Effects of Concentrated Long-Chain Omega-3 Polyunsaturated Fatty Acid Supplementation on Quality of Life after Radical Prostatectomy: A Phase II Randomized Placebo-Controlled Trial (RCT-EPA)
Abstract
1. Introduction
2. Materials and Methods
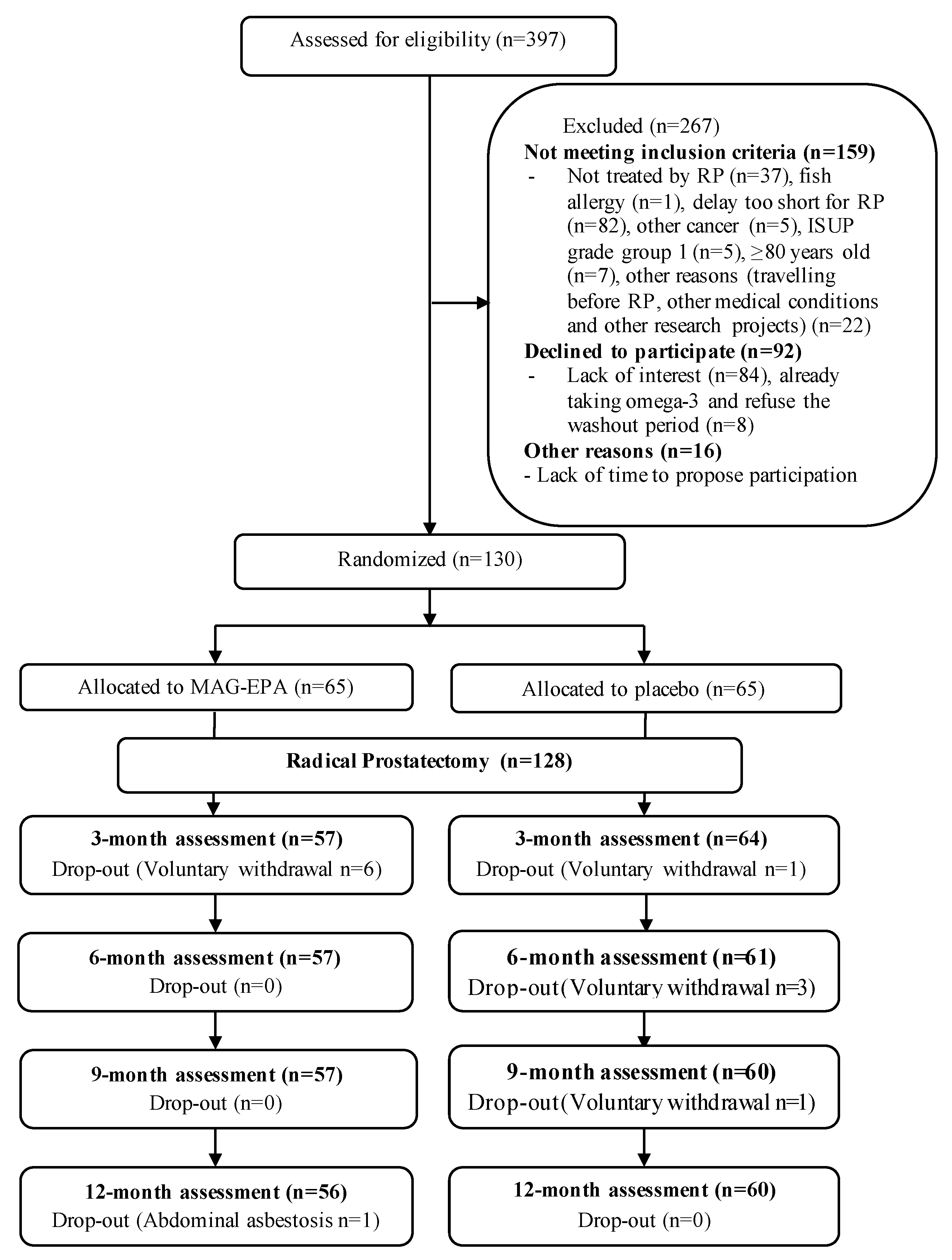
2.1. Study Population and Design
2.2. Intervention
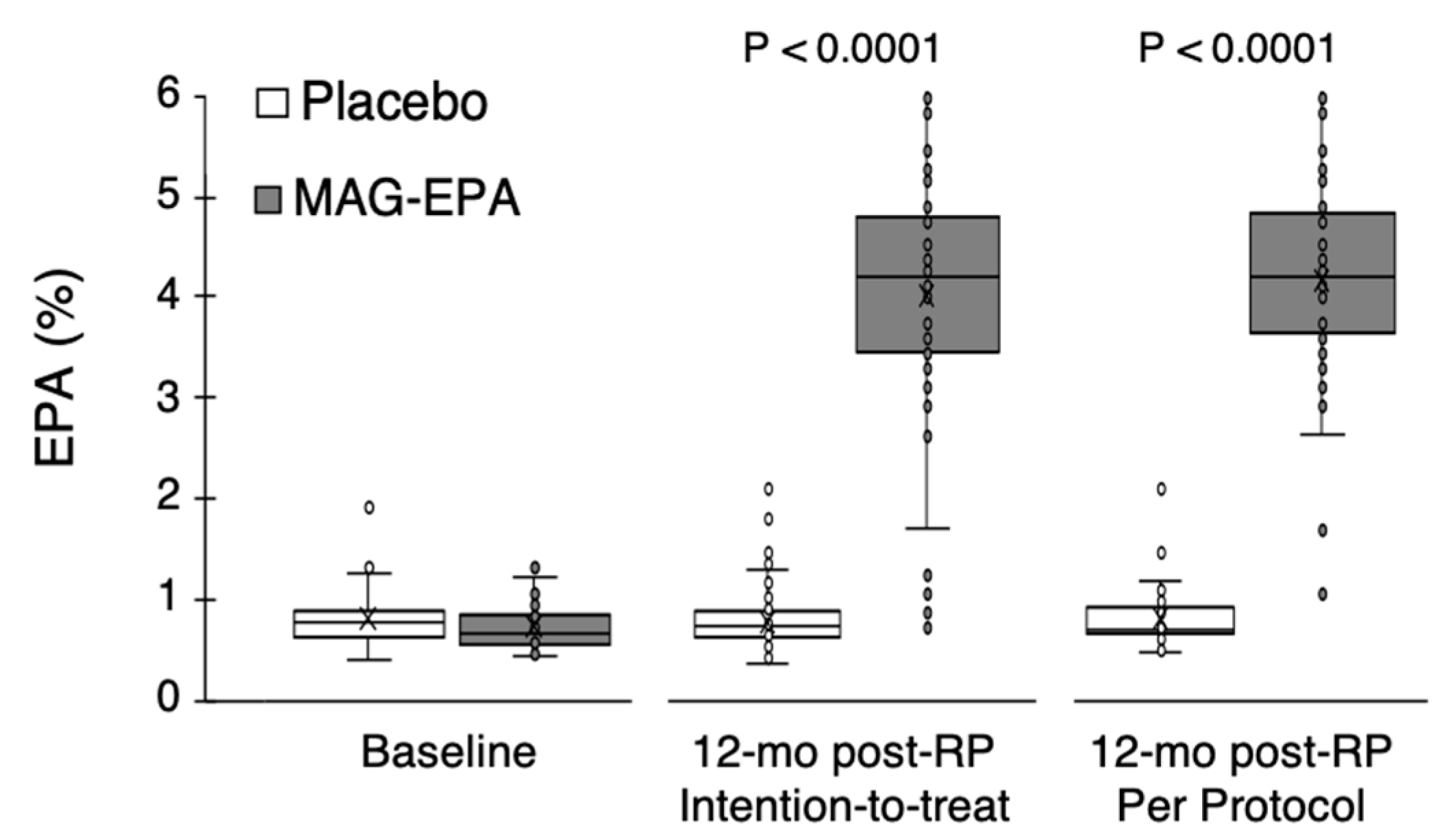
2.3. Fatty Acid Profiles
2.4. Prostate-Specific Quality of Life
2.5. Potential Confounders
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fradet, Y.; Klotz, L.; Trachtenberg, J.; Zlotta, A. The burden of prostate cancer in Canada. Can. Urol. Assoc. J.=J. L’association Urol. Can. 2009, 3, S92–S100. [Google Scholar] [CrossRef]
- Statistiques sur le Cancer de la Prostate—Société Canadienne du Cancer. Available online: https://cancer.ca/fr/cancer-information/cancer-types/prostate/statistics (accessed on 9 February 2023).
- Grover, S.A.; Coupal, L.; Zowall, H. The economic burden of prostate cancer in Canada: Forecasts from the Montreal Prostate Cancer Model. CMAJ Can. Med. Assoc. J.=J. L’association Med. Can. 2000, 162, 987–992. [Google Scholar]
- Donovan, J.L.; Hamdy, F.C.; Lane, J.A. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N. Engl. J. Med. 2016, 375, 1425–1437. [Google Scholar] [CrossRef] [PubMed]
- Fossa, S.D.; Bengtsson, T.; Borre, M. Reduction of quality of life in prostate cancer patients: Experience among 6200 men in the Nordic countries. Scand. J. Urol. 2016, 50, 330–337. [Google Scholar] [CrossRef]
- Statistiques de Survie Pour le Cancer de la Prostate—Société Canadienne du Cancer. Available online: https://www.cancer.ca/fr-ca/cancer-information/cancer-type/prostate/prognosis-and-survival/survival-statistics/?region=qc (accessed on 31 August 2021).
