The Complex Effects of Light on Metabolism in Humans
Abstract
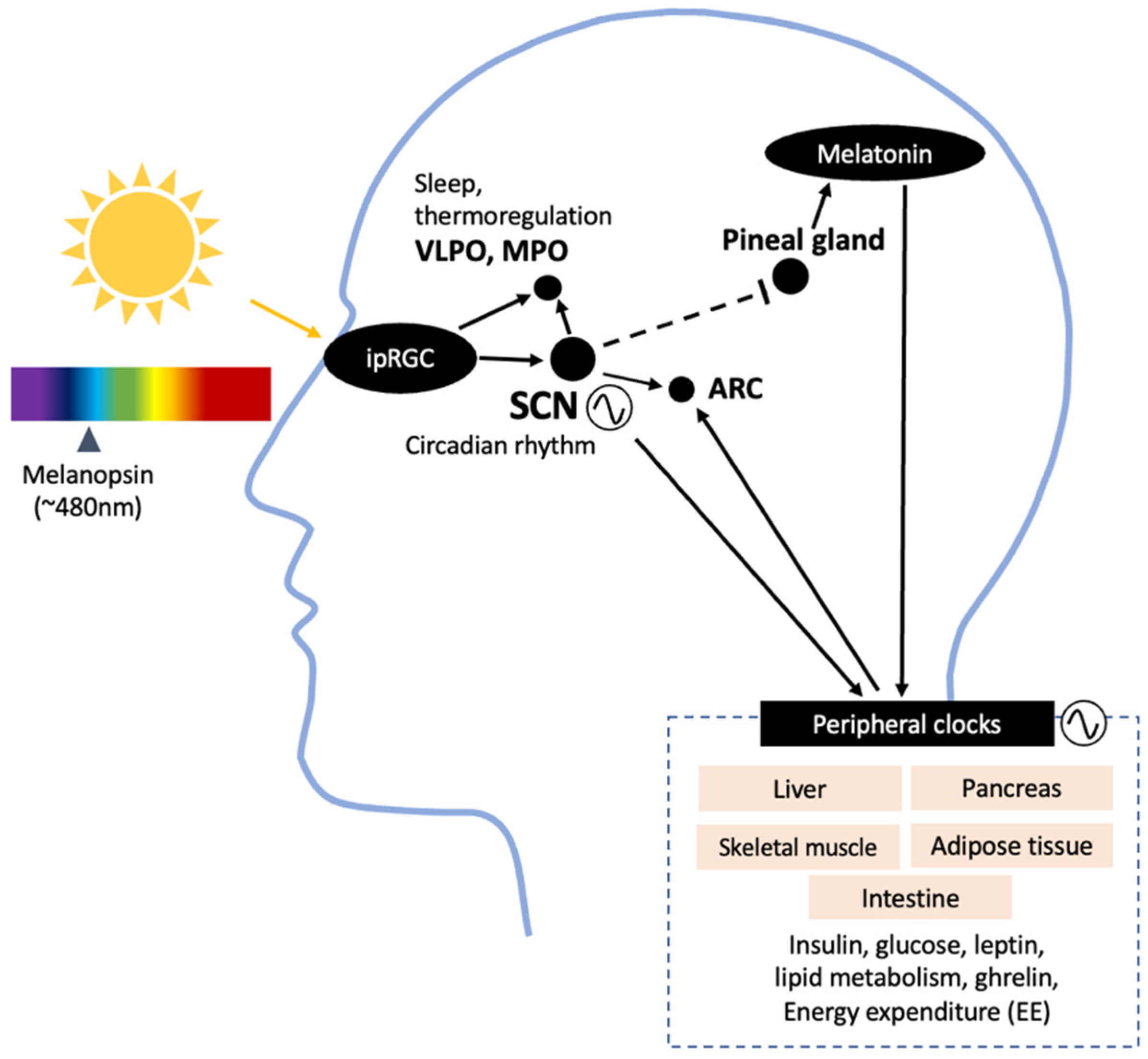
1. Introduction
2. Sleep and Circadian Impact on Metabolism
3. Properties of Light Affecting Metabolism
3.1. Intensity of Light
3.2. Duration of Light
3.3. Exposure Timing
3.3.1. Morning Light Exposure
3.3.2. Daytime Light Exposure
3.3.3. Evening Light Exposure
3.3.4. Evening Light Exposure and Food Intake
3.4. Spectral Composition of Light
3.5. Other Properties of Light-Influencing Metabolism
4. Melatonin’s Contribution to Metabolism
4.1. Exogenous Melatonin Administration
4.2. Melatonin-Enriched Food on Sleep
4.2.1. Fruits
4.2.2. Milk
4.2.3. Grain and Other Food Sources
5. Variability in Response to Light
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fonken, L.K.; Nelson, R.J. The effects of light at night on circadian clocks and metabolism. Endocr. Rev. 2014, 35, 648–670. [Google Scholar] [CrossRef] [PubMed]
- LeGates, T.A.; Fernandez, D.C.; Hattar, S. Light as a central modulator of circadian rhythms, sleep and affect. Nat. Rev. Neurosci. 2014, 15, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.F.; Czeisler, C.A. Effect of Light on Human Circadian Physiology. Sleep Med. Clin. 2009, 4, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; White, A.J.; Jackson, C.L.; Weinberg, C.R.; Sandler, D.P. Association of Exposure to Artificial Light at Night While Sleeping With Risk of Obesity in Women. JAMA Intern. Med. 2019, 179, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Plano, S.A.; Casiraghi, L.P.; Garcia Moro, P.; Paladino, N.; Golombek, D.A.; Chiesa, J.J. Circadian and Metabolic Effects of Light: Implications in Weight Homeostasis and Health. Front. Neurol. 2017, 8, 558. [Google Scholar] [CrossRef]
- Karlsson, B.; Knutsson, A.; Lindahl, B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup. Environ. Med. 2001, 58, 747–752. [Google Scholar] [CrossRef]
- Obayashi, K.; Saeki, K.; Iwamoto, J.; Okamoto, N.; Tomioka, K.; Nezu, S.; Ikada, Y.; Kurumatani, N. Exposure to light at night, nocturnal urinary melatonin excretion, and obesity/dyslipidemia in the elderly: A cross-sectional analysis of the HEIJO-KYO study. J. Clin. Endocrinol. Metab. 2013, 98, 337–344. [Google Scholar] [CrossRef]
- van Amelsvoort, L.G.; Schouten, E.G.; Kok, F.J. Duration of shiftwork related to body mass index and waist to hip ratio. Int. J. Obes. Relat. Metab. Disord. 1999, 23, 973–978. [Google Scholar] [CrossRef]
- Borbély, A.A.; Daan, S.; Wirz-Justice, A.; Deboer, T. The two-process model of sleep regulation: A reappraisal. J. Sleep Res. 2016, 25, 131–143. [Google Scholar] [CrossRef]
- Aschoff, J.; von Goetz, C.; Wildgruber, C.; Wever, R.A. Meal timing in humans during isolation without time cues. J. Biol. Rhythms. 1986, 1, 151–162. [Google Scholar] [CrossRef]
- Fleury, G.; Masis-Vargas, A.; Kalsbeek, A. Metabolic Implications of Exposure to Light at Night: Lessons from Animal and Human Studies. Obesity 2020, 28, S18–S28. [Google Scholar] [CrossRef] [PubMed]
- Lockley, S.W.; Brainard, G.C.; Czeisler, C.A. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J. Clin. Endocrinol. Metab. 2003, 88, 4502–4505. [Google Scholar] [CrossRef] [PubMed]
- Rupp, A.C.; Ren, M.; Altimus, C.M.; Fernandez, D.C.; Richardson, M.; Turek, F.; Hattar, S.; Schmidt, T.M. Distinct ipRGC subpopulations mediate light’s acute and circadian effects on body temperature and sleep. eLife 2019, 8, e44358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Beier, C.; Weil, T.; Hattar, S. The retinal ipRGC-preoptic circuit mediates the acute effect of light on sleep. Nat. Commun. 2021, 12, 5115. [Google Scholar] [CrossRef]
- Aranda, M.L.; Schmidt, T.M. Diversity of intrinsically photosensitive retinal ganglion cells: Circuits and functions. Cell Mol. Life Sci. 2021, 78, 889–907. [Google Scholar] [CrossRef]
- Reid, K.J. Assessment of Circadian Rhythms. Neurol. Clin. 2019, 37, 505–526. [Google Scholar] [CrossRef]
- Jung, C.M.; Melanson, E.L.; Frydendall, E.J.; Perreault, L.; Eckel, R.H.; Wright, K.P. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J. Physiol. 2011, 589, 235–244. [Google Scholar] [CrossRef]
- Kayaba, M.; Park, I.; Iwayama, K.; Seya, Y.; Ogata, H.; Yajima, K.; Satoh, M.; Tokuyama, K. Energy metabolism differs between sleep stages and begins to increase prior to awakening. Metabolism 2017, 69, 14–23. [Google Scholar] [CrossRef]
- Kalsbeek, A.; Scheer, F.A.; Perreau-Lenz, S.; La Fleur, S.E.; Yi, C.X.; Fliers, E.; Buijs, R.M. Circadian disruption and SCN control of energy metabolism. FEBS Lett. 2011, 585, 1412–1426. [Google Scholar] [CrossRef]
- Kalsbeek, A.; la Fleur, S.; Fliers, E. Circadian control of glucose metabolism. Mol. Metab. 2014, 3, 372–383. [Google Scholar] [CrossRef]
- Ruiter, M.; La Fleur, S.E.; van Heijningen, C.; van der Vliet, J.; Kalsbeek, A.; Buijs, R.M. The daily rhythm in plasma glucagon concentrations in the rat is modulated by the biological clock and by feeding behavior. Diabetes 2003, 52, 1709–1715. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Gong, Y.; Eckel-Mahan, K.L.; Sun, Z. Central Circadian Clock Regulates Energy Metabolism. Adv. Exp. Med. Biol. 2018, 1090, 79–103. [Google Scholar] [CrossRef] [PubMed]
- Zitting, K.M.; Vujovic, N.; Yuan, R.K.; Isherwood, C.M.; Medina, J.E.; Wang, W.; Buxton, O.M.; Williams, J.S.; Czeisler, C.A.; Duffy, J.F. Human Resting Energy Expenditure Varies with Circadian Phase. Curr. Biol. 2018, 28, 3685–3690.e3. [Google Scholar] [CrossRef] [PubMed]
