Effects of Omega-3 Fatty Acids on Postoperative Inflammatory Response: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Intervention and Control
2.4. Outcomes
2.5. Data Extraction
2.6. Subgroup Analysis
2.7. Statistical Analysis
3. Results
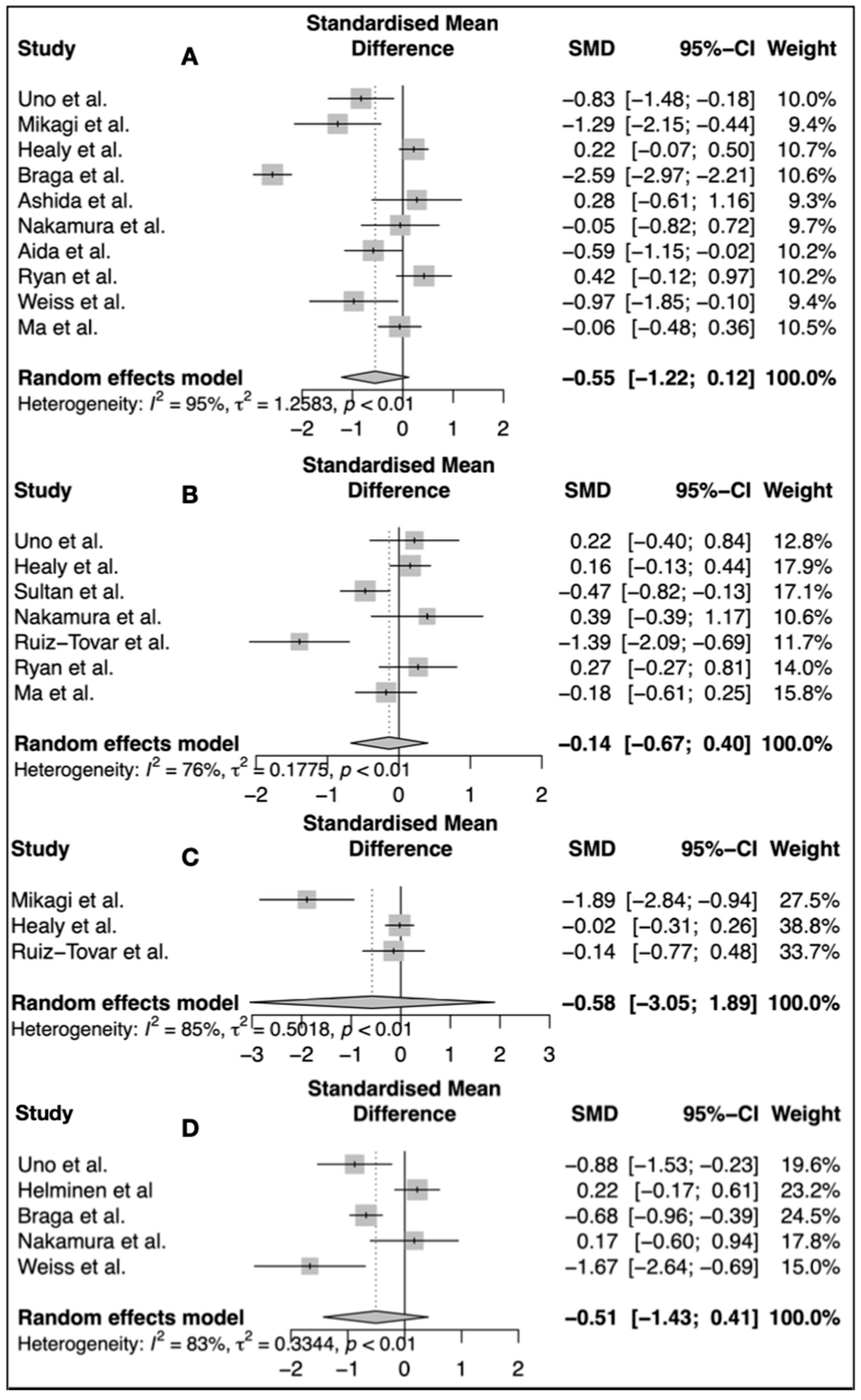
3.1. Interleukin 6
3.2. C-Reactive Protein
3.3. White Blood Count
3.4. Length of Hospital Stay
| No. | Author | Country | Surgical Procedure | Sample Size (Control/Intervention) | Definition of Control | Age (Control/Intervention) | Dosis of Omega 3 g/d | Route of Administratio | Time of Initiation (Day 0: Operation) | Duration of Therapy | LOS Control/Intervention |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Uno et al. [30] | Japan | Major hepatobiliary resection | 40 (20/20) | No supplementation | 66.4/65.5 | 2.6 | Enteral | −5 | −5 d–>0 d | Control: 53.9 ± 5 Intervention: 36.9 ± 3.3 |
| 2 | Mikagi et al. [31] | Japan | Segmentectomy or extensive hepatectomy | 26 (13/13) | No supplementation | 61.5/67.5 | 3.1 | Enteral | −5 | −5 d–>0 d | Control: 14.5 Intervention: 16.3 |
| 3 * | Helminen et al. [42] | Finland | Gastrointestinal cancer operations | 100 (50/50) | No supplementation | 63/58 | 3 | Enteral | −5 | −5 d–>+5 d | Control: 9 ± 5 Intervention: 10 ± 4 |
| 4 ** | Healy et al. [32] | Ireland | Esophagectomy | 191 (94/97) | No supplementation | 62/62 | 2.2 | Enteral | −5 | −1 d–>+30 d | No mentioned data |
| 5 | Braga et al. [33] | Italy | Colorectal cancer surgery | 200 (100/100) | 50 no supplementation 50 isocaloric, isonitrogenous nutrition | No supplementation 62.6: control. 61.8 only preOp Omega 3 FA 63: Pre and postOp 60.5 | 3.3 | Enteral | −5 | preOp: −5 d–>0 d Pre + PostOp: −5 d–>+8 d | No supp.: 12.2 ± 3.9 Control Supp.: 12.0 ± 4.5 preOp Omega 3: 9.5 ± 2.9 pre and postOp: 9.8 ± 3.1 |
| 6 | Ashida et al. [34] | Japan | Pancreatoduodenectomy | 20 (9/11) | Isocaloric isonitrogenous nutrition | 69/64 | 2 | Enteral | −7 | −7 d–>0 d | No mentioned data |
| 7 *** | Sultan et al. [40] | UK | Esophagogastric cancer surgery | 195 (129/66) | 66 no supplementation 63 Standard enteral nutrition without immunonutrients | No supplementation: 66 Enteral nutrition: 60 Intervention: 67 | 4.92 | Enteral | −7 | −7 d–>+7 d | Conventional: 16 (11–34) Control: 16 (11–116) Intervention: 18 (4–141) |
| 8 | Nakamura et al. [39] | Japan | Bile duct cancer/pancreatic cancer, gastric cancer, esophageal cancer | 26 (14/12) | No supplementation | 60.75/65 | 4 | Enteral | −5 | −5 d–>0 d | Control: 46.1 ± 15 Intervention: 49.0 ± 18.3 |
| 9 | Aida et al. [35] | Japan | Pancreatoduodenectomy | 50 (25/25) | No supplementation | 65.1/66.4 | 4 | Enteral | −5 | −5 d–>0 d | No mentioned data |
| 10 *** | Ruiz-Tovar et al. [41] | Spain | Roux-en-Y gastric bypass | 40 (20/20) | balanced energy high-protein formula | 46.4/45.5 | 4.26 | Enteral | −10 | −10 d–>0 d | Control: 2 Intervention: 2 |
