus, increase the hepatic conversion of cholesterol into bile acids leading to augmented LDL uptake by the liver [141]. A low-GI diet reduces acute adverse effects on arterial stiffness compared to high-GI food in young, healthy adults (n = 40, consuming breakfast with various GI). The high GI was related to increased AIx and heart rate; however, stratifying data by sex, this interaction remained significant for AIx and increased pressure only in males [101]. Thus, changes in arterial stiffness vary between meals with high and low glycemic indexes. Artery stiffness increases immediately after food intake [96]. Breakfast consumption (~600 kcal), including carbohydrates characterized by various amounts of fiber and glycemic index—GI (fibre amount-4 vs. 20 grammes and GI-44 vs.70,resepctively)—differs from endothelial function. The high-fiber, low-GI diet significantly increased flow-mediated dilation (FMD) four hours after meal ingestion [102]. It is worth mentioning that a low-GI diet can also have a beneficial influence on fetus vascular health in women at risk of gestational diabetes. Low-GI meals were shown to influence the birth weight of the offspring, the length of the birth, and the thickness of the arterial wall in early childhood [142].
Not every simple sugar has a deleterious impact on arterial health. Recently, many studies have underlined the crucial effect of isomaltose on vascular health. Isomaltose is a naturally occurring beet disaccharide and can be commercially produced from sucrose on an industrial scale. It is used in various food and drink additives and clinical formula diets as an alternative sugar [143]. Isomaltose breaks down slower than sucrose in the small intestine, which influences glycemia after ingestion. Compared to other low-glycemic sugars (e.g., tagatose or psicose), isomaltulose is unique because it does not escape digestion in the small intestine and is yet a fully available low-glycemic carbohydrate that provides sustained glucose release [103,144]. The study analyzing the influence of this sugar has shown that brachial-ankle PWV (baPWV) did not increase in 30, 60, and 90 min after 25 g of isomaltulose ingestion compared to the state before ingestion; however, it increased after 25 g of sucrose intake (n = 10, healthy middle-aged and older adult) [103]. Furthermore, a similar study showed an increase in baPWV at 30, 60, and 90 min after ingestion of 25 g glucose, and an increase in the cardio-ankle vascular index was greater at 60 min than at baseline after ingestion of 25 g glucose [103]. Thus, isomaltulose intake inhibits an acute increase in arterial stiffness compared with the consumption of other carbohydrates, such as sucrose and glucose, which creates a possibility of using this sugar in healthy food production [96,103,144].
5.5. The Role of Sodium and Potassium in Arterial Stiffness
A critical dietary factor modulating arterial stiffness is dietary sodium intake. A high-salt diet leads to hypertension and increases incidences of cardiovascular disease [106,145,146]. An increase in blood pressure is associated with decreased arterial elasticity and thus influences arterial stiffness. This inclination tempts many scientists to analyze the influence of sodium on vascular dilation in various groups of patients. For example, the cohort study of the Chinese population (n = 36,324 subjects, aged 49.10 ± 12.57 years) analyzed the influence of various amounts of sodium intake (low sodium <6 g, medium 6–10 g, and high >10 g daily) on arterial stiffness. The high sodium intake (>10 g/day) revealed a deleterious effect on arterial stiffness (baPWV ≥ 1400 cm/s) [105]. Conversely, reduced sodium intake decreases blood pressure and alleviates arterial stiffness. Such changes are observed independently of antihypertensive effects, which suggests that daily salt overconsumption may directly affect arterial stiffness [107]. The beneficial effect of a low-salt diet was proved by a meta-analysis, which showed that an average reduction of 89.3 mmol/day in sodium intake in different populations is associated with a 2.84% reduction in PWV [108].
Sodium influences vascular health (increases arterial stiffness) in both the normotensive and hypertensive groups of patients. The data regarding normotensive subjects have shown that high-sodium meal increases the AIx [106] and raises wave reflection and carotid blood pressure after a high-salt diet used for two weeks [145]. In addition, increased salt intake has a deleterious effect on healthy young and middle-aged adults. A seven-day high-sodium diet used by both groups caused an increase in central SBP (measured by radial artery applanation tonometry). The growing SBP can increase wave amplitudes and is one of the crucial mechanisms that regulate arterial pressure and worsen cardiovascular risk [146]. Similar results are observed in older people. Gates et al. have shown that two weeks of dietary sodium restriction influences arterial pressure in older adults, which results in decreased arterial stiffness. In that group of patients, sodium restriction has rapidly improved compliance with the large elastic arteries and AIx, causing a decrease in systolic hypertension [109]. However, Garcia-Ortiz noticed that the relationship between arterial stiffness parameters and carotid intima-media thickness (C-IMT evaluated by ultrasonography) with quartiles of sodium intake resembles a J-shaped curve, illustrating that both very high and low intake ratios correlate with increased arterial stiffness [113]. Decreased salt intake can improve blood pressure in newly diagnosed hypertension. The salt restriction for 6 weeks in whites, blacks, and Asians with untreated mildly raised blood pressure has caused a significant decrease in blood pressure, urinary albumin, albumin/creatinine ratio, and carotid-femoral PWV [110].
Since the blood sodium and potassium concentrations influence blood pressure, the analysis of sodium intake is usually performed in the context of potassium supplementation. The available data regarding the role of potassium in arterial stiffness are inconsistent. Data for high potassium supplementation (with an additional 20 or 40 mmol K(+)/d from fruit and vegetables) do not show changes in arterial stiffness and endothelial function compared to a low-potassium diet used in the early stages of hypertension [111]. Some data indicate that higher potassium excretion can be associated with lower aortic AIx and lower carotid-femoral PWV [113]. However, most studies prove the beneficial effect of adequate potassium intake on vascular and heart health. For example, the study that analyzed potassium chloride and potassium bicarbonate supplementation showed significantly improved endothelial function (measured by flow-mediated dilatation of the brachial artery flow-mediated dilatation) and increased arterial compliance (assessed by carotid-femoral PWV) [112]. The survey by Lennon-Edwards et al. in a group of healthy young adults revealed that low potassium intake in the diet is associated with greater reflection of waves and arterial stiffness. Notably, the beneficial influence of potassium on blood pressure is observed even with high sodium consumption [147].
Since both macroelements (sodium and potassium) influence blood pressure and arterial stiffness, some data suggest that their ratio can be used as a reliable marker of vascular health. For example, a cross-sectional study by Garcia-Ortiz et al. showed that the sodium-to-potassium intake ratio is associated with the aortic AIx and arterial stiffness parameters (including PWV) [113]. Some data even report that the sodium-to-potassium ratio is more strongly associated with hypertension and/or SBP and DBP outcomes than either sodium or potassium alone [148,149,150].
The influence of sodium on endothelial function is strictly related to the ability of sodium elimination from urine. The study by Del Giorno has shown that low daytime urinary sodium excretion is associated with increased arterial stiffness and central blood pressure [151]. Analysis of the relationship between urine sodium, urinary sodium to potassium (Na/K) ratio, and arterial stiffness in the group of hypertensive patients without antihypertensive treatment showed that urinary sodium and urinary Na/K ratio are independently related to baPWV [152].
Hypertensive patients using high-sodium and low-potassium diet are encouraged to implement the DASH diet (Dietary Approaches to Stop hypertension). Such diets can also have a beneficial influence on arterial stiffness. DASH diet is rich in vegetables, fruits, and low-fat dairy products, which results in low sodium intake. The DASH diet reduces blood pressure in subjects with and without hypertension, regardless of race and gender. Compared to the control diet, which is typical for the United States, and is characterized by a high sodium level, the DASH diet with a low sodium amount caused a lower mean SBP of 7.1 mmHg in normotensive participants and a decrease of approximately 11.5 mmHg in hypertensive patients with SBP [153]. Therefore, the DASH diet should be recommended for hypertension treatment and prevention.
5.6. Vitamin Intake and Arterial Stiffness
Since supplementation of vitamins can improve endothelial function, many studies recently analyzed the influence of these molecules on vascular health. Intake of ADEC vitamins decreases inflammatory and oxidative stress processes, which are deleterious for endothelial function and arterial wall changes (e.g., collagen deposition, smooth muscle cell proliferation, and elastin fragmentation) [154]. Antioxidative vitamins (C, E, and β-carotene) in fruits and vegetables scavenge free radicals. Furthermore, vitamin C protects the membranes from peroxidation by regenerating their α-tocopherol components [155,156]. Vitamin C influences vascular beds and supports endothelial cells. It scavenges radical species, inhibits apoptosis, and spares endothelial cell-derived nitric oxide helping modulate blood flow. Thus, it prevents endothelial dysfunction, which is the initial sign of arterial stiffness [155]. Ascorbic acid also improves flow-mediated dilation, reduces central blood pressure, and decreases asymmetric dimethylarginine (ADMA). ADMA is an endogenous eNOS inhibitor and vascular toxin that is associated with arterial stiffening [120]. ADMA can cause endothelial dysfunction through eNOS inhibition, raise systemic vascular resistance [157], and increases intima-media thickness [158]. Furthermore, ADMA enhances vascular stiffness and decreases cerebral perfusion in healthy subjects [121]. Thus, vitamin C can benefit arterial stiffness by inhibiting ADMA.