- Prayag, A.S.; Münch, M.; Aeschbach, D.; Chellappa, S.L.; Gronfier, C. Light Modulation of Human Clocks, Wake, and Sleep. Clocks Sleep 2019, 1, 193–208. [Google Scholar] [CrossRef]
- Wirz-Justice, A.; Skene, D.J.; Münch, M. The relevance of daylight for humans. Biochem. Pharmacol. 2021, 191, 114304. [Google Scholar] [CrossRef]
- Bhandary, S.K.; Dhakal, R.; Sanghavi, V.; Verkicharla, P.K. Ambient light level varies with different locations and environmental conditions: Potential to impact myopia. PLoS ONE 2021, 16, e0254027. [Google Scholar] [CrossRef]
- Brown, T.M.; Brainard, G.C.; Cajochen, C.; Czeisler, C.A.; Hanifin, J.P.; Lockley, S.W.; Lucas, R.J.; Münch, M.; O’Hagan, J.B.; Peirson, S.N.; et al. Recommendations for daytime, evening, and nighttime indoor light exposure to best support physiology, sleep, and wakefulness in healthy adults. PLoS Biol. 2022, 20, e3001571. [Google Scholar] [CrossRef]
- Fonken, L.K.; Workman, J.L.; Walton, J.C.; Weil, Z.M.; Morris, J.S.; Haim, A.; Nelson, R.J. Light at night increases body mass by shifting the time of food intake. Proc. Natl. Acad. Sci. USA 2010, 107, 18664–18669. [Google Scholar] [CrossRef]
- Boivin, D.B.; Duffy, J.F.; Kronauer, R.E.; Czeisler, C.A. Dose-response relationships for resetting of human circadian clock by light. Nature 1996, 379, 540–542. [Google Scholar] [CrossRef]
- Cajochen, C.; Zeitzer, J.M.; Czeisler, C.A.; Dijk, D.J. Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav. Brain Res. 2000, 115, 75–83. [Google Scholar] [CrossRef]
- Zeitzer, J.M.; Dijk, D.J.; Kronauer, R.; Brown, E.; Czeisler, C. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J. Physiol. 2000, 526, 695–702. [Google Scholar] [CrossRef] [PubMed]
- McFadden, E.; Jones, M.E.; Schoemaker, M.J.; Ashworth, A.; Swerdlow, A.J. The relationship between obesity and exposure to light at night: Cross-sectional analyses of over 100,000 women in the Breakthrough Generations Study. Am. J. Epidemiol. 2014, 180, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, K.; Saeki, K.; Iwamoto, J.; Ikada, Y.; Kurumatani, N. Independent associations of exposure to evening light and nocturnal urinary melatonin excretion with diabetes in the elderly. Chronobiol. Int. 2014, 31, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, K.; Saeki, K.; Kurumatani, N. Ambient Light Exposure and Changes in Obesity Parameters: A Longitudinal Study of the HEIJO-KYO Cohort. J. Clin. Endocrinol. Metab. 2016, 101, 3539–3547. [Google Scholar] [CrossRef]
- Obayashi, K.; Yamagami, Y.; Kurumatani, N.; Saeki, K. Bedroom lighting environment and incident diabetes mellitus: A longitudinal study of the HEIJO-KYO cohort. Sleep Med. 2020, 65, 1–3. [Google Scholar] [CrossRef]
- Cho, C.H.; Lee, H.J.; Yoon, H.K.; Kang, S.G.; Bok, K.N.; Jung, K.Y.; Kim, L.; Lee, E.I. Exposure to dim artificial light at night increases REM sleep and awakenings in humans. Chronobiol. Int. 2016, 33, 117–123. [Google Scholar] [CrossRef]
- Cho, C.H.; Yoon, H.K.; Kang, S.G.; Kim, L.; Lee, E.I.; Lee, H.J. Impact of Exposure to Dim Light at Night on Sleep in Female and Comparison with Male Subjects. Psychiatry Investig. 2018, 15, 520–530. [Google Scholar] [CrossRef]
- Ursino, G.; Coppari, R. Insulin under the influence of light. Swiss. Med. Wkly. 2020, 150, w20273. [Google Scholar] [CrossRef]
- Mason, I.C.; Grimaldi, D.; Reid, K.J.; Warlick, C.D.; Malkani, R.G.; Abbott, S.M.; Zee, P.C. Light exposure during sleep impairs cardiometabolic function. Proc. Natl. Acad. Sci. USA 2022, 119, e2113290119. [Google Scholar] [CrossRef]
- Gil-Lozano, M.; Hunter, P.M.; Behan, L.A.; Gladanac, B.; Casper, R.F.; Brubaker, P.L. Short-term sleep deprivation with nocturnal light exposure alters time-dependent glucagon-like peptide-1 and insulin secretion in male volunteers. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E41–E50. [Google Scholar] [CrossRef]
- Srivastava, S.; Donaldson, L.F.; Rai, D.; Melichar, J.K.; Potokar, J. Single bright light exposure decreases sweet taste threshold in healthy volunteers. J. Psychopharmacol. 2013, 27, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.J.; Peirson, S.N.; Berson, D.M.; Brown, T.M.; Cooper, H.M.; Czeisler, C.A.; Figueiro, M.G.; Gamlin, P.D.; Lockley, S.W.; O’Hagan, J.B.; et al. Measuring and using light in the melanopsin age. Trends Neurosci. 2014, 37, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.J.K.; Vidafar, P.; Burns, A.C.; McGlashan, E.M.; Anderson, C.; Rajaratnam, S.M.W.; Lockley, S.W.; Cain, S.W. High sensitivity and interindividual variability in the response of the human circadian system to evening light. Proc. Natl. Acad. Sci. USA 2019, 116, 12019–12024. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.M.; Santhi, N.; St Hilaire, M.; Gronfier, C.; Bradstreet, D.S.; Duffy, J.F.; Lockley, S.W.; Kronauer, R.E.; Czeisler, C.A. Human responses to bright light of different durations. J. Physiol. 2012, 590, 3103–3112. [Google Scholar] [CrossRef]
- Pattinson, C.L.; Allan, A.C.; Staton, S.L.; Thorpe, K.J.; Smith, S.S. Environmental Light Exposure Is Associated with Increased Body Mass in Children. PLoS ONE 2016, 11, e0143578. [Google Scholar] [CrossRef]
- Dennison, B.A.; Erb, T.A.; Jenkins, P.L. Television viewing and television in bedroom associated with overweight risk among low-income preschool children. Pediatrics 2002, 109, 1028–1035. [Google Scholar] [CrossRef]
- Haghjoo, P.; Siri, G.; Soleimani, E.; Farhangi, M.A.; Alesaeidi, S. Screen time increases overweight and obesity risk among adolescents: A systematic review and dose-response meta-analysis. BMC Prim. Care 2022, 23, 161. [Google Scholar] [CrossRef]
- Zeitzer, J.M.; Ruby, N.F.; Fisicaro, R.A.; Heller, H.C. Response of the human circadian system to millisecond flashes of light. PLoS ONE 2011, 6, e22078. [Google Scholar] [CrossRef]
- Najjar, R.P.; Zeitzer, J.M. Temporal integration of light flashes by the human circadian system. J. Clin. Investig. 2016, 126, 938–947. [Google Scholar] [CrossRef]
- Khalsa, S.B.; Jewett, M.E.; Cajochen, C.; Czeisler, C.A. A phase response curve to single bright light pulses in human subjects. J. Physiol. 2003, 549, 945–952. [Google Scholar] [CrossRef]
- Campbell, P.D.; Miller, A.M.; Woesner, M.E. Bright Light Therapy: Seasonal Affective Disorder and Beyond. Einstein. J. Biol. Med. 2017, 32, E13–E25. [Google Scholar]
- Gaist, P.A.; Obarzanek, E.; Skwerer, R.G.; Duncan, C.C.; Shultz, P.M.; Rosenthal, N.E. Effects of bright light on resting metabolic rate in patients with seasonal affective disorder and control subjects. Biol. Psychiatry 1990, 28, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Putilov, A.A.; Danilenko, K.V. The sympatho-adrenal and energy-regulating systems in winter depression. In Biologic Effects of Light 1998; Springer: Berlin/Heidelberg, Germany, 1999; pp. 455–458. [Google Scholar]
- Ivanova, I.A.; Danilenko, K.V.; Aftanas, L.I. Investigation of an Immediate Effect of Bright Light on Oxygen Consumption, Heart Rate, Cortisol, and alpha-Amylase in Seasonal Affective Disorder Subjects and Healthy Controls. Neuropsychobiology 2016, 74, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Lam, R.W.; Goldner, E.M.; Solyom, L.; Remick, R.A. A controlled study of light therapy for bulimia nervosa. Am. J. Psychiatry 1994, 151, 744–750. [Google Scholar] [CrossRef]
- Braun, D.L.; Sunday, S.R.; Fornari, V.M.; Halmi, K.A. Bright light therapy decreases winter binge frequency in women with bulimia nervosa: A double-blind, placebo-controlled study. Compr. Psychiatry 1999, 40, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Roy, T.; Lloyd, C.E. Epidemiology of depression and diabetes: A systematic review. J. Affect. Disord. 2012, 142, S8–S21. [Google Scholar] [CrossRef]
- Nieuwenhuis, R.F.; Spooren, P.F.; Tilanus, J.J. Less need for insulin, a surprising effect of phototherapy in insulin-dependent diabetes mellitus. Tijdschr. Psychiatr. 2009, 51, 693–697. [Google Scholar]
- Allen, N.H.; Kerr, D.; Smythe, P.J.; Martin, N.; Osola, K.; Thompson, C. Insulin sensitivity after phototherapy for seasonal affective disorder. Lancet 1992, 339, 1065–1066. [Google Scholar] [CrossRef]
- Brouwer, A.; van Raalte, D.H.; Nguyen, H.T.; Rutters, F.; van de Ven, P.M.; Elders, P.J.M.; Moll, A.C.; Van Someren, E.J.W.; Snoek, F.J.; Beekman, A.T.F.; et al. Effects of Light Therapy on Mood and Insulin Sensitivity in Patients With Type 2 Diabetes and Depression: Results From a Randomized Placebo-Controlled Trial. Diabetes Care 2019, 42, 529–538. [Google Scholar] [CrossRef]
- Bylesjö, E.I.; Boman, K.; Wetterberg, L. Obesity treated with phototherapy: Four case studies. Int. J. Eat. Disord. 1996, 20, 443–446. [Google Scholar] [CrossRef]
- Versteeg, R.I.; Stenvers, D.J.; Visintainer, D.; Linnenbank, A.; Tanck, M.W.; Zwanenburg, G.; Smilde, A.K.; Fliers, E.; Kalsbeek, A.; Serlie, M.J.; et al. Acute Effects of Morning Light on Plasma Glucose and Triglycerides in Healthy Men and Men with Type 2 Diabetes. J. Biol. Rhythms. 2017, 32, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Danilenko, K.V.; Mustafina, S.V.; Pechenkina, E.A. Bright light for weight loss: Results of a controlled crossover trial. Obes. Facts. 2013, 6, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Dunai, A.; Novak, M.; Chung, S.A.; Kayumov, L.; Keszei, A.; Levitan, R.; Shapiro, C.M. Moderate exercise and bright light treatment in overweight and obese individuals. Obesity 2007, 15, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Ru, T.; Li, S.; Li, Y.; Zhou, G. Shine light on sleep: Morning bright light improves nocturnal sleep and next morning alertness among college students. J. Sleep Res. 2022, 2022, e13724. [Google Scholar] [CrossRef]
- Ma, J.; Kim, M.; Kim, J.; Hong, G.; Namgung, E.; Park, S.; Lim, S.M.; Lyoo, I.K.; Yoon, S. Decreased functional connectivity within the salience network after two-week morning bright light exposure in individuals with sleep disturbances: A preliminary randomized controlled trial. Sleep Med. 2020, 74, 66–72. [Google Scholar] [CrossRef]
- Rubiño, J.A.; Gamundí, A.; Akaarir, M.; Canellas, F.; Rial, R.; Nicolau, M.C. Bright Light Therapy and Circadian Cycles in Institutionalized Elders. Front. Neurosci. 2020, 14, 359. [Google Scholar] [CrossRef]
- Liu, C.R.; Liou, Y.M.; Jou, J.H. Ambient bright lighting in the morning improves sleep disturbances of older adults with dementia. Sleep Med. 2022, 89, 1–9. [Google Scholar] [CrossRef]
- Dumont, M.; Carrier, J. Daytime sleep propensity after moderate circadian phase shifts induced with bright light exposure. Sleep 1997, 20, 11–17. [Google Scholar] [CrossRef]
- Jewett, M.E.; Rimmer, D.W.; Duffy, J.F.; Klerman, E.B.; Kronauer, R.E.; Czeisler, C.A. Human circadian pacemaker is sensitive to light throughout subjective day without evidence of transients. Am. J. Physiol. 1997, 273, R1800–R1809. [Google Scholar] [CrossRef]
- Duffy, J.F.; Wright, K.P., Jr. Entrainment of the human circadian system by light. J. Biol. Rhythms. 2005, 20, 326–338. [Google Scholar] [CrossRef]
- Melanson, E.L.; Ritchie, H.K.; Dear, T.B.; Catenacci, V.; Shea, K.; Connick, E.; Moehlman, T.M.; Stothard, E.R.; Higgins, J.; McHill, A.W.; et al. Daytime bright light exposure, metabolism, and individual differences in wake and sleep energy expenditure during circadian entrainment and misalignment. Neurobiol. Sleep Circadian Rhythms. 2018, 4, 49–56. [Google Scholar] [CrossRef]
- Harmsen, J.F.; Wefers, J.; Doligkeit, D.; Schlangen, L.; Dautzenberg, B.; Rense, P.; van Moorsel, D.; Hoeks, J.; Moonen-Kornips, E.; Gordijn, M.C.M.; et al. The influence of bright and dim light on substrate metabolism, energy expenditure and thermoregulation in insulin-resistant individuals depends on time of day. Diabetologia 2022, 65, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Pinchasov, B.B.; Shurgaja, A.M.; Grischin, O.V.; Putilov, A.A. Mood and energy regulation in seasonal and non-seasonal depression before and after midday treatment with physical exercise or bright light. Psychiatry Res. 2000, 94, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Sone, Y.; Hyun, K.J.; Nishimura, S.; Lee, Y.A.; Tokura, H. Effects of dim or bright-light exposure during the daytime on human gastrointestinal activity. Chronobiol. Int. 2003, 20, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Benedito-Silva, A.A.; Evans, S.; Viana Mendes, J.; Castro, J.; Gonçalves, B.; Ruiz, F.S.; Beijamini, F.; Evangelista, F.S.; Vallada, H.; Krieger, J.E.; et al. Association between light exposure and metabolic syndrome in a rural Brazilian town. PLoS ONE 2020, 15, e0238772. [Google Scholar] [CrossRef]
- Coomans, C.P.; van den Berg, S.A.; Houben, T.; van Klinken, J.B.; van den Berg, R.; Pronk, A.C.; Havekes, L.M.; Romijn, J.A.; van Dijk, K.W.; Biermasz, N.R.; et al. Detrimental effects of constant light exposure and high-fat diet on circadian energy metabolism and insulin sensitivity. FASEB J. 2013, 27, 1721–1732. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.Q.; Ansari, T.S.; McGuinness, O.P.; Wasserman, D.H.; Johnson, C.H. Circadian disruption leads to insulin resistance and obesity. Curr. Biol. 2013, 23, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Schernhammer, E.S.; Thompson, C.A. Light at night and health: The perils of rotating shift work. Occup. Environ. Med. 2011, 68, 310–311. [Google Scholar] [CrossRef]
- Reid, K.J.; Santostasi, G.; Baron, K.G.; Wilson, J.; Kang, J.; Zee, P.C. Timing and intensity of light correlate with body weight in adults. PLoS ONE 2014, 9, e92251. [Google Scholar] [CrossRef]
- Wang, X.S.; Armstrong, M.E.; Cairns, B.J.; Key, T.J.; Travis, R.C. Shift work and chronic disease: The epidemiological evidence. Occup. Med. 2011, 61, 78–89. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, J.; Duan, P.; Liu, B.; Li, T.; Wang, C.; Li, H.; Yang, T.; Gan, Y.; Wang, X.; et al. Is shift work associated with a higher risk of overweight or obesity? A systematic review of observational studies with meta-analysis. Int. J. Epidemiol. 2018, 47, 1956–1971. [Google Scholar] [CrossRef] [PubMed]
- Brum, M.C.B.; Dantas Filho, F.F.; Schnorr, C.C.; Bertoletti, O.A.; Bottega, G.B.; da Costa Rodrigues, T. Night shift work, short sleep and obesity. Diabetol. Metab. Syndr. 2020, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.P.; Jen, H.J. BMI differences between different genders working fixed day shifts and rotating shifts: A literature review and meta-analysis. Chronobiol. Int. 2020, 37, 1754–1765. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Gan, T.; Jiang, L.; Yu, L.; Tang, D.; Wang, Y.; Li, X.; Ding, G. Association between shift work and risk of type 2 diabetes mellitus: A systematic review and dose-response meta-analysis of observational studies. Chronobiol. Int. 2020, 37, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Cheung, I.N.; Zee, P.C.; Shalman, D.; Malkani, R.G.; Kang, J.; Reid, K.J. Morning and Evening Blue-Enriched Light Exposure Alters Metabolic Function in Normal Weight Adults. PLoS ONE 2016, 11, e0155601. [Google Scholar] [CrossRef]
- Sooriyaarachchi, P.; Jayawardena, R.; Pavey, T.; King, N.A. Shift work and the risk for metabolic syndrome among healthcare workers: A systematic review and meta-analysis. Obes. Rev. 2022, 23, e13489. [Google Scholar] [CrossRef]
- Karlsson, B.H.; Knutsson, A.K.; Lindahl, B.O.; Alfredsson, L.S. Metabolic disturbances in male workers with rotating three-shift work. Results of the WOLF study. Int. Arch. Occup. Environ. Health 2003, 76, 424–430. [Google Scholar] [CrossRef]
- Ishihara, A.; Park, I.; Suzuki, Y.; Yajima, K.; Cui, H.; Yanagisawa, M.; Sano, T.; Kido, J.; Tokuyama, K. Metabolic responses to polychromatic LED and OLED light at night. Sci. Rep. 2021, 11, 12402. [Google Scholar] [CrossRef]
- Choi, Y.; Nakamura, Y.; Akazawa, N.; Park, I.; Kwak, H.B.; Tokuyama, K.; Maeda, S. Effects of nocturnal light exposure on circadian rhythm and energy metabolism in healthy adults: A randomized crossover trial. Chronobiol. Int. 2022, 39, 602–612. [Google Scholar] [CrossRef]
- Nakamura, Y.; Choi, Y.; Akazawa, N.; Park, I.; Kawana, F.; Satoh, M.; Tokuyama, K.; Maeda, S. Effect of bright-light exposure before sleep on human urine metabolome. J. Phys. Fitness Sports Med. 2019, 8, 89–96. [Google Scholar] [CrossRef]
- Lewy, A.J.; Wehr, T.A.; Goodwin, F.K.; Newsome, D.A.; Markey, S.P. Light suppresses melatonin secretion in humans. Science 1980, 210, 1267–1269. [Google Scholar] [CrossRef]
- Tapia, M.; Wulff-Zottele, C.; De Gregorio, N.; Lang, M.; Varela, H.; Josefa Serón-Ferré, M.; Vivaldi, E.A.; Araneda, O.F.; Silva-Urra, J.; Gunga, H.C.; et al. Melatonin Relations With Respiratory Quotient Weaken on Acute Exposure to High Altitude. Front. Physiol. 2018, 9, 798. [Google Scholar] [CrossRef] [PubMed]
- Hirota, N.; Sone, Y.; Tokura, H. Effect of evening exposure to dim or bright light on the digestion of carbohydrate in the supper meal. Chronobiol. Int. 2003, 20, 853–862. [Google Scholar] [CrossRef]
- Stokkan, K.A.; Yamazaki, S.; Tei, H.; Sakaki, Y.; Menaker, M. Entrainment of the circadian clock in the liver by feeding. Science 2001, 291, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Nakamura, K.; Ogata, H.; Miyashita, A.; Nagasaka, S.; Omi, N.; Yamaguchi, S.; Hibi, M.; Umeda, T.; Nakaji, S.; et al. Acute effect of late evening meal on diurnal variation of blood glucose and energy metabolism. Obes. Res. Clin. Pract. 2011, 5, e169–e266. [Google Scholar] [CrossRef] [PubMed]
- Albreiki, M.S.; Middleton, B.; Hampton, S.M. A single night light exposure acutely alters hormonal and metabolic responses in healthy participants. Endocr. Connect. 2017, 6, 100–110. [Google Scholar] [CrossRef]
- Kinsey, A.W.; Ormsbee, M.J. The health impact of nighttime eating: Old and new perspectives. Nutrients 2015, 7, 2648–2662. [Google Scholar] [CrossRef]
- Cain, S.W.; Filtness, A.J.; Phillips, C.L.; Anderson, C. Enhanced preference for high-fat foods following a simulated night shift. Scand. J. Work Environ. Health 2015, 41, 288–293. [Google Scholar] [CrossRef]
- Chen, Y.; Lauren, S.; Chang, B.P.; Shechter, A. Objective Food Intake in Night and Day Shift Workers: A Laboratory Study. Clocks Sleep 2018, 1, 42–49. [Google Scholar] [CrossRef]
- AlBreiki, M.; Middleton, B.; Ebajemito, J.; Hampton, S. The effect of light on appetite in healthy young individuals. Proc. Nutr. Soc. 2015, 74, E4. [Google Scholar] [CrossRef]
- Chang, A.M.; Aeschbach, D.; Duffy, J.F.; Czeisler, C.A. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc. Natl. Acad. Sci. USA 2015, 112, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Cajochen, C.; Münch, M.; Kobialka, S.; Kräuchi, K.; Steiner, R.; Oelhafen, P.; Orgül, S.; Wirz-Justice, A. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J. Clin. Endocrinol. Metab. 2005, 90, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Pilorz, V.; Tam, S.K.; Hughes, S.; Pothecary, C.A.; Jagannath, A.; Hankins, M.W.; Bannerman, D.M.; Lightman, S.L.; Vyazovskiy, V.V.; Nolan, P.M.; et al. Melanopsin Regulates Both Sleep-Promoting and Arousal-Promoting Responses to Light. PLoS Biol. 2016, 14, e1002482. [Google Scholar] [CrossRef]
- Bourgin, P.; Hubbard, J. Alerting or Somnogenic Light: Pick Your Color. PLoS Biol. 2016, 14, e2000111. [Google Scholar] [CrossRef]
- Figueiro, M.G.; Plitnick, B.; Rea, M.S. Light modulates leptin and ghrelin in sleep-restricted adults. Int. J. Endocrinol. 2012, 2012, 530726. [Google Scholar] [CrossRef]
- Kayaba, M.; Iwayama, K.; Ogata, H.; Seya, Y.; Kiyono, K.; Satoh, M.; Tokuyama, K. The effect of nocturnal blue light exposure from light-emitting diodes on wakefulness and energy metabolism the following morning. Environ. Health Prev. Med. 2014, 19, 354–361. [Google Scholar] [CrossRef]
- Hasenbeck, A.; Cho, S.; Meullenet, J.F.; Tokar, T.; Yang, F.; Huddleston, E.A.; Seo, H.S. Color and illuminance level of lighting can modulate willingness to eat bell peppers. J. Sci. Food Agric. 2014, 94, 2049–2056. [Google Scholar] [CrossRef]
- Suk, H.; Park, G.; Kim, Y. Bon Appétit! An Investigation About the Best and Worst Color Combinations of Lighting and Food. J. Lit. Art Stud. 2012, 2, 559–566. [Google Scholar]
- Wansink, B.; Chandon, P. Slim by design: Redirecting the accidental drivers of mindless overeating. J. Consum. Psychol. 2014, 24, 413–431. [Google Scholar] [CrossRef]
- Biswas, D.; Szocs, C.; Wansink, B.; Chacko, R. Shining Light on Atmospherics: How Ambient Light Influlences Food Choices. J. Mark. Res. 2017, 54, 111–123. [Google Scholar]
- Cho, S.; Han, A.; Taylor, M.H.; Huck, A.C.; Mishler, A.M.; Mattal, K.L.; Barker, C.A.; Seo, H.S. Blue lighting decreases the amount of food consumed in men, but not in women. Appetite 2015, 85, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Nagare, R.; Plitnick, B.; Figueiro, M.G. Does the iPad Night Shift mode reduce melatonin suppression? Light. Res. Technol. 2019, 51, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Driller, M.W.; Jacobson, G.; Uiga, L. Hunger hormone and sleep responses to the built-in blue-light filter on an electronic device: A pilot study. Sleep Sci. 2019, 12, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.; Parikh, R.; Michael, K.; Bikovski, L.; Barnabas, G.; Mardamshina, M.; Hemi, R.; Manich, P.; Goldstein, N.; Malcov-Brog, H.; et al. Food-seeking behavior is triggered by skin ultraviolet exposure in males. Nat. Metab. 2022, 4, 883–900. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Ding, X.; Hong, C.; Pang, Y.; Chen, L.; Liu, Q.; Zhang, X.; Xin, H.; Wang, X. Several biological benefits of the low color temperature light-emitting diodes based normal indoor lighting source. Sci. Rep. 2019, 9, 7560. [Google Scholar] [CrossRef]
- Arguelles-Prieto, R.; Madrid, J.A.; Rol, M.A.; Bonmati-Carrion, M.A. Correlated color temperature and light intensity: Complementary features in non-visual light field. PLoS ONE 2021, 16, e0254171. [Google Scholar] [CrossRef]
- Yasukouchi, A.; Yasukouchi, Y.; Ishibashi, K. Effects of color temperature of fluorescent lamps on body temperature regulation in a moderately cold environment. J. Physiol. Anthropol. Appl. Hum. Sci. 2000, 19, 125–134. [Google Scholar] [CrossRef]