| 11 | Ryan et al. [36] | Ireland | Esophagectomy | 53 (25/28) | isocaloric isonitrogenous standard nutritional feed | 65.7/62 | 2.2 | Enteral | −5 | −5 d–>+21 d | No mentioned data |
| 12 | Weiss et al. [37] | DE | Extended abdominal surgery | 23 (11/12) | Parenteral nutrition with glucose, amino acids and fat | 61.6/57.4 | 4 | Intravenous | −1 | −1 d–>+5 d | Control: 23.5 Intervention: 17.8 |
| 13 **** | Ma et al. [38] | Taiwan | Gastrointestinal cancer operations | 86 (41/45) | Soybean oil and medium-chain triglycerides | 62.85/61.55 | 6.5 g mean weight | Intravenous | −1 | −1 d–>+7 d | No mentioned data |
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ni Choileain, N. Cell Response to Surgery. Arch. Surg. 2006, 141, 1132. [Google Scholar] [CrossRef]
- Sido, B.; Teklote, J.-R.; Hartel, M.; Friess, H.; Büchler, M.W. Inflammatory Response after Abdominal Surgery. Best Pract. Res. Clin. Anaesthesiol. 2004, 18, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Szczepanik, A.M.; Scislo, L.; Scully, T.; Walewska, E.; Siedlar, M.; Kolodziejczyk, P.; Lenart, M.; Rutkowska, M.; Galas, A.; Czupryna, A.; et al. IL-6 Serum Levels Predict Postoperative Morbidity in Gastric Cancer Patients. Gastric Cancer 2011, 14, 266–273. [Google Scholar] [CrossRef]
- Rettig, T.C.D.; Verwijmeren, L.; Dijkstra, I.M.; Boerma, D.; van de Garde, E.M.W.; Noordzij, P.G. Postoperative Interleukin-6 Level and Early Detection of Complications After Elective Major Abdominal Surgery. Ann. Surg. 2016, 263, 1207–1212. [Google Scholar] [CrossRef]
- Watt, D.G.; Horgan, P.G.; McMillan, D.C. Routine Clinical Markers of the Magnitude of the Systemic Inflammatory Response after Elective Operation: A Systematic Review. Surgery 2015, 157, 362–380. [Google Scholar] [CrossRef]
- Santonocito, C.; De Loecker, I.; Donadello, K.; Moussa, M.D.; Markowicz, S.; Gullo, A.; Vincent, J.-L. C-Reactive Protein Kinetics After Major Surgery. Anesth. Analg. 2014, 119, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Polyunsaturated Fatty Acids, Inflammatory Processes and Inflammatory Bowel Diseases. Mol. Nutr. Food Res. 2008, 52, 885–897. [Google Scholar] [CrossRef]
- Chan, E.J.; Cho, L. What Can We Expect from Omega-3 Fatty Acids? CCJM 2009, 76, 245–251. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, Y.; Yang, P.; Wan, H.; Wu, X. Safety and Efficacy of Fish Oil–Enriched Parenteral Nutrition Regimen on Postoperative Patients Undergoing Major Abdominal Surgery: A Meta-Analysis of Randomized Controlled Trials. JPEN J. Parenter. Enter. Nutr. 2010, 34, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Immunonutrition in Surgical and Critically Ill Patients. Br. J. Nutr. 2007, 98, S133–S139. [Google Scholar] [CrossRef]
- Moosavian, S.P.; Arab, A.; Mehrabani, S.; Moradi, S.; Nasirian, M. The Effect of Omega-3 and Vitamin E on Oxidative Stress and Inflammation: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Vitam. Nutr. Res. 2020, 90, 553–563. [Google Scholar] [CrossRef]