Recently available data on vitamin influence on endothelial function (strictly related to the stiffness of the artery) is controversial. A meta-analysis by Saz-Lara et al. [114] (22 studies; n = 1318 participants in the orally supplemented vitamin group vs. n = 1115 subjects in the placebo group) showed no statistically significant effects on arterial stiffness (in both pairwise comparison and frequentist network meta-analysis). However, if oral vitamin supplementation was longer than 12 weeks, vitamin D3 showed a significant reduction in central arterial stiffness, compared with the placebo (−60.0% m/s). Other studies confirm the beneficial effect of vitamin D on cardiovascular risk. Both forms of vitamin D, circulating ergocalciferol (25-hydroxyvitamin) and its active form (1,25-dihydroxy-vitamin D, cholecalciferol), influence endothelial function via endothelial vitamin D receptors (VDR) and enzymes converting circulating vitamin D to the active form. In vitamin D deficiency, an increased risk of CVD and arterial stiffness is observed [115,116]. Vitamin D also reveals antioxidative properties. This function is crucial in the case of exacerbated oxidative and pro-inflammatory processes, which cause vascular injury by increasing the proliferation and migration of vascular smooth muscle cells (VSCM) in vitro [115]. Wu-Wong identified genes regulated by VDR (via DNA microarray technology), which participate in the regulation of human coronary artery VSCM growth and therefore can influence arterial stiffness [116].
However, some data do not confirm the influence of vitamin D on arterial stiffness. Two large meta-analyses have shown that vitamin D supplementation is associated with improved endothelial function but does not affect arterial stiffness [117,118]. Similarly, a systematic review and meta-analysis of Upala et al. have shown that short implementation of vitamin D (from 1000 IU/day to 120,000 IU/month of cholecalciferol) for 2 to 12 months does not improve aortic PWV and AIx [119].
The effect of B vitamins on arterial stiffness is mediated by their influence on homocysteine metabolism, which requires the proper intake of folic acid contents, B12, vitamin B6, and riboflavin. Insufficiency of these vitamins increases the concentration of homocysteine, a molecule that causes endothelial dysfunction [122]. The 10-year analysis of cardiovascular risk in the general population has shown that homocysteine activity revealed its mutual effect with uric acid on arterial stiffness. This analysis proved that high levels of homocysteine and uric acid are associated with the highest baPWV compared to the group characterized by low levels of homocysteine and low levels of uric acid (UA) [123].
Recent data confirm the hypothesis that arterial stiffness is one of the possible pathways between hyperuricemia and cardiovascular disease. High serum UA concentrations impair the generation of vasoactive mediators in endothelial cells, including nitric oxide (NO) [159,160]. The decreased NO bioavailability promotes endothelial dysfunction, increases vascular tone, and contributes to arterial stiffness [161]. A decrease in serum nitric oxide rises after lowering UA levels [162]. UA also causes vasoconstrictive dysregulation by inducing oxidative stress. Pro-oxidant molecules stimulate endothelin-1 synthesis, a potent vasoconstrictor that increases arterial stiffness [163]. Besides this, UA stimulates VSMCs proliferation and ROS by stimulating the vascular renin-angiotensin system (RAS) [161]. RAS is responsible for increased stiffness of the cytoskeleton in endothelial cells and extracellular matrix fibrosis [164,165]. Subsequently, vascular hypertrophy, impaired NO synthesis, and arterial stiffness is observed [162,165,166]. Thus, high UA levels stimulate oxidative stress, induce endothelial VSMCs proliferation, and increase endothelin level leading to vascular dysfunction [162]. Effective treatment to prevent or reduce serum UA concentration should be implemented in each hyperuricemic patient to decrease ROS generation, prevent endothelial dysfunction, and reduce arterial stiffening.
Recent data also underline the potential roles of folate and/or related B vitamins in protecting against CVD (especially stroke), certain cancers, cognitive impairment, and osteoporosis [167]. Nicotinic acid (niacin) is known for its lipid-lowering properties and thus the reduction in myocardial infarction, stroke, and atherosclerosis. However, this vitamin also has anti-inflammatory and potentially anti-atherosclerotic properties independent of its effects on lipid regulation. For example, niacin inhibits vascular inflammation by decreasing endothelial ROS production and subsequent LDL oxidation. As a result, it decreases inflammatory cytokine production in cultured human aortic endothelial cells [168].
The intake of fruits and vegetables has a beneficial influence on arterial stiffness and supports the recommendation to consume more than five servings of fruits and vegetables per day, reducing cardiovascular risk, which is partially related to vitamin consumption [153]. The protective effects of fruits and vegetables are related to their antioxidant properties, which result from a high concentration of antioxidant vitamins, minerals, and metabolically active vitamins (e.g., vitamins from group B) [169]. Therefore, products containing specific vitamins should be included in the daily diet (Table 2).

Table 2.
Dietary recommendation to decrease cardiovascular risk and arterial stiffness.
5.7. Phytochemicals and Arterial Stiffness
Phytochemicals include a large group of active compounds with antioxidative, antiproliferative, anti-inflammatory, and inhibitory angiogenesis molecules important in preventing chronic diseases. They reduce neointimal thickening by inhibiting smooth muscle cell proliferation, thus improving endothelium-dependent vasorelaxation. Phytochemicals also modulate the bioavailability of nitric oxide and voltage-gated ion channels [178]. The main phytochemicals groups are polyphenols, flavonoids, isoflavonoids, anthocyanidins, phytoestrogens, terpenoids, carotenoids, limonoids, phytosterols, glucosinolates, and fibers [172].
Polyphenols are the natural components of various fruits, vegetables, cereals, and beverages, which may reduce arterial stiffness. About 100 g of fresh grapes, apples, pears, cherries, and berries deliver up to 200–300 mg of polyphenols. They are also present in red wine, tea, and coffee and in smaller amounts in cereals, dry legumes, and chocolate [173,174]. Flavonoids are the most abundant polyphenols in the human diet [176]. Flavonoids (flavonols, flavones, and isoflavones) have antioxidative, anti-inflammatory, and antithrombotic properties. Plant food, including apples, berries, grapes, and onions, are the main dietary sources of these molecules [176,177,182]. Flavonoids inhibit lipid peroxidation, promoting vascular relaxation and preventing atherosclerosis. In the study by Dohadwala et al., chronic consumption of cranberry juice (including a high dose of polyphenols) reduced carotid-femoral PWV; however, only an immediate effect of such consumption was observed and without chronic influence on vasodilator endothelial function [124].
Other nutrients that seem to benefit arterial stiffness are phytoestrogens [125,183]. Phytoestrogens (isoflavonoids genistein, daidzein), flavonoids, and anthocyanins bind to estrogen receptors in physiological concentrations, increasing eNOS activity and NO synthesis [184]. Phytoestrogens, such as isoflavones and lignans, benefit vascular beds and reduce PWV [128]. Soy isoflavones induced nitrite/nitrate levels, decreased endothelin (ET-1) levels, and alleviated arterial stiffness in men and postmenopausal women [178]. Therefore, diets with a high intake of soy isoflavones benefit arterial stiffness by reducing PWV and improving arterial compliance. All these effects can partially explain the decreed CVD risk in populations with a high soy intake [125]. Furthermore, isoflavone intake shows improved arterial stiffness (decreased PWV) [126,127]. Still, some studies did not confirm such influence (no effect on PWV and AIx) [129,130]. Differences in these studies can result from isoflavone intake (different forms and amounts) and specific populations (characterized by various ages, sex, cardiovascular risk, and comorbidities).
Similarly to flavonoids, beneficial antioxidative effects have carotenoids. They reveal antiatherogenic properties by reducing inflammation and ROS formation within the arterial wall. Carotenoids with many conjugated double bonds demonstrate their antioxidant potential. They also improve endothelial function [179] and decrease arterial stiffness [180]. One of the most popular carotenoids is lycopene, found in tomatoes, watermelon, papaya, red grapefruits, and guava. Its antioxidant properties are very high among carotenoids [180]. Lycopene also effectively reduces cardiovascular risk, making it one of the most important dietary components in the daily diet of the general population [181]. An inverse relationship between circulating lycopene and brachial-ankle PWV was observed in healthy women (n = 264, 31–75 years), which showed a significant decrease in baPWV with increased lycopene concentration [131]. A similar inverse correlation was found between PWV and serum lycopene in Korean men (n = 299) after considering confounding factors such as blood pressure, insulin resistance, and oxidative stress [132]. On the contrary, the study of healthy overweight volunteers (n = 255, aged 40–65 years) showed that a relatively high daily consumption of tomato products (32–50 mg lycopene/day) or lycopene supplements (10 mg/day) was ineffective in reducing conventional cardiovascular risk markers and arterial stiffness in middle-aged individuals [133]. Thus, the lycopene effect can vary between the analyzed population, body fat content, and related diseases.
6. Dietary Recommendations to Prevent Arterial Stiffness
Since an ageing society characterizes the general population, it is crucial to control the progression of arterial stiffness using a well-balanced diet. Such a diet should contain adequate energy intake to maintain proper body mass. In the case of overweight and obesity, caloric restriction should be considered. A well-balanced diet is one of the most important recommendations for preventing arterial stiffens. Such a diet should contain a proper quantity and quality of protein, fat, and carbohydrate (Table 2).
Two diets can have beneficial effects on vascular health—the DASH diet and the Mediterranean diet. Both diets are rich in vegetables, fruits, low-fat dairy products, and minimal sodium intake. They are widely recommended to diminish or prevent hypertension and cardiovascular events and minimize the risk of arterial stiffness; furthermore, these types of diets decrease inflammation, improve endothelial function, and increase flow-mediated dilatation [89,153,175].
Adequate fat intake should include a low amount of trans and saturated fats, possibly replacing them with MUFA and PUFA (Figure 2). Fermented dairy products (without butter), fish, and lean meat are good protein products that can decrease arterial stiffness. Since low glycemic index products benefit postprandial vascular responses, they should be considered when planning daily meals. Isomaltose (bee sugar) can be used to sweeten some meals because it does not have a deleterious effect on postprandial hyperglycemia. Reducing excessive salt intake in the diet is important for overall vascular health and blood pressure. Products including potassium and low sodium: potassium intake ratio is required to decrease blood pressure and reduce arterial stiffness. Active antioxidative, antiproliferative, and anti-inflammatory elements of the diet, such as vitamins and phytochemicals, are crucial for the prevention of chronic diseases and can protect against atherosclerosis and arterial degeneration through an effect on arterial walls.
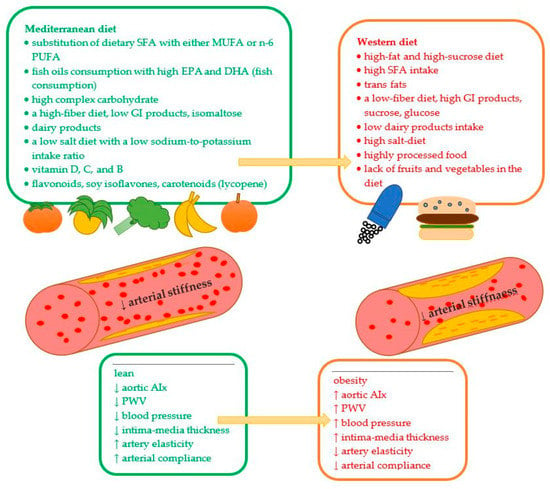
Figure 2.
The influence of selective nutrients on arterial stiffness.↓—decrease; ↑—increase; AIx—augmentation index; PWV—brachial-ankle pulse wave velocity; DHA—docosahexaenoic acid; EFA—essential fatty acids; EPA—eicosapentaenoic acid; GI—glycemic index; MUFA—monounsaturated fatty acids; PUFA—polyunsaturated fatty acids; SFA—saturated fatty acids.
7. Conclusions
Since endothelial dysfunction and increased arterial stiffness are predictors of CVD, a well-balanced diet should be the first step to maintaining vascular health. This review gathers the main dietary recommendations supported by multiple studies. It underlines the role of nutritional habits in overweight or obesity and suggests healthy behavior which can help reduce body mass and improve endothelial function. Striving for a correct body weight through a negative energy balance should be the first element of prophylaxis in excessive body mass and arterial stiffness prevention. Low-fat and high-fiber products (low-energy and food characterized by low GI index) in the diet are crucial for proper body mass achievement.
Unfortunately, the Western diet (rich in energy overconsumption and a large amount of SFA, trans fats, red meat, simple sugars, and sodium) is the leading cause of obesity and arterial stiffness. High-fat and high-sucrose diets typical for quickly available food not only increase body mass but also have harmful effects on vascular health. Replacing red meat with fish, margarine with olive oils, and sweets with vegetables can be the first step in decreasing adiposity. In addition, normalization of the metabolic state by weight loss returns arterial stiffness and blood pressure to normal. Patients (independently of co-existed diseases) should know the basic dietary recommendations to maintain general and vascular health. The role of nutritional education, in this case, is invaluable. Heightened awareness of the adverse consequences of obesity and obesity-related complications (hypertension, diabetes mellitus) should prompt healthcare providers to institute early and aggressive lifestyle interventions in overweight and obese patients. Thus, there is a need for the development of early detection and prevention programs to mitigate this potentially serious health problem.
Future research should examine arterial stiffness and its influence on cardiometabolic health outcomes in various populations characterized by a specific age, body mass (overweight and obesity), and co-morbidities (diabetes mellitus, cardiac events, metabolic syndrome). Such data can provide valuable insights into the role of particular nutrients and eating patterns on arterial stiffness and cardiometabolic health. The not-fully defined role of specific products in arterial stiffness (e.g., milk, non-fermented products, potassium-rich meals) should be carefully analyzed. Considering the epidemic incidence of obesity worldwide, caused mainly due to excessive consumption of a high-energy diet, arterial stiffness could represent a novel therapeutic target to prevent obesity-associated cardiovascular complications. Początek formularza.
Author Contributions
A.S., conceptualization, writing—original draft preparation, writing—review and editing; B.G.-G., writing—original draft preparation, writing—review and editing, visualization; K.B.-T., writing—original draft preparation, visualization; A.C., supervision and review; W.M., supervision; S.Z., writing—original draft preparation. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
We used PubMed and Web of Science to screen articles for this narrative review. We did not report any data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO. World Obesity Day 2022-Accelerating Action to Stop Obesity. Available online: https://www.who.int/news/item/04-03-2022-world-obesity-day-2022-accelerating-action-to-stop-obesity (accessed on 9 January 2023).
- Boutari, C.; Mantzoros, C.S. A 2022 update on the epidemiology of obesity and a call to action: As its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 2022, 133, 55217. [Google Scholar] [CrossRef] [PubMed]
- Stanek, A.; Brożyna-Tkaczyk, K.; Myśliński, W. The Role of Obesity-Induced Perivascular Adipose Tissue (PVAT) Dysfunction in Vascular Homeostasis. Nutrients 2021, 13, 3843. [Google Scholar] [CrossRef] [PubMed]
- Stanek, A.; Brożyna-Tkaczyk, K.; Myśliński, W. Oxidative Stress Markers among Obstructive Sleep Apnea Patients. Oxid. Med. Cell Longev. 2021, 2021, 9681595. [Google Scholar] [CrossRef] [PubMed]
- Rahban, M.; Stanek, A.; Hooshmand, A.; Khamineh, Y.; Ahi, S.; Kazim, S.N.; Ahmad, F.; Muronetz, V.; Abousenna, M.S.; Zolghadri, S.; et al. Infection of Human Cells by SARS-CoV-2 and Molecular Overview of Gastrointestinal, Neurological, and Hepatic Problems in COVID-19 Patients. J. Clin. Med. 2021, 10, 4802. [Google Scholar] [CrossRef]
- Safar, M.E.; Czernichow, S.; Blacher, J. Obesity, arterial stiffness, and cardiovascular risk. J. Am. Soc. Nephrol. 2006, 17, S109–S111. [Google Scholar] [CrossRef]
- Femia, R.; Kozakova, M.; Nannipieri, M.; Gonzales-Villalpando, C.; Stern, M.P.; Haffner, S.M. Carotid intima-media thickness in confirmed prehypertensive subjects: Predictors and progression. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2244–2249. [Google Scholar] [CrossRef]
- Kim, H.L.; Ahn, D.W.; Kim, S.H.; Lee, D.S.; Yoon, S.H.; Zo, J.H.; Kim, M.A.; Jeong, J.B. Association between body fat parameters and arterial stiffness. Sci. Rep. 2021, 11, 20536. [Google Scholar] [CrossRef]
- Melo E Silva, F.V.; Almonfrey, F.B.; Freitas, C.M.N.; Fonte, F.K.; Sepulvida, M.B.C.; Almada-Filho, C.M.; Cendoroglo, M.S.; Quadrado, E.B.; Amodeo, C.; Povoa, R.; et al. Association of Body Composition with Arterial Stiffness in Long-lived People. Arq. Bras. Cardiol. 2021, 117, 457–462. [Google Scholar] [CrossRef]
- Peto, R.; Whitlock, G.; Jha, P. Effects of obesity and smoking on US life expectancy. N. Engl. J. Med. 2010, 362, 855–856. [Google Scholar] [CrossRef]
- Fernhall, B.; Agiovlasitis, S. Arterial function in youth: Window into cardiovascular risk. J. Appl. Physiol. 1985, 105, 325–333. [Google Scholar] [CrossRef]
- Starzak, M.; Stanek, A.; Jakubiak, G.K.; Cholewka, A.; Cieślar, G. Arterial Stiffness Assessment by Pulse Wave Velocity in Patients with Metabolic Syndrome and Its Components: Is It a Useful Tool in Clinical Practice. Int. J. Environ. Res. Public Health 2022, 19, 10368. [Google Scholar] [CrossRef]
- Urbina, E.M.; Kimball, T.R.; Khoury, P.R.; Daniels, S.R.; Dolan, L.M. Increased arterial stiffness is found in adolescents with obesity or obesity-related type 2 diabetes mellitus. J. Hypertens. 2010, 28, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Farmer, J. Arterisal Stiffness as a Risk Factor for Coronary Artery Disease. Curr. Atheroscler. Rep. 2014, 16, 387. [Google Scholar] [CrossRef] [PubMed]
- Manrique, C.; Lastra, G.; Ramirez-Perez, F.I.; Haertling, D.; DeMarco, V.G.; Aroor, A.R.; Jia, G.; Chen, D.; Barron, B.J.; Garro, M.; et al. Endothelial estrogen receptor-α does not protect against vascular stiffness induced by Western diet in female mice. Endocrinology 2016, 157, 1590–1600. [Google Scholar] [CrossRef] [PubMed]
- Amore, L.; Alghisi, F.; Pancaldi, E.; Pascariello, G.; Cersosimo, A.; Cimino, G.; Bernardi, N.; Calvi, E.; Lombardi, C.M.; Sciatti, E.; et al. Study of endothelial function and vascular stiffness in patients affected by dilated cardiomyopathy on treatment with sacubitril/valsartan. Am. J. Cardiovasc. Dis. 2022, 12, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Dengo, A.L.; Dennis, E.A.; Orr, J.S.; Marinik, E.L.; Ehrlich, E.; Davy, B.M.; Davy, K.P. Arterial destiffening with weight loss in overweight and obese middle-aged and older adults. Hypertension 2010, 55, 855–8561. [Google Scholar] [CrossRef] [PubMed]
- Aroor, A.R.; Jia, G.; Sowers, J.R. Cellular mechanisms underlying obesity-induced arterial stiffness. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R387–R398. [Google Scholar] [CrossRef]
- Bagheri, S.; Zolghadri, S.; Stanek, A. Beneficial Effects of Anti-Inflammatory Diet in Modulating Gut Microbiota and Controlling Obesity. Nutrients 2022, 14, 3985. [Google Scholar] [CrossRef]
- Stanek, A.; Brożyna-Tkaczyk, K.; Zolghadri, S.; Cholewka, A.; Myśliński, W. The Role of Intermittent Energy Restriction Diet on Metabolic Profile and Weight Loss among Obese Adults. Nutrients 2022, 14, 1509. [Google Scholar] [CrossRef]
- Recio-Rodriguez, J.I.; Gomez-Marcos, M.A.; Patino-Alonso, M.C.; Agudo-Conde, C.; Rodriguez-Sanchez, E.; Garcia-Ortiz, L. Vasorisk group. Abdominal Obesity vs General Obesity for Identifying Arterial Stiffness, Subclinical Atherosclerosis and Wave Reflection in Healthy, Diabetics and Hypertensive. BMC Cardiovasc. Disord. 2012, 12, 3. [Google Scholar] [CrossRef]
- Fu, S.; Luo, L.; Ye, P.; Liu, Y.; Zhu, B.; Zheng, J.; Bai, Y.; Bai, J. Overall and Abdominal Obesity Indicators Had Different Association with Central Arterial Stiffness and Hemodynamics Independent of Age, Sex, Blood Pressure, Glucose, and Lipids in Chinese Community-Dwelling Adults. Clin. Interv. Aging 2013, 8, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Cildir, G.; Akıncılar, S.C.; Tergaonkar, V. Chronic Adipose Tissue Inflammation: All Immune Cells on the Stage. Trends. Mol. Med. 2013, 19, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.F.; Braga, V.D.A.; Silva, M.E.S.D.F.; Cruz, J.D.C.; Santos, S.H.S.; Monteiro, M.M.D.O.; Balarini, C.D.M. Adipokines, Diabetes and Atherosclerosis: An Inflammatory Association. Front. Physiol. 2015, 6, 304. [Google Scholar] [CrossRef]
- Jonas, M.I.; Kurylowicz, A.; Bartoszewicz, Z.; Lisik, W.; Jonas, M.; Wierzbicki, Z.; Chmura, A.; Pruszczyk, P.; Puzianowska-Kuznicka, M. Interleukins 6 and 15 Levels Are Higher in Subcutaneous Adipose Tissue, but Obesity Is Associated with Their Increased Content in Visceral Fat Depots. Int. J. Mol. Sci. 2015, 16, 25817–25830. [Google Scholar] [CrossRef]
- Watanabe, N.; Ikeda, U. Matrix Metalloproteinases and Atherosclerosis. Curr. Atheroscler. Rep. 2004, 6, 112–120. [Google Scholar] [CrossRef]
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Shankar-Hari, M.; Vale, C.L.; Godolphin, P.J.; Fisher, D.; Higgins, J.P.T.; Spiga, F.; Savovic, J.; Tierney, J.; Baron, G.; et al. Association between Administration of IL-6 Antagonists and Mortality among Patients Hospitalized for COVID-19: A Meta-Analysis. JAMA 2021, 326, 499–518. [Google Scholar] [CrossRef]
- Protogerou, A.D.; Zampeli, E.; Fragiadaki, K.; Stamatelopoulos, K.; Papamichael, C.; Sfikakis, P.P. A pilot study of endothelial dysfunction and aortic stiffness after interleukin-6 receptor inhibition in rheumatoid arthritis. Atherosclerosis 2011, 219, 734–736. [Google Scholar] [CrossRef]
- Nishimoto, N.; Kanakura, Y.; Aozasa, K.; Johkoh, T.; Nakamura, M.; Nakano, S.; Nakano, N.; Ikeda, Y.; Sasaki, T.; Nishioka, K.; et al. Humanized Anti-Interleukin-6 Receptor Antibody Treatment of Multicentric Castleman Disease. Blood 2005, 106, 2627–2632. [Google Scholar] [CrossRef]
- Urschel, K.; Cicha, I. TNF-alpha; in the Cardiovascular System: From Physiology to Therapy. Int. J. Interf. Cytokine Mediat. Res. 2015, 7, 9–25. [Google Scholar] [CrossRef]
- Li, Z.; Yang, S.; Lin, H.; Huang, J.; Watkins, P.A.; Moser, A.B.; Desimone, C.; Song, X.; Diehl, A.M. Probiotics and Antibodies to TNF Inhibit Inflammatory Activity and Improve Nonalcoholic Fatty Liver Disease. Hepatology 2003, 37, 343–350. [Google Scholar] [CrossRef]
- Wascher, T.C.; Lindeman, J.H.; Sourij, H.; Kooistra, T.; Pacini, G.; Roden, M. Chronic TNF-α Neutralization Does Not Improve Insulin Resistance or Endothelial Function in “Healthy” Men with Metabolic Syndrome. Mol. Med. 2011, 17, 189–193. [Google Scholar] [CrossRef]
- Sudhakar, M.; Silambanan, S.; Chandran, A.S.; Prabhakaran, A.A.; Ramakrishnan, R. C-Reactive Protein (CRP) and Leptin Receptor in Obesity: Binding of Monomeric CRP to Leptin Receptor. Front. Immunol. 2018, 9, 1167. [Google Scholar] [CrossRef]
- Gnacińska, M.; Małgorzewicz, S.; Guzek, M.; Lysiak-Szydłowska, W.; Sworczak, K. Adipose Tissue Activity in Relation to Overweight or Obesity. Endokrynol. Polska 2010, 61, 160–168. [Google Scholar]
- Jia, G.; Aroor, A.R.; Sowers, J.R. Arterial Stiffness: A Nexus between Cardiac and Renal Disease. Cardiorenal. Med. 2014, 4, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Aroor, A.R.; Jia, G.; Sowers, J.R. The Role of Tissue Renin-Angiotensin-Aldosterone System in the Development of Endothelial Dysfunction and Arterial Stiffness. Front. Endocrinol. 2013, 4, 161. [Google Scholar] [CrossRef] [PubMed]
- Bredt, D.S.; Snyder, S.H. Isolation of Nitric Oxide Synthetase, a Calmodulin-Requiring Enzyme. Proc. Natl. Acad. Sci. USA 1990, 87, 682–685. [Google Scholar] [CrossRef]
- Kim, J.A.; Jang, H.J.; Martinez-Lemus, L.A.; Sowers, J.R. Activation of MTOR/P70S6 Kinase by ANG II Inhibits Insulin-Stimulated Endothelial Nitric Oxide Synthase and Vasodilation. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E201–E208. [Google Scholar] [CrossRef]
- Kane, M.O.; Etienne-Selloum, N.; Madeira, S.V.F.; Sarr, M.; Walter, A.; Dal-Ros, S.; Schott, C.; Chataigneau, T.; Schini-Kerth, V.B. Endothelium-Derived Contracting Factors Mediate the Ang II-Induced Endothelial Dysfunction in the Rat Aorta: Preventive Effect of Red Wine Polyphenols. Pflugers Arch.-Eur. J. Physiol. 2010, 459, 671–679. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nogami, T.; Taguchi, K.; Matsumoto, T.; Kamata, K. Diabetic State, High Plasma Insulin and Angiotensin II Combine to Augment Endothelin-1-Induced Vasoconstriction via ETA Receptors and ERK. Br. J. Pharmacol. 2008, 155, 974–983. [Google Scholar] [CrossRef]
- Kushibiki, M.; Yamada, M.; Oikawa, K.; Tomita, H.; Osanai, T.; Okumura, K. Aldosterone Causes Vasoconstriction in Coronary Arterioles of Rats via Angiotensin II Type-1 Receptor: Influence of Hypertension. Eur. J. Pharmacol. 2007, 572, 182–188. [Google Scholar] [CrossRef]
- Aghamohammadzadeh, R.; Unwin, R.D.; Greenstein, A.S.; Heagerty, A.M. Effects of Obesity on Perivascular Adipose Tissue. Vasorelaxant Function: Nitric Oxide, Inflammation and Elevated Systemic Blood Pressure. J. Vasc. Res. 2016, 52, 299–305. [Google Scholar] [CrossRef]
- Schroeter, M.R.; Eschholz, N.; Herzberg, S.; Jerchel, I.; Leifheit-Nestler, M.; Czepluch, F.S.; Chalikias, G.; Konstantinides, S.; Schäfer, K. Leptin-Dependent and Leptin-Independent Paracrine Effects of Perivascular Adipose Tissue on Neointima Formation. Arter. Thromb. Vasc. Biol. 2013, 33, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Almabrouk, T.A.M.; White, A.D.; Ugusman, A.B.; Skiba, D.S.; Katwan, O.J.; Alganga, H.; Guzik, T.J.; Touyz, R.M.; Salt, I.P.; Kennedy, S. High Fat Diet Attenuates the Anticontractile Activity of Aortic PVAT via a Mechanism Involving AMPK and Reduced Adiponectin Secretion. Front. Physiol. 2018, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, A.; Feely, J. Adiponectin and Arterial Stiffness. Am. J. Hypertens. 2005, 18, 1543–1548. [Google Scholar] [CrossRef]
- Tontonoz, P.; Spiegelman, B.M. Fat and Beyond: The Diverse Biology of PPAR. Annu. Rev. Biochem. 2008, 77, 289–312. [Google Scholar] [CrossRef] [PubMed]
- Korda, M.; Kubant, R.; Patton, S.; Malinski, T. Leptin-Induced Endothelial Dysfunction in Obesity. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1514–H1521. [Google Scholar] [CrossRef] [PubMed]
- Opatrilova, R.; Caprnda, M.; Kubatka, P.; Valentova, V.; Uramova, S.; Nosal, V.; Gaspar, L.; Zachar, L.; Mozos, I.; Petrovic, D.; et al. Adipokines in Neurovascular Diseases. Biomed. Pharmacother. 2018, 98, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Gairolla, J.; Kler, R.; Modi, M.; Khurana, D. Leptin and Adiponectin: Pathophysiological Role and Possible Therapeutic Target of Inflammation in Ischemic Stroke. Rev. Neurosci. 2017, 28, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Wing, R.R.; Lang, W.; Wadden, T.A.; Safford, M.; Knowler, W.C.; Bertoni, A.G.; Hill, J.O.; Brancati, F.L.; Peters, A.; Wagenknecht, L. Look AHEAD Research Group. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011, 34, 1481–1486. [Google Scholar] [CrossRef]
- Horvath, K.; Jeitler, K.; Siering, U.; Stich, A.K.; Skipka, G.; Gratzer, T.W.; Siebenhofer, A. Long-term effects of weight-reducing interventions in hypertensive patients: Systematic review and meta-analysis. Arch. Intern. Med. 2008, 168, 571–580. [Google Scholar] [CrossRef]
- Sacks, F.M.; Bray, G.A.; Carey, V.J.; Smith, S.R.; Ryan, D.H.; Anton, S.D.; McManus, K.; Champagne, C.M.; Bishop, L.M.; Laranjo, N.; et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N. Engl. J. Med. 2009, 360, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.D.; Stefanick, M.L.; Williams, P.T.; Haskell, W.L. The effects on plasma lipoproteins of a prudent weight-reducing diet, with or without exercise, in overweight men and women. N. Engl. J. Med. 1991, 325, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Vega-López, S.; Venn, B.J.; Slavin, J.L. Relevance of the glycemic index and glycemic load for body weight, diabetes, and cardiovascular disease. Nutrients 2018, 10, 1361. [Google Scholar] [CrossRef]
- Sjöström, L.; Lindroos, A.K.; Peltonen, M.; Torgerson, J.; Bouchard, C.; Carlsson, B.; Dahlgren, S.; Larsson, B.; Narbro, K.; Sjöström, C.D.; et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N. Engl. J. Med. 2004, 351, 2683–2693. [Google Scholar] [CrossRef] [PubMed]
- Cunha, P.G.; Cotter, J.; Oliveira, P.; Vila, I.; Boutouyrie, P.; Laurent, S.; Nilsson, P.M.; Scuteri, A.; Sousa, N. Pulse wave velocity distribution in a cohort study: From arterial stiffness to early vascular aging. J. Hypertens. 2015, 33, 1438–1445. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortal- ity with arterial stiffness: A systematic review and meta-analysis. J. Hypertens. 2015, 33, 1438–1445. [Google Scholar] [CrossRef]
- Beckman, J.A.; Creager, M.A.; Libby, P. Diabetes and atherosclerosis: Epidemiology, pathophysiology, and management. JAMA 2002, 287, 2570–2581. [Google Scholar] [CrossRef]
- Ahmet, I.; Tae, H.J.; de Cabo, R.; Lakatta, E.G.; Talan, M.I. Effects of calorie restriction on cardioprotection and cardiovascular health. J. Mol. Cell. Cardiol. 2011, 51, 263–271. [Google Scholar] [CrossRef]
- Fornieri, C.; Taparelli, F.; Quaglino, D., Jr.; Contri, M.B.; Davidson, J.M.; Algeri, S.; Ronchetti, I.P. The effect of s restriction on the aortic tissue of aging rats. Connect. Tissue Res. 1999, 40, 131–143. [Google Scholar] [CrossRef]
- Donato, A.J.; Walker, A.E.; Magerko, K.A.; Bramwell, R.C.; Black, A.D.; Henson, G.D.; Lawson, B.R.; Lesniewski, L.A.; Seals, D.R. Life-long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell 2013, 12, 772–783. [Google Scholar] [CrossRef]
- Wilkinson, I.B.; Qasem, A.; McEniery, C.M.; Webb, D.J.; Avolio, A.P.; Cockcroft, J.R. Nitric oxide regulates local arterial distensibility in vivo. Circulation 2002, 105, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Weisbrod, R.M.; Shiang, T.; Al Sayah, L.; Fry, J.L.; Bajpai, S.; Reinhart-King, C.A.; Lob, H.E.; Santhanam, L.; Mitchell, G.; Cohen, R.A.; et al. Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension 2013, 2, 1105–11010. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.S.; Blanch, N.; Keogh, J.B.; Clifton, P.M. Effect of weight loss on pulse wave velocity: Systematic review and meta-analysis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Vicil, F.; Sanchez-Gonzalez, M.A.; Wong, A.; Ormsbee, M.J.; Hooshmand, S.; Daggy, B. Effects of diet and/or low-intensity resistance exercise training on arterial stiffness, adiposity, and lean mass in obese postmenopausal women. Am. J. Hypertens. 2013, 26, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Redman, L.M.; Smith, S.R.; Burton, J.H.; Martin, C.K.; Il’yasova, D.; Ravussin, E. Metabolic Slowing and Reduced Oxidative Damage with Sustained Caloric Restriction Support the Rate of Living and Oxidative Damage Theories of Aging. Cell Metab. 2018, 27, 805–815. [Google Scholar] [CrossRef]
- Guasch-Ferre, M.; Babio, N.; Martinez-Gonzalez, M.A.; Corella, D.; Ros, E.; Martín-Peláez, S.; Estruch, R.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease. Am. J. Clin. Nutr. 2015, 102, 1563–1573. [Google Scholar] [CrossRef]
- Hooper, L.; Martin, N.; Abdelhamid, A.; Davey Smith, G. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst. Rev. 2015, 6, CD011737. [Google Scholar] [CrossRef]
- Blekkenhorst, L.C.; Prince, R.L.; Hodgson, J.M.; Lim, W.H.; Zhu, K.; Devine, A.; Thompson, P.L.; Lewis, J.R. Dietary saturated fat intake and atherosclerotic vascular disease mortality in elderly women: A prospective cohort study. Am. J. Clin. Nutr. 2015, 101, 1263–1268. [Google Scholar] [CrossRef]
- Vaccaro, J.A.; Huffman, F.G. Monounsaturated fatty acid, carbohydrate intake, and diabetes status are associated with arterial pulse pressure. Nutr. J. 2011, 10, 126. [Google Scholar] [CrossRef]
- Hall, W.L. Dietary saturated and unsaturated fats as determinants of blood pressure and vascular function. Nutr. Res. Rev. 2009, 22, 18–38. [Google Scholar] [CrossRef]
- FAO; WHO. Fats and fatty acids in human nutrition. Proceedings of the Joint FAO/WHO Expert Consultation, 10–14 November 2008, Geneva, Switzerland. Ann. Nutr. Metab. 2009, 55, 5–300. [Google Scholar] [CrossRef]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. American Heart Association. Dietary Fats and Cardiovascular Disease: A Presidential Advisory From the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef] [PubMed]
- Mita, T.; Someya, Y.; Osonoi, Y.; Osonoi, T.; Saito, M.; Nakayama, S.; Ishida, H.; Sato, H.; Gosho, M.; Watada, H. Lower intake of saturated fatty acids is associated with persistently higher arterial stiffness in patients with type 2 diabetes. J. Diabetes Investig. 2021, 12, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Willett, W.C. Optimal diets for prevention of coronary heart disease. JAMA 2002, 288, 2569–2578. [Google Scholar] [CrossRef]
- Alonso, A.; Ruiz-Gutierrez, V.; Martínez-González, M.A. Monounsaturated fatty acids, olive oil and blood pressure: Epidemiological, clinical and experimental evidence. Public Health Nutr. 2006, 9, 251–257. [Google Scholar] [CrossRef]
- Baylin, A.; Kabagambe, E.K.; Ascherio, A.; Spiegelman, D.; Campos, H. Adipose tissue alpha-linolenic acid and nonfatal acute myocardial infarction in Costa Rica. Circulation 2003, 107, 1586–1591. [Google Scholar] [CrossRef]
- Lemaitre, R.N.; King, I.; Mozaffarian, D.; Kuller, L.H.; Tracy, R.P.; Siscovick, D.S. Plasma phospholipid n-3 polyunsaturated PUFAs, fatal ischemic heart disease, and nonfatal myocardial infarction in older adults: The Cardiovascular Health Study. Am. J. Clin. Nutr. 2003, 77, 319–325. [Google Scholar] [CrossRef]
- Vafeiadou, K.; Weech, M.; Altowaijri, H.; Todd, S.; Yaqoob, P.; Jackson, K.G.; Lovegrove, J.A. Replacement of saturated with unsaturated fats had no impact on vascular function but beneficial effects on lipid biomarkers, E-selectin, and blood pressure: Results from the randomized, controlled Dietary Intervention and VAScular function (DIVAS) study. Am. J. Clin. Nutr. 2015, 102, 40–48. [Google Scholar] [CrossRef]
- Jayedi, A.; Zargar, M.S.; Shab-Bidar, S. Fish consumption and risk of myocardial infarction: A systematic review and dose-response meta-analysis suggests a regional difference. Nutr. Res. 2019, 62, 1–12. [Google Scholar] [CrossRef]
- Yang, B.; Shi, M.Q.; Li, Z.H.; Yang, J.J.; Li, D. Fish, Long-Chain n-3 PUFA and Incidence of Elevated Blood Pressure: A Meta-Analysis of Prospective Cohort Studies. Nutrients 2016, 8, 58. [Google Scholar] [CrossRef]
- Zhang, B.; Xiong, K.; Cai, J.; Ma, A. Fish Consumption and Coronary Heart Disease: A Meta-Analysis. Nutrients 2020, 12, 2278. [Google Scholar] [CrossRef] [PubMed]
- Iso, H.; Kobayashi, M.; Ishihara, J.; Sasaki, S.; Okada, K.; Kita, Y.; Kokubo, Y.; Tsugane, S. JPHC Study Group. Intake of fish and n3 fatty acids and risk of coronary heart disease among Japanese: The Japan Public Health Center-Based (JPHC) Study Cohort I. Circulation 2006, 113, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Strong, J.P.; Ishii, T.; Ueno, T.; Koyama, M.; Wagayama, H.; Shimizu, A.; Sakai, T.; Malcom, G.T.; Guzman, M.A. Atherosclerosis and omega-3 fatty acids in the populations of a fishing village and a farming village in Japan. Atherosclerosis 2000, 153, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Monahan, K.D.; Feehan, R.P.; Blaha, C.; McLaughlin, D.J. Effect of omega-3 polyunsaturated fatty acid supplementation on central arterial stiffness and arterial wave reflections in young and older healthy adults. Physiol. Rep. 2015, 3, e12438. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.T.; Chan, D.C.; Barrett, P.H.; Adams, L.A.; Watts, G.F. Supplementation with n3 fatty acid ethyl esters increases large and small artery elasticity in obese adults on a weight loss diet. J. Nutr. 2013, 143, 437–441. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Becerra-Tomás, N.; García-Gavilán, J.F.; Bulló, M.; Barrubés, L. Mediterranean Diet and Cardiovascular Disease Prevention: What Do We Know? Prog. Cardiovasc. Dis. 2018, 61, 62–67. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Lagiou, P. Healthy traditional Mediterranean diet: An expression of culture, history, and lifestyle. Nutr. Rev. 1997, 55, 383–389. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Mediterranean dietary pattern, inflammation and endothelial function: A systematic review and meta-analysis of intervention trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Navarro, J.; Antoniazzi, L.; Oki, A.; Bonfim, M.C.; Hong, V.; Acosta-Cardenas, P.; Strunz, C.; Brunoro, E.; Miname, M.H.; Filho, W.S.; et al. Reduced subclinical carotid vascular disease and arterial stiffness in vegetarian men: The CARVOS Study. Int. J. Cardiol. 2017, 230, 562–566. [Google Scholar] [CrossRef]
- Mayra, S.T.; Johnston, C.S. Arterial stiffness and cardiometabolic health in omnivores and vegetarians: A cross-sectional pilot study. BMC Res. Notes 2022, 15, 69. [Google Scholar] [CrossRef]
- Diez-Fernández, A.; Álvarez-Bueno, C.; Martínez-Vizcaíno, V.; Sotos-Prieto, M.; Recio-Rodríguez, J.I.; Cavero-Redondo, I. Total Dairy, Cheese and Milk Intake and Arterial Stiffness: A Systematic Review and Meta-Analysis of Cross-sectional Studies. Nutrients 2019, 11, 741. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, K.M.; Lovegrove, J.A.; Cockcroft, J.R.; Elwood, P.C.; Pickering, J.E.; Givens, D.I. Does dairy food intake predict arterial stiffness and blood pressure in men?: Evidence from the Caerphilly Prospective Study. Hypertension 2013, 61, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Jauhiainen, T.; Rönnback, M.; Vapaatalo, H.; Wuolle, K.; Kautiainen, H.; Groop, P.H.; Korpela, R. Long-term intervention with Lactobacillus helveticus fermented milk reduces augmentation index in hypertensive subjects. Eur. J. Clin. Nutr. 2010, 64, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.S.; Keogh, J.B.; Meikle, P.J.; Garg, M.L.; Clifton, P.M. Dietary predictors of arterial stiffness in a cohort with type 1 and type 2 diabetes. Atherosclerosis 2015, 238, 175–1781. [Google Scholar] [CrossRef]
- Tsuboi, A.; Ito, C.; Fujikawa, R.; Yamamoto, H.; Kihara, Y. Association between the Postprandial Glucose Levels and Arterial Stiffness Measured According to the Cardio-ankle Vascular Index in Non-diabetic Subjects. Intern. Med. 2015, 54, 1961–1969. [Google Scholar] [CrossRef]
- Gordin, D.; Saraheimo, M.; Tuomikangas, J.; Soro-Paavonen, A.; Forsblom, I.; Paavonen, K.; Steckel-Hamann, B.; Vandenhende, F.; Nicolaou, L.; Pavo, I.; et al. Influence of Postprandial Hyperglycemic Conditions on Arterial Stiffness in Patients with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 1134–1143. [Google Scholar] [CrossRef]
- Stern, L.; Iqbal, N.; Seshadri, P.; Chicano, K.L.; Daily, D.A.; McGrory, J.; Williams, M.; Gracely, E.J.; Samaha, F.F. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: One-year follow-up of a randomized trial. Ann. Int. Med. 2004, 140, 778–785. [Google Scholar] [CrossRef]
- Wing, R.R. Look AHEAD Research Group.Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: Four-year results of the Look AHEAD trial. Arch. Int. Med. 2010, 170, 1566–1575. [Google Scholar] [CrossRef]
- Syed-Abdul, M.M.; Hu, Q.; Jacome-Sosa, M.; Padilla, J.; Manrique-Acevedo, C.; Heimowitz, C.; Parks, E.J. Effect of carbohydrate restriction-induced weight loss on aortic pulse wave velocity in overweight men and women. Appl. Physiol. Nutr. Metab. 2018, 43, 1247–1256. [Google Scholar] [CrossRef]
- Sanchez-Aguadero, N.; Patino-Alonso, M.C.; Mora-Simon, S.; Gomez-Marcos, M.A.; Alonso-Dominguez, R.; Sanchez-Salgado, B.; Recio-Rodriguez, J.I.; Garcia-Ortiz, L. Postprandial Effects of Breakfast Glycemic Index on Vascular Function among Young Healthy Adults: A Crossover Clinical Trial. Nutrients 2017, 9, 712. [Google Scholar] [CrossRef]
- Gaesser, G.A.; Rodriguez, J.; Patrie, J.T.; Whisner, C.M.; Angadi, S.S. Effects of Glycemic Index and Cereal Fiber on Postprandial Endothelial Function, Glycemia, and Insulinemia in Healthy Adults. Nutrients 2019, 11, 2387. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, R.; Sakazaki, M.; Nagai, Y.; Asaki, K.; Hashiguchi, T.; Negoro, H. Effects of Different Types of Carbohydrates on Arterial Stiffness: A Comparison of Isomaltulose and Sucrose. Nutrients 2021, 13, 4493. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, R.; Sato, K.; Sakazaki, M.; Nagai, Y.; Iwanuma, S.; Ohashi, N.; Hashiguchi, T. Acute effects of difference in glucose intake on arterial stiffness in healthy subjects. Cardiol. J. 2021, 28, 446–452. [Google Scholar] [CrossRef]
- Jin, M.; Miao, C.; An, L.; Guo, L.; Yang, X.; Zheng, M.; Hong, J.; Wu, S.; Su, Q. Association between Perceived Salt Intake and Arterial Stiffness. Biomed. Res. Int. 2022, 2022, 9072082. [Google Scholar] [CrossRef]
- Dickinson, K.M.; Clifton, P.M.; Burrell, L.M.; Barrett, P.H.; Keogh, J.B. Postprandial effects of a high salt meal on serum sodium, arterial stiffness, markers of nitric oxide production and markers of endothelial function. Atherosclerosis 2014, 232, 211–216. [Google Scholar] [CrossRef]
- Safar, M.E.; Thuilliez, C.; Richard, V.; Benetos, A. Pressure-independent contribution of sodium to large artery structure and function in hypertension. Cardiovasc. Res. 2000, 46, 269–276. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, L.; Rossi, G.; di Cola, M.S.; Savino, I.; Galletti, F.; Strazzullo, P. Meta-Analysis of the Effect of Dietary Sodium Restriction with or without Concomitant Renin-Angiotensin-Aldosterone System-Inhibiting Treatment on Albuminuria. Clin. J. Am. Soc. Nephrol. 2015, 10, 1542–1552. [Google Scholar] [CrossRef]
- Gates, P.E.; Tanaka, H.; Hiatt, W.R.; Seals, D.R. Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension 2004, 44, 35–41. [Google Scholar] [CrossRef]
- He, F.J.; Marciniak, M.; Visagie, E.; Markandu, N.D.; Anand, V.; Dalton, R.N.; MacGregor, G.A. Effect of modest salt reduction on blood pressure, urinary albumin, and pulse wave velocity in white, black, and Asian mild hypertensives. Hypertension 2009, 54, 482–488. [Google Scholar] [CrossRef]
- Berry, S.E.; Mulla, U.Z.; Chowienczyk, P.J.; Sanders, T.A. Increased potassium intake from fruit and vegetables or supplements does not lower blood pressure or improve vascular function in UK men and women with early hypertension: A randomised controlled trial. Br. J. Nutr. 2010, 104, 1839–1847. [Google Scholar] [CrossRef]
- He, F.J.; Marciniak, M.; Carney, C.; Markandu, N.D.; Anand, V.; Fraser, W.D.; Dalton, R.N.; Kaski, J.C.; MacGregor, G.A. Effects of potassium chloride and potassium bicarbonate on endothelial function, cardiovascular risk factors, and bone turnover in mild hypertensives. Hypertension 2010, 55, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ortiz, L.; Recio-Rodriguez, J.I.; Rodriguez-Sanchez, E.; Patino-Alonso, M.C.; Agudo-Conde, C.; Rodriguez-Martin, C.; Castano-Sanchez, C.; Runkle, I.; Gomez-Marcos, M.A. Sodium and potassium intake present a J-shaped relationship with arterial stiffness and carotid intima-media thickness. Atherosclerosis 2012, 225, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Saz-Lara, A.; Cavero-Redondo, I.; Martínez-Vizcaíno, V.; Martínez-Ortega, I.A.; Notario-Pacheco, B.; Pascual-Morena, C. The Comparative Effects of Different Types of Oral Vitamin Supplements on Arterial Stiffness: A Network Meta-Analysis. Nutrients 2022, 14, 1009. [Google Scholar] [CrossRef] [PubMed]
- Raymond, M.A.; Désormeaux, A.; Labelle, A.; Soulez, M.; Soulez, G.; Langelier, Y.; Pshezhetsky, A.V.; Hébert, M.J. Endothelial stress induces the release of vitamin D-binding protein, a novel growth factor. Biochem. Biophys. Res. Commun. 2005, 338, 1374–1382. [Google Scholar] [CrossRef]