- Lin, P.H.; Aronson, W.; Freedland, S.J. Nutrition, dietary interventions and prostate cancer: The latest evidence. BMC Med. 2015, 13, 3. [Google Scholar] [CrossRef]
- Caughey, G.E.; Mantzioris, E.; Gibson, R.A. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am. J. Clin. Nutr. 1996, 63, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Kiecolt-Glaser, J.K.; Belury, M.A.; Andridge, R. Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: A randomized controlled trial. Brain Behav. Immun. 2012, 26, 988–995. [Google Scholar] [CrossRef]
- Muralidharan, J.; Papandreou, C.; Sala-Vila, A. Fatty Acids Composition of Blood Cell Membranes and Peripheral Inflammation in the PREDIMED Study: A Cross-Sectional Analysis. Nutrients 2019, 11, 576. [Google Scholar] [CrossRef] [PubMed]
- Haghiac, M.; Yang, X.H.; Presley, L. Dietary Omega-3 Fatty Acid Supplementation Reduces Inflammation in Obese Pregnant Women: A Randomized Double-Blind Controlled Clinical Trial. PLoS ONE 2015, 10, e0137309. [Google Scholar] [CrossRef]
- Esposito, K.; Giugliano, F.; Di Palo, C. Effect of lifestyle changes on erectile dysfunction in obese men: A randomized controlled trial. JAMA 2004, 291, 2978–2984. [Google Scholar] [CrossRef]
- Yafi, F.A.; Jenkins, L.; Albersen, M. Erectile dysfunction. Nat. Rev. Dis. Primers 2016, 2, 16003. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Vincent, A.; Taylor, A.W. Lower Urinary Tract Symptoms, Depression, Anxiety and Systemic Inflammatory Factors in Men: A Population-Based Cohort Study. PLoS ONE 2015, 10, e0137903. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wang, Z.; Liu, G. Metabolic syndrome, inflammation and lower urinary tract symptoms: Possible translational links. Prostate Cancer Prostatic Dis. 2016, 19, 7–13. [Google Scholar] [CrossRef]
- Hung, S.F.; Chung, S.D.; Kuo, H.C. Increased serum C-reactive protein level is associated with increased storage lower urinary tract symptoms in men with benign prostatic hyperplasia. PLoS ONE 2014, 9, e85588. [Google Scholar] [CrossRef]
- Hsiao, S.-M.; Lin, H.-H.; Kuo, H.-C. The role of serum C-reactive protein in women with lower urinary tract symptoms. Int. Urogynecol. J. 2012, 23, 935–940. [Google Scholar] [CrossRef]
- Bauer, S.R.; Van Blarigan, E.L.; Stampfer, M.J. Mediterranean diet after prostate cancer diagnosis and urinary and sexual functioning: The health professionals follow-up study. Prostate 2018, 78, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, W.; Zhou, L. Relationship among diet habit and lower urinary tract symptoms and sexual function in outpatient-based males with LUTS/BPH: A multiregional and cross-sectional study in China. BMJ Open 2016, 6, e010863. [Google Scholar] [CrossRef]
- Esposito, K.; Ciotola, M.; Giugliano, F. Mediterranean diet improves erectile function in subjects with the metabolic syndrome. Int. J. Impot. Res. 2006, 18, 405–410. [Google Scholar] [CrossRef]
- Suzuki, S.; Platz, E.A.; Kawachi, I. Intakes of energy and macronutrients and the risk of benign prostatic hyperplasia. Am. J. Clin. Nutr. 2002, 75, 689–697. [Google Scholar] [CrossRef]
- Epstein, J.I.; Egevad, L.; Amin, M.B. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Guertin, M.H.; Robitaille, K.; Pelletier, J.F. Effects of concentrated long-chain omega-3 polyunsaturated fatty acid supplementation before radical prostatectomy on prostate cancer proliferation, inflammation, and quality of life: Study protocol for a phase IIb, randomized, double-blind, placebo-controlled trial. BMC Cancer 2018, 18, 64. [Google Scholar]
- Vrijens, B.; De Geest, S.; Hughes, D.A. A new taxonomy for describing and defining adherence to medications. Br. J. Clin. Pharmacol. 2012, 73, 691–705. [Google Scholar] [CrossRef]
- Pot, G.K.; Brouwer, I.A.; Enneman, A. No effect of fish oil supplementation on serum inflammatory markers and their interrelationships: A randomized controlled trial in healthy, middle-aged individuals. Eur. J. Clin. Nutr. 2009, 63, 1353–1359. [Google Scholar] [CrossRef]
- De Roos, B.; Geelen, A.; Ross, K. Identification of potential serum biomarkers of inflammation and lipid modulation that are altered by fish oil supplementation in healthy volunteers. Proteomics 2008, 8, 1965–1974. [Google Scholar] [CrossRef] [PubMed]
- Ottestad, I.; Vogt, G.; Retterstol, K. Oxidised fish oil does not influence established markers of oxidative stress in healthy human subjects: A randomised controlled trial. Br. J. Nutr. 2012, 108, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Gevariya, N.; Besançon, M.; Robitaille, K. Omega-3 fatty acids decrease prostate cancer progression associated with an anti-tumor immune response in eugonadal and castrated mice. Prostate 2019, 79, 9–20. [Google Scholar] [CrossRef]
- Szymanski, K.M.; Wei, J.T.; Dunn, R.L. Development and validation of an abbreviated version of the expanded prostate cancer index composite instrument for measuring health-related quality of life among prostate cancer survivors. Urology 2010, 76, 1245–1250. [Google Scholar] [CrossRef]
- Vigneault, E.; Savard, J.; Ivers, H. Validation of a French-Canadian Version of the Expanded Prostate Cancer Index Composite Instrument. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, E531–E532. [Google Scholar] [CrossRef]
- Galán, M.L.; Borda, A.P.; Osés, E.M.; González, L.L.; Sánchez, A.B. The validity of the IPSS questionnaire in a sample of 262 patients with benign prostatichyperplasia. Arch. Esp. Urol. 1997, 50, 847–853. [Google Scholar]
- Axcrona, K.; Nilsson, R.; Brennhovd, B.; Sørebø, Ø.; Fosså, S.D.; Dahl, A.A. Psychometric properties of the expanded prostate cancer index composite—26 instrument in a cohort of radical prostatectomy patients: Theoretical and practical examinations. BMC Urol. 2017, 17, 111. [Google Scholar] [CrossRef]
- Labonté, M.È.; Cyr, A.; Baril-Gravel, L. Validity and reproducibility of a web-based, self-administered food frequency questionnaire. Eur. J. Clin. Nutr. 2012, 66, 166–173. [Google Scholar] [CrossRef]
- Skolarus, T.A.; Dunn, R.L.; Sanda, M.G. Minimally important difference for the Expanded Prostate Cancer Index Composite Short Form. Urology 2015, 85, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.L.; Zhou, Z.; Yang, Y. Efficacy and Safety of Medium-to-long-term Use of Tolterodine Extended Release with or without Tamsulosin in Patients with Benign Prostate Hyperplasia and Larger Prostate Size: A Double-blind, Placebo-controlled, Randomized Clinical Trial. Chin. Med. J. 2016, 129, 2899–2906. [Google Scholar] [CrossRef]
- Marzorati, C.; Monzani, D.; Mazzocco, K. Predicting trajectories of recovery in prostate cancer patients undergone Robot-Assisted Radical Prostatectomy (RARP). PLoS ONE. 2019, 14, e0214682. [Google Scholar] [CrossRef] [PubMed]
- Yonguc, T.; Sefik, E.; Inci, I. Randomized, controlled trial of fesoterodine fumarate for overactive bladder in Parkinson’s disease. World J. Urol. 2020, 38, 2013–2019. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed]
| Variables | MAG-EPA (n = 65) | Placebo (n = 65) | p-Value |
|---|---|---|---|
| Socio-demographic characteristics | |||