- Natto, Z.S.; Yaghmoor, W.; Alshaeri, H.K.; Van Dyke, T.E. Omega-3 Fatty Acids Effects on Inflammatory Biomarkers and Lipid Profiles among Diabetic and Cardiovascular Disease Patients: A Systematic Review and Meta-Analysis. Sci. Rep. 2019, 9, 18867. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi; Wallace; Calder. Dietary Lipids Modify the Cytokine Response to Bacterial Lipopolysaccharide in Mice. Immunology 1999, 96, 404–410. [Google Scholar] [CrossRef]
- Billiar, T.R.; Bankey, P.E.; Svingen, B.A.; Curran, R.D.; West, M.A.; Holman, R.T.; Simmons, R.L.; Cerra, F.B. Fatty Acid Intake and Kupffer Cell Function: Fish Oil Alters Eicosanoid and Monokine Production to Endotoxin Stimulation. Surgery 1988, 104, 343–349. [Google Scholar]
- Calder, P.C. Omega-3 Polyunsaturated Fatty Acids and Inflammatory Processes: Nutrition or Pharmacology?: Omega-3 Fatty Acids and Inflammation. Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Kiecolt-Glaser, J.K.; Belury, M.A.; Andridge, R.; Malarkey, W.B.; Hwang, B.S.; Glaser, R. Omega-3 Supplementation Lowers Inflammation in Healthy Middle-Aged and Older Adults: A Randomized Controlled Trial. Brain Behav. Immun. 2012, 26, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Habicht, I.; Mohsen, G.; Eichhorn, L.; Frede, S.; Weisheit, C.; Hilbert, T.; Treede, H.; Güresir, E.; Dewald, O.; Duerr, G.D.; et al. DHA Supplementation Attenuates MI-Induced LV Matrix Remodeling and Dysfunction in Mice. Oxidative Med. Cell. Longev. 2020, 2020, 7606938. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 Fatty Acids and Inflammatory Processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef]
- Żebrowska, A.; Hall, B.; Stolecka-Warzecha, A.; Stanula, A.; Sadowska-Krępa, E. The Effect of Omega-3 Fatty Acid Supplementation on Serum Adipocytokines, Lipid Profile and Biochemical Markers of Inflammation in Recreational Runners. Nutrients 2021, 13, 456. [Google Scholar] [CrossRef]
- Tutino, V.; De Nunzio, V.; Caruso, M.G.; Veronese, N.; Lorusso, D.; Di Masi, M.; Benedetto, M.L.; Notarnicola, M. Elevated AA/EPA Ratio Represents an Inflammatory Biomarker in Tumor Tissue of Metastatic Colorectal Cancer Patients. Int. J. Mol. Sci. 2019, 20, 2050. [Google Scholar] [CrossRef]
- Kitagawa, M.; Haji, S.; Amagai, T. Elevated Serum AA/EPA Ratio as a Predictor of Skeletal Muscle Depletion in Cachexic Patients with Advanced Gastro-Intestinal Cancers. In Vivo 2018, 31, 1003–1009. [Google Scholar] [CrossRef]
- Nelson, J.R.; Raskin, S. The Eicosapentaenoic Acid:Arachidonic Acid Ratio and Its Clinical Utility in Cardiovascular Disease. Postgrad. Med. 2019, 131, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Marine Omega-3 Fatty Acids and Inflammatory Processes: Effects, Mechanisms and Clinical Relevance. Biochim. Biophys. Acta BBA—Mol. Cell Biol. Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- Lee, J.Y.; Sohn, K.H.; Rhee, S.H.; Hwang, D. Saturated Fatty Acids, but Not Unsaