Green Tea and Benign Gynecologic Disorders: A New Trick for An Old Beverage?
Abstract
1. Introduction
2. Methods
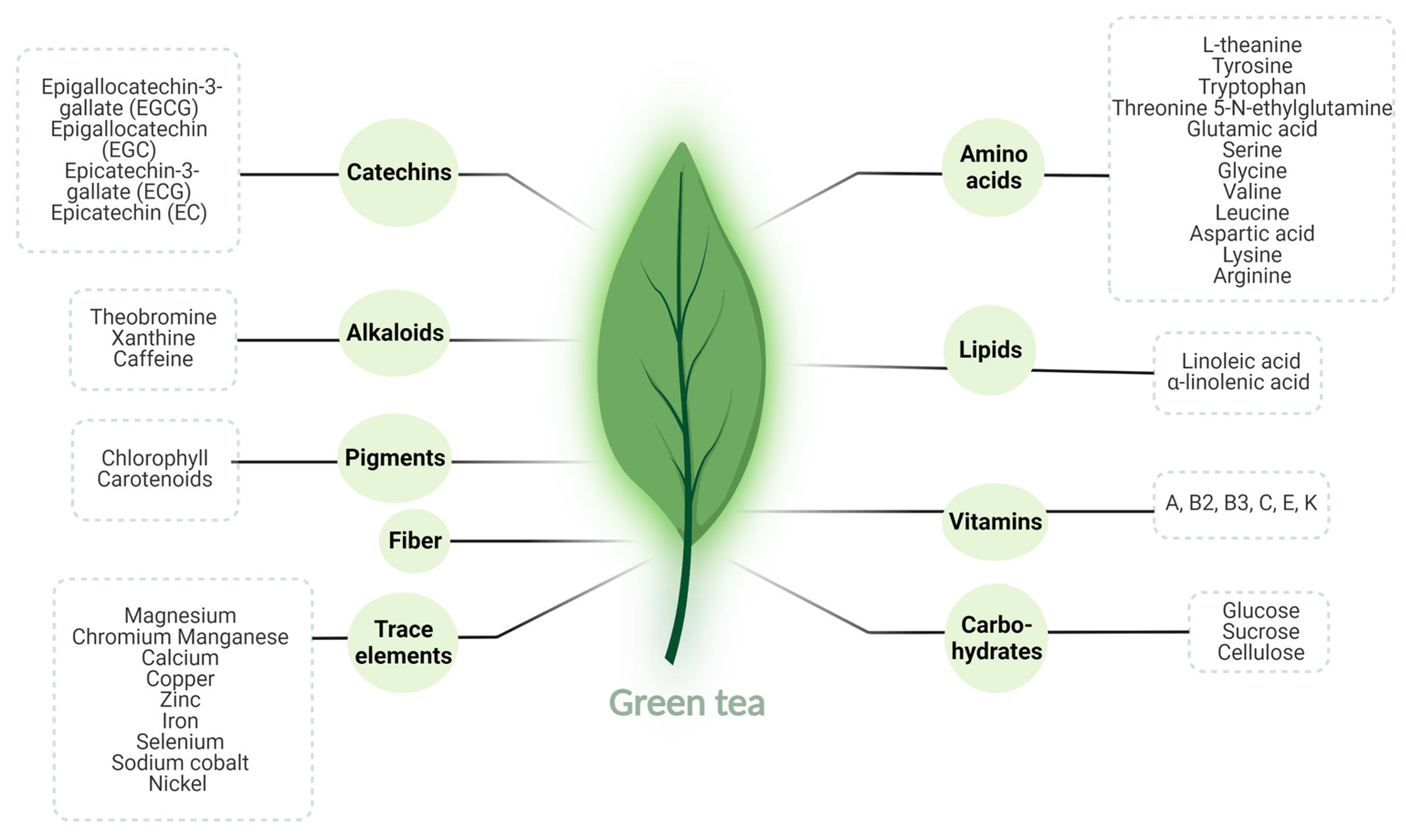
3. Green Tea
3.1. Green Tea: Overview
3.2. Green Tea: Pharmacokinetics and Bioavailability
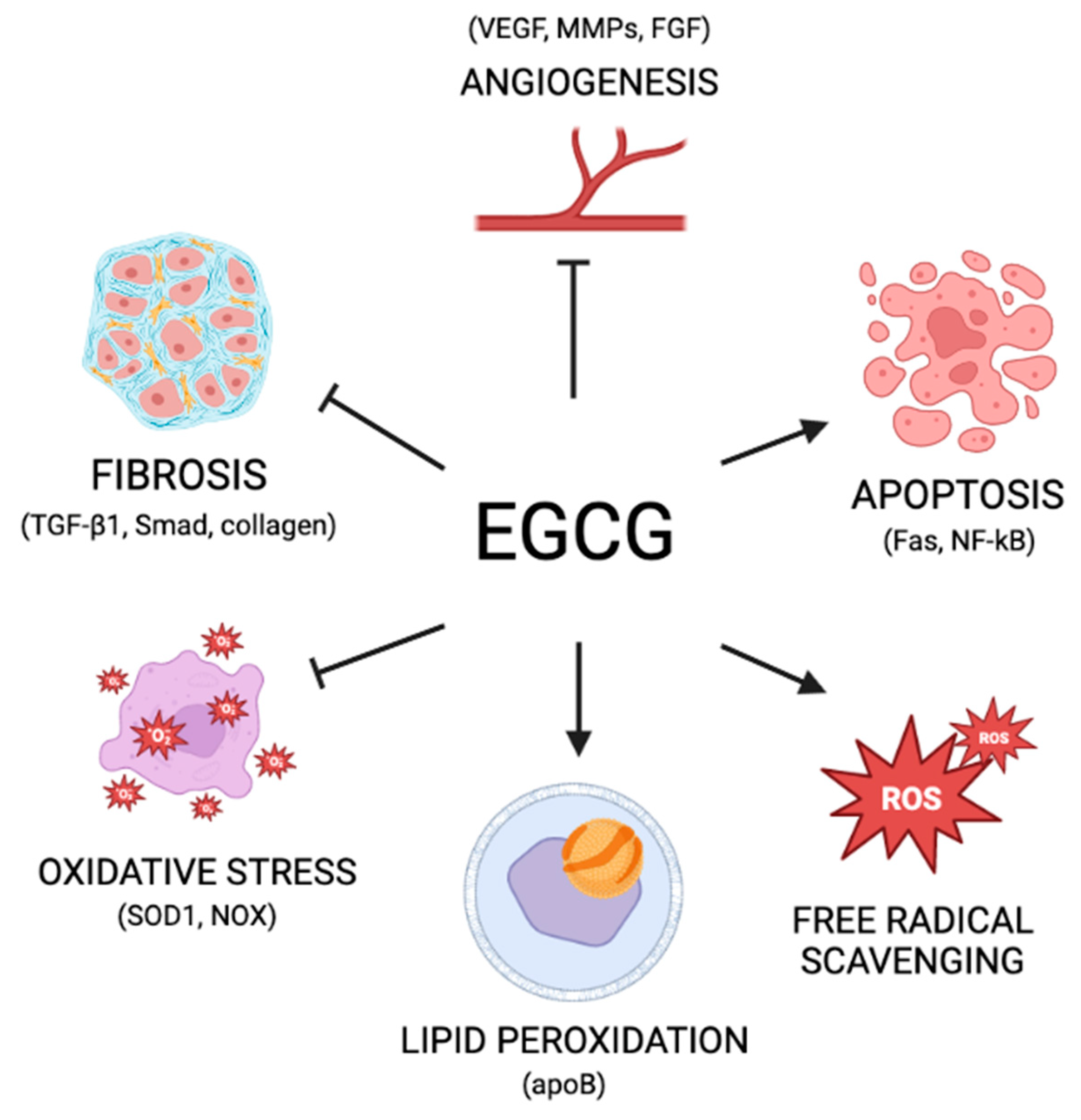
3.3. Green Tea: Mechanisms of Action
4. Role of Green Tea against Benign Gynecological Disorders
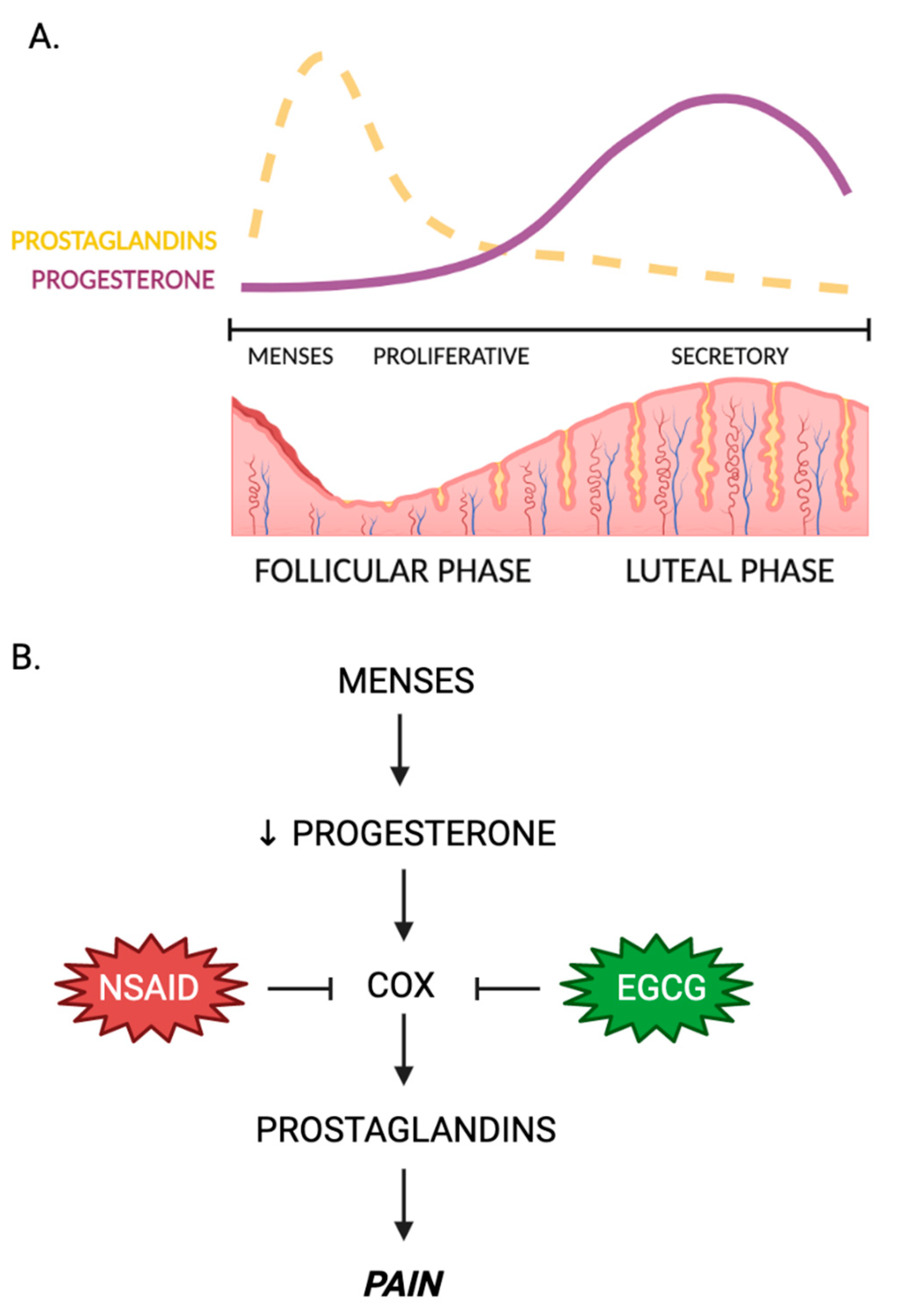
4.1. Green Tea and Dysmenorrhea
4.2. Green Tea and Infertility
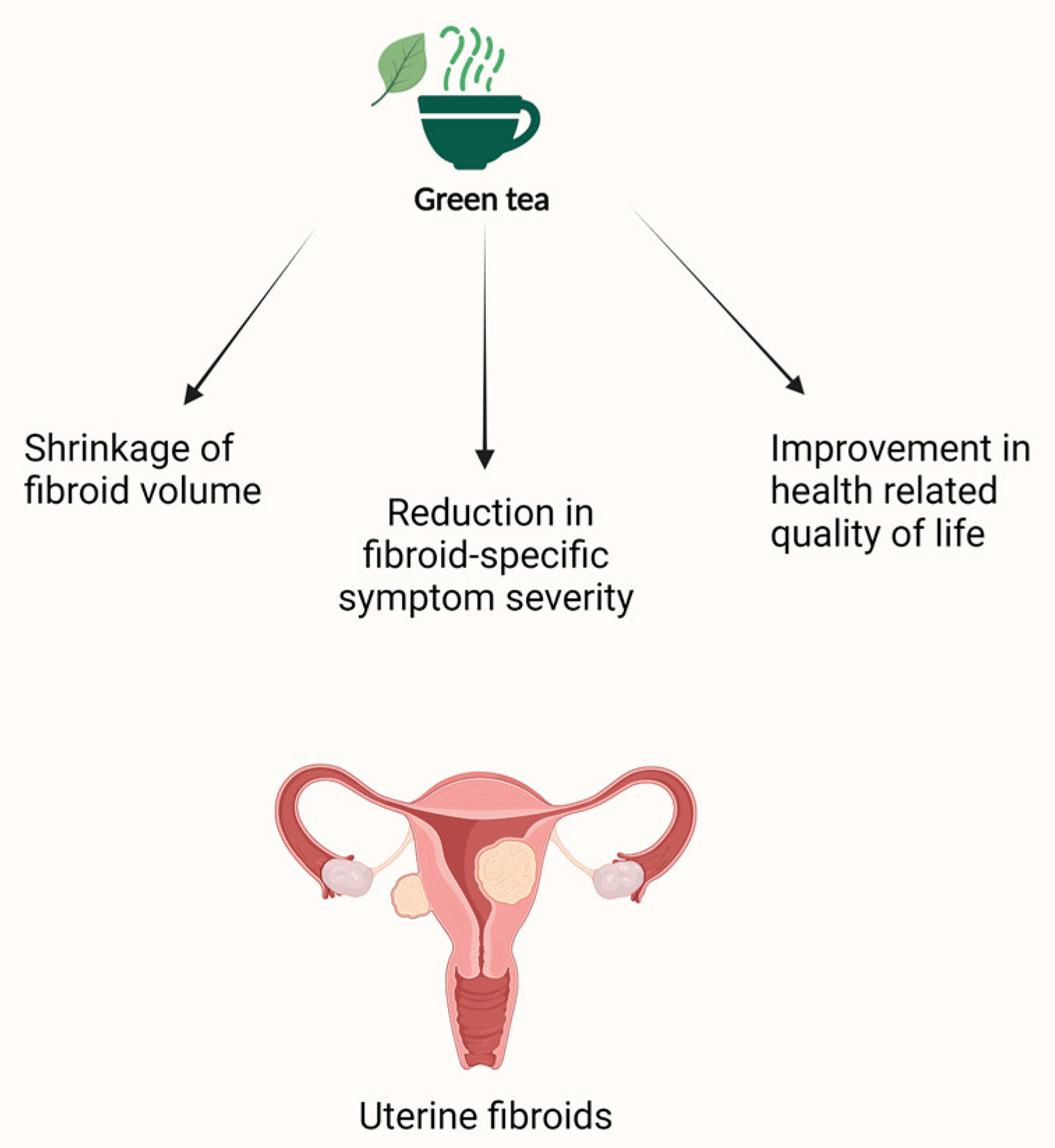
4.3. Green Tea and Uterine Fibroids
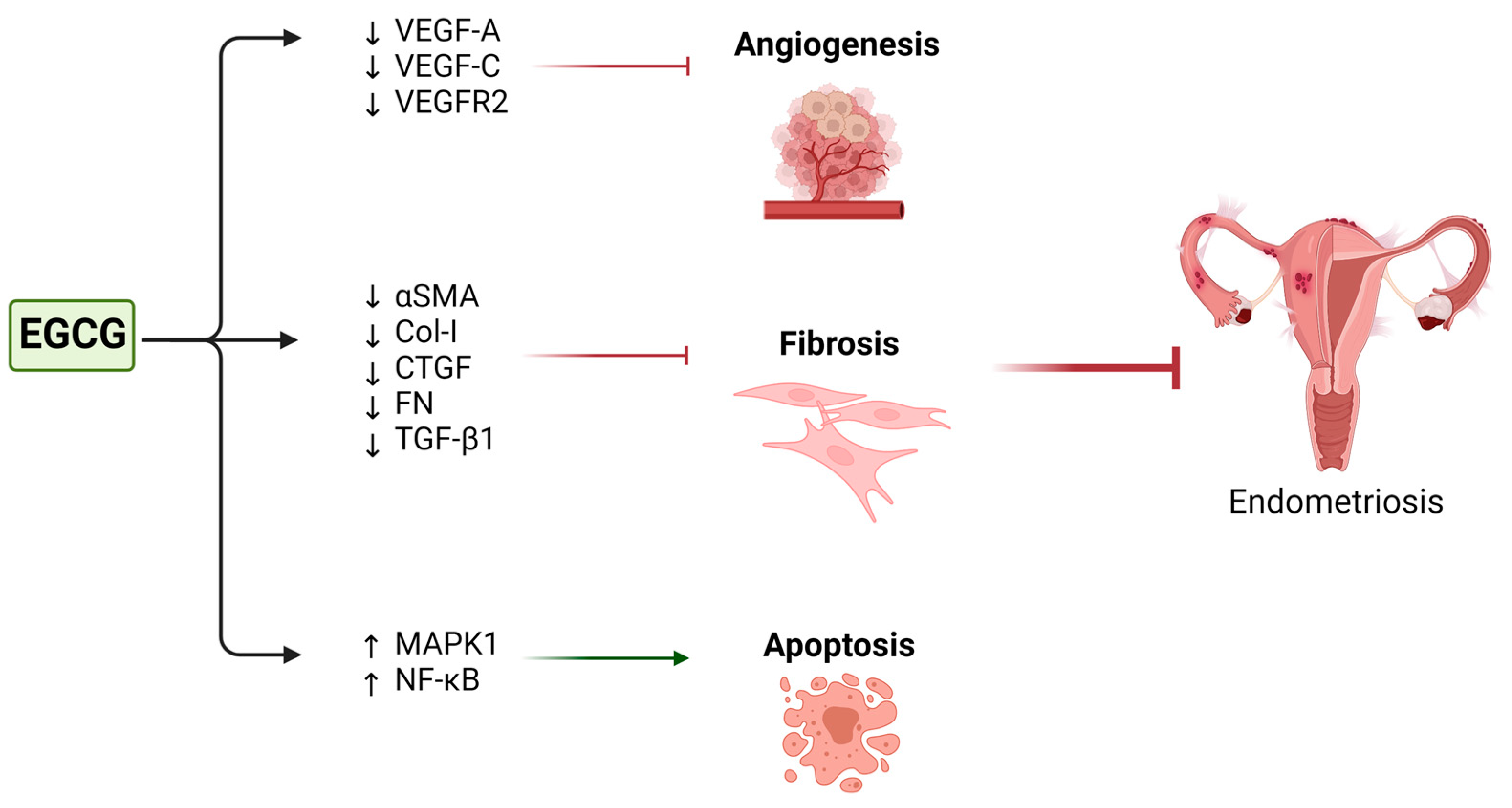
4.4. Green Tea and Endometriosis
| Study and Year | Cell Lines | Green Tea Extract/EGCG Concentrations | Mechanism of Action | Effect | |
|---|---|---|---|---|---|
| Fibroids | Zhang et al. (2010) [141] | HuLM | 0, 0.1, 1.0, 10, 50, 100, and 200 µM | Upregulation of genes from the TGF-b and stress pathways, inhibition of the survival pathway and NFκB–dependent inflammatory pathway Decreased expression of PCNA, cdk4, and bcl2 Increased the expression BAX | Dose-dependent and time-dependent inhibition of cellular proliferation Increase in apoptosis |
| Zhang et al. (2010) [142] | ELT3 | 200 μmol/L | Decreased expression of PCNA and Cdk4 | Inhibition of cellular proliferation Induction of apoptosis | |
| Zhang et al. (2014) [147] | WT-HuLM and COMT-shRNA-HuLM | 100 μM | Decreased COMT expression and enzyme activity Decreased expression of PCNA and Cdk4 | Decreased proliferation of WT-HuLM cells but not COMT-shRNA-HuLM | |
| Endometriosis | Laschke et al. (2008) [163] | Isolated hamster endometrial stromal and glandular cells | 1. 40 µM EGCG. 2. 1 µM 17β-estradiol 3. 40 µM EGCG + 1 µM 17β-estradiol | Suppressed estrogen-stimulated activation, proliferation, and VEGF expression | Prevention of the development of new endometriotic lesions |
| Xu et al. (2011) [161] | Human microvascular endothelial cells | 10–50 µM | Suppressed VEGF-C expression and reduced VEGFR-2 and ERK activation | Inhibition of angiogenesis | |
| Ricci et al. (2013) [160] | Primary cultures of human endometrial epithelial cells from patients with endometriosis | 0, 20, 40, 80, and 100 µM | Reduction in cell proliferation and increase in apoptosis | ||
| Matsuzaki et al. (2014) [162] | Isolated endometrial and endometriotic stromal cells | 50 or 100 µM | Decreased expression of the fibrotic markers αSMA, Col-I, CTGF, and FN Inhibition of TGF-β1-stimulated activation of MAPK and Smad signaling pathways | Inhibition of fibrosis in endometriosis | |
| Infertility | Huang et al. (2021) [125] | Porcine cumulus oocyte complexes | 5, 10, 20 µM | Reduction in MDA and ROS levels Increased GSH concentrations Increased expression of superoxide dismutase, catalase, and glutathione peroxidase Reduction of bax and caspase-3 expression Upregulation of bcl-2 expression Increase in blastocyst and cleavage rate | Increased oocyte maturation and antioxidant capacity |
| Wang et al. (2007) [126] | Bovine cumulus oocyte complexes | 15, 20 µM of green tea extract | Increased blastocyst formation Increased GSH levels | Increased oocyte maturation Improvements in embryonic development | |
| Barakat et al. (2014) [127] | Sheep cumulus oocyte complexes | 0.3 mg/mL | Increased oocyte development to metaphase II Increased blastocyst formation | Increased nuclear maturation and embryo development | |
| Spinaci et al. (2008) [128] | Pig cumulus oocytes complexes | 0 to 25 μg/ml | Decreased blastocyst formation at 25 μg/mL Inhibition of progesterone production | Inhibition of steroidogenesis | |
| Basini et al. (2005) [129] | Swine granulosa cells | 5, 50 μg/ml | Decreased E2 and P4 production Decreased VEGF Increased H2O2 and superoxide dismutase activity | Inhibition of cellular proliferation, steroidogenesis, and angiogenesis | |
| Balasi et al. (2019) [130] | Rabbit ovarian fragments | 0, 1, 10, 100 μg/ml | Upregulation of caspase-3 Inhibition of P4 and testosterone | Inhibition of steroidogenesis Increase in apoptosis |
| Study and Year | Animal Model | Green Tea Extract/EGCG Concentrations | Mechanism of Action | Effect | Side Effects | |
|---|---|---|---|---|---|---|
| Fibroids | Zhang et al. (2010) [142] | Female athymic nude mice | 1.25-mg EGCG/mouse/day for 4 and 8 weeks | Decreased expression of PCNA and Cdk4 | Reduction of fibroid tumor size and weight | None reported |
| Ozercan et al. (2008) [143] |
Japanese quail (Coturnix coturnix japonica) | 200 or 400 mg EGCG/kg for 12 months | Decreased serum and liver malondialdehyde and TNF-α concentrations | Decreased incidence and size of leiomyomas | None reported | |
| Endometriosis | Laschke et al. (2008) [163] | Syrian golden hamster dorsal skinfold chamber model | 65 mg/kg EGCG for 14 days | Decreased microvessel number and density Decreased centerline red blood cell velocity and volumetric blood flow | Reduced angiogenesis and blood perfusion Induced regression of endometriotic lesions | None reported |
| Xu et al. (2011) [161] | SCID mice | 50 mg/kg/day EGCG for 3 weeks | Suppressed VEGFC/VEGFR2 expression and signaling pathway through c-JUN, interferon-γ, matrix metalloproteinase 9, and chemokine (C-X-C motif) ligand 3 pathway | Inhibition of angiogenesis | None reported | |
| Guan et al. (2020) [164] | BALB/c female nude mice | 8.333 mg/mL EGCG for 16 days | Increased E-cadherin expression Reduced DNA methylation of the E-cadherin promoter region | Inhibition of endometrial lesion growth | None reported | |
| Ricci et al. (2013) [160] | BALB/c mouse model | 1.20 or 100 mg/kg EGCG for 4 weeks | Decreased cell proliferation, reduced vascular density, and increased apoptosis within the lesions | Inhibition of the development and reduction in the size of endometriotic lesions | None reported | |
| Matsuzaki et al. (2014) [162] | Female nude mice | 50 mg/kg/day EGCG for 21 or 8 days | Decreased scores for Sirius Red and Masson trichrome staining in EGCG-treated mice. Lower staining score for human CD10-positive cells in untreated mice than in treated mice | Prevention of fibrosis progression in endometriosis | None reported | |
| Xu et al. (2009) [159] | SCID mice | 50 mg/kg/day EGCG for 2 weeks | Down-regulation of VEGF-A expression Up-regulation of Nuclear factor-kappa B and mitogen-activated protein kinase 1 expression Reduction in mean total number and size of endometrial microvessels | Inhibition of angiogenesis Increase in apoptosis | None reported | |
| Wang et al. (2013) [62] | NOD-SCID mice transplanted with endometrial tissues from CMV-Luc mic | 50 mg/kg pro-EGCG (EGCG octaacetate). or 50 mg/kg EGCG for 4 weeks | Increased total apoptotic cell numbers in the lesions Decreased microvessel parameters Decrease in both the CD31-positively and αSMA-negatively stained new microvessel numbers and the CD31-positively and αSMA-positively stained old microvessel numbers in the lesion | Inhibition of growth and angiogenesis Increase in apoptosis | None reported | |
| Adenomyosis | Chen et al. (2013) [165] | Mice treated with 1 mg/kg tamoxifen to induce adenomyosis | 5 or 50 mg/kg EGCG for 3 weeks | Decreased plasma corticosterone levels Reduced uterine contractility and suppressed myometrial infiltration | Improvement in adenomyosis- induced pain, stress, and uterine hyperactivity | None reported |
| Chen et al. (2014) [166] | Mice treated with 1 mg/kg tamoxifen to induce adenomyosis | 5 or 50 mg/kg EGCG for 3 weeks | Increase in GABAergic inhibition manifested by an increase in glutamate decarboxylase (GAD) 65-expressing neurons in the brainstem nucleus raphe magnus (NRM) Decreased expression of p-p65, cyclooxygenase 2, oxytocin receptor, collagen I and IV, and transient receptor potential vanilloid type 1 in ectopic endometrium or myometrium Reduced number of macrophages infiltrating into the ectopic endometrium Increased expression of progesterone receptor isoform B(PR-B) |
Attenuated stress response and improved hyperalgesia through the GABAergic system in the brain Reduced myometrial infiltration | None reported | |
| Infertility | Balazi et al. (2019) [130] | Nulliparous rabbit | Food enriched with 5 g and 20 g of green tea for 302 days | Increased ovarian length Decreased conception and kindling rates Decreased number of liveborn and weaned pups |
Inhibition of steroidogenesis Increase in apoptosis | Decrease in platelet distribution width |
| PCOS | Ghafurniyan et al. (2015) [167] | PCOS rats | 50, 100, 200 mg/kg of hydro-alcoholic extract containing 200 g of dried green tea leaves powder for 10 days |
Decreased weight and insulin resistance Decreased testosterone level | None reported |
| Study | Study Design | Intervention vs. Control | Outcomes | Treatment Duration | Results | Side Effects | |
|---|---|---|---|---|---|---|---|
| Fibroids | Roshdy et al. [144] | Randomized, double-blinded clinical trial N = 33 22 treated and 11 control | Intervention: 800 mg of green tea extract daily Control: placebo (800 mg of brown rice) daily | Primary outcome: total fibroid volume Secondary outcome: mean change in fibroid specific symptom severity and health-related quality of life (UFS-QOL) | 4 months |
32.6% reduction in fibroid volume in the group treated with green tea extract vs. 24.3% increase in the placebo group. 32.4% reduction in fibroid-specific symptom severity and significant improvement (18.53%) in health-related quality of life in the treatment group compared to the placebo group. | None reported |
| Grandi et al. [108] | Pilot prospective daily-dairy based study N = 16 | A combination of EGCG (300 mg), Vitamin B6 (10 mg), and VD (50 mg) daily No control | Primary outcome: reduction of uterine fibroid (UF) size Secondary outcomes:
| 3 months | 17.8% reduction in UF’s median size compared to baseline. No significant changes in health-related quality of life or sexual life after treatment. Significant decrease in menstrual flow length (0.9 day). No change in cycle length, menstrual flow intensity, or menstrual pain intensity. | None reported | |
| Porcaro et al. [145] | Pilot trial N = 30 15 treated and 15 control | Intervention: one tablet of 25 μg vitamin D + 150 mg EGCG + 5 mg vitamin B6 twice daily Control: no treatment | Primary outcome: change of fibroid volume Secondary outcomes: variation of the number of fibroids, distress caused by heavy menstrual bleeding, pelvic pressure, fatigue, quality of life (QoL), and the severity of symptoms (SS) | 4 months | 34.7% decrease in fibroid volume in the treated group vs. 6.9% increase in the control group. Improvement in the QoL of women in the treatment group vs. a slight decrease in QoL in the control group. Reduction of the SS in the treatment group vs. no variation in the control group. | None reported | |
| Miriello et al. [146] | N = 95 41 treated and 54 control | Intervention: one tablet of 25 μg vitamin D + 150 mg EGCG + 5 mg vitamin B6 twice daily Control: no treatment | Primary outcome: change of fibroid volume Secondary outcomes: variation of myomas color score, distress by heavy menstrual bleeding, pelvic pain, and quality of life | 4 months | 37.9% decrease in fibroid total volume in the treated group vs. 5.5% increase in the control group. Decrease in the peripherical fibroid vascularization (color score 2) in 7.7% of treated patients vs. 5.5% increase in color score 2 in the control group. Significant improvement in pelvic pain in the treated group vs. no change in the control group. The decrease in heavy bleeding in the treated group compared to the control group was not statistically significant. Significant improvement in the quality of life of treated women compared to their pre-treatment baseline. | None reported | |
| Infertility | NCT05364008 | Pilot trial N = 200 | Intervention: 1650 mg low caffeine green tea extract Control: 1650 mg placebo matching smell, taste, color, and texture | Primary outcome: cumulative live birth rate Secondary outcomes: conception rate, miscarriage rate, change of fibroid volume, change of fibroid symptom severity score, change of health-related quality-of-life questionnaire score, and time of conception | 6 months | Ongoing trial | None reported |
| Westphal et al. [131] | Double blind placebo-controlled study N = 93 53 were treated and 40 control | Intervention: FertilityBlend Control: placebo | Outcomes: changes in progesterone levels, basal body temperature, length of menstrual cycle, pregnancy rate, and incidence of side effects | 3 months | Increase in mean mid-luteal progesterone, basal body temperature, and normalization of the menstrual cycle in the treated group compared to the control group. 14 of the 53 women in the intervention group were pregnant. No significant side effects. | None reported | |
| Menopause | Rondanelli et al. [168] | Double-blind placebo-controlled randomized trial N = 28 14 were treated and 14 control | Intervention: 150 mg Greenselect Phytosome Control: placebo matching size, shape, color, odor, and taste | Primary outcomes: respiratory quotient (RQ), percentages of carbohydrates (% CHO) and fat oxidation (%FAT), and resting energy expenditure (REE) Secondary outcomes: body composition, glucose and lipid profiles, inflammatory state, liver and kidney functions, and hormonal and catecholamines status | 60 days | Statistically significant decrease in RQ, in % CHO and fat oxidation, in waist circumference, in insulin, as well as in inflammatory markers in the treated group compared to the control group. Statistically significant increase in adiponectin, noradrenalin, MB, % LIP, and the adiponectin/leptin ratio in the treated group compared to the control group. | None reported |
| PCOS | Tehrani et al. [169] | Double-blind randomized trial N = 60 30 were treated and 30 control | Intervention:500 mg green tea tablet Control: placebo | Outcomes: free testosterone, fasting insulin levels, and weight | 12 weeks | Decrease in free testosterone as well as insulin levels and weight in the treated group compared to the control group. | Gastrointestinal side effects |
| Mombaini et al. [170] | Randomized double-blind placebo-controlled clinical trial N = 50 25 were treated and 25 control | Intervention: 500 mg green tea tablet Control: placebo corn starch tablets | Outcomes: anthropometric indices and inflammatory markers | 45 days | Significant decrease in BMI, waist circumference, and body fat percentage in the treated group compared to the control group. No significant difference in inflammatory markers (IL-6, CRP, and TNF- α) between the treated and control groups. | Gastrointestinal side effects | |
| Chan et al. [171] | Randomized placebo-controlled clinical trial N = 34 18 were treated and 16 control | Intervention: capsules containing 2% freeze-dried tea powder Control: placebo | Outcomes: anthropometric measurements, biochemical and hormonal profiles | 3 months | No statistically significant difference in BMI, body weight, and body fat, fasting insulin, glucose, cholesterol, HDL, LDL, testosterone, SHBG, free androgen, androstenedione, DHEA-S, LH, and FSH between the intervention and control groups. | No significant side effects |
4.5. Green Tea and Adenomyosis
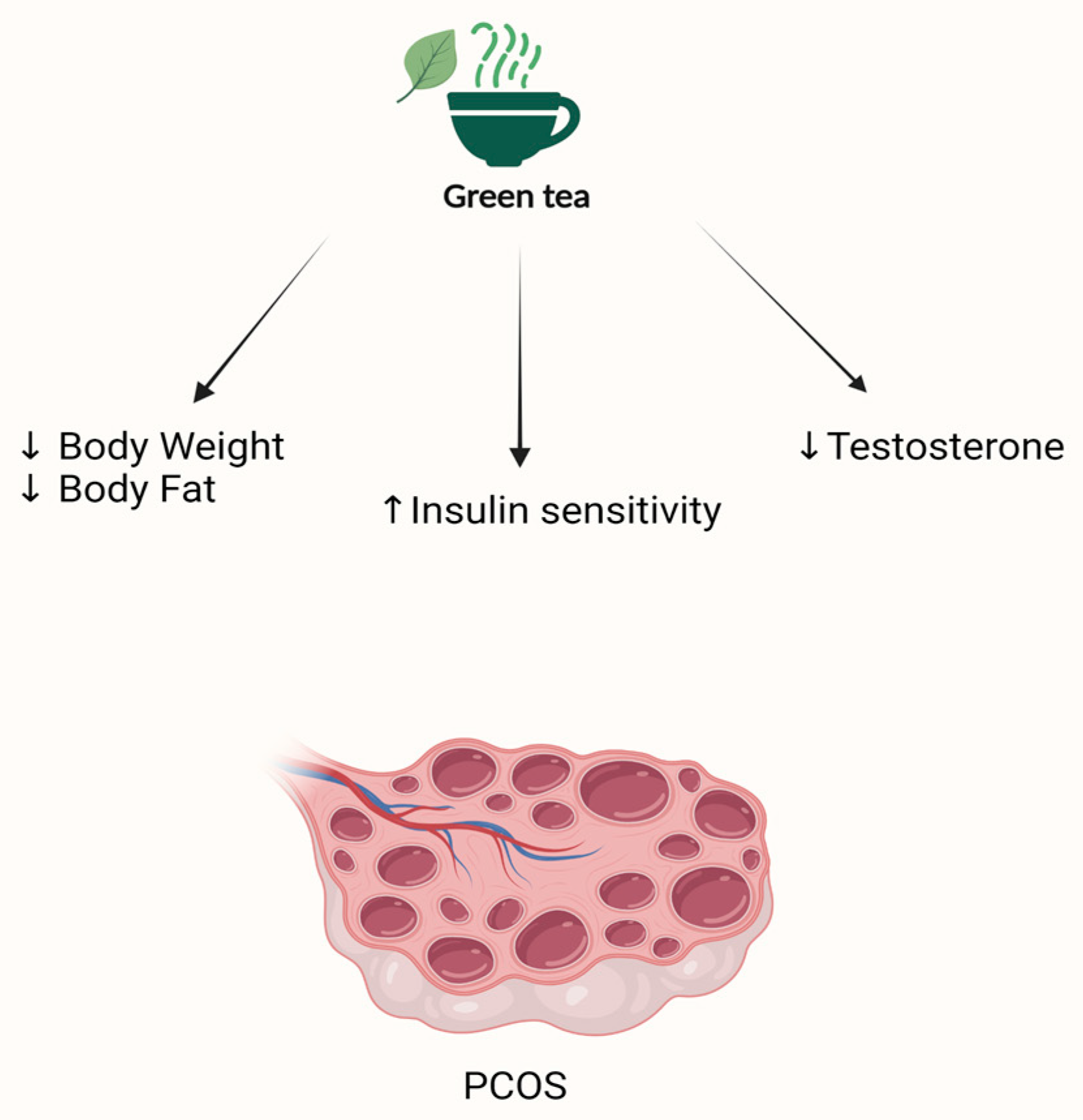
4.6. Green Tea and PCOS
4.7. Green Tea and Menopause
5. EGCG: Safety and Side Effects
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Prasanth, M.I.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. A review of the role of green tea (Camellia sinensis) in antiphotoaging, stress resistance, neuroprotection, and autophagy. Nutrients 2019, 11, 474. [Google Scholar] [CrossRef]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M.A.-O. Beneficial properties of green tea catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef] [PubMed]
- Brusselmans, K.; De Schrijver, E.; Heyns, W.; Verhoeven, G.; Swinnen, J.V. Epigallocatechin-3-gallate is a potent natural inhibitor of fatty acid synthase in intact cells and selectively induces apoptosis in prostate cancer cells. Int. J. Cancer 2003, 106, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Duan, W.; Owusu, L.; Wu, D.; Xin, Y. Epigallocatechin-3-gallate induces the apoptosis of hepatocellular carcinoma LM6 cells but not non-cancerous liver cells. Int. J. Mol. Med. 2015, 35, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Dixon, D.A.; Muga, S.J.; Smith, T.J.; Wargovich, M.J. Green tea polyphenol (−)-epigallocatechin-3-gallate inhibits cyclooxygenase-2 expression in colon carcinogenesis. Mol. Carcinog. 2006, 45, 309–319. [Google Scholar] [CrossRef]
- Parish, M.; Massoud, G.; Hazimeh, D.; Segars, J.; Islam, M.S. Green tea in reproductive cancers: Could treatment be as simple? Cancers 2023, 15, 862. [Google Scholar] [CrossRef]
- Kim, H.S.; Quon, M.J.; Kim, J.A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef]
- Li, L.; Stillemark-Billton, P.; Beck, C.; Boström, P.; Andersson, L.; Rutberg, M.; Ericsson, J.; Magnusson, B.; Marchesan, D.; Ljungberg, A.; et al. Epigallocatechin gallate increases the formation of cytosolic lipid droplets and decreases the secretion of apoB-100 VLDL. J. Lipid Res. 2006, 47, 67–77. [Google Scholar] [CrossRef]
- Xu, R.; Yang, K.; Li, S.; Dai, M.; Chen, G. Effect of green tea consumption on blood lipids: A systematic review and meta-analysis of randomized controlled trials. Nutr. J. 2020, 19, 48. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, T.; Ito, K.; Ikeda, Y.; Kizaki, M. Green tea component, catechin, induces apoptosis of human malignant B cells via production of reactive oxygen species. Clin. Cancer Res. 2005, 11, 6040–6049. [Google Scholar] [CrossRef]
- Smolarz, B.; Szyłło, K.; Romanowicz, H. Endometriosis: Epidemiology, classification, pathogenesis, treatment and genetics (review of literature). Int. J. Mol. Sci. 2021, 22, 10554. [Google Scholar] [CrossRef]
- Marsh, E.E.; Al-Hendy, A.; Kappus, D.; Galitsky, A.; Stewart, E.A.; Kerolous, M. Burden, Prevalence, and treatment of uterine fibroids: A survey of U.S. Women. J. Womens Health 2018, 27, 1359–1367. [Google Scholar] [CrossRef]
- Deswal, R.; Narwal, V.; Dang, A.; Pundir, C.S. The prevalence of polycystic ovary syndrome: A brief systematic review. J. Hum. Reprod. Sci. 2020, 13, 261–271. [Google Scholar] [CrossRef]
- Black, K.I.; Fraser, I.S. The burden of health associated with benign gynecological disorders in low-resource settings. Int. J. Gynaecol. Obstet. 2012, 119 (Suppl. 1), S72–S75. [Google Scholar] [CrossRef]
- Chen, D.; Dou, Q.P. Tea polyphenols and their roles in cancer prevention and chemotherapy. Int. J. Mol. Sci. 2008, 9, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Kamal, D.A.-O.; Salamt, N.; Zaid, S.S.M.; Mokhtar, M.A.-O. Beneficial effects of green tea catechins on female reproductive disorders: A review. Molecules 2021, 26, 2675. [Google Scholar] [CrossRef]
- Lee, A.H.; Su, D.; Pasalich, M.; Binns, C.W. Tea consumption reduces ovarian cancer risk. Cancer Epidemiol. 2013, 37, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Yan, J.; Cui, J.; Mao, S.; Li, M.; Liao, X.; Tong, H. Dynamic changes in amino acids, catechins, caffeine and gallic acid in green tea during withering. J. Food Compost. Anal. 2018, 66, 98–108. [Google Scholar] [CrossRef]
- Cabrera, C.; Giménez, R.; López, M.C. Determination of tea components with antioxidant activity. J. Agric. Food Chem. 2003, 51, 4427–4435. [Google Scholar] [CrossRef]
- Roy, M.K.; Koide, M.; Rao, T.P.; Okubo, T.; Ogasawara, Y.; Juneja, L.R. ORAC and DPPH assay comparison to assess antioxidant capacity of tea infusions: Relationship between total polyphenol and individual catechin content. Int. J. Food Sci. Nutr. 2010, 61, 109–124. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea polyphenols for health promotion. Life Sci. 2007, 81, 519–533. [Google Scholar] [CrossRef]
- Salah, N.; Miller, N.J.; Paganga, G.; Tijburg, L.; Bolwell, G.P.; Riceevans, C. Polyphenolic flavanols as scavengers of aqueous phase radicals and as chain-breaking antioxidants. Arch. Biochem. Biophys. 1995, 322, 339–346. [Google Scholar] [CrossRef]
- Rice-evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic. Res. 1995, 22, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Szeto, Y.T. Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J. Agric. Food Chem. 1999, 47, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Vinson, J.; Dabbagh, Y.; Serry, M.; Jang, J. Plant flavonoids, especially tea flavonols, are powerful antioxidants using an in vitro oxidation model for heart disease. J. Agric. Food Chem. 1995, 43, 2800–2802. [Google Scholar] [CrossRef]
- Vinson, J.A.; Dabbagh, Y.A. Tea phenols: Antioxidant effectiveness of teas, tea components, tea fractions and their binding with lipoproteins. Nutr. Res. 1998, 18, 1067–1075. [Google Scholar] [CrossRef]
- Cardoso, R.R.; Neto, R.O.; dos Santos D’Almeida, C.T.; do Nascimento, T.P.; Pressete, C.G.; Azevedo, L.; Martino, H.S.D.; Cameron, L.C.; Ferreira, M.S.L.; Barros, F.A.R. Kombuchas from green and black teas have different phenolic profile, which impacts their antioxidant capacities, antibacterial and antiproliferative activities. Food Res. Int. 2020, 128, 108782. [Google Scholar] [CrossRef] [PubMed]
- Komes, D.; Horžić, D.; Belščak, A.; Ganić, K.K.; Vulić, I. Green tea preparation and its influence on the content of bioactive compounds. Food Res. Int. 2010, 43, 167–176. [Google Scholar] [CrossRef]
- Seeram, N.P.; Henning, S.M.; Niu, Y.; Lee, R.; Scheuller, H.S.; Heber, D. Catechin and caffeine content of green tea dietary supplements and correlation with antioxidant capacity. J. Agric. Food Chem. 2006, 54, 1599–1603. [Google Scholar] [CrossRef]
- Yanagimoto, K.; Ochi, H.; Lee, K.-G.; Shibamoto, T. Antioxidative activities of volatile extracts from green tea, oolong tea, and black tea. J. Agric. Food Chem. 2003, 51, 7396–7401. [Google Scholar] [CrossRef]
- Zeng, L.; Ma, M.; Li, C.; Luo, L. Stability of tea polyphenols solution with different pH at different temperatures. Int. J. Food Prop. 2017, 20, 983605. [Google Scholar] [CrossRef]
- Henning, S.M.; Choo, J.J.; Heber, D. Nongallated compared with gallated flavan-3-ols in green and black tea are more bioavailable. J. Nutr. 2008, 138, 1529S–1534S. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, J.B.; Walle, T. Cellular uptake and efflux of the tea flavonoid (−)epicatechin-3-gallate in the human intestinal cell line Caco-2. J. Pharmacol. Exp. Ther. 2003, 307, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Lambert, J.D.; Lee, S.H.; Sinko, P.J.; Yang, C.S. Involvement of multidrug resistance-associated proteins in regulating cellular levels of (−)-epigallocatechin-3-gallate and its methyl metabolites. Biochem. Biophys. Res. Commun. 2003, 310, 222–227. [Google Scholar] [CrossRef]
- Lin, L.C.; Wang, M.N.; Tsai, T.H. Food-drug interaction of (−)-epigallocatechin-3-gallate on the pharmacokinetics of irinotecan and the metabolite SN-38. Chem. Biol. Interact. 2008, 174, 177–182. [Google Scholar] [CrossRef]
- Abdelkawy, K.S.; Abdelaziz, R.M.; Abdelmageed, A.M.; Donia, A.M.; El-Khodary, N.M. Effects of green tea extract on atorvastatin pharmacokinetics in healthy volunteers. Eur. J. Drug Metab. Pharmacokinet. 2020, 45, 351–360. [Google Scholar] [CrossRef]
- Misaka, S.; Yatabe, J.; Muller, F.; Takano, K.; Kawabe, K.; Glaeser, H.; Yatabe, M.S.; Onoue, S.; Werba, J.P.; Watanabe, H.; et al. Green tea ingestion greatly reduces plasma concentrations of nadolol in healthy subjects. Clin. Pharmacol. Ther. 2014, 95, 432–438. [Google Scholar] [CrossRef]
- Green, R.J.; Murphy, A.S.; Schulz, B.; Watkins, B.A.; Ferruzzi, M.G. Common tea formulations modulate in vitro digestive recovery of green tea catechins. Mol. Nutr. Food Res. 2007, 51, 1152–1162. [Google Scholar] [CrossRef]
- Peters, C.M.; Green, R.J.; Janle, E.M.; Ferruzzi, M.G. Formulation with ascorbic acid and sucrose modulates catechin bioavailability from green tea. Food Res. Int. 2010, 43, 95–102. [Google Scholar] [CrossRef]
- Shim, S.-M.; Yoo, S.-H.; Ra, C.-S.; Kim, Y.-K.; Chung, J.-O.; Lee, S.-J. Digestive stability and absorption of green tea polyphenols: Influence of acid and xylitol addition. Food Res. Int. 2012, 45, 204–210. [Google Scholar] [CrossRef]
- Schramm, D.D.; Karim, M.; Schrader, H.R.; Holt, R.R.; Kirkpatrick, N.J.; Polagruto, J.A.; Ensunsa, J.L.; Schmitz, H.H.; Keen, C.L. Food effects on the absorption and pharmacokinetics of cocoa flavanols. Life Sci. 2003, 73, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Naumovski, N.; Blades, B.L.; Roach, P.D. Food inhibits the oral bioavailability of the major green tea antioxidant epigallocatechin gallate in humans. Antioxidants 2015, 4, 373–393. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Okuda, S.; Miyazawa, T. Dose-dependent incorporation of tea catechins, (−)-epigallocatechin-3-gallate and (−)-epigallocatechin, into human plasma. Biosci. Biotechnol. Biochem. 1997, 61, 1981–1985. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Wang, Z.Y.; Li, H.; Chen, L.; Sun, Y.; Gobbo, S.; Balentine, D.A.; Yang, C.S. Analysis of plasma and urinary tea polyphenols in human subjects. Cancer Epidemiol. Biomark. Prev. 1995, 4, 393–399. [Google Scholar]
- Lee, M.J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1025–1032. [Google Scholar]
- Pervin, M.; Unno, K.; Nakagawa, A.; Takahashi, Y.; Iguchi, K.; Yamamoto, H.; Hoshino, M.; Hara, A.; Takagaki, A.; Nanjo, F.; et al. Blood brain barrier permeability of (−)-epigallocatechin gallate, its proliferation-enhancing activity of human neuroblastoma SH-SY5Y cells, and its preventive effect on age-related cognitive dysfunction in mice. Biochem. Biophys. Rep. 2017, 9, 180–186. [Google Scholar] [CrossRef]
- Lin, L.C.; Wang, M.N.; Tseng, T.Y.; Sung, J.S.; Tsai, T.H. Pharmacokinetics of (−)-epigallocatechin-3-gallate in conscious and freely moving rats and its brain regional distribution. J. Agric. Food Chem. 2007, 55, 1517–1524. [Google Scholar] [CrossRef]
- Wang, P.; Aronson, W.J.; Huang, M.; Zhang, Y.; Lee, R.P.; Heber, D.; Henning, S.M. Green tea polyphenols and metabolites in prostatectomy tissue: Implications for cancer prevention. Cancer Prev. Res. 2010, 3, 985–993. [Google Scholar] [CrossRef]
- Zhu, B.T.; Patel, U.K.; Cai, M.X.; Lee, A.J.; Conney, A.H. Rapid conversion of tea catechins to monomethylated products by rat liver cytosolic catechol-O-methyltransferase. Xenobiotica 2001, 31, 879–890. [Google Scholar] [CrossRef]
- Kim, S.; Lee, M.J.; Hong, J.; Li, C.; Smith, T.J.; Yang, G.Y.; Seril, D.N.; Yang, C.S. Plasma and tissue levels of tea catechins in rats and mice during chronic consumption of green tea polyphenols. Nutr. Cancer 2000, 37, 41–48. [Google Scholar] [CrossRef]
- Lambert, J.D.; Lee, M.J.; Lu, H.; Meng, X.; Hong, J.J.; Seril, D.N.; Sturgill, M.G.; Yang, C.S. Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice. J. Nutr. 2003, 133, 4172–4177. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Dionisi, F.; Renouf, M. Flavanols from green tea and phenolic acids from coffee: Critical quantitative evaluation of the pharmacokinetic data in humans after consumption of single doses of beverages. Mol. Nutr. Food Res. 2011, 55, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Schantz, M.; Erk, T.; Richling, E. Metabolism of green tea catechins by the human small intestine. Biotechnol. J. 2010, 5, 1050–1059. [Google Scholar] [CrossRef]
- Van’t Slot, G.; Humpf, H.U. Degradation and metabolism of catechin, epigallocatechin-3-gallate (EGCG), and related compounds by the intestinal microbiota in the pig cecum model. J. Agric. Food Chem. 2009, 57, 8041–8048. [Google Scholar] [CrossRef]
- Van der Hooft, J.J.; de Vos, R.C.; Mihaleva, V.; Bino, R.J.; Ridder, L.; de Roo, N.; Jacobs, D.M.; van Duynhoven, J.P.; Vervoort, J. Structural elucidation and quantification of phenolic conjugates present in human urine after tea intake. Anal. Chem. 2012, 84, 7263–7271. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Chen, L.; Lee, M.J.; Balentine, D.; Kuo, M.C.; Schantz, S.P. Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidemiol. Biomark. Prev. 1998, 7, 51–54. [Google Scholar]
- Janle, E.M.; Morré, D.M.; Morré, D.J.; Zhou, Q.; Zhu, Y. Pharmacokinetics of green tea catechins in extract and sustained-release preparations. J. Diet. Suppl. 2009, 5, 248–263. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.H.; Cai, Y.; Alberts, D.S.; Hakim, I.; Dorr, R.; Shahi, F.; Crowell, J.A.; Yang, C.S.; Hara, Y. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol. Biomark. Prev. 2001, 10, 53–58. [Google Scholar]
- Clifford, M.N.; van der Hooft, J.J.J.; Crozier, A. Human studies on the absorption, distribution, metabolism, and excretion of tea polyphenols. Am. J. Clin. Nutr. 2013, 98, 1619S–1630S. [Google Scholar] [CrossRef]
- Lam, W.H.; Kazi, A.; Kuhn, D.J.; Chow, L.M.C.; Chan, A.S.C.; Ping Dou, Q.; Chan, T.H. A potential prodrug for a green tea polyphenol proteasome inhibitor: Evaluation of the peracetate ester of (−)-epigallocatechin gallate [(−)-EGCG]. Bioorg. Med. Chem. 2004, 12, 5587–5593. [Google Scholar] [CrossRef]
- Lambert, J.D.; Sang, S.; Hong, J.; Kwon, S.-J.; Lee, M.-J.; Ho, C.-T.; Yang, C.S. Peracetylation as a means of enhancing in vitro bioactivity and bioavailability of epigallocatechin-3-gallate. Drug Metab. Dispos. 2006, 34, 2111–2116. [Google Scholar] [CrossRef]
- Wang, C.C.; Xu, H.; Man, G.C.W.; Zhang, T.; Chu, K.O.; Chu, C.Y.; Cheng, J.T.Y.; Li, G.; He, Y.X.; Qin, L.; et al. Prodrug of green tea epigallocatechin-3-gallate (Pro-EGCG) as a potent anti-angiogenesis agent for endometriosis in mice. Angiogenesis 2013, 16, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.S.; Liu, G.; Renzetti, A.; Farshi, P.; Yang, H.; Soave, C.; Saed, G.; El-Ghoneimy, A.A.; El-Banna, H.A.; Foldes, R.; et al. Biological and mechanistic characterization of novel prodrugs of green tea polyphenol epigallocatechin gallate analogs in human leiomyoma cell lines. J. Cell Biochem. 2016, 117, 2357–2369. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.; Sharma, M.; Sharma, P.D.; Singh, T.V. Design, semisynthesis, and evaluation of o-acyl derivatives of (−)-epigallocatechin-3-gallate as antitumor agents. J. Agric. Food Chem. 2007, 55, 6319–6324. [Google Scholar] [CrossRef]
- Landis-Piwowar, K.R.; Huo, C.; Chen, D.; Milacic, V.; Shi, G.; Chan, T.H.; Dou, Q.P. A novel prodrug of the green tea polyphenol (−)-epigallocatechin-3-gallate as a potential anticancer agent. Cancer Res. 2007, 67, 4303–4310. [Google Scholar] [CrossRef] [PubMed]
- Chiou, Y.-S.; Sang, S.; Cheng, K.-H.; Ho, C.-T.; Wang, Y.-J.; Pan, M.-H. Peracetylated (−)-epigallocatechin-3-gallate (AcEGCG) potently prevents skin carcinogenesis by suppressing the PKD1-dependent signaling pathway in CD34 + skin stem cells and skin tumors. Carcinogenesis 2013, 34, 1315–1322. [Google Scholar] [CrossRef]
- Chiou, Y.-S.; Ma, N.J.-L.; Sang, S.; Ho, C.-T.; Wang, Y.-J.; Pan, M.-H. Peracetylated (−)-Epigallocatechin-3-gallate (AcEGCG) Potently Suppresses Dextran Sulfate Sodium-Induced Colitis and Colon Tumorigenesis in Mice. J. Agric. Food Chem. 2012, 60, 3441–3451. [Google Scholar] [CrossRef]
- Meeran, S.M.; Patel, S.N.; Chan, T.-H.; Tollefsbol, T.O. A novel prodrug of epigallocatechin-3-gallate: Differential epigenetic hTERT repression in human breast cancer cells. Cancer Prev. Res. 2011, 4, 1243–1254. [Google Scholar] [CrossRef]
- Ermakova, S.P.; Kang, B.S.; Choi, B.Y.; Choi, H.S.; Schuster, T.F.; Ma, W.Y.; Bode, A.M.; Dong, Z. (−)-Epigallocatechin gallate overcomes resistance to etoposide-induced cell death by targeting the molecular chaperone glucose-regulated protein 78. Cancer Res. 2006, 66, 9260–9269. [Google Scholar] [CrossRef]
- Tachibana, H.; Koga, K.; Fujimura, Y.; Yamada, K. A receptor for green tea polyphenol EGCG. Nat. Struct. Mol. Biol. 2004, 11, 380–381. [Google Scholar] [CrossRef]
- Urusova, D.V.; Shim, J.H.; Kim, D.J.; Jung, S.K.; Zykova, T.A.; Carper, A.; Bode, A.M.; Dong, Z. Epigallocatechin-gallate suppresses tumorigenesis by directly targeting Pin1. Cancer Prev. Res. 2011, 4, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Kim, T.J.; Peng, D.H.; Duan, D.; Gibbons, D.L.; Yamauchi, M.; Jackson, J.R.; Le Saux, C.J.; Calhoun, C.; Peters, J.; et al. Fibroblast-specific inhibition of TGF-β1 signaling attenuates lung and tumor fibrosis. J. Clin. Investig. 2017, 127, 3675–3688. [Google Scholar] [CrossRef]
- Sazuka, M.; Itoi, T.; Suzuki, Y.; Odani, S.; Koide, T.; Isemura, M. Evidence for the interaction between (−)-epigallocatechin gallate and human plasma proteins fibronectin, fibrinogen, and histidine-rich glycoprotein. Biosci. Biotechnol. Biochem. 1996, 60, 1317–1319. [Google Scholar] [CrossRef]
- Ermakova, S.; Choi, B.Y.; Choi, H.S.; Kang, B.S.; Bode, A.M.; Dong, Z. The intermediate filament protein vimentin is a new target for epigallocatechin gallate. J. Biol. Chem. 2005, 280, 16882–16890. [Google Scholar] [CrossRef]
- Sazuka, M.; Imazawa, H.; Shoji, Y.; Mita, T.; Hara, Y.; Isemura, M. Inhibition of collagenases from mouse lung carcinoma cells by green tea catechins and black tea theaflavins. Biosci. Biotechnol. Biochem. 1997, 61, 1504–1506. [Google Scholar] [CrossRef] [PubMed]
- Demeule, M.; Brossard, M.; Pagé, M.; Gingras, D.; Béliveau, R. Matrix metalloproteinase inhibition by green tea catechins. Biochim. Biophys. Acta 2000, 1478, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Wei, D.; Zhang, Q.; Yang, S. Modulation of P-glycoprotein function and reversal of multidrug resistance by (−)-epigallocatechin gallate in human cancer cells. Biomed. Pharmacother. 2005, 59, 64–69. [Google Scholar] [CrossRef]
- Frenzel, A.; Grespi, F.; Chmelewskij, W.; Villunger, A. Bcl2 family proteins in carcinogenesis and the treatment of cancer. Apoptosis 2009, 14, 584–596. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xu, L.; Yang, L.; Wang, X. Epigallocatechin gallate is the most effective catechin against antioxidant stress via hydrogen peroxide and radical scavenging activity. Med. Sci. Monit. 2018, 24, 8198–8206. [Google Scholar] [CrossRef]
- Leone, M.; Zhai, D.; Sareth, S.; Kitada, S.; Reed, J.C.; Pellecchia, M. Cancer prevention by tea polyphenols is linked to their direct inhibition of antiapoptotic Bcl-2-family proteins. Cancer Res. 2003, 63, 8118–8121. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef] [PubMed]
- Zwolak, I. Epigallocatechin gallate for management of heavy metal-induced oxidative stress: Mechanisms of action, efficacy, and concerns. Int. J. Mol. Sci. 2021, 22, 4027. [Google Scholar] [CrossRef] [PubMed]
- Mokra, D.; Adamcakova, J.; Mokry, J. Green tea polyphenol (−)-epigallocatechin-3-gallate (egcg): A time for a new player in the treatment of respiratory diseases? Antioxidants 2022, 11, 1566. [Google Scholar] [CrossRef]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
- Suzuki-Sugihara, N.; Kishimoto, Y.; Saita, E.; Taguchi, C.; Kobayashi, M.; Ichitani, M.; Ukawa, Y.; Sagesaka, Y.M.; Suzuki, E.; Kondo, K. Green tea catechins prevent low-density lipoprotein oxidation via their accumulation in low-density lipoprotein particles in humans. Nutr. Res. 2016, 36, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Yee, W.L.; Wang, Q.; Agdinaoay, T.; Dang, K.; Chang, H.; Grandinetti, A.; Franke, A.A.; Theriault, A. Green tea catechins decrease apolipoprotein B-100 secretion from HepG2 cells. Mol. Cell. Biochem. 2002, 229, 85–92. [Google Scholar] [CrossRef]
- Ouyang, J.; Zhu, K.; Liu, Z.; Huang, J. Prooxidant effects of epigallocatechin-3-gallate in health benefits and potential adverse effect. Oxid. Med. Cell. Longev. 2020, 2020, 9723686. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, P.; Ling, T.; Wang, Y.; Dong, R.; Zhang, C.; Zhang, L.; Han, M.; Wang, D.; Wan, X.; et al. Certain (−)-epigallocatechin-3-gallate (EGCG) auto-oxidation products (EAOPs) retain the cytotoxic activities of EGCG. Food Chem. 2016, 204, 218–226. [Google Scholar] [CrossRef]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell. Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef]
- Timmer, T.; de Vries, E.G.; de Jong, S. Fas receptor-mediated apoptosis: A clinical application? J. Pathol. 2002, 196, 125–134. [Google Scholar] [CrossRef]
- Wu, P.P.; Kuo, S.C.; Huang, W.W.; Yang, J.S.; Lai, K.C.; Chen, H.J.; Lin, K.L.; Chiu, Y.J.; Huang, L.J.; Chung, J.G. (−)-Epigallocatechin gallate induced apoptosis in human adrenal cancer NCI-H295 cells through caspase-dependent and caspase-independent pathway. Anticancer Res. 2009, 29, 1435–1442. [Google Scholar] [PubMed]
- Hayakawa, S.; Saeki, K.; Sazuka, M.; Suzuki, Y.; Shoji, Y.; Ohta, T.; Kaji, K.; Yuo, A.; Isemura, M. Apoptosis induction by epigallocatechin gallate involves its binding to Fas. Biochem. Biophys. Res. Commun. 2001, 285, 1102–1106. [Google Scholar] [CrossRef]
- Lin, H.Y.; Hou, S.C.; Chen, S.C.; Kao, M.C.; Yu, C.C.; Funayama, S.; Ho, C.T.; Way, T.D. (−)-Epigallocatechin gallate induces Fas/CD95-mediated apoptosis through inhibiting constitutive and IL-6-induced JAK/STAT3 signaling in head and neck squamous cell carcinoma cells. J. Agric. Food Chem. 2012, 60, 2480–2489. [Google Scholar] [CrossRef]
- Basu, A.; Haldar, S. Combinatorial effect of epigallocatechin-3-gallate and TRAIL on pancreatic cancer cell death. Int. J. Oncol. 2009, 34, 281–286. [Google Scholar] [CrossRef]
- Kuo, P.L.; Lin, C.C. Green tea constituent (−)-epigallocatechin-3-gallate inhibits Hep G2 cell proliferation and induces apoptosis through p53-dependent and Fas-mediated pathways. J. Biomed. Sci. 2003, 10, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.P.; Grazul-Bilska, A.T.; Redmer, D.A. Angiogenesis in the female reproductive organs: Pathological implications. Int. J. Exp. Pathol. 2002, 83, 151–163. [Google Scholar] [CrossRef]
- Cao, Y.; Cao, R. Angiogenesis inhibited by drinking tea. Nature 1999, 398, 381. [Google Scholar] [CrossRef] [PubMed]
- Fassina, G.; Venè, R.; Morini, M.; Minghelli, S.; Benelli, R.; Noonan, D.M.; Albini, A. Mechanisms of inhibition of tumor angiogenesis and vascular tumor growth by epigallocatechin-3-gallate. Clin. Cancer Res. 2004, 10, 4865–4873. [Google Scholar] [CrossRef]
- Basini, G.; Bianco, F.; Grasselli, F. EGCG, a major component of green tea, inhibits VEGF production by swine granulosa cells. Biofactors 2005, 23, 25–33. [Google Scholar] [CrossRef]
- Sukhthankar, M.; Yamaguchi, K.; Lee, S.H.; McEntee, M.F.; Eling, T.E.; Hara, Y.; Baek, S.J. A green tea component suppresses posttranslational expression of basic fibroblast growth factor in colorectal cancer. Gastroenterology 2008, 134, 1972–1980. [Google Scholar] [CrossRef] [PubMed]
- Sartippour, M.R.; Heber, D.; Zhang, L.; Beatty, P.; Elashoff, D.; Elashoff, R.; Go, V.L.; Brooks, M.N. Inhibition of fibroblast growth factors by green tea. Int. J. Oncol. 2002, 21, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of matrix metalloproteinases in angiogenesis and cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, L.; Wang, M.; Zhou, S.; Lu, Y.; Cui, H.; Racanelli, A.C.; Zhang, L.; Ye, T.; Ding, B.; et al. Targeting fibrosis, mechanisms and cilinical trials. Signal Transduct. Target. Ther. 2022, 7, 206. [Google Scholar] [CrossRef]
- Hu, H.H.; Chen, D.Q.; Wang, Y.N.; Feng, Y.L.; Cao, G.; Vaziri, N.D.; Zhao, Y.Y. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem. Biol. Interact. 2018, 292, 76–83. [Google Scholar] [CrossRef]
- Tsai, M.J.; Chang, W.A.; Liao, S.H.; Chang, K.F.; Sheu, C.C.; Kuo, P.L. The Effects of epigallocatechin gallate (egcg) on pulmonary fibroblasts of idiopathic pulmonary fibrosis (IPF)-a next-generation sequencing and bioinformatic approach. Int. J. Mol. Sci. 2019, 20, 1958. [Google Scholar] [CrossRef] [PubMed]
- Tipoe, G.L.; Leung, T.M.; Liong, E.C.; Lau, T.Y.; Fung, M.L.; Nanji, A.A. Epigallocatechin-3-gallate (EGCG) reduces liver inflammation, oxidative stress and fibrosis in carbon tetrachloride (CCl4)-induced liver injury in mice. Toxicology 2010, 273, 45–52. [Google Scholar] [CrossRef]
- Grandi, G.; Del Savio, M.C.; Melotti, C.; Feliciello, L.; Facchinetti, F. Vitamin D and green tea extracts for the treatment of uterine fibroids in late reproductive life: A pilot, prospective, daily-diary based study. Gynecol. Endocrinol. 2022, 38, 63–67. [Google Scholar] [CrossRef]
- Kanlaya, R.; Thongboonkerd, V. Protective effects of epigallocatechin-3-gallate from green tea in various kidney diseases. Adv. Nutr. 2019, 10, 112–121. [Google Scholar] [CrossRef]
- Klass, B.R.; Branford, O.A.; Grobbelaar, A.O.; Rolfe, K.J. The effect of epigallocatechin-3-gallate, a constituent of green tea, on transforming growth factor-beta1-stimulated wound contraction. Wound Repair Regen. 2010, 18, 80–88. [Google Scholar] [CrossRef]
- Sriram, N.; Kalayarasan, S.; Manikandan, R.; Arumugam, M.; Sudhandiran, G. Epigallocatechin gallate attenuates fibroblast proliferation and excessive collagen production by effectively intervening TGF-β1 signalling. Clin. Exp. Pharmacol. Physiol. 2015, 42, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, M.; Lazzeri, L.; Perelli, F.; Reis, F.M.; Petraglia, F. Dysmenorrhea and related disorders. F1000Res 2017, 6, 1645. [Google Scholar] [CrossRef] [PubMed]
- McKenna, K.A.; Fogleman, C.D. Dysmenorrhea. Am. Fam. Physician. 2021, 104, 164–170. [Google Scholar]
- Dawood, M.Y. Nonsteroidal anti-inflammatory drugs and changing attitudes toward dysmenorrhea. Am. J. Med. 1988, 84, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.L.; Botting, R.M.; Hla, T. Cyclooxygenase isozymes: The biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 2004, 56, 387–437. [Google Scholar] [CrossRef] [PubMed]
- Harada, T. Dysmenorrhea and endometriosis in young women. Yonago Acta Med. 2013, 56, 81–84. [Google Scholar]
- Barcikowska, Z.; Rajkowska-Labon, E.; Grzybowska, M.E.; Hansdorfer-Korzon, R.; Zorena, K. Inflammatory markers in dysmenorrhea and therapeutic options. Int. J. Environ. Res. Public Health 2020, 17, 1191. [Google Scholar] [CrossRef]
- Wong, C.L.; Farquhar, C.; Roberts, H.; Proctor, M. Oral contraceptive pill for primary dysmenorrhoea. Cochrane Database Syst. Rev. 2009, 2009, CD002120. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, R.; Chen, D.; Huang, R.; Tian, Y.; Zhang, P.; Zhang, J. Association of tea drinking and dysmenorrhoea among reproductive-age women in Shanghai, China (2013–2015): A cross-sectional study. BMJ Open 2019, 9, e026643. [Google Scholar] [CrossRef]
- Koeberle, A.; Bauer, J.; Verhoff, M.; Hoffmann, M.; Northoff, H.; Werz, O. Green tea epigallocatechin-3-gallate inhibits microsomal prostaglandin E(2) synthase-1. Biochem. Biophys. Res. Commun. 2009, 388, 350–354. [Google Scholar] [CrossRef]
- Hedbrant, A.; Persson, I.; Erlandsson, A.; Wijkander, J. Green, black and rooibos tea inhibit prostaglandin e2 formation in human monocytes by inhibiting expression of enzymes in the prostaglandin E2 pathway. Molecules 2022, 27, 397. [Google Scholar] [CrossRef] [PubMed]
- CDC. Infertility and Public Health. Available online: https://www.cdc.gov/reproductivehealth/infertility/index.htm#:~:text=Genetic%20disorders&text=Although%20advanced%20age%20plays%20a,use%20(opioids%2C%20marijuana) (accessed on 28 January 2023).