- te Kulve, M.; Schlangen, L.; Lichtenbelt, W.V. Interactions between the perception of light and temperature. Indoor Air 2018, 28, 881–891. [Google Scholar] [CrossRef]
- Huebner, G.M.; Shipworth, D.T.; Gauthier, S.; Witzel, C.; Raynham, P.; Chan, W. Saving energy with light? Experimental studies assessing the impact of colour temperature on thermal comfort. Energy Res. Soc. Sci. 2016, 15, 45–57. [Google Scholar] [CrossRef]
- Omidvar, A.; Brambilla, A. A novel theoretical method for predicting the effects of lighting colour temperature on physiological responses and indoor thermal perception. Build. Environ. 2021, 203, e108062. [Google Scholar] [CrossRef]
- Dewan, K.; Benloucif, S.; Reid, K.; Wolfe, L.F.; Zee, P.C. Light-induced changes of the circadian clock of humans: Increasing duration is more effective than increasing light intensity. Sleep 2011, 34, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Peuhkuri, K.; Sihvola, N.; Korpela, R. Diet promotes sleep duration and quality. Nutr. Res. 2012, 32, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Burgess, H.J.; Fogg, L.F. Individual differences in the amount and timing of salivary melatonin secretion. PLoS ONE 2008, 3, e3055. [Google Scholar] [CrossRef] [PubMed]
- Arendt, J. Melatonin and the pineal gland: Influence on mammalian seasonal and circadian physiology. Rev. Reprod. 1998, 3, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Kvetnoy, I.; Ivanov, D.; Mironova, E.; Evsyukova, I.; Nasyrov, R.; Kvetnaia, T.; Polyakova, V. Melatonin as the Cornerstone of Neuroimmunoendocrinology. Int. J. Mol. Sci. 2022, 23, 1835. [Google Scholar] [CrossRef]
- Cipolla-Neto, J.; Amaral, F.G.; Afeche, S.C.; Tan, D.X.; Reiter, R.J. Melatonin, energy metabolism, and obesity: A review. J. Pineal. Res. 2014, 56, 371–381. [Google Scholar] [CrossRef]
- Cipolla-Neto, J.; Amaral, F.G.D. Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr. Rev. 2018, 39, 990–1028. [Google Scholar] [CrossRef]
- Milcu, I.; Nanu, L.; Marcean, R.; Sitaru, S. The Action of Pineal Extract and Epiphysectomy on Hepatic and Muscular Glycogen after Prolonged Infusion of Glucose. Stud. Cercet. Endocrinol. 1963, 14, 651–655. [Google Scholar]
- Peschke, E.; Bähr, I.; Mühlbauer, E. Melatonin and pancreatic islets: Interrelationships between melatonin, insulin and glucagon. Int. J. Mol. Sci. 2013, 14, 6981–7015. [Google Scholar] [CrossRef]
- Tutuncu, N.B.; Batur, M.K.; Yildirir, A.; Tutuncu, T.; Deger, A.; Koray, Z.; Erbas, B.; Kabakci, G.; Aksoyek, S.; Erbas, T. Melatonin levels decrease in type 2 diabetic patients with cardiac autonomic neuropathy. J. Pineal. Res. 2005, 39, 43–49. [Google Scholar] [CrossRef]
- Mäntele, S.; Otway, D.T.; Middleton, B.; Bretschneider, S.; Wright, J.; Robertson, M.D.; Skene, D.J.; Johnston, J.D. Daily rhythms of plasma melatonin, but not plasma leptin or leptin mRNA, vary between lean, obese and type 2 diabetic men. PLoS ONE 2012, 7, e37123. [Google Scholar] [CrossRef] [PubMed]
- Robeva, R.; Kirilov, G.; Tomova, A.; Kumanov, P. Melatonin-insulin interactions in patients with metabolic syndrome. J. Pineal. Res. 2008, 44, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Koziróg, M.; Poliwczak, A.R.; Duchnowicz, P.; Koter-Michalak, M.; Sikora, J.; Broncel, M. Melatonin treatment improves blood pressure, lipid profile, and parameters of oxidative stress in patients with metabolic syndrome. J. Pineal. Res. 2011, 50, 261–266. [Google Scholar] [CrossRef]
- Wakatsuki, A.; Okatani, Y.; Ikenoue, N.; Kaneda, C.; Fukaya, T. Effects of short-term melatonin administration on lipoprotein metabolism in normolipidemic postmenopausal women. Maturitas 2001, 38, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Nachtigal, M.C.; Patterson, R.E.; Stratton, K.L.; Adams, L.A.; Shattuck, A.L.; White, E. Dietary supplements and weight control in a middle-age population. J. Altern. Complement. Med. 2005, 11, 909–915. [Google Scholar] [CrossRef]
- Garfinkel, D.; Zorin, M.; Wainstein, J.; Matas, Z.; Laudon, M.; Zisapel, N. Efficacy and safety of prolonged-release melatonin in insomnia patients with diabetes: A randomized, double-blind, crossover study. Diabetes Metab. Syndr. Obes. 2011, 4, 307–313. [Google Scholar] [CrossRef]
- Marqueze, E.C.; Nogueira, L.F.R.; Vetter, C.; Skene, D.J.; Cipolla-Neto, J.; Moreno, C.R.C. Exogenous melatonin decreases circadian misalignment and body weight among early types. J. Pineal. Res. 2021, 71, e12750. [Google Scholar] [CrossRef]
- Folkard, S.; Arendt, J.; Clark, M. Can melatonin improve shift workers’ tolerance of the night shift? Some preliminary findings. Chronobiol. Int. 1993, 10, 315–320. [Google Scholar] [CrossRef]
- Sack, R.L.; Lewy, A.J. Melatonin as a chronobiotic: Treatment of circadian desynchrony in night workers and the blind. J. Biol. Rhythms. 1997, 12, 595–603. [Google Scholar] [CrossRef]
- Nogueira, L.F.R.; Crispim, C.A.; Cipolla-Neto, J.; de Castro Moreno, C.R.; Marqueze, E.C. The Effect of Exogenous Melatonin on Eating Habits of Female Night Workers with Excessive Weight. Nutrients 2022, 14, 3420. [Google Scholar] [CrossRef]
- Albreiki, M.S.; Shamlan, G.H.; BaHammam, A.S.; Alruwaili, N.W.; Middleton, B.; Hampton, S.M. Acute impact of light at night and exogenous melatonin on subjective appetite and plasma leptin. Front. Nutr. 2022, 9, 1079453. [Google Scholar] [CrossRef] [PubMed]
- Albreiki, M.S.; Middleton, B.; Hampton, S.M. The effect of melatonin on glucose tolerance, insulin sensitivity and lipid profiles after a late evening meal in healthy young males. J. Pineal. Res. 2021, 71, e12770. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Sastre, P.; Scheer, F.A.; Gómez-Abellán, P.; Madrid, J.A.; Garaulet, M. Acute melatonin administration in humans impairs glucose tolerance in both the morning and evening. Sleep 2014, 37, 1715–1719. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, A.; Ohta, Y.; Ohashi, K. Melatonin improves metabolic syndrome induced by high fructose intake in rats. J. Pineal. Res. 2012, 52, 403–413. [Google Scholar] [CrossRef]
- Agil, A.; Rosado, I.; Ruiz, R.; Figueroa, A.; Zen, N.; Fernández-Vázquez, G. Melatonin improves glucose homeostasis in young Zucker diabetic fatty rats. J. Pineal. Res. 2012, 52, 203–210. [Google Scholar] [CrossRef]
- Balzer, I.; Hardeland, R. Photoperiodism and effects of indoleamines in a unicellular alga, Gonyaulax polyedra. Science 1991, 253, 795–797. [Google Scholar] [CrossRef]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J. Pineal. Res. 1995, 18, 28–31. [Google Scholar] [CrossRef]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar]
- Paredes, S.D.; Korkmaz, A.; Manchester, L.C.; Tan, D.X.; Reiter, R.J. Phytomelatonin: A review. J. Exp. Bot. 2009, 60, 57–69. [Google Scholar] [CrossRef]
- Navarro-Alarcón, M.; Ruiz-Ojeda, F.J.; Blanca-Herrera, R.M.; MM, A.S.; Acuña-Castroviejo, D.; Fernández-Vázquez, G.; Agil, A. Melatonin and metabolic regulation: A review. Food Funct. 2014, 5, 2806–2832. [Google Scholar] [CrossRef]
- Meng, X.; Li, Y.; Li, S.; Zhou, Y.; Gan, R.Y.; Xu, D.P.; Li, H.B. Dietary Sources and Bioactivities of Melatonin. Nutrients 2017, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.N.; Turi, C.E.; Shipley, P.R.; Murch, S.J. Comparisons of large (Vaccinium macrocarpon Ait.) and small (Vaccinium oxycoccos L., Vaccinium vitis-idaea L.) cranberry in British Columbia by phytochemical determination, antioxidant potential, and metabolomic profiling with chemometric analysis. Planta Med. 2012, 78, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, A.; Giridhar, P.; Sankar, K.U.; Ravishankar, G.A. Melatonin and serotonin profiles in beans of Coffea species. J. Pineal. Res. 2012, 52, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Murch, S.J.; Simmons, C.B.; Saxena, P.K. Melatonin in feverfew and other medicinal plants. Lancet 1997, 350, 1598–1599. [Google Scholar] [CrossRef]
- Tan, D.X.; Zanghi, B.M.; Manchester, L.C.; Reiter, R.J. Melatonin identified in meats and other food stuffs: Potentially nutritional impact. J. Pineal. Res. 2014, 57, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.; Naufel, M.F.; Ribeiro, E.B.; Tufik, S.; Hachul, H. Influence of Dietary Sources of Melatonin on Sleep Quality: A Review. J. Food Sci. 2020, 85, 5–13. [Google Scholar] [CrossRef]
- Kelley, D.S.; Adkins, Y.; Laugero, K.D. A Review of the Health Benefits of Cherries. Nutrients 2018, 10, 368. [Google Scholar] [CrossRef]
- Howatson, G.; Bell, P.G.; Tallent, J.; Middleton, B.; McHugh, M.P.; Ellis, J. Effect of tart cherry juice (Prunus cerasus) on melatonin levels and enhanced sleep quality. Eur. J. Nutr. 2012, 51, 909–916. [Google Scholar] [CrossRef]
- Pigeon, W.R.; Carr, M.; Gorman, C.; Perlis, M.L. Effects of a tart cherry juice beverage on the sleep of older adults with insomnia: A pilot study. J. Med. Food 2010, 13, 579–583. [Google Scholar] [CrossRef]
- Losso, J.N.; Finley, J.W.; Karki, N.; Liu, A.G.; Prudente, A.; Tipton, R.; Yu, Y.; Greenway, F.L. Pilot Study of the Tart Cherry Juice for the Treatment of Insomnia and Investigation of Mechanisms. Am. J. Ther. 2018, 25, e194–e201. [Google Scholar] [CrossRef]
- Garrido, M.; González-Gómez, D.; Lozano, M.; Barriga, C.; Paredes, S.D.; Rodríguez, A.B. A Jerte valley cherry product provides beneficial effects on sleep quality. Influence on aging. J. Nutr. Health Aging 2013, 17, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.; Paredes, S.D.; Cubero, J.; Lozano, M.; Toribio-Delgado, A.F.; Muñoz, J.L.; Reiter, R.J.; Barriga, C.; Rodriguez, A.B. Jerte Valley cherry-enriched diets improve nocturnal rest and increase 6-sulfatoxymelatonin and total antioxidant capacity in the urine of middle-aged and elderly humans. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 909–914. [Google Scholar] [CrossRef]
- Sae-Teaw, M.; Johns, J.; Johns, N.P.; Subongkot, S. Serum melatonin levels and antioxidant capacities after consumption of pineapple, orange, or banana by healthy male volunteers. J. Pineal. Res. 2013, 55, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Valtonen, M.; Niskanen, L.; Kangas, A.P.; Koskinen, T. Effect of melatonin-rich night-time milk on sleep and activity in elderly institutionalized subjects. Nord. J. Psychiatry 2005, 59, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Jeong, J.; Jeon, H.; Bang, Y.; Yoon, I. Effects of melatonin-rich milk on mild insomnia symptoms. Korean Soc. Sleep Med. 2016, 7, 60–67. [Google Scholar] [CrossRef]
- Sekartini, R.; Chandra, D.N.; Arsianti, T.; Bardosono, S.; Wiguna, T.; Schaafsma, A. An evening milk drink can affect word recall in Indonesian children with decreased sleep efficiency: A randomized controlled trial. Nutr. Neurosci. 2018, 21, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Takada, M.; Nishida, K.; Gondo, Y.; Kikuchi-Hayakawa, H.; Ishikawa, H.; Suda, K.; Kawai, M.; Hoshi, R.; Kuwano, Y.; Miyazaki, K.; et al. Beneficial effects of Lactobacillus casei strain Shirota on academic stress-induced sleep disturbance in healthy adults: A double-blind, randomised, placebo-controlled trial. Benef. Microbes 2017, 8, 153–162. [Google Scholar] [CrossRef] [PubMed]
