Selenium, Stroke, and Infection: A Threefold Relationship; Where Do We Stand and Where Do We Go?
Abstract
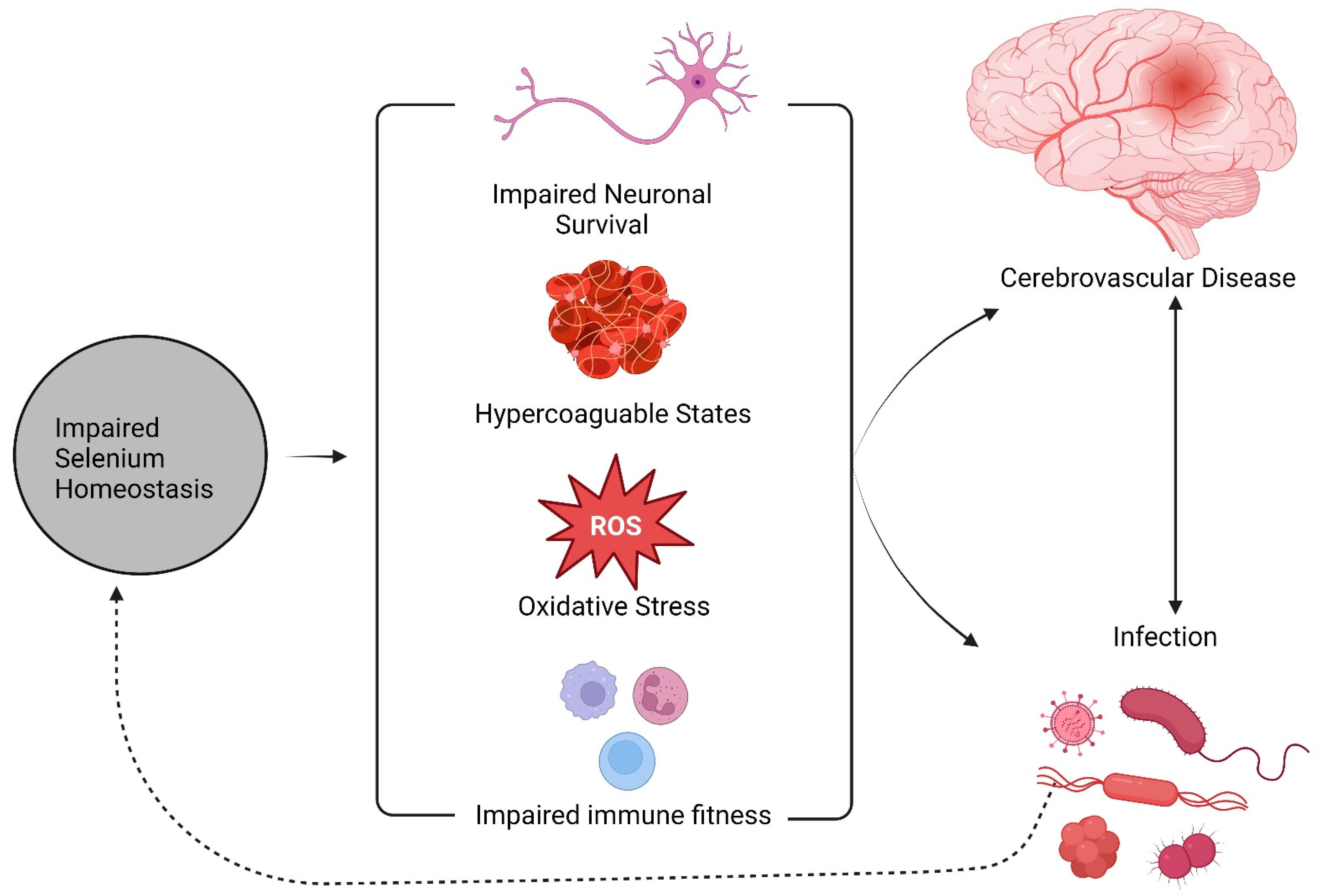
1. Background
1.1. Selenium Biology
1.2. Functional Classification of the Selenoproteome
1.3. The Selenoproteome and Immunity
1.4. Selenium and Coagulation
2. The Role of Selenium on Cerebrovascular Risk
3. Selenium, Stroke, and Infection: From Clinical Observations to Molecular Mechanisms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Global, Regional, and National Life Expectancy, All-Cause Mortality, and Cause-Specific Mortality for 249 Causes of Death, 1980–2015: A Systematic Analysis for the Global Burden of Disease Study 2015—The Lancet. Available online: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(16)31012-1/fulltext (accessed on 12 September 2022).
- Campbell, B.C.V.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic Stroke. Nat. Rev. Dis. Primer 2019, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, L.B.; Adams, R.; Becker, K.; Furberg, C.D.; Gorelick, P.B.; Hademenos, G.; Hill, M.; Howard, G.; Howard, V.J.; Jacobs, B.; et al. Primary Prevention of Ischemic Stroke: A Statement for Healthcare Professionals from the Stroke Council of the American Heart Association. Stroke 2001, 32, 280–299. [Google Scholar] [CrossRef]
- Krishnamurthi, R.V.; Feigin, V.L.; Forouzanfar, M.H.; Mensah, G.A.; Connor, M.; Bennett, D.A.; Moran, A.E.; Sacco, R.L.; Anderson, L.M.; Truelsen, T.; et al. Global Burden of Diseases, Injuries, Risk Factors Study 2010 (GBD 2010); GBD Stroke Experts Group. Global and Regional Burden of First-Ever Ischaemic and Haemorrhagic Stroke during 1990-2010: Findings from the Global Burden of Disease Study 2010. Lancet Glob. Health 2013, 1, e259–e281. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Roth, G.A.; Naghavi, M.; Parmar, P.; Krishnamurthi, R.; Chugh, S.; Mensah, G.A.; Norrving, B.; Shiue, I.; Ng, M.; et al. Global Burden of Diseases, Injuries and Risk Factors Study 2013 and Stroke Experts Writing Group. Global Burden of Stroke and Risk Factors in 188 Countries, during 1990–2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016, 15, 913–924. [Google Scholar] [CrossRef]
- Xian, Y.; Holloway, R.G.; Smith, E.E.; Schwamm, L.H.; Reeves, M.J.; Bhatt, D.L.; Schulte, P.J.; Cox, M.; Olson, D.M.; Hernandez, A.F.; et al. Racial/Ethnic Differences in Process of Care and Outcomes among Patients Hospitalized with Intracerebral Hemorrhage. Stroke 2014, 45, 3243–3250. [Google Scholar] [CrossRef] [PubMed]
- Owolabi, M.; Sarfo, F.; Howard, V.J.; Irvin, M.R.; Gebregziabher, M.; Akinyemi, R.; Bennett, A.; Armstrong, K.; Tiwari, H.K.; Akpalu, A.; et al. SIREN-REGARDS Collaboration (Stroke Investigative Research and Educational Network–Reasons for Geographic and Racial Differences in Stroke). Stroke in Indigenous Africans, African Americans, and European Americans: Interplay of Racial and Geographic Factors. Stroke 2017, 48, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Katan, M.; Luft, A. Global Burden of Stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef]
- Rayman, M.P. The Importance of selenium to Human Health. Lancet Lond. Engl. 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Sakr, Y.; Reinhart, K.; Bloos, F.; Marx, G.; Russwurm, S.; Bauer, M.; Brunkhorst, F. Time Course and Relationship between Plasma selenium Concentrations, Systemic Inflammatory Response, Sepsis, and Multiorgan Failure. Br. J. Anaesth. 2007, 98, 775–784. [Google Scholar] [CrossRef]
- Martinez, S.S.; Huang, Y.; Acuna, L.; Laverde, E.; Trujillo, D.; Barbieri, M.A.; Tamargo, J.; Campa, A.; Baum, M.K. Role of selenium in Viral Infections with a Major Focus on SARS-CoV-2. Int. J. Mol. Sci. 2021, 23, 280. [Google Scholar] [CrossRef]
- Hogan, C.; Perkins, A.V. Selenoproteins in the Human Placenta: How Essential Is selenium to a Healthy Start to Life? Nutrients 2022, 14, 628. [Google Scholar] [CrossRef] [PubMed]
- Reilly, C. Selenium in Food and Health; Blackie Academic & Professional: London, UK; New York, NY, USA, 1996. [Google Scholar]
- Stoffaneller, R.; Morse, N.L. A Review of Dietary selenium Intake and selenium Status in Europe and the Middle East. Nutrients 2015, 7, 1494–1537. [Google Scholar] [CrossRef] [PubMed]
- Zeece, M. Chapter Five—Vitamins and Minerals. In Introduction to the Chemistry of Food; Zeece, M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 163–212. [Google Scholar] [CrossRef]
- Dietary Reference Values for Food Energy and Nutrients for the United Kingdom. Report of the Panel on Dietary Reference Values of the Committee on Medical Aspects of Food Policy. Rep. Health Soc. Subj. 1991, 41, 1–210.
- Ross, A.C.; Caballero, B.H.; Cousins, R.J.; Tucker, K.L.; Ziegler, T.R. Modern Nutrition in Health and Disease, 11th ed.; Wolters Kluwer Health Adis (ESP): London, UK, 2012. [Google Scholar]
- Ashton, K.; Hooper, L.; Harvey, L.J.; Hurst, R.; Casgrain, A.; Fairweather-Tait, S.J. Methods of Assessment of selenium Status in Humans: A Systematic Review. Am. J. Clin. Nutr. 2009, 89, 2025S–2039S. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Lee, J.; Wu, C.; Guo, X.; Lee, B.J.; Chun, J.-S.; Kim, J.-H. The Role of selenium Metabolism and Selenoproteins in Cartilage Homeostasis and Arthropathies. Exp. Mol. Med. 2020, 52, 1198–1208. [Google Scholar] [CrossRef]
- Hill, K.E.; Zhou, J.; McMahan, W.J.; Motley, A.K.; Atkins, J.F.; Gesteland, R.F.; Burk, R.F. Deletion of Selenoprotein P Alters Distribution of selenium in the Mouse. J. Biol. Chem. 2003, 278, 13640–13646. [Google Scholar] [CrossRef]
- McConnell, K.P.; Portman, O.W. Excretion of Dimethyl Selenide by the Rat. J. Biol. Chem. 1952, 195, 277–282. [Google Scholar] [CrossRef]
- Byard, J.L. Trimethyl Selenide. A Urinary Metabolite of Selenite. Arch. Biochem. Biophys. 1969, 130, 556–560. [Google Scholar] [CrossRef]
- Hadrup, N.; Ravn-Haren, G. Absorption, Distribution, Metabolism and Excretion (ADME) of Oral selenium from Organic and Inorganic Sources: A Review. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. GMS 2021, 67, 126801. [Google Scholar] [CrossRef]
- Xu, X.-M.; Carlson, B.A.; Irons, R.; Mix, H.; Zhong, N.; Gladyshev, V.N.; Hatfield, D.L. Selenophosphate Synthetase 2 Is Essential for Selenoprotein Biosynthesis. Biochem. J. 2007, 404, 115–120. [Google Scholar] [CrossRef]
- Lee, B.J.; Worland, P.J.; Davis, J.N.; Stadtman, T.C.; Hatfield, D.L. Identification of a Selenocysteyl-TRNA(Ser) in Mammalian Cells That Recognizes the Nonsense Codon, UGA. J. Biol. Chem. 1989, 264, 9724–9727. [Google Scholar] [CrossRef] [PubMed]
- Holman, K.M.; Puppala, A.K.; Lee, J.W.; Lee, H.; Simonović, M. Insights into Substrate Promiscuity of Human Seryl-TRNA Synthetase. RNA N. Y. N 2017, 23, 1685–1699. [Google Scholar] [CrossRef]
- Carlson, B.A.; Xu, X.-M.; Kryukov, G.V.; Rao, M.; Berry, M.J.; Gladyshev, V.N.; Hatfield, D.L. Identification and Characterization of Phosphoseryl-TRNA[Ser]Sec Kinase. Proc. Natl. Acad. Sci. USA 2004, 101, 12848–12853. [Google Scholar] [CrossRef]
- Palioura, S.; Herkel, J.; Simonović, M.; Lohse, A.W.; Söll, D. Human SepSecS or SLA/LP: Selenocysteine Formation and Autoimmune Hepatitis. Biol. Chem. 2010, 391, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, K.S.; Crnković, A.; Söll, D. Versatility of Synthetic TRNAs in Genetic Code Expansion. Genes 2018, 9, 537. [Google Scholar] [CrossRef] [PubMed]
- Papp, L.V.; Lu, J.; Striebel, F.; Kennedy, D.; Holmgren, A.; Khanna, K.K. The Redox State of SECIS Binding Protein 2 Controls Its Localization and Selenocysteine Incorporation Function. Mol. Cell. Biol. 2006, 26, 4895–4910. [Google Scholar] [CrossRef]
- Shetty, S.P.; Shah, R.; Copeland, P.R. Regulation of Selenocysteine Incorporation into the selenium Transport Protein, Selenoprotein P. J. Biol. Chem. 2014, 289, 25317–25326. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and Human Health. Lancet Lond. Engl. 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Avery, J.C.; Hoffmann, P.R. selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef]
- Kyung, L.; Daewon, J. Bimodal Actions of selenium Essential for Antioxidant and Toxic Pro-Oxidant Activities: The selenium Paradox (Review). Mol. Med. Rep. 2012, 5, 299–304. [Google Scholar] [CrossRef]
- Ip, C.; Thompson, H.J.; Zhu, Z.; Ganther, H.E. In Vitro and in Vivo Studies of Methylseleninic Acid: Evidence That a Monomethylated selenium Metabolite Is Critical for Cancer Chemoprevention. Cancer Res. 2000, 60, 2882–2886. [Google Scholar] [PubMed]
- Kryukov, G.V.; Castellano, S.; Novoselov, S.V.; Lobanov, A.V.; Zehtab, O.; Guigó, R.; Gladyshev, V.N. Characterization of Mammalian Selenoproteomes. Science 2003, 300, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Papp, L.V.; Lu, J.; Holmgren, A.; Khanna, K.K. From selenium to Selenoproteins: Synthesis, Identity, and Their Role in Human Health. Antioxid. Redox Signal. 2007, 9, 775–806. [Google Scholar] [CrossRef]
- Reeves, M.A.; Hoffmann, P.R. The Human Selenoproteome: Recent Insights into Functions and Regulation. Cell. Mol. Life Sci. CMLS 2009, 66, 2457–2478. [Google Scholar] [CrossRef]
- Arteel, G.E.; Klotz, L.-O.; Buchczyk, D.P.; Sies, H. Selenoprotein P. Protein Sensors and Reactive Oxygen Species—Part A: Selenoproteins and Thioredoxin. In Methods in Enzymology; Sies, H., Packer, L., Eds.; Academic Press: Cambridge, MA, USA, 2002; Volume 347, pp. 121–125. [Google Scholar] [CrossRef]
- Hill, K.E.; Dasouki, M.; Phillips, J.A.; Burk, R.F. Human Selenoprotein P Gene Maps to 5q31. Genomics 1996, 36, 550–551. [Google Scholar] [CrossRef] [PubMed]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular Pathways and Physiological Roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef] [PubMed]
- Damdimopoulos, A.E.; Miranda-Vizuete, A.; Treuter, E.; Gustafsson, J.-A.; Spyrou, G. An Alternative Splicing Variant of the Selenoprotein Thioredoxin Reductase Is a Modulator of Estrogen Signaling. J. Biol. Chem. 2004, 279, 38721–38729. [Google Scholar] [CrossRef]
- Santesmasses, D.; Gladyshev, V.N. Pathogenic Variants in Selenoproteins and Selenocysteine Biosynthesis Machinery. Int. J. Mol. Sci. 2021, 22, 11593. [Google Scholar] [CrossRef]
- Méplan, C. selenium and Chronic Diseases: A Nutritional Genomics Perspective. Nutrients 2015, 7, 3621–3651. [Google Scholar] [CrossRef]
- Gladyshev, V.N.; Arnér, E.S.; Berry, M.J.; Brigelius-Flohé, R.; Bruford, E.A.; Burk, R.F.; Carlson, B.A.; Castellano, S.; Chavatte, L.; Conrad, M.; et al. Selenoprotein Gene Nomenclature. J. Biol. Chem. 2016, 291, 24036–24040. [Google Scholar] [CrossRef]
- Méplan, C.; Rohrmann, S.; Steinbrecher, A.; Schomburg, L.; Jansen, E.; Linseisen, J.; Hesketh, J. Polymorphisms in Thioredoxin Reductase and Selenoprotein K Genes and selenium Status Modulate Risk of Prostate Cancer. PLoS ONE 2012, 7, e48709. [Google Scholar] [CrossRef]
- Peters, U.; Chatterjee, N.; Hayes, R.B.; Schoen, R.E.; Wang, Y.; Chanock, S.J.; Foster, C.B. Variation in the Selenoenzyme Genes and Risk of Advanced Distal Colorectal Adenoma. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2008, 17, 1144–1154. [Google Scholar] [CrossRef]
- Méplan, C.; Hughes, D.J.; Pardini, B.; Naccarati, A.; Soucek, P.; Vodickova, L.; Hlavatá, I.; Vrána, D.; Vodicka, P.; Hesketh, J.E. Genetic Variants in Selenoprotein Genes Increase Risk of Colorectal Cancer. Carcinogenesis 2010, 31, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Polymorphisms in the Selenoprotein S and 15-kDa Selenoprotein Genes Are Associated with Altered Susceptibility to Colorectal Cancer—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/21052528/ (accessed on 26 November 2022).
- Mukhtar, M.; Ashfield, N.; Vodickova, L.; Vymetalkova, V.; Levy, M.; Liska, V.; Bruha, J.; Bendova, P.; O’Sullivan, J.; Doherty, G.; et al. The Associations of Selenoprotein Genetic Variants with the Risks of Colorectal Adenoma and Colorectal Cancer: Case-Control Studies in Irish and Czech Populations. Nutrients 2022, 14, 2718. [Google Scholar] [CrossRef]
- Yang, P.; Bamlet, W.R.; Ebbert, J.O.; Taylor, W.R.; de Andrade, M. Glutathione Pathway Genes and Lung Cancer Risk in Young and Old Populations. Carcinogenesis 2004, 25, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, K.; Gupta, S.; Durda, K.; Muszyńska, M.; Sukiennicki, G.; Jaworowska, E.; Grodzki, T.; Sulikowski, M.; Waloszczyk, P.; Wójcik, J.; et al. A Low selenium Level Is Associated with Lung and Laryngeal Cancers. PLoS ONE 2013, 8, e59051. [Google Scholar] [CrossRef]
- Jablonska, E.; Gromadzinska, J.; Sobala, W.; Reszka, E.; Wasowicz, W. Lung Cancer Risk Associated with selenium Status Is Modified in Smoking Individuals by Sep15 Polymorphism. Eur. J. Nutr. 2008, 47, 47–54. [Google Scholar] [CrossRef]
- Hamanishi, T.; Furuta, H.; Kato, H.; Doi, A.; Tamai, M.; Shimomura, H.; Sakagashira, S.; Nishi, M.; Sasaki, H.; Sanke, T.; et al. Functional Variants in the Glutathione Peroxidase-1 (GPx-1) Gene Are Associated with Increased Intima-Media Thickness of Carotid Arteries and Risk of Macrovascular Diseases in Japanese Type 2 Diabetic Patients. Diabetes 2004, 53, 2455–2460. [Google Scholar] [CrossRef]
- Cox, A.J.; Lehtinen, A.B.; Xu, J.; Langefeld, C.D.; Freedman, B.I.; Carr, J.J.; Bowden, D.W. Polymorphisms in the Selenoprotein S Gene and Subclinical Cardiovascular Disease in the Diabetes Heart Study. Acta Diabetol. 2013, 50, 391–399. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Hoffmann, P.R.; Berry, M.J. The Influence of selenium on Immune Responses. Mol. Nutr. Food Res. 2008, 52, 1273–1280. [Google Scholar] [CrossRef]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The Role of selenium in Inflammation and Immunity: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid. Redox Signal. 2012, 16, 705–743. [Google Scholar] [CrossRef]
- Tsuji, P.A.; Carlson, B.A.; Anderson, C.B.; Seifried, H.E.; Hatfield, D.L.; Howard, M.T. Dietary selenium Levels Affect Selenoprotein Expression and Support the Interferon-γ and IL-6 Immune Response Pathways in Mice. Nutrients 2015, 7, 6529–6549. [Google Scholar] [CrossRef] [PubMed]
- Bentley-Hewitt, K.L.; Chen, R.K.-Y.; Lill, R.E.; Hedderley, D.I.; Herath, T.D.; Matich, A.J.; McKenzie, M.J. Consumption of selenium-Enriched Broccoli Increases Cytokine Production in Human Peripheral Blood Mononuclear Cells Stimulated Ex Vivo, a Preliminary Human Intervention Study. Mol. Nutr. Food Res. 2014, 58, 2350–2357. [Google Scholar] [CrossRef]
- Buonacera, A.; Stancanelli, B.; Colaci, M.; Malatino, L. Neutrophil to Lymphocyte Ratio: An Emerging Marker of the Relationships between the Immune System and Diseases. Int. J. Mol. Sci. 2022, 23, 3636. [Google Scholar] [CrossRef] [PubMed]
- Regolo, M.; Vaccaro, M.; Sorce, A.; Stancanelli, B.; Colaci, M.; Natoli, G.; Russo, M.; Alessandria, I.; Motta, M.; Santangelo, N.; et al. Neutrophil-to-Lymphocyte Ratio (NLR) Is a Promising Predictor of Mortality and Admission to Intensive Care Unit of COVID-19 Patients. J. Clin. Med. 2022, 11, 2235. [Google Scholar] [CrossRef]
- Cataudella, E.; Giraffa, C.M.; Di Marca, S.; Pulvirenti, A.; Alaimo, S.; Pisano, M.; Terranova, V.; Corriere, T.; Ronsisvalle, M.L.; Di Quattro, R.; et al. Neutrophil-To-Lymphocyte Ratio: An Emerging Marker Predicting Prognosis in Elderly Adults with Community-Acquired Pneumonia. J. Am. Geriatr. Soc. 2017, 65, 1796–1801. [Google Scholar] [CrossRef]
- Corriere, T.; Di Marca, S.; Cataudella, E.; Pulvirenti, A.; Alaimo, S.; Stancanelli, B.; Malatino, L. Neutrophil-to-Lymphocyte Ratio Is a Strong Predictor of Atherosclerotic Carotid Plaques in Older Adults. Nutr. Metab. Cardiovasc. Dis. NMCD 2018, 28, 23–27. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, F.; Chen, F.; Li, P.; He, Y.; Wu, J.; Dong, L.; Wang, C.; Wang, X.; Zhang, W.; et al. Micronutrient Level Is Negatively Correlated with the Neutrophil-Lymphocyte Ratio in Patients with Severe COVID-19. Int. J. Clin. Pract. 2022, 2022, 6498794. [Google Scholar] [CrossRef]
- Razaghi, A.; Poorebrahim, M.; Sarhan, D.; Björnstedt, M. selenium Stimulates the Antitumour Immunity: Insights to Future Research. Eur. J. Cancer Oxf. Engl. 1990 2021, 155, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Eisenmann, C.J.; Miller, R.K. The Effect of selenium Compounds (Selenite, Selenate, Ebselen) on the Production of Thromboxane and Prostacyclin by the Human Term Placenta in Vitro. Toxicol. Appl. Pharmacol. 1995, 135, 18–24. [Google Scholar] [CrossRef]