- Pandey, A.N.; Chaube, S.K. A moderate increase of hydrogen peroxide level is beneficial for spontaneous resumption of meiosis from diplotene arrest in rat oocytes cultured in vitro. BioRes. Open Access 2014, 3, 183–191. [Google Scholar] [CrossRef]
- Tiwari, M.; Chaube, S.K. Moderate increase of reactive oxygen species triggers meiotic resumption in rat follicular oocytes. J. Obstet. Gynaecol. Res. 2016, 42, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Li, C.; Gao, F.; Fan, Y.; Zeng, F.; Meng, L.; Li, L.; Zhang, S.; Wei, H. Epigallocatechin-3-gallate promotes the in vitro maturation and embryo development following IVF of porcine oocytes. Drug Des. Dev. Ther. 2021, 15, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.G.; Yu, S.D.; Xu, Z.R. Improvement in bovine embryo production in vitro by treatment with green tea polyphenols during in vitro maturation of oocytes. Anim. Reprod. Sci. 2007, 100, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Barakat, I.A.H.; Al-Himaidi, A.R.; Rady, A.M. Antioxidant effect of green tea leaves extract on in vitro production of sheep embryos. Pakistan J. Zool. 2014, 46, 167–175. [Google Scholar]
- Spinaci, M.; Volpe, S.; De Ambrogi, M.; Tamanini, C.; Galeati, G. Effects of epigallocatechin-3-gallate (EGCG) on in vitro maturation and fertilization of porcine oocytes. Theriogenology 2008, 69, 877–885. [Google Scholar] [CrossRef]
- Basini, G.; Bianco, F.; Grasselli, F. Epigallocatechin-3-gallate from green tea negatively affects swine granulosa cell function. Domest. Anim. Endocrinol. 2005, 28, 243–256. [Google Scholar] [CrossRef]
- Balazi, A.; Sirotkin, A.V.; Foldesiova, M.; Makovicky, P.; Chrastinova, L.; Makovicky, P.; Chrenek, P. Green tea can supress rabbit ovarian functions in vitro and in vivo. Theriogenology 2019, 127, 72–79. [Google Scholar] [CrossRef]
- Westphal, L.M.; Polan, M.L.; Trant, A.S. Double-blind, placebo-controlled study of Fertilityblend: A nutritional supplement for improving fertility in women. Clin. Exp. Obstet. Gynecol. 2006, 33, 205–208. [Google Scholar]
- Day Baird, D.; Dunson, D.B.; Hill, M.C.; Cousins, D.; Schectman, J.M. High cumulative incidence of uterine leiomyoma in black and white women: Ultrasound evidence. Am. J. Obstet. Gynecol. 2003, 188, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Sinai Talaulikar, V. Medical therapy for fibroids: An overview. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 46, 48–56. [Google Scholar] [CrossRef]
- Holdsworth-Carson, S.J.; Zaitseva, M.; Vollenhoven, B.J.; Rogers, P.A.W. Clonality of smooth muscle and fibroblast cell populations isolated from human fibroid and myometrial tissues. Mol. Hum. Reprod. 2014, 20, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Lethaby, A.; Vollenhoven, B. Fibroids (uterine myomatosis, leiomyomas). BMJ Clin. Evid. 2007, 2007, 814. [Google Scholar]
- Fritton, K.; Borahay, M.A. New and emerging therapies for uterine fibroids. Semin. Reprod. Med. 2017, 35, 549–559. [Google Scholar] [CrossRef]
- Pavone, D.; Clemenza, S.; Sorbi, F.; Fambrini, M.; Petraglia, F. Epidemiology and risk factors of uterine fibroids. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 46, 3–11. [Google Scholar] [CrossRef]
- Laughlin, S.K.; Schroeder, J.C.; Baird, D.D. New directions in the epidemiology of uterine fibroids. Semin. Reprod. Med. 2010, 28, 204–217. [Google Scholar] [CrossRef]
- Filippini, T.; Malavolti, M.; Borrelli, F.; Izzo, A.A.; Fairweather-Tait, S.J.; Horneber, M.; Vinceti, M. Green tea (Camellia sinensis) for the prevention of cancer. Cochrane Database Syst. Rev. 2020, 3, CD005004. [Google Scholar] [CrossRef]
- Huang, Y.J.; Wang, K.A.-O.; Chen, H.Y.; Chiang, Y.F.; Hsia, S.A.-O. Protective effects of epigallocatechin gallate (EGCG) on endometrial, breast, and ovarian cancers. Biomolecules 2020, 10, 1481. [Google Scholar] [CrossRef]
- Zhang, D.; Al-Hendy, M.; Richard-Davis, G.; Montgomery-Rice, V.; Rajaratnam, V.; Al-Hendy, A. Antiproliferative and proapoptotic effects of epigallocatechin gallate on human leiomyoma cells. Fertil. Steril. 2010, 94, 1887–1893. [Google Scholar] [CrossRef]
- Zhang, D.; Al-Hendy, M.; Richard-Davis, G.; Montgomery-Rice, V.; Sharan, C.; Rajaratnam, V.; Khurana, A.; Al-Hendy, A. Green tea extract inhibits proliferation of uterine leiomyoma cells in vitro and in nude mice. Am. J. Obstet. Gynecol. 2010, 202, 289.e1–289.e9. [Google Scholar] [CrossRef]
- Ozercan, I.H.; Sahin, N.; Akdemir, F.; Onderci, M.; Seren, S.; Sahin, K.; Kucuk, O. Chemoprevention of fibroid tumors by [−]-epigallocatechin-3-gallate in quail. Nutr. Res. 2008, 28, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Roshdy, E.; Rajaratnam, V.; Maitra, S.; Sabry, M.; Allah, A.S.A.; Al-Hendy, A. Treatment of symptomatic uterine fibroids with green tea extract: A pilot randomized controlled clinical study. Int. J. Womens Health 2013, 7, 477–486. [Google Scholar] [CrossRef]
- Porcaro, G.; Santamaria, A.; Giordano, D.; Angelozzi, P. Vitamin D plus epigallocatechin gallate: A novel promising approach for uterine myomas. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3344–3351. [Google Scholar] [CrossRef]
- Miriello, D.; Galanti, F.; Cignini, P.; Antonaci, D.; Schiavi, M.C.; Rago, R. Uterine fibroids treatment: Do we have new valid alternative? Experiencing the combination of vitamin D plus epigallocatechin gallate in childbearing age affected women. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2843–2851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Rajaratnam, V.; Al-Hendy, O.; Halder, S.; Al-Hendy, A. Green tea extract inhibition of human leiomyoma cell proliferation is mediated via catechol-O-methyltransferase. Gynecol. Obstet. Investig. 2014, 78, 109–118. [Google Scholar] [CrossRef]
- Salama, S.A.; Ho, S.L.; Wang, H.-Q.; Tenhunen, J.; Tilgmann, C.; Al-Hendy, A. Hormonal regulation of catechol-O-methyl transferase activity in women with uterine leiomyomas. Fertil. Steril. 2006, 86, 259–262. [Google Scholar] [CrossRef]
- Salama, S.A.; Nasr, A.B.; Dubey, R.K.; Al-Hendy, A. Estrogen metabolite 2-methoxyestradiol induces apoptosis and inhibits cell proliferation and collagen production in rat and human leiomyoma cells: A potential medicinal treatment for uterine fibroids. J. Soc. Gynecol. Investig. 2006, 13, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Bariani, M.V.; Yang, Q.; Al-Hendy, A. Understanding the impact of uterine fibroids on human endometrium function. Front. Cell. Dev. Biol. 2021, 9, 633180. [Google Scholar] [CrossRef]
- Doherty, L.F.; Taylor, H.S. Leiomyoma-derived transforming growth factor-β impairs bone morphogenetic protein-2-mediated endometrial receptivity. Fertil. Steril. 2015, 103, 845–852. [Google Scholar] [CrossRef]
- Sinclair, D.C.; Mastroyannis, A.; Taylor, H.S. Leiomyoma simultaneously impair endometrial BMP-2-mediated decidualization and anticoagulant expression through secretion of TGF-β3. J. Clin. Endocrinol. Metab. 2011, 96, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Viganò, P. Endometriosis. Nat. Rev. Dis. Primers 2018, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Viganò, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Rafique, S.; Decherney, A.H. Medical management of endometriosis. Clin. Obstet. Gynecol. 2017, 60, 485–496. [Google Scholar] [CrossRef]
- Afrin, S.A.-O.; AlAshqar, A.A.-O.; El Sabeh, M.A.-O.; Miyashita-Ishiwata, M.; Reschke, L.; Brennan, J.T.; Fader, A.; Borahay, M.A.-O.X. Diet and nutrition in gynecological disorders: A focus on clinical studies. Nutrients 2021, 13, 1747. [Google Scholar] [CrossRef]
- Spinella, F.; Rosanò, L.; Di Castro, V.; Decandia, S.; Albini, A.; Nicotra, M.R.; Natali, P.G.; Bagnato, A. Green tea polyphenol epigallocatechin-3-gallate inhibits the endothelin axis and downstream signaling pathways in ovarian carcinoma. Mol. Cancer Ther. 2006, 5, 1483–1492. [Google Scholar] [CrossRef]
- Asif Siddiqui, F.; Naim, M.; Islam, N. Apoptotic effect of green tea polyphenol (EGCG) on cervical carcinoma cells. Diagn. Cytopathol. 2011, 39, 500–504. [Google Scholar] [CrossRef]
- Xu, H.; Lui, W.T.; Chu, C.Y.; Ng, P.S.; Wang, C.C.; Rogers, M.S. Anti-angiogenic effects of green tea catechin on an experimental endometriosis mouse model. Hum. Reprod. 2009, 24, 608–618. [Google Scholar] [CrossRef]
- Ricci, A.G.; Olivares, C.N.; Bilotas, M.A.; Bastón, J.I.; Singla, J.J.; Meresman, G.F.; Barañao, R.I. Natural therapies assessment for the treatment of endometriosis. Hum. Reprod. 2013, 28, 178–188. [Google Scholar] [CrossRef]
- Xu, H.; Becker, C.M.; Lui, W.T.; Chu, C.Y.; Davis, T.N.; Kung, A.L.; Birsner, A.E.; D’Amato, R.J.; Wai Man, G.C.; Wang, C.C. Green tea epigallocatechin-3-gallate inhibits angiogenesis and suppresses vascular endothelial growth factor C/vascular endothelial growth factor receptor 2 expression and signaling in experimental endometriosis in vivo. Fertil. Steril. 2011, 96, 1021–1028. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Darcha, C. Antifibrotic properties of epigallocatechin-3-gallate in endometriosis. Hum. Reprod. 2014, 29, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Laschke, M.W.; Schwender, C.; Scheuer, C.; Vollmar, B.; Menger, M.D. Epigallocatechin-3-gallate inhibits estrogen-induced activation of endometrial cells in vitro and causes regression of endometriotic lesions in vivo. Hum. Reprod. 2008, 23, 2308–2318. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.-H.; Shi, W.-J.; Zhou, L.-S.; Tao, A.-L.; Li, L. Effect of epigallocatechin-3-gallate on the status of DNA methylation of E-cadherin promoter region on endometriosis mouse. J. Obstet. Gynaecol. Res. 2020, 46, 2076–2083. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, B.; Zhang, H.; Liu, X.; Guo, S.W. Epigallocatechin-3-gallate reduces myometrial infiltration, uterine hyperactivity, and stress levels and alleviates generalized hyperalgesia in mice induced with adenomyosis. Reprod. Sci. 2013, 20, 1478–1491. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, B.; Zhang, H.; Ding, D.; Liu, X.; Guo, S.W. Possible loss of GABAergic inhibition in mice with induced adenomyosis and treatment with epigallocatechin-3-gallate attenuates the loss with improved hyperalgesia. Reprod. Sci. 2014, 21, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Ghafurniyan, H.; Azarnia, M.; Nabiuni, M.; Karimzadeh, L. The effect of green tea extract on reproductive improvement in estradiol valerate-induced polycystic ovarian syndrome in rat. Iran. J. Pharm. Res. 2015, 14, 1215–1233. [Google Scholar]
- Rondanelli, M.; Gasparri, C.; Perna, S.; Petrangolini, G.; Allegrini, P.; Fazia, T.; Bernardinelli, L.; Cavioni, A.; Mansueto, F.; Oberto, L.; et al. A 60-day green tea extract supplementation counteracts the dysfunction of adipose tissue in overweight post-menopausal and class I obese women. Nutrients 2022, 14, 5209. [Google Scholar] [CrossRef]
- Tehrani, H.G.; Allahdadian, M.; Zarre, F.; Ranjbar, H.; Allahdadian, F. Effect of green tea on metabolic and hormonal aspect of polycystic ovarian syndrome in overweight and obese women suffering from polycystic ovarian syndrome: A clinical trial. J. Educ. Health Promot. 2017, 6, 36. [Google Scholar] [CrossRef]
- Mombaini, E.; Jafarirad, S.; Husain, D.; Haghighizadeh, M.H.; Padfar, P. The impact of green tea supplementation on anthropometric indices and inflammatory cytokines in women with polycystic ovary syndrome. Phytother. Res. 2017, 31, 747–754. [Google Scholar] [CrossRef]
- Chan, C.C.; Koo, M.W.; Ng, E.H.; Tang, O.S.; Yeung, W.S.; Ho, P.C. Effects of Chinese green tea on weight, and hormonal and biochemical profiles in obese patients with polycystic ovary syndrome--a randomized placebo-controlled trial. J. Soc. Gynecol. Investig. 2006, 13, 63–68. [Google Scholar] [CrossRef]
- Bird, C.C.; McElin, T.W.; Manalo-Estrella, P. The elusive adenomyosis of the uterus--revisited. Am. J. Obstet. Gynecol. 1972, 112, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Peric, H.; Fraser, I.S. The symptomatology of adenomyosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Wouk, N.; Helton, M. Abnormal uterine bleeding in premenopausal women. Am. Fam. Physician 2019, 99, 435–443. [Google Scholar] [PubMed]
- Harada, T.; Khine, Y.M.; Kaponis, A.; Nikellis, T.; Decavalas, G.; Taniguchi, F. The impact of adenomyosis on women’s fertility. Obstet. Gynecol. Surv. 2016, 71, 557–568. [Google Scholar] [CrossRef]
- CDC. PCOS (Polycystic Ovary Syndrome) and Diabetes. Available online: https://www.cdc.gov/diabetes/basics/pcos.html (accessed on 30 January 2023).
- Tadayon, M.; Movahedi, S.; Abedi, P.; Syahpoosh, A. Impact of green tea extract on serum lipid of postmenopausal women: A randomized controlled trial. J. Tradit. Complement. Med. 2018, 8, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Wang, P.; Guerrieri, J.; Yeh, J.K.; Wang, J.S. Protective effect of green tea polyphenols on bone loss in middle-aged female rats. Osteoporos. Int. 2008, 19, 979–990. [Google Scholar] [CrossRef]
- Chen, C.-H.; Ho, M.-L.; Chang, J.-K.; Hung, S.-H.; Wang, G.-J. Green tea catechin enhances osteogenesis in a bone marrow mesenchymal stem cell line. Osteoporos. Int. 2005, 16, 2039–2045. [Google Scholar] [CrossRef]
- Lin, R.-W.; Chen, C.-H.; Wang, Y.-H.; Ho, M.-L.; Hung, S.-H.; Chen, I.-S.; Wang, G.-J. (−)-Epigallocatechin gallate inhibition of osteoclastic differentiation via NF-κB. Biochem. Biophys. Res. Commun. 2009, 379, 1033–1037. [Google Scholar] [CrossRef]
- Song, D.; Gan, M.; Zou, J.; Zhu, X.; Shi, Q.; Zhao, H.; Luo, Z.; Zhang, W.; Li, S.; Niu, J.; et al. Effect of (−)-epigallocatechin-3-gallate in preventing bone loss in ovariectomized rats and possible mechanisms. Int. J. Clin. Exp. Med. 2014, 7, 4183–4190. [Google Scholar]
- Muraki, S.; Yamamoto, S.; Ishibashi, H.; Oka, H.; Yoshimura, N.; Kawaguchi, H.; Nakamura, K. Diet and lifestyle associated with increased bone mineral density: Cross-sectional study of Japanese elderly women at an osteoporosis outpatient clinic. J. Orthop. Sci. 2007, 12, 317–320. [Google Scholar] [CrossRef]
- Ni, S.; Wang, L.; Wang, G.; Lin, J.; Ma, Y.; Zhao, X.; Ru, Y.; Zheng, W.; Zhang, X.; Zhu, S. Drinking tea before menopause is associated with higher bone mineral density in postmenopausal women. Eur. J. Clin. Nutr. 2021, 75, 1454–1464. [Google Scholar] [CrossRef]
- Shen, C.-L.; Yeh, J.K.; Cao, J.J.; Wang, J.-S. Green tea and bone metabolism. Nutr. Res. 2009, 29, 437–456. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.H.; Arakawa, K.; Stanczyk, F.Z.; Van Den Berg, D.; Koh, W.-P.; Yu, M.C. Tea and circulating estrogen levels in postmenopausal Chinese women in Singapore. Carcinogenesis 2005, 26, 976–980. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Webster, D.; Cao, J.; Shao, A. The safety of green tea and green tea extract consumption in adults—Results of a systematic review. Regul. Toxicol. Pharmacol. 2018, 95, 412–433. [Google Scholar] [CrossRef]
- Ullmann, U.; Haller, J.; Decourt, J.P.; Girault, N.; Girault, J.; Richard-Caudron, A.S.; Pineau, B.; Weber, P. A single ascending dose study of epigallocatechin gallate in healthy volunteers. J. Int. Med. Res. 2003, 31, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Siblini, H.; Al-Hendy, A.; Segars, J.; González, F.; Taylor, H.S.; Singh, B.; Flaminia, A.; Flores, V.A.; Christman, G.M.; Huang, H.; et al. Assessing the hepatic safety of epigallocatechin gallate (EGCG) in reproductive-aged women. Nutrients 2023, 15, 320. [Google Scholar] [CrossRef]
- Cerbin-Koczorowska, M.; Waszyk-Nowaczyk, M.; Bakun, P.; Goslinski, T.; Koczorowski, T. Current view on green tea catechins formulations, their interactions with selected drugs, and prospective applications for various health conditions. Appl. Sci. 2021, 11, 4905. [Google Scholar] [CrossRef]
- Oketch-Rabah, H.A.; Roe, A.L.; Rider, C.V.; Bonkovsky, H.L.; Giancaspro, G.I.; Navarro, V.; Paine, M.F.; Betz, J.M.; Marles, R.J.; Casper, S. United States Pharmacopeia (USP) comprehensive review of the hepatotoxicity of green tea extracts. Toxicol. Rep. 2020, 7, 386–402. [Google Scholar] [CrossRef] [PubMed]
- Dekant, W.; Fujii, K.; Shibata, E.; Morita, O.; Shimotoyodome, A. Safety assessment of green tea based beverages and dried green tea extracts as nutritional supplements. Toxicol. Lett. 2017, 277, 104–108. [Google Scholar] [CrossRef]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547852/ (accessed on 25 January 2023).
- Hayat, K.; Iqbal, H.; Malik, U.; Bilal, U.; Mushtaq, S. Tea and its consumption: Benefits and risks. Crit. Rev. Food Sci. Nutr. 2015, 55, 939–954. [Google Scholar] [CrossRef]
- Yu, Z.; Samavat, H.; Dostal, A.M.; Wang, R.; Torkelson, C.J.; Yang, C.S.; Butler, L.M.; Kensler, T.W.; Wu, A.H.; Kurzer, M.S. Effect of green tea supplements on liver enzyme elevation: Results from a randomized intervention study in the United States. Cancer Prev. Res. 2017, 10, 571–579. [Google Scholar] [CrossRef] [PubMed]
| Nutrition Facts | |
|---|---|
| Serving size (g) | 100 |
| Energy (kcal) | 1 |
| Total Carbs (g) | 0 |
| Protein (g) | 0.22 |
| Fats (g) | 0 |
| Vitamin B1 (thiamine) (mg) | 0.007 |
| Vitamin B2 (riboflavin) (mg) | 0.058 |
| Vitamin B3 (niacin) (mg) | 0.03 |
| Vitamin B6 (mg) | 0.005 |
| Vitamin C (mg) | 0.3 |
| Iron (mg) | 0.02 |
| Potassium (mg) | 8 |
| Sodium (mg) | 1 |
| Magnesium (mg) | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazimeh, D.; Massoud, G.; Parish, M.; Singh, B.; Segars, J.; Islam, M.S. Green Tea and Benign Gynecologic Disorders: A New Trick for An Old Beverage? Nutrients 2023, 15, 1439. https://doi.org/10.3390/nu15061439
Hazimeh D, Massoud G, Parish M, Singh B, Segars J, Islam MS. Green Tea and Benign Gynecologic Disorders: A New Trick for An Old Beverage? Nutrients. 2023; 15(6):1439. https://doi.org/10.3390/nu15061439
Chicago/Turabian StyleHazimeh, Dana, Gaelle Massoud, Maclaine Parish, Bhuchitra Singh, James Segars, and Md Soriful Islam. 2023. "Green Tea and Benign Gynecologic Disorders: A New Trick for An Old Beverage?" Nutrients 15, no. 6: 1439. https://doi.org/10.3390/nu15061439
APA StyleHazimeh, D., Massoud, G., Parish, M., Singh, B., Segars, J., & Islam, M. S. (2023). Green Tea and Benign Gynecologic Disorders: A New Trick for An Old Beverage? Nutrients, 15(6), 1439. https://doi.org/10.3390/nu15061439