- dela Peña, I.J.; Hong, E.; de la Peña, J.B.; Kim, H.J.; Botanas, C.J.; Hong, Y.S.; Hwang, Y.S.; Moon, B.S.; Cheong, J.H. Milk Collected at Night Induces Sedative and Anxiolytic-Like Effects and Augments Pentobarbital-Induced Sleeping Behavior in Mice. J. Med. Food 2015, 18, 1255–1261. [Google Scholar] [CrossRef]
- Yoneyama, S.; Sakurai, M.; Nakamura, K.; Morikawa, Y.; Miura, K.; Nakashima, M.; Yoshita, K.; Ishizaki, M.; Kido, T.; Naruse, Y.; et al. Associations between rice, noodle, and bread intake and sleep quality in Japanese men and women. PLoS ONE 2014, 9, e105198. [Google Scholar] [CrossRef] [PubMed]
- Nisar, M.; Mohammad, R.M.; Arshad, A.; Hashmi, I.; Yousuf, S.M.; Baig, S. Influence of Dietary Intake on Sleeping Patterns of Medical Students. Cureus 2019, 11, e4106. [Google Scholar] [CrossRef]
- Bravo, R.; Matito, S.; Cubero, J.; Paredes, S.D.; Franco, L.; Rivero, M.; Rodríguez, A.B.; Barriga, C. Tryptophan-enriched cereal intake improves nocturnal sleep, melatonin, serotonin, and total antioxidant capacity levels and mood in elderly humans. Age 2013, 35, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Helmert, D.; Spinweber, C.L. Evaluation of L-tryptophan for treatment of insomnia: A review. Psychopharmacology 1986, 89, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hudson, C.; Hudson, S.P.; Hecht, T.; MacKenzie, J. Protein source tryptophan versus pharmaceutical grade tryptophan as an efficacious treatment for chronic insomnia. Nutr. Neurosci. 2005, 8, 121–127. [Google Scholar] [CrossRef]
- Fukushige, H.; Fukuda, Y.; Tanaka, M.; Inami, K.; Wada, K.; Tsumura, Y.; Kondo, M.; Harada, T.; Wakamura, T.; Morita, T. Effects of tryptophan-rich breakfast and light exposure during the daytime on melatonin secretion at night. J. Physiol. Anthropol. 2014, 33, 33. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Yata, S.; Akimitsu, O.; Krejci, M.; Noji, T.; Nakade, M.; Takeuchi, H.; Harada, T. A tryptophan-rich breakfast and exposure to light with low color temperature at night improve sleep and salivary melatonin level in Japanese students. J. Circadian Rhythms. 2013, 11, 4. [Google Scholar] [CrossRef]
- Wang, W.; Liu, L.; Tian, Z.; Han, T.; Sun, C.; Li, Y. Dietary Tryptophan and the Risk of Metabolic Syndrome: Total Effect and Mediation Effect of Sleep Duration. Nat. Sci. Sleep 2021, 13, 2141–2151. [Google Scholar] [CrossRef] [PubMed]
- Karasek, M. Melatonin, human aging, and age-related diseases. Exp. Gerontol. 2004, 39, 1723–1729. [Google Scholar] [CrossRef]
- Cain, S.W.; Dennison, C.F.; Zeitzer, J.M.; Guzik, A.M.; Khalsa, S.B.; Santhi, N.; Schoen, M.W.; Czeisler, C.A.; Duffy, J.F. Sex differences in phase angle of entrainment and melatonin amplitude in humans. J. Biol. Rhythms. 2010, 25, 288–296. [Google Scholar] [CrossRef]
- Santhi, N.; Lazar, A.S.; McCabe, P.J.; Lo, J.C.; Groeger, J.A.; Dijk, D.J. Sex differences in the circadian regulation of sleep and waking cognition in humans. Proc. Natl. Acad. Sci. USA 2016, 113, E2730–E2739. [Google Scholar] [CrossRef]
- Chellappa, S.L.; Steiner, R.; Oelhafen, P.; Cajochen, C. Sex differences in light sensitivity impact on brightness perception, vigilant attention and sleep in humans. Sci. Rep. 2017, 7, 14215. [Google Scholar] [CrossRef]
- Lockley, S.W.; Arendt, J.; Skene, D.J. Visual impairment and circadian rhythm disorders. Dialogues Clin. Neurosci. 2007, 9, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Quera Salva, M.A.; Hartley, S.; Léger, D.; Dauvilliers, Y.A. Non-24-Hour Sleep-Wake Rhythm Disorder in the Totally Blind: Diagnosis and Management. Front. Neurol. 2017, 8, 686. [Google Scholar] [CrossRef] [PubMed]
- Lewy, A.J.; Emens, J.S.; Lefler, B.J.; Yuhas, K.; Jackman, A.R. Melatonin entrains free-running blind people according to a physiological dose-response curve. Chronobiol. Int. 2005, 22, 1093–1106. [Google Scholar] [CrossRef] [PubMed]
- Aubin, S.; Kupers, R.; Ptito, M.; Jennum, P. Melatonin and cortisol profiles in the absence of light perception. Behav. Brain Res. 2017, 317, 515–521. [Google Scholar] [CrossRef]
- Yang, F.; Yang, C.; Liu, Y.; Peng, S.; Liu, B.; Gao, X.; Tan, X. Associations between Body Mass Index and Visual Impairment of School Students in Central China. Int. J. Environ. Res. Public Health 2016, 13, 1024. [Google Scholar] [CrossRef]
- Minella, C.; Coliat, P.; Amé, S.; Neuberger, K.; Stora, A.; Mathelin, C.; Reix, N. Protective role of melatonin in breast cancer: What we can learn from women with blindness. Cancer Causes Control 2022, 33, 1–13. [Google Scholar] [CrossRef]
- Grivas, T.B.; Savvidou, O.D. Melatonin the “light of night” in human biology and adolescent idiopathic scoliosis. Scoliosis 2007, 2, 6. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishihara, A.; Courville, A.B.; Chen, K.Y. The Complex Effects of Light on Metabolism in Humans. Nutrients 2023, 15, 1391. https://doi.org/10.3390/nu15061391
Ishihara A, Courville AB, Chen KY. The Complex Effects of Light on Metabolism in Humans. Nutrients. 2023; 15(6):1391. https://doi.org/10.3390/nu15061391
Chicago/Turabian StyleIshihara, Asuka, Amber B. Courville, and Kong Y. Chen. 2023. "The Complex Effects of Light on Metabolism in Humans" Nutrients 15, no. 6: 1391. https://doi.org/10.3390/nu15061391
APA StyleIshihara, A., Courville, A. B., & Chen, K. Y. (2023). The Complex Effects of Light on Metabolism in Humans. Nutrients, 15(6), 1391. https://doi.org/10.3390/nu15061391