- Perona, G.; Schiavon, R.; Guidi, G.C.; Veneri, D.; Minuz, P. selenium Dependent Glutathione Peroxidase: A Physiological Regulatory System for Platelet Function. Thromb. Haemost. 1990, 64, 312–318. [Google Scholar] [PubMed]
- Irani, K. Oxidant Signaling in Vascular Cell Growth, Death, and Survival: A Review of the Roles of Reactive Oxygen Species in Smooth Muscle and Endothelial Cell Mitogenic and Apoptotic Signaling. Circ. Res. 2000, 87, 179–183. [Google Scholar] [CrossRef]
- Jin, R.C.; Mahoney, C.E.; Coleman Anderson, L.; Ottaviano, F.; Croce, K.; Leopold, J.A.; Zhang, Y.-Y.; Tang, S.-S.; Handy, D.E.; Loscalzo, J. Glutathione Peroxidase-3 Deficiency Promotes Platelet-Dependent Thrombosis in Vivo. Circulation 2011, 123, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Loscalzo, J. Nitric Oxide Insufficiency, Platelet Activation, and Arterial Thrombosis. Circ. Res. 2001, 88, 756–762. [Google Scholar] [CrossRef]
- Kong, L.; Wu, Q.; Liu, B. The impact of selenium administration on severe sepsis or septic shock: A meta-analysis of randomized controlled trials. Afr. Health Sci. 2021, 21, 277–285. [Google Scholar] [CrossRef] [PubMed]
- French, L.; Temblett, P. Selenium levels in patients with different sources of sepsis. Crit. Care 2013, 17, P247. [Google Scholar] [CrossRef]
- Hughes, D.J.; Duarte-Salles, T.; Hybsier, S.; Trichopoulou, A.; Stepien, M.; Aleksandrova, K.; Overvad, K.; Tjønneland, A.; Olsen, A.; Affret, A.; et al. Prediagnostic selenium Status and Hepatobiliary Cancer Risk in the European Prospective Investigation into Cancer and Nutrition Cohort. Am. J. Clin. Nutr. 2016, 104, 406–414. [Google Scholar] [CrossRef]
- Chan, S.-Y.; Hsu, C.-P.; Ou Yang, C.-H.; Wang, C.-C.; Wu, Y.-T.; Fu, C.-Y.; Hsieh, C.-H.; Cheng, C.-T.; Lin, W.-C.; Huang, J.-F.; et al. The Impact on the Clinical Prognosis of Low Serum selenium Level in Patients with Severe Trauma: Systematic Review and Meta-Analysis. Nutrients 2022, 14, 1295. [Google Scholar] [CrossRef]
- Wang, Q.; Zennadi, R. Oxidative Stress and Thrombosis during Aging: The Roles of Oxidative Stress in RBCs in Venous Thrombosis. Int. J. Mol. Sci. 2020, 21, 4259. [Google Scholar] [CrossRef]
- Hu, X.F.; Stranges, S.; Chan, L.H.M. Circulating selenium Concentration Is Inversely Associated With the Prevalence of Stroke: Results From the Canadian Health Measures Survey and the National Health and Nutrition Examination Survey. J. Am. Heart Assoc. 2019, 8, e012290. [Google Scholar] [CrossRef]
- Hu, X.F.; Sharin, T.; Chan, H.M. Dietary and Blood selenium Are Inversely Associated with the Prevalence of Stroke among Inuit in Canada. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. J. Trace Elem. Med. Biol. 2017, 44, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Huang, S.; Zhang, Y.; Zhang, H.; Zhou, L.; Li, D.; Xie, C.; Lv, Z.; Guo, Y.; Ke, Y.; et al. Associations of Multiple Plasma Metals with the Risk of Ischemic Stroke: A Case-Control Study. Environ. Int. 2019, 125, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Bi, C.; Lin, T.; Liu, L.; Song, Y.; Wang, B.; Wang, P.; Zhou, Z.; Fang, C.; Ma, H.; et al. Sex Difference in the Association between Plasma selenium and First Stroke: A Community-Based Nested Case-Control Study. Biol. Sex Differ. 2021, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhang, Y. Relationship between the Circulating selenium Level and Stroke: A Meta-Analysis of Observational Studies. J. Am. Nutr. Assoc. 2022, 41, 444–452. [Google Scholar] [CrossRef]
- Mirończuk, A.; Kapica-Topczewska, K.; Socha, K.; Soroczyńska, J.; Jamiołkowski, J.; Kułakowska, A.; Kochanowicz, J. selenium, Copper, Zinc Concentrations and Cu/Zn, Cu/Se Molar Ratios in the Serum of Patients with Acute Ischemic Stroke in Northeastern Poland-A New Insight into Stroke Pathophysiology. Nutrients 2021, 13, 2139. [Google Scholar] [CrossRef]
- Fang, H.; Liu, W.; Zhang, L.; Pei, L.; Gao, Y.; Zhao, L.; Zhang, R.; Yang, J.; Song, B.; Xu, Y. A Bidirectional Mendelian Randomization Study of selenium Levels and Ischemic Stroke. Front. Genet. 2022, 13, 782691. [Google Scholar] [CrossRef]
- Wei, W.-Q.; Abnet, C.C.; Qiao, Y.-L.; Dawsey, S.M.; Dong, Z.-W.; Sun, X.-D.; Fan, J.-H.; Gunter, E.W.; Taylor, P.R.; Mark, S.D. Prospective Study of Serum selenium Concentrations and Esophageal and Gastric Cardia Cancer, Heart Disease, Stroke, and Total Death. Am. J. Clin. Nutr. 2004, 79, 80–85. [Google Scholar] [CrossRef]
- Merrill, P.D.; Ampah, S.B.; He, K.; Rembert, N.J.; Brockman, J.; Kleindorfer, D.; McClure, L.A. Association between trace elements in the environment and stroke risk: The reasons for geographic and racial differences in stroke (REGARDS) study. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. 2017, 42, 45–49. [Google Scholar] [CrossRef]
- Wu, Q.; Sun, X.; Chen, Q.; Zhang, X.; Zhu, Y. Genetically Predicted selenium Is Negatively Associated with Serum TC, LDL-C and Positively Associated with HbA1C Levels. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. J. Trace Elem. Med. Biol. 2021, 67, 126785. [Google Scholar] [CrossRef]
- Rayman, M.P.; Stranges, S. Epidemiology of selenium and Type 2 Diabetes: Can We Make Sense of It? Free Radic. Biol. Med. 2013, 65, 1557–1564. [Google Scholar] [CrossRef]
- Yang, J.; Chen, E.; Choi, C.; Chan, K.; Yang, Q.; Rana, J.; Yang, B.; Huang, C.; Yang, A.; Lo, K. Cross-Sectional Association of Blood selenium with Glycemic Biomarkers among U.S. Adults with Normoglycemia in the National Health and Nutrition Examination Survey 2013-2016. Nutrients 2022, 14, 3972. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Razavi, A.; Karimi, N.; Jafarpour, H. Evaluation of selenium Supplementation in Acute Ischemic Stroke Outcome: An Outcome Assessor Blind, Randomized, Placebo-Controlled, Feasibility Study. Neurol. India 2022, 70, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, M.; Simani, L.; Abedi, S.; Pakdaman, H. Is selenium Supplementation Beneficial in Acute Ischemic Stroke? Neurologist 2021, 27, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Elkind, M.S.V.; Boehme, A.K.; Smith, C.J.; Meisel, A.; Buckwalter, M.S. Infection as a Stroke Risk Factor and Determinant of Outcome After Stroke. Stroke 2020, 51, 3156–3168. [Google Scholar] [CrossRef]
- Antoniak, S. The Coagulation System in Host Defense. Res. Pract. Thromb. Haemost. 2018, 2, 549–557. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, M.; Mao, Z.; Yuan, R.; Wang, L.; Hu, X.; Zhou, F.; Kang, H. The Clinical Outcomes of selenium Supplementation on Critically Ill Patients. Medicine 2019, 98, e15473. [Google Scholar] [CrossRef]
- Leong, P.K.; Wong, H.S.; Chen, J.; Chan, W.M.; Leung, H.Y.; Ko, K.M. Differential Action between Schisandrin A and Schisandrin B in Eliciting an Anti-Inflammatory Action: The Depletion of Reduced Glutathione and the Induction of an Antioxidant Response. PLoS ONE 2016, 11, e0155879. [Google Scholar] [CrossRef]
- Ishibashi, N.; Prokopenko, O.; Reuhl, K.R.; Mirochnitchenko, O. Inflammatory Response and Glutathione Peroxidase in a Model of Stroke. J. Immunol. 2002, 168, 1926–1933. [Google Scholar] [CrossRef]
- Tamburrano, S.; Rhodes, S.; Mosneag, I.-E.; Roberts, L.; Hurry, M.E.D.; Grainger, J.R.; Shaw, T.N.; Smith, C.J.; Allan, S.M. Do Concentration or Activity of Selenoproteins Change in Acute Stroke Patients? A Systematic Review and Meta-Analyses. Cerebrovasc. Dis. Basel Switz. 2022, 51, 461–472. [Google Scholar] [CrossRef]
- Taylor, E.W.; Ruzicka, J.A.; Premadasa, L.; Zhao, L. Cellular Selenoprotein MRNA Tethering via Antisense Interactions with Ebola and HIV-1 MRNAs May Impact Host selenium Biochemistry. Curr. Top. Med. Chem. 2016, 16, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
- Premadasa, L.S.; Dailey, G.P.; Ruzicka, J.A.; Taylor, E.W. Selenium-Dependent Read Through of the Conserved 3′-Terminal UGA Stop Codon of HIV-1 nef. Am. J. Biopharmacy Pharm. Sci. 2021, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Copeland, P.R. Functional analysis of the interplay between translation termination, selenocysteine codon context, and selenocysteine insertion sequence-binding protein 2. J. Biol. Chem. 2007, 282, 36797–36807. [Google Scholar] [CrossRef] [PubMed]
- Vindry, C.; Guillin, O.; Mangeot, P.E.; Ohlmann, T.; Chavatte, L. A Versatile Strategy to Reduce UGA-Selenocysteine Recoding Efficiency of the Ribosome Using CRISPR-Cas9-Viral-Like-Particles Targeting Selenocysteine-TRNA[Ser]Sec Gene. Cells 2019, 8, E574. [Google Scholar] [CrossRef]
- Vavougios, G.D.; Zarogiannis, S.G.; Krogfelt, K.A.; Stamoulis, G.; Gourgoulianis, K.I. Epigenetic Regulation of Apoptosis via the PARK7 Interactome in Peripheral Blood Mononuclear Cells Donated by Tuberculosis Patients vs. Healthy Controls and the Response to Treatment: A Systems Biology Approach. Tuberc. Edinb. Scotl. 2020, 123, 101938. [Google Scholar] [CrossRef]
- Schwarz, M.; Lossow, K.; Schirl, K.; Hackler, J.; Renko, K.; Kopp, J.F.; Schwerdtle, T.; Schomburg, L.; Kipp, A.P. Copper Interferes with Selenoprotein Synthesis and Activity. Redox Biol. 2020, 37, 101746. [Google Scholar] [CrossRef]
- Ravoori, S.; Srinivasan, C.; Pereg, D.; Robertson, L.W.; Ayotte, P.; Gupta, R.C. Protective Effects of selenium against DNA Adduct Formation in Inuit Environmentally Exposed to PCBs. Environ. Int. 2010, 36, 980–986. [Google Scholar] [CrossRef]
- Lai, I.K.; Chai, Y.; Simmons, D.; Watson, W.H.; Tan, R.; Haschek, W.M.; Wang, K.; Wang, B.; Ludewig, G.; Robertson, L.W. Dietary selenium as a Modulator of PCB 126–Induced Hepatotoxicity in Male Sprague-Dawley Rats. Toxicol. Sci. 2011, 124, 202–214. [Google Scholar] [CrossRef]
- Sugiura, Y.; Tamai, Y.; Tanaka, H. selenium Protection against Mercury Toxicity: High Binding Affinity of Methylmercury by selenium-Containing Ligands in Comparison with Sulfur-Containing Ligands. Bioinorg. Chem. 1978, 9, 167–180. [Google Scholar] [CrossRef]
- Limaye, A.; Yu, R.-C.; Chou, C.-C.; Liu, J.-R.; Cheng, K.-C. Protective and Detoxifying Effects Conferred by Dietary selenium and Curcumin against AFB1-Mediated Toxicity in Livestock: A Review. Toxins 2018, 10, 25. [Google Scholar] [CrossRef]
- Cai, H.; Harrison, D.G. Endothelial Dysfunction in Cardiovascular Diseases: The Role of Oxidant Stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.V.; Bartosch, B.; Isaguliants, M.G. Oxidative Stress in Infection and Consequent Disease. Oxid. Med. Cell. Longev. 2017, 2017, 3496043. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Ahmad, A.S.; Ahmad, M.; Salim, S.; Yousuf, S.; Ishrat, T.; Islam, F. selenium Protects Cerebral Ischemia in Rat Brain Mitochondria. Biol. Trace Elem. Res. 2004, 101, 73–86. [Google Scholar] [CrossRef]
- Zimmermann, C.; Winnefeld, K.; Streck, S.; Roskos, M.; Haberl, R.L. Antioxidant Status in Acute Stroke Patients and Patients at Stroke Risk. Eur. Neurol. 2004, 51, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Angelova, E.A.; Atanassova, P.A.; Chalakova, N.T.; Dimitrov, B.D. Associations between Serum selenium and Total Plasma Homocysteine during the Acute Phase of Ischaemic Stroke. Eur. Neurol. 2008, 60, 298–303. [Google Scholar] [CrossRef]
- Song, J.; Park, J.; Oh, Y.; Lee, J.E. Glutathione Suppresses Cerebral Infarct Volume and Cell Death after Ischemic Injury: Involvement of FOXO3 Inactivation and Bcl2 Expression. Oxid. Med. Cell. Longev. 2015, 2015, 426069. [Google Scholar] [CrossRef]
- Morris, D.; Khurasany, M.; Nguyen, T.; Kim, J.; Guilford, F.; Mehta, R.; Gray, D.; Saviola, B.; Venketaraman, V. Glutathione and Infection. Biochim. Biophys. Acta 2013, 1830, 3329–3349. [Google Scholar] [CrossRef]
- Guerra, C.; Morris, D.; Sipin, A.; Kung, S.; Franklin, M.; Gray, D.; Tanzil, M.; Guilford, F.; Khasawneh, F.T.; Venketaraman, V. Glutathione and Adaptive Immune Responses against Mycobacterium Tuberculosis Infection in Healthy and HIV Infected Individuals. PLoS ONE 2011, 6, e28378. [Google Scholar] [CrossRef]
- Bizerea-Moga, T.O.; Pitulice, L.; Bizerea-Spiridon, O.; Moga, T.V. Evaluation of Serum selenium Status by Age and Gender: A Retrospective Observational Cohort Study in Western Romania. Nutrients 2021, 13, 1497. [Google Scholar] [CrossRef]
- Muntau, A.C.; Streiter, M.; Kappler, M.; Röschinger, W.; Schmid, I.; Rehnert, A.; Schramel, P.; Roscher, A.A. Age-Related Reference Values for Serum selenium Concentrations in Infants and Children. Clin. Chem. 2002, 48, 555–560. [Google Scholar] [CrossRef]
- Alehagen, U.; Johansson, P.; Björnstedt, M.; Rosén, A.; Post, C.; Aaseth, J. Relatively High Mortality Risk in Elderly Swedish Subjects with Low selenium Status. Eur. J. Clin. Nutr. 2016, 70, 91–96. [Google Scholar] [CrossRef] [PubMed]
- González, S.; Huerta, J.M.; Fernández, S.; Patterson, E.M.; Lasheras, C. Food Intake and Serum selenium Concentration in Elderly People. Ann. Nutr. Metab. 2006, 50, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Smith, A.M.; Failla, M.L.; Hill, K.E.; Yu, Z. Estrogen Status Alters Tissue Distribution and Metabolism of selenium in Female Rats. J. Nutr. Biochem. 2012, 23, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, N.M.; Stewart, R.D.; Robinson, M.F. The Metabolism of [75Se]Selenomethionine in Four Women. Br. J. Nutr. 1976, 35, 373–382. [Google Scholar] [CrossRef]
- Janghorbani, M.; Christensen, M.J.; Nahapetian, A.; Young, V.R. selenium Metabolism in Healthy Adults: Quantitative Aspects Using the Stable Isotope 74SeO32−. Am. J. Clin. Nutr. 1982, 35, 647–654. [Google Scholar] [CrossRef]
- Bratakos, M.S.; Zafiropoulos, T.F.; Siskos, P.A.; Ioannou, P.V. selenium Losses on Cooking Greek Foods. Int. J. Food Sci. Technol. 1988, 23, 585–590. [Google Scholar] [CrossRef]
- Li, A.; Zhou, Q.; Mei, Y.; Zhao, J.; Zhao, M.; Xu, J.; Ge, X.; Xu, Q. Novel Strategies for Assessing Associations Between selenium Biomarkers and Cardiometabolic Risk Factors: Concentration, Visit-to-Visit Variability, or Individual Mean? Evidence from a Repeated-Measures Study of Older Adults With High selenium. Front. Nutr. 2022, 9, 838613. [Google Scholar] [CrossRef]
- Elkind, M.S.V.; Carty, C.L.; O’Meara, E.S.; Lumley, T.; Lefkowitz, D.; Kronmal, R.A.; Longstreth, W.T. Hospitalization for Infection and Risk of Acute Ischemic Stroke: The Cardiovascular Health Study. Stroke 2011, 42, 1851–1856. [Google Scholar] [CrossRef]
- Cowan, L.T.; Alonso, A.; Pankow, J.S.; Folsom, A.R.; Rosamond, W.D.; Gottesman, R.F.; Lakshminarayan, K. Hospitalized Infection as a Trigger for Acute Ischemic Stroke: The Atherosclerosis Risk in Communities Study. Stroke 2016, 47, 1612–1617. [Google Scholar] [CrossRef]
- Smeeth, L.; Thomas, S.L.; Hall, A.J.; Hubbard, R.; Farrington, P.; Vallance, P. Risk of myocardial infarction and stroke after acute infection or vaccination. N. Engl. J. Med. 2004, 351, 2611–2618. [Google Scholar] [CrossRef]
- Sebastian, S.; Stein, L.K.; Dhamoon, M.S. Infection as a Stroke Trigger. Stroke 2019, 50, 2216–2218. [Google Scholar] [CrossRef] [PubMed]
- Boehme, A.K.; Ranawat, P.; Luna, J.; Kamel, H.; Elkind, M.S.V. Risk of Acute Stroke After Hospitalization for Sepsis. Stroke 2017, 48, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Shao, I.Y.; Elkind, M.S.V.; Boehme, A.K. Risk Factors for Stroke in Patients With Sepsis and Bloodstream Infections. Stroke 2019, 50, 1046–1051. [Google Scholar] [CrossRef]
- Miller, E.C.; Elkind, M.S.V. Infection and Stroke: An Update on Recent Progress. Curr. Neurol. Neurosci. Rep. 2016, 16, 2. [Google Scholar] [CrossRef]
- Harms, H.; Halle, E.; Meisel, A. Post-Stroke Infections—Diagnosis, Prediction, Prevention and Treatment to Improve Patient Outcomes. Eur. Neurol. Rev. 2011, 5, 39–43. [Google Scholar] [CrossRef]
- Yuan, Y.; Xiao, Y.; Feng, W.; Liu, Y.; Yu, Y.; Zhou, L.; Qiu, G.; Wang, H.; Liu, B.; Liu, K.; et al. Plasma Metal Concentrations and Incident Coronary Heart Disease in Chinese Adults: The Dongfeng-Tongji Cohort. Environ. Health Perspect. 2017, 125, 107007. [Google Scholar] [CrossRef]
- Salonen, J.; Alfthan, G.; Huttunen, J.; Pikkarainen, J.; Puska, P. Association between cardiovascular death and myocardial infarction and serum selenium in matched-pair longitudinal study. Lancet 1982, 320, 175–179. [Google Scholar] [CrossRef]
- Blankenberg, S.; Rupprecht, H.J.; Bickel, C.; Torzewski, M.; Hafner, G.; Tiret, L.; Smieja, M.; Cambien, F.; Meyer, J.; Lackner, K.J. Glutathione Peroxidase 1 Activity and Cardiovascular Events in Patients with Coronary Artery Disease. N. Engl. J. Med. 2003, 349, 1605–1613. [Google Scholar] [CrossRef]
- Laclaustra, M.; Navas-Acien, A.; Stranges, S.; Ordovas, J.M.; Guallar, E. Serum selenium Concentrations and Hypertension in the US Population. Circ. Cardiovasc. Qual. Outcomes 2009, 2, 369–376. [Google Scholar] [CrossRef]
- Bodnar, M.; Szczyglowska, M.; Konieczka, P.; Namiesnik, J. Methods of selenium Supplementation: Bioavailability and Determination of selenium Compounds. Crit. Rev. Food Sci. Nutr. 2016, 56, 36–55. [Google Scholar] [CrossRef]
- Bhaskar, A.; Munshi, M.; Khan, S.Z.; Fatima, S.; Arya, R.; Jameel, S.; Singh, A. Measuring Glutathione Redox Potential of HIV-1-Infected Macrophages. J. Biol. Chem. 2015, 290, 1020–1038. [Google Scholar] [CrossRef] [PubMed]
- Turck, N.; Robin, X.; Walter, N.; Fouda, C.; Hainard, A.; Sztajzel, R.; Wagner, G.; Hochstrasser, D.F.; Montaner, J.; Burkhard, P.R.; et al. Blood Glutathione S-Transferase-π as a Time Indicator of Stroke Onset. PLoS ONE 2012, 7, e43830. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liampas, A.; Zis, P.; Hadjigeorgiou, G.; Vavougios, G.D. Selenium, Stroke, and Infection: A Threefold Relationship; Where Do We Stand and Where Do We Go? Nutrients 2023, 15, 1405. https://doi.org/10.3390/nu15061405
Liampas A, Zis P, Hadjigeorgiou G, Vavougios GD. Selenium, Stroke, and Infection: A Threefold Relationship; Where Do We Stand and Where Do We Go? Nutrients. 2023; 15(6):1405. https://doi.org/10.3390/nu15061405
Chicago/Turabian StyleLiampas, Andreas, Panagiotis Zis, Georgios Hadjigeorgiou, and George D. Vavougios. 2023. "Selenium, Stroke, and Infection: A Threefold Relationship; Where Do We Stand and Where Do We Go?" Nutrients 15, no. 6: 1405. https://doi.org/10.3390/nu15061405
APA StyleLiampas, A., Zis, P., Hadjigeorgiou, G., & Vavougios, G. D. (2023). Selenium, Stroke, and Infection: A Threefold Relationship; Where Do We Stand and Where Do We Go? Nutrients, 15(6), 1405. https://doi.org/10.3390/nu15061405