- Wu-Wong, J.R.; Nakane, M.; Ma, J.; Ruan, X.; Kroeger, P.E. Effects of Vitamin D analogs on gene expression profiling in human coronary artery smooth muscle cells. Atherosclerosis 2006, 186, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Karimi, E.; Rezaie, P.; Vatanparast, H. The impact of vitamin D supplement intake on vascular endothelial function; a systematic review and meta-analysis of randomized controlled trials. Food Nutr. Res. 2017, 61, 1273574. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, R.; Vakili, S.; Lankarani, K.B.; Akbari, M.; Jamilian, M.; Mahdizadeh, Z.; Mirhosseini, N.; Asemi, Z. Effects of Vitamin D Supplementation on Markers Related to Endothelial Function Among Patients with Metabolic Syndrome and Related Disorders: A Systematic Review and Meta-Analysis of Clinical Trials. A Systematic Review and Meta-Analysis of Clinical Trials. Horm. Metab. Res. 2018, 50, 587–596. [Google Scholar] [CrossRef]
- Upala, S.; Sanguankeo, A.; Congrete, S.; Jaruvongvanich, V. Effect of cholecalciferol supplementation on arterial stiffness: A systematic review and meta-analysis. Scand. Cardiovasc. J. 2016, 50, 230–235. [Google Scholar] [CrossRef]
- Gillis, K.; Stevens, K.K.; Bell, E.; Patel, R.K.; Jardine, A.G.; Morris, S.T.W.; Schneider, M.P.; Delles, C.; Mark, P.B. Ascorbic acid lowers central blood pressure and asymmetric dimethylarginine in chronic kidney disease. Clin. Kidney J. 2018, 11, 532–539. [Google Scholar] [CrossRef]
- Kielstein, J.T.; Donnerstag, F.; Gasper, S.; Menne, J.; Kielstein, A.; Martens-Lobenhoffer, J.; Scalera, F.; Cooke, J.P.; Fliser, D.; Bode-Böger, S.M. ADMA increases arterial stiffness and decreases cerebral blood flow in humans. Stroke 2006, 37, 2024–2029. [Google Scholar] [CrossRef]
- Esse, R.; Barroso, M.; Tavares de Almeida, I.; Castro, R. The Contribution of Homocysteine Metabolism Disruption to Endothelial Dysfunction: State-of-the-Art. Int. J. Mol. Sci. 2019, 20, 867. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, H.; Li, Z.; Li, H.; Miao, X.; Pan, H.; Wang, J.; Liu, X.; Kang, X.; Li, X.; et al. Mutual effect of homocysteine and uric acid on arterial stiffness and cardiovascular risk in the context of predictive, preventive, and personalized medicine. EPMA J. 2022, 13, 581–595. [Google Scholar] [CrossRef]
- Dohadwala, M.M.; Holbrook, M.; Hamburg, N.M.; Shenouda, S.M.; Chung, W.B.; Titas, M.; Kluge, M.A.; Wang, N.; Palmisano, J.; Milbury, P.E.; et al. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am. J. Clin. Nutr. 2011, 93, 934–940. [Google Scholar] [CrossRef]
- Pase, M.P.; Grima, N.A.; Sarris, J. The effects of dietary and nutrient interventions on arterial stiffness: A systematic review. Am. J. Clin. Nutr. 2011, 93, 446–454. [Google Scholar] [CrossRef]
- Teede, H.J.; McGrath, B.P.; DeSilva, L.; Cehun, M.; Fassoulakis, A.; Nestel, P.J. Isoflavones reduce arterial stiffness: A placebo-controlled study in men and postmenopausal women. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Hazim, S.; Curtis, P.J.; Schär, M.Y.; Ostertag, L.M.; Kay, C.D.; Minihane, A.M.; Cassidy, A. Acute benefits of the microbial-derived isoflavone metabolite equol on arterial stiffness in men prospectively recruited according to equol producer phenotype: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 694–702. [Google Scholar] [CrossRef] [PubMed]
- van der Schouw, Y.T.; Pijpe, A.; Lebrun, C.E.; Bots, M.L.; Peeters, P.H.; van Staveren, W.A.; Lamberts, S.W.; Grobbee, D.E. Higher usual dietary intake of phytoestrogens is associated with lower aortic stiffness in postmenopausal women. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1316–1322. [Google Scholar] [CrossRef]
- Hoshida, S.; Miki, T.; Nakagawa, T.; Shinoda, Y.; Inoshiro, N.; Terada, K.; Adachi, T. Different effects of isoflavones on vascular function in premenopausal and postmenopausal smokers and nonsmokers: NYMPH study. Heart Vessels 2011, 26, 590–595. [Google Scholar] [CrossRef]
- Richter, C.K.; Skulas-Ray, A.C.; Fleming, J.A.; Link, C.J.; Mukherjea, R.; Krul, E.S.; Kris-Etherton, P.M. Effects of isoflavone-containing soya protein on ex vivo cholesterol efflux, vascular function and blood markers of CVD risk in adults with moderately elevated blood pressure: A dose-response randomised controlled trial. Br. J. Nutr. 2017, 117, 1403–1413. [Google Scholar] [CrossRef]
- Kim, O.Y.; Yoe, H.Y.; Kim, H.J.; Park, J.Y.; Kim, J.Y.; Lee, S.H.; Lee, J.H.; Lee, K.P.; Jang, Y.; Lee, J.H. Independent inverse relationship between serum lycopene concentration and arterial stiffness. Atherosclerosis 2010, 208, 581–586. [Google Scholar] [CrossRef]
- Yeo, H.Y.; Kim, O.Y.; Lim, H.H.; Kim, J.Y.; Lee, J.H. Association of serum lycopene and brachial-ankle pulse wave velocity with metabolic syndrome. Metabolism 2011, 60, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Thies, F.; Masson, L.F.; Rudd, A.; Vaughan, N.; Tsang, C.; Brittenden, J.; Simpson, W.G.; Duthie, S.; Horgan, G.W.; Duthie, G. Effect of a tomato-rich diet on markers of cardiovascular disease risk in moderately overweight, disease-free, middle-aged adults: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Cavani, C.; Petracci, M.; Trocino, A.; Xiccato, G. Advances in Research on Poultry and Rabbit Meat Quality. Ital. J. Anim. Sci. 2009, 8 (Suppl. 2), 741–750. [Google Scholar] [CrossRef]
- Barroeta, A. Nutritive value of poultry meat: Relationship between vitamin E and PUFA. World’s Poult. Sci. J. 2007, 63, 277–284. [Google Scholar] [CrossRef]
- Dehghan, M.; Mente, A.; Rangarajan, S.; Sheridan, P.; Mohan, V.; Iqbal, R.; Gupta, R.; Lear, S.; Wentzel-Viljoen, E.; Avezum, A.; et al. Prospective Urban Rural Epidemiology (PURE) study investigators. Association of dairy intake with cardiovascular disease and mortality in 21 countries from five continents (PURE): A prospective cohort study. Lancet 2018, 392, 2288–2297. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fox, C.S.; Troy, L.M.; Mckeown, N.M.; Jacques, P.F. Longitudinal association of dairy consumption with the changes in blood pressure and the risk of incident hypertension: The Framingham Heart Study. Br. J. Nutr. 2015, 114, 1887–1899. [Google Scholar] [CrossRef]
- Tognon, G.; Nilsson, L.M.; Shungin, D.; Lissner, L.; Jansson, J.H.; Renström, F.; Wennberg, M.; Winkvist, A.; Johansson, I. Nonfermented milk and other dairy products: Associations with all-cause mortality. Am. J. Clin. Nutr. 2017, 105, 1502–1511. [Google Scholar] [CrossRef]
- Drouin-Chartier, J.P.; Brassard, D.; Tessier-Grenier, M.; Côté, J.A.; Labonté, M.È.; Desroches, S.; Couture, P.; Lamarche, B. Systematic Review of the Association between Dairy Product Consumption and Risk of Cardiovascular-Related Clinical Outcomes. Adv. Nutr. 2016, 7, 1026–1040. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Farrés, X.; Luque, X.; Narejos, S.; Borrell, M.; Basora, J.; Anguera, A.; Torres, F.; Bulló, M.; Balanza, R. and Group, Fiber in Obesity-Study. Effect of two doses of a mixture of soluble fibres on body weight and metabolic variables in overweight or obese patients: A randomised trial. Br. J. Nutr. 2008, 99, 1380–1387. [Google Scholar] [CrossRef]
- Theuwissen, E.; Mensink, R.P. Water-soluble dietary fibers and cardiovascular disease. Physiol. Behav. 2008, 94, 85–92. [Google Scholar] [CrossRef]
- Kizirian, N.V.; Kong, Y.; Muirhead, R.; Brodie, S.; Garnett, S.P.; Petocz, P.; Sim, K.A.; Celermajer, D.S.; Louie, J.C.; Markovic, T.P.; et al. Effects of a low-glycemic index diet during pregnancy on offspring growth, body composition, and vascular health: A pilot randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 1073–1082. [Google Scholar] [CrossRef]
- Maresch, C.C.; Petry, S.F.; Theis, S.; Bosy-Westphal, A.; Linn, T. Low Glycemic Index Prototype Isomaltulose-Update of Clinical Trials. Nutrients 2017, 9, 381. [Google Scholar] [CrossRef]
- Holub, I.; Gostner, A.; Theis, S.; Nosek, L.; Kudlich, T.; Melcher, R.; Scheppach, W. Novel findings on the metabolic effects of the low glycaemic carbohydrate isomaltulose (Palatinose). Br. J. Nutr. 2010, 103, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Starmans-Kool, M.J.; Stanton, A.V.; Xu, Y.Y.; Thom, S.A.M.; Parker, K.H.; Hughes, A.D. High dietary salt intake increases carotid blood pressure and wave reflection in normotensive healthy young men. J. Appl. Physiol. 2011, 110, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Muth, B.J.; Brian, M.S.; Chirinos, J.A.; Lennon, S.L.; Farquhar, W.B.; Edwards, D.G. Central systolic blood pressure and aortic stiffness response to dietary sodium in young and middle-aged adults. J. Am. Soc. Hypertens. 2017, 11, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Lennon-Edwards, S.; Allman, B.R.; Schellhardt, T.A.; Ferreira, C.R.; Farquhar, W.B.; Edwards, D.G. Lower potassium intake is associated with increased wave reflection in young healthy adults. Nutr. J. 2014, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Mente, A.; O’Donnell, M.J.; Rangarajan, S.; McQueen, M.J.; Poirier, P.; Wielgosz, A.; Morrison, H.; Li, W.; Wang, X.; Di, C.; et al. Association of urinary sodium and potassium excretion with blood pressure. N. Engl. J. Med. 2014, 371, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Huggins, C.E.; O’Reilly, S.; Brinkman, M.; Hodge, A.; Giles, G.G.; English, D.R.; Nowson, C.A. Relationship of urinary sodium and sodium-to-potassium ratio to blood pressure in older adults in Australia. Med. J. Aust. 2011, 195, 128–132. [Google Scholar] [CrossRef]
- Schröder, H.; Schmelz, E.; Marrugat, J. Relationship between diet and blood pressure in a representative Mediterranean population. Eur. J. Nutr. 2002, 41, 161–167. [Google Scholar] [CrossRef]
- Del Giorno, R.; Ceresa, C.; Gabutti, S.; Troiani, C.; Gabutti, L. Arterial Stiffness and Central Hemodynamics are Associated with Low Diurnal Urinary Sodium Excretion. Diabetes Metab. Syndr. Obes. 2020, 13, 3289–3299. [Google Scholar] [CrossRef]
- Han, W.; Han, X.; Sun, N.; Chen, Y.; Jiang, S.; Li, M. Relationships between urinary electrolytes excretion and central hemodynamics, and arterial stiffness in hypertensive patients. Hypertens. Res. 2017, 40, 746–751. [Google Scholar] [CrossRef]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R., 3rd; Simons-Morton, D.G.; et al. DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, R.; Larson, M.G.; Dupuis, J.; Lunetta, K.L.; Lipinska, I.; Meigs, J.B.; Yin, X.; Rong, J.; Vita, J.A.; Newton-Cheh, C.; et al. Relations of inflammatory biomarkers and common genetic variants with arterial stiffness and wave reflection. Hypertension 2008, 51, 1651–1657. [Google Scholar] [CrossRef]
- May, J.M.; Harrison, F.E. Role of vitamin C in the function of the vascular endothelium. Antioxid. Redox Signal. 2013, 19, 2068–2083. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef]
- Achan, V.; Broadhead, M.; Malaki, M.; Whitley, G.; Leiper, J.; MacAllister, R.; Vallance, P. Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1455–1459. [Google Scholar] [CrossRef]
- Zoccali, C.; Benedetto, F.A.; Maas, R.; Mallamaci, F.; Tripepi, G. Asymmetric dimethylarginine, C-reactive protein, and carotid intima-media thickness in end-stage renal disease. J. Am. Soc. Nephrol. 2002, 13, 490–496. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Yoon, Y.; Lee, K.-Y. Uric acid induces endothelial dysfunction by vascular insulin resistance associated with the impairment of nitric oxide synthesis. FASEB J. 2014, 28, 3197–3204. [Google Scholar] [CrossRef]
- Zharikov, S.; Krotova, K.; Hu, H.; Baylis, C.; Johnson, R.J.; Block, E.R.; Patel, J. Uric acid decreases NO production and increases arginase activity in cultured pulmonary artery endothelial cells. Am. J. Physiol. Cell. Physiol. 2008, 295, C1183–C1190. [Google Scholar] [CrossRef]
- Corry, D.B.; Eslami, P.; Yamamoto, K.; Nyby, M.D.; Makino, H.; Tuck, M.L. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J. Hypertens. 2008, 26, 269–275. [Google Scholar] [CrossRef]
- Khosla, U.M.; Zharikov, S.; Finch, J.L.; Nakagawa, T.; Roncal, C.; Mu, W.; Krotova, K.; Block, E.R.; Prabhakar, S.; Johnson, R.J. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005, 67, 1739–1742. [Google Scholar] [CrossRef]
- du Plooy, C.S.; Mels, C.M.; Huisman, H.W.; Kruger, R. The Association of Endothelin-1 with Markers of Arterial Stiffness in Black South African Women: The SABPA Study. J. Amino Acids. 2015, 2015, 481517. [Google Scholar] [CrossRef]
- Drüppel, V.; Kusche-Vihrog, K.; Grossmann, C.; Gekle, M.; Kasprzak, B.; Brand, E.; Pavenstädt, H.; Oberleithner, H.; Kliche, K. Long-term application of the aldosterone antagonist spironolactone prevents stiff endothelial cell syndrome. FASEB J. 2013, 27, 3652–3659. [Google Scholar] [CrossRef]
- Izzo, J.L., Jr. Systolic hypertension, arterial stiffness, and vascular damage: Role of the renin-angiotensin system. Blood Press Monit. 2000, 5 (Suppl. 2), S7–S11. [Google Scholar] [CrossRef]
- Suzuki, T. Nitrosation of uric acid induced by nitric oxide under aerobic conditions. Nitric Oxide 2007, 16, 266–273. [Google Scholar] [CrossRef]
- McNulty, H.; Scott, J.M. Intake and status of folate and related B-vitamins: Considerations and challenges in achieving optimal status. Br. J. Nutr. 2008, 99, S48–S54. [Google Scholar] [CrossRef]
- Ganji, S.H.; Qin, S.; Zhang, L.; Kamanna, V.S.; Kashyap, M.L. Niacin inhibits vascular oxidative stress, redox-sensitive genes, and monocyte adhesion to human aortic endothelial cells. Atherosclerosis 2009, 202, 68–75. [Google Scholar] [CrossRef]
- Hariri, M.; Darvishi, L.; Maghsoudi, Z.; Khorvash, F.; Aghaei, M.; Iraj, B.; Ghiasvand, R.; Askari, G. Intakes of Vegetables and Fruits are Negatively Correlated with Risk of Stroke in Iran. Int. J. Prev. Med. 2013, 4 (Suppl. 2), S300–S305. [Google Scholar]
- Rees, K.; Takeda, A.; Martin, N.; Ellis, L.; Wijesekara, D.; Vepa, A.; Das, A.; Hartley, L.; Stranges, S. Mediterranean-style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2019, 3, CD009825. [Google Scholar] [CrossRef]
- Dhaka, V.; Gulia, N.; Ahlawat, K.S.; Khatkar, B.S. Trans fats-sources, health risks and alternative approach. J. Food. Sci. Technol. 2011, 48, 534–541. [Google Scholar] [CrossRef]
- Prakash, B. Functional and Preservative Properties of Phytochemicals, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128196861. [Google Scholar]
- Spencer, J.P.E.; El Mohsen, M.M.A.; Minihane, A.-M.; Mathers, J.C. Biomarkers of the intake of dietary polyphenols: Strengths, limitations and application in nutrition research. Br. J. Nutr. 2008, 99, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Kizhakekuttu, T.J.; Widlansky, M.E. Natural antioxidants and hypertension: Promise and challenges. Cardiovasc. Ther. 2010, 28, e20–e32. [Google Scholar] [CrossRef]
- Upadhyay, S.; Dixit, M. Role of Polyphenols and Other Phytochemicals on Molecular Signaling. Oxid. Med. Cell. Longev. 2015, 2015, 504253. [Google Scholar] [CrossRef]
- Hozawa, A.; Jacobs, D.R., Jr.; Steffes, M.W.; Gross, M.D.; Steffen, L.M.; Lee, D.H. Relationships of circulating carotenoid concentrations with several markers of inflammation, oxidative stress, and endothelial dysfunction: The coronary artery risk development in young adults (CARDIA)/Young adult longitudinal trends in antioxidants (YALTA) study. Clin. Chem. 2007, 53, 447–455. [Google Scholar] [CrossRef]
- Markovits, N.; Ben Amotz, A.; Levy, Y. The effect of tomato-derived lycopene on low carotenoids and enhanced systemic inflammation and oxidation in severe obesity. Isr. Med. Assoc. J. 2009, 11, 598–601. [Google Scholar]
- Sesso, H.D.; Buring, J.E.; Norkus, E.P.; Gaziano, J.M. Plasma lycopene, other carotenoids, and retinol and the risk of cardiovascular disease in women. Am. J. Clin. Nutr. 2004, 79, 47–53. [Google Scholar] [CrossRef]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef]
- Teede, H.J.; Giannopoulos, D.; Dalais, F.S.; Hodgson, J.; McGrath, B.P. Randomised, controlled, cross-over trial of soy protein with isoflavones on blood pressure and arterial function in hypertensive subjects. J. Am. Coll. Nutr. 2006, 25, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Goszcz, K.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Bioactive polyphenols and cardiovascular disease: Chemical antagonists, pharmacological agents or xenobiotics that drive an adaptive response? Br. J. Pharmacol. 2017, 174, 1209–1225. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).