| Age (years) | 0.13 1 | ||
| Mean (SD) | 64.34 (6.31) | 62.50 (7.36) | |
| Median (Q1–Q3) | 65.0 (60.0–70.0) | 64.0 (58.0–67.0) | |
| BMI (Kg/m2), n (%) | 0.06 3 | ||
| <25 | 7 (10.77) | 16 (24.62) | |
| 25–29 | 34 (52.31) | 32 (49.23) | |
| >= 30 | 23 (35.38) | 15 (23.08) | |
| Missing | 1 (1.54) | 2 (3.08) | |
| Smoking status n (%) | 0.30 3 | ||
| Current | 4 (6.15) | 8 (12.31) | |
| Former | 34 (52.31) | 27 (41.54) | |
| Never | 26 (40.00) | 30 (46.15) | |
| Missing | 1 (1.54) | 0 (0) | |
| Education, n (%) | 0.42 3 | ||
| Secondary school or less | 24 (36.92) | 19 (29.23) | |
| Postsecondary diploma | 18 (27.69) | 25 (38.46) | |
| University degree | 21 (32.31) | 20 (30.77) | |
| Missing | 2 (3.08) | 1 (1.54) | |
| Physical activity n (%) | 0.74 3 | ||
| Active | 24 (36.92) | 27 (41.54) | |
| Inactive | 37 (56.92) | 37 (56.92) | |
| Missing | 4 (6.15) | 1 (1.54) | |
| Marital status n (%) | 0.84 3 | ||
| Married or common-law | 54 (83.08) | 54 (83.08) | |
| Single or not married | 10 (15.38) | 11 (16.92) | |
| Missing | 1 (1.54) | 0 (0) | |
| Medical characteristics | |||
| PSA (ng/mL) | 0.19 2 | ||
| Mean (SD) | 8.74 (9.33) | 6.64 (5.53) | |
| Median (Q1–Q3) | 6.00 (4.40–8.70) | 5.70 (4.00–7.00) | |
| Grade group n (%) | 0.03 3 | ||
| 2 (3 + 4) | 31 (47.69) | 41 (63.08) | |
| 3 (4 + 3) | 17 (26.15) | 18 (27.69) | |
| >=4 (8 and 9) | 17 (26.15) | 6 (9.23) | |
| Cancer Stage n (%) | 0.17 3 | ||
| T2a or less | 52 (80.00) | 59 (90.77) | |
| T2b or T2c | 4 (6.15) | 3 (4.62) | |
| T3 or more | 9 (13.85) | 3 (4.62) | |
| NCCN risk, n (%) | 0.04 3 | ||
| Intermediate risk (2) | 45 (69.23) | 55 (84.62) | |
| High risk (3) | 20 (30.77) | 10 (15.38) | |
| Comorbidity index n (%) | 0.62 3 | ||
| 0 | 41 (63.08) | 39 (60.00) | |
| 1 | 10 (15.38) | 15 (23.08) | |
| ≥2 | 10 (15.38) | 11 (16.92) | |
| Missing | 4 (6.15) | 0 (0) | |
| RBC fatty acid profile (%) * | |||
| Total n3 | 0.98 1 | ||
| Mean (SD) | 7.40 (1.17) | 7.40 (1.02) | |
| Median (Q1–Q3) | 7.25 (6.52–8.09) | 7.32 (6.74–7.96) | |
| LCn3 | 0.94 1 | ||
| Mean (SD) | 7.10 (1.16) | 7.11 (1.02) | |
| Median (Q1–Q3) | 6.90 (6.25–7.75) | 7.05 (6.50–7.66) | |
| EPA | 0.03 2 | ||
| Mean (SD) | 0.73 (0.23) | 0.80 (0.25) | |
| Median (Q1–Q3) | 0.65 (0.55–0.82) | 0.77 (0.62–0.89) | |
| DHA | 0.60 1 | ||
| Mean (SD) | 3.92 (0.96) | 3.83 (0.82) | |
| Median (Q1–Q3) | 3.79 (3.25–4.61) | 3.73 (3.34–4.48) | |
| Total n6 | 0.49 1 | ||
| Mean (SD) | 26.44 (1.48) | 26.61 (1.39) | |
| Median (Q1–Q3) | 26.69 (25.43–27.40) | 26.63 (25.69–27.50) | |
| n6/n3 ratio | 0.99 1 | ||
| Mean (SD) | 3.68 (0.71) | 3.68 (0.65) | |
| Median (Q1–Q3) | 3.69 (3.20–4.26) | 3.61 (3.28–4.04) | |
| Quality of life characteristics | |||
| EPIC-26 | |||
| Urinary incontinence | 0.30 2 | ||
| Mean (SD) | 92.61 (13.30) | 93.76 (13.39) | |
| Median (Q1–Q3) | 100.00 (91.75–100.00) | 100.00 (100.00–100.00) | |
| Missing n | 4 (6.15) | 3 (4.61) | |
| Urinary irritation | 0.17 1 | ||
| Mean (SD) | 83.97 (15.66) | 87.50 (12.75) | |
| Median (Q1–Q3) | 87.50 (75.00–93.75) | 87.50 (81.25–100.00) | |
| Missing n | 3 (4.61) | 4 (6.15) | |
| Sexual | 0.26 1 | ||
| Mean (SD) | 62.45 (27.93) | 68.05 (27.75) | |
| Median (Q1–Q3) | 58.33 (40.33–87.50) | 77.08 (48.66–87.50) | |
| Missing n | 2 (3.07) | 3 (4.61) | |
| Hormonal | 0.42 2 | ||
| Mean (SD) | 89.07 (16.06) | 89.19 (12.45) | |
| Median (Q1–Q3) | 95.00 (80.00–100.00) | 90.00 (85.00–100.00) | |
| Missing n | 3 (4.61) | 3 (4.61) | |
| Bowel | 0.08 2 | ||
| Mean (SD) | 90.71 (13.40) | 93.88 (11.08) | |
| Median (Q1–Q3) | 95.83 (87.50–100.00) | 100 (91.66–100.00) | |
| Missing n | 4 (6.15) | 3 (4.61) | |
| IPSS | |||
| Mean (SD) | 8.70 (6.72) | 8.22 (5.98) | 0.59 1 |
| Median (Q1–Q3) | 7.00 (3.00–13.00) | 7.00 (4.00–11.00) | |
| Missing n | 1 (1.53) | 2 (3.07) |
| Variables | Placebo | MAG-EPA | Difference between Group | |||||
|---|---|---|---|---|---|---|---|---|
| Mean (SE) | MD (95% CI) | p-Value * | Mean (SE) | MD (95% CI) | p-Value * | MD (95% CI) | p-Value ** | |
| EPIC-26 | ||||||||
| Urinary Incontinence | ||||||||
| Randomization | 94.7 (2.0) | 0.0 | 94.5 (1.9) | 0.0 | ||||
| 3 Months Post-RP | 47.9 (3.4) | −46.8 (−54.3; −39.4) | <0.0001 | 36.8 (3.3) | −57.6 (−65.1; −50.1) | <0.0001 | −9.8 (−19.2; −0.4) | 0.04 |
| 6 Months Post-RP | 66.7 (3.6) | −28.0 (−36.0; −20.1) | <0.0001 | 58.6 (3.7) | −35.8 (−43.9; −27.8) | <0.0001 | −6.6 (−16.6; 3.5) | 0.20 |
| 9 Months Post-RP | 69.3 (3.5) | −25.4 (−32.8; −18.1) | <0.0001 | 67.0 (3.5) | −27.4 (−35.0; −19.9) | <0.0001 | −0.5 (−10.0; 9.0) | 0.92 |
| 12 Months Post-RP | 73.2 (3.5) | −21.5 (−28.7; −14.3) | <0.0001 | 66.5 (3.6) | −27.9 (−35.4; −20.5) | <0.0001 | −3.7 (−13.4; 6.0) | 0.45 |
| Urinary irritation | ||||||||
| Randomization | 85.6 (2.1) | 0.0 | 83.1 (2.0) | 0.0 | ||||
| 3 Months Post-RP | 78.5 (2.1) | −7.1 (−11.9; −2.2) | 0.005 | 79.1 (2.0) | −4.1 (−8.9; 0.8) | 0.10 | 1.6 (−3.9; 7.1) | 0.57 |
| 6 Months Post-RP | 85.3 (1.8) | −0.3 (0.1; 8.8) | 0.90 | 87.8 (1.8) | 4.7 (0.4; 8.9) | 0.03 | 2.6 (−1.8; 7.0) | 0.24 |
| 9 Months Post-RP | 86.9 (1.8) | 1.4 (−2.3; 5.1) | 0.47 | 87.3 (1.8) | 4.1 (0.4; 7.8) | 0.03 | 1.7 (−2.4; 5.8) | 0.42 |
| 12 Months Post-RP | 86.6 (1.7) | 1.0 (−3.0; 5.1) | 0.62 | 88.8 (1.7) | 5.6 (1.5; 9.6) | 0.007 | 3.5 (−0.8; 7.8) | 0.11 |
| Sexual function | ||||||||
| Randomization | 65.7 (3.7) | 0.0 | 62.8 (3.6) | 0.0 | ||||
| 3 Months Post-RP | 19.6 (2.8) | −46.1 (−53.4; −38.9) | <0.0001 | 17.7 (2.8) | −45.1 (−52.3; −37.8) | <0.0001 | −1.8 (−9.0; 5.4) | 0.62 |
| 6 Months Post-RP | 26.3 (3.3) | −39.4 (−46.8; −31.9) | <0.0001 | 26.2 (3.3) | −36.6 (−44.1; −29.2) | <0.0001 | −0.4 (−8.6; 7.9) | 0.93 |
| 9 Months Post-RP | 32.0 (3.5) | −33.7 (−40.8; −26.6) | <0.0001 | 30.5 (3.5) | −32.3 (−39.5; −25.2) | <0.0001 | −1.8 (−10.4; 6.7) | 0.67 |
| 12 Months Post-RP | 36.0 (3.8) | −29.7 (−37.2; −22.3) | <0.0001 | 31.5 (3.8) | −31.3 (−38.8; −23.8) | <0.0001 | −4.5 (−14.0; 5.0) | 0.35 |
| Bowel function | ||||||||
| Randomization | 92.8 (1.7) | 0.0 | 90.2 (1.6) | 0.0 | ||||
| 3 Months Post-RP | 91.3 (1.6) | −1.5 (−5.2; 2.2) | 0.42 | 89.7 (1.7) | −0.5 (−4.2; 3.2) | 0.77 | −0.8 (−5.2; 3.7) | 0.72 |
| 6 Months Post-RP | 93.0 (1.4) | 0.2 (−2.8; 3.2) | 0.90 | 91.4 (1.3) | 1.2 (−1.8; 4.3) | 0.43 | −1.0 (−4.3; 2.3) | 0.55 |
| 9 Months Post-RP | 93.0 (1.7) | 0.2 (−3.4; 3.8) | 0.93 | 90.8 (1.7) | 0.6 (−3.2; 4.2) | 0.76 | −0.4 (−4.5; 3.7) | 0.84 |
| 12 Months Post-RP | 95.2 (1.3) | 2.4 (−0.5; 5.2) | 0.11 | 94.0 (1.2) | 3.8 (0.9; 6.7) | 0.009 | −0.6 (−3.4; 2.2) | 0.69 |
| Hormonal function | ||||||||
| Randomization | 85.9 (2.0) | 0.0 | 88.3 (1.9) | 0.0 | ||||
| 3 Months Post-RP | 85.8 (1.9) | −0.1 (−3.8; 3.6) | 0.96 | 88.2 (1.8) | −0.1 (−3.8; 3.7) | 0.97 | 0.9 (−3.4; 5.3) | 0.66 |
| 6 Months Post-RP | 88.1 (1.6) | 2.2 (−1.3; 5.8) | 0.21 | 88.2 (1.5) | −0.1 (−3.6; 3.4) | 0.96 | −0.3 (−3.9; 3.4) | 0.89 |
| 9 Months Post-RP | 87.5 (1.6) | 1.6 (−1.7; 4.9) | 0.33 | 89.4 (1.5) | 1.2 (−2.2; 4.5) | 0.49 | 1.4 (−2.2; 5.0) | 0.43 |
| 12 Months Post-RP | 86.6 (1.7) | 0.7 (−2.5; 4.0) | 0.66 | 89.2 (1.7) | 1.0 (−2.2; 4.2) | 0.55 | 1.6 (−2.0; 5.3) | 0.36 |
| IPSS | ||||||||
| Voiding and storage Problem | ||||||||
| Randomization | 9.0 (0.9) | 0.0 | 8.8 (0.8) | 0.0 | ||||
| 3 Months Post-RP | 11.2 (0.9) | 2.2 (0.1; 4.3) | 0.04 | 10.3 (0.9) | 1.5 (−0.6; 3.6) | 0.17 | −0.9 (−3.2; 1.4) | 0.43 |
| 6 Months Post-RP | 7.7 (0.7) | −1.2 (−3.0; 0.5) | 0.17 | 7.8 (0.7) | −1.0 (−2.7; 0.8) | 0.28 | 0.1 (−1.6; 1.8) | 0.91 |
| 9 Months Post-RP | 7.3 (0.7) | −1.7 (−3.3; 0.0) | 0.05 | 6.5 (0.7) | −2.3 (−4.0; −0.6) | 0.007 | −0.9 (−2.7; 0.8) | 0.30 |
| 12 Months Post-RP | 6.9 (0.7) | −2.1 (−3.8; −0.4) | 0.02 | 5.9 (0.6) | −2.9 (−4.6; −1.2) | 0.0008 | −1.1 (−2.8; 0.5) | 0.17 |
| Variables | Placebo | MAG-EPA | Difference between Group | |||||
|---|---|---|---|---|---|---|---|---|
| Mean (SE) | MD (95% CI) | p-Value * | Mean (SE) | MD (95% CI) | p-Value * | MD (95% CI) | p-Value ** | |
| EPIC-26 | ||||||||
| Urinary Incontinence | ||||||||
| Randomization | 94.5 (2.0) | 0.0 | 94.2 (1.9) | 0.0 | ||||
| 3 Months Post-RP | 47.3 (3.7) | −47.2 (−55.3; −39.2) | <0.0001 | 37.1 (3.8) | −57.2 (−65.3; −48.9) | <0.0001 | −7.2 (−17.9; 3.4) | 0.18 |
| 6 Months Post-RP | 66.4 (4.1) | −28.1 (−36.9; −19.3) | <0.0001 | 58.3 (4.3) | −35.9 (−45.2; −26.7) | <0.0001 | −4.7 (−16.5; 7.2) | 0.44 |
| 9 Months Post-RP | 70.8 (3.8) | −23.7 (−31.6; −15.7) | <0.0001 | 65.7 (4.1) | −28.5 (−37.1; −19.9) | <0.0001 | −1.4 (−12.6; 9.7) | 0.80 |
| 12 Months Post-RP | 74.4 (3.9) | −19.1 (−28.0; −12.1) | <0.0001 | 65.8 (4.1) | −28.6 (−37.1; −20.2) | <0.0001 | −4.4 (−15.9; 7.2) | 0.45 |
| Urinary irritation | ||||||||
| Randomization | 85.2 (2.1) | 0.0 | 82.5 (2.0) | 0.0 | ||||
| 3 Months Post-RP | 78.9 (2.3) | −6.3 (−11.4; −1.3) | 0.01 | 77.0 (2.3) | −5.4 (−10.5; −0.3) | 0.04 | 0.3 (−5.9; 6.4) | 0.93 |
| 6 Months Post-RP | 85.1 (2.0) | −0.1 (−4.5; 4.3) | 0.95 | 85.8 (2.0) | 3.3 (−1.2; 7.7) | 0.15 | 2.1 (−2.9; 7.0) | 0.41 |
| 9 Months Post-RP | 87.5 (1.9) | 2.3 (−1.3; 6.0) | 0.21 | 85.3 (1.9) | 2.9 (−0.8; 6.5) | 0.12 | 0.7 (−3.8; 5.1) | 0.77 |
| 12 Months Post-RP | 84.4 (2.0) | −0.8 (−5.0; 3.5) | 0.72 | 87.3 (2.0) | 4.9 (0.6; 9.2) | 0.03 | 5.5 (0.4; 10.6) | 0.03 |
| Sexual function | ||||||||
| Randomization | 66.3 (3.6) | 0.0 | 63.2 (3.5) | 0.0 | ||||
| 3 Months Post-RP | 19.0 (3.0) | −47.3 (−54.6; −40.0) | <0.0001 | 18.8 (3.0) | −44.4 (−51.7; −37.1) | <0.0001 | −0.7 (−8.5; 7.1) | 0.86 |
| 6 Months Post-RP | 28.1 (3.6) | −38.2 (−45.8; −30.6) | <0.0001 | 27.4 (3.8) | −35.8 (−43.6; −28.0) | <0.0001 | −1.2 (−10.6; 8.2) | 0.80 |
| 9 Months Post-RP | 33.0 (3.8) | −33.3 (−40.8; −25.8) | <0.0001 | 31.4 (4.0) | −31.8 (−39.6; −24.1) | <0.0001 | −2.3 (−12.2; 7.6) | 0.65 |
| 12 Months Post-RP | 36.2 (4.3) | −30.1 (−38.1; −22.2) | <0.0001 | 32.4 (4.5) | −30.8 (−39.0; −22.6) | <0.0001 | −3.8 (−15.1; 7.5) | 0.50 |
| Bowel function | ||||||||
| Randomization | 93.1 (1.7) | 0.0 | 90.3 (1.6) | 0.0 | ||||
| 3 Months Post-RP | 92.8 (1.8) | −0.3 (−4.3; 3.7) | 0.86 | 88.5 (1.8) | −1.8 (−5.8; 2.2) | 0.38 | −3.2 (−8.2; 1.7) | 0.20 |
| 6 Months Post-RP | 93.6 (1.4) | 0.5 (−2.6; 3.7) | 0.75 | 91.5 (1.4) | 1.2 (−1.9; 4.5) | 0.45 | −1.6 (−5.2; 2.0) | 0.37 |
| 9 Months Post-RP | 94.3 (1.7) | 1.2 (−2.8; 5.2) | 0.56 | 89.9 (1.8) | −0.4 (−4.5; 3.7) | 0.85 | −2.4 (−6.8; 2.0) | 0.28 |
| 12 Months Post-RP | 96.2 (1.3) | 3.0 (−0.3; 6.4) | 0.07 | 93.9 (1.3) | 3.6 (0.3; 6.9) | 0.03 | −1.0 (−4.4; 2.4) | 0.55 |
| Hormonal function | ||||||||
| Randomization | 86.3 (2.0) | 0.0 | 87.9 (1.9) | 0.0 | ||||
| 3 Months Post-RP | 88.0 (1.9) | 1.6 (−1.4; 4.6) | 0.29 | 87.2 (1.9) | −0.7 (−3.8; 2.4) | 0.64 | −1.2 (−5.2; 2.9) | 0.57 |
| 6 Months Post-RP | 88.7 (1.5) | 2.4 (−0.3; 5.1) | 0.08 | 89.2 (1.6) | 1.3 (−1.4; 3.9) | 0.35 | −0.2 (−3.2; 2.8) | 0.90 |
| 9 Months Post-RP | 87.8 (1.6) | 1.4 (−1.8; 4.6) | 0.38 | 88.6 (1.7) | 0.7 (−2.5; 3.9) | 0.66 | 0.5 (−3.3; 4.3) | 0.80 |
| 12 Months Post-RP | 88.3 (1.7) | 1.9 (−1.3; 5.1) | 0.24 | 90.4 (1.7) | 2.5 (−0.7; 5.7) | 0.12 | 1.7 (−2.2; 5.6) | 0.38 |
| IPSS | ||||||||
| Voiding and storage Problem | ||||||||
| Randomization | 9.1 (0.9) | 0.0 | 9.0 (0.9) | 0.0 | ||||
| 3 Months Post-RP | 11.1 (1.0) | 1.9 (−0.3; 4.2) | 0.08 | 11.1 (1.0) | 2.2 (−0.1; 4.4) | 0.06 | −0.3 (−2.9; 2.4) | 0.85 |
| 6 Months Post-RP | 7.8 (0.7) | −1.3 (−3.0; 0.5) | 0.15 | 8.4 (0.8) | −0.6 (−2.4; 1.1) | 0.48 | 0.2 (−1.6; 2.0) | 0.81 |
| 9 Months Post-RP | 7.0 (0.7) | −2.2 (−3.8; −0.5) | 0.009 | 7.2 (0.8) | −1.8 (−3.5; −0.2) | 0.03 | −0.1 (−1.9; 1.7) | 0.91 |
| 12 Months Post-RP | 7.0 (0.7) | −2.1 (−3.8; −0.5) | 0.01 | 6.4 (0.7) | −2.6 (−4.2; −0.9) | 0.002 | −0.9 (−2.8; 0.9) | 0.30 |
| Placebo (n = 65) * n (%) | MAG-EPA (n = 63) * n (%) | |
|---|---|---|
| Diarrhea | 1 (1.5) | 3 (4.8) |
| Skin rash | 3 (4.6) | 1 (1.6) |
| Nausea | 1 (1.5) | 3 (4.8) |
| Heartburn | 0 | 1 (1.6) |
| Digestive problems | 1 (1.5) | 1 (1.6) |
| Change of stool frequency | 1 (1.5) | 1 (1.6) |
| Headaches | 0 | 1 (1.6) |
| Total of patients with events | 7 (10.8) | 10 (15.9) |
| Withdrawal because of adverse events | 1 (1.5) | 5 (7.9) |
| Placebo (n = 65) | MAG-EPA (n = 63) | |
|---|---|---|
| Adherence to study drug for the entire study (%) * | 91.9 | 93.3 |
| Adherence to study drug until withdrawal or end of the study ** | 91.4 | 92.9 |
| Adherence to study drug for all patients who underwent RP § | 86.3 | 83.4 |
| Adherence for patients who underwent RP and took at least 80% of study drug † | 93.4 | 94.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moussa, H.; Robitaille, K.; Pelletier, J.-F.; Tourigny, R.; Fradet, Y.; Lacombe, L.; Toren, P.; Lodde, M.; Tiguert, R.; Dujardin, T.; et al. Effects of Concentrated Long-Chain Omega-3 Polyunsaturated Fatty Acid Supplementation on Quality of Life after Radical Prostatectomy: A Phase II Randomized Placebo-Controlled Trial (RCT-EPA). Nutrients 2023, 15, 1369. https://doi.org/10.3390/nu15061369
Moussa H, Robitaille K, Pelletier J-F, Tourigny R, Fradet Y, Lacombe L, Toren P, Lodde M, Tiguert R, Dujardin T, et al. Effects of Concentrated Long-Chain Omega-3 Polyunsaturated Fatty Acid Supplementation on Quality of Life after Radical Prostatectomy: A Phase II Randomized Placebo-Controlled Trial (RCT-EPA). Nutrients. 2023; 15(6):1369. https://doi.org/10.3390/nu15061369
Chicago/Turabian StyleMoussa, Hanane, Karine Robitaille, Jean-François Pelletier, Roxane Tourigny, Yves Fradet, Louis Lacombe, Paul Toren, Michele Lodde, Rabi Tiguert, Thierry Dujardin, and et al. 2023. "Effects of Concentrated Long-Chain Omega-3 Polyunsaturated Fatty Acid Supplementation on Quality of Life after Radical Prostatectomy: A Phase II Randomized Placebo-Controlled Trial (RCT-EPA)" Nutrients 15, no. 6: 1369. https://doi.org/10.3390/nu15061369
APA StyleMoussa, H., Robitaille, K., Pelletier, J.-F., Tourigny, R., Fradet, Y., Lacombe, L., Toren, P., Lodde, M., Tiguert, R., Dujardin, T., Caumartin, Y., Duchesne, T., Julien, P., Savard, J., Diorio, C., & Fradet, V. (2023). Effects of Concentrated Long-Chain Omega-3 Polyunsaturated Fatty Acid Supplementation on Quality of Life after Radical Prostatectomy: A Phase II Randomized Placebo-Controlled Trial (RCT-EPA). Nutrients, 15(6), 1369. https://doi.org/10.3390/nu15061369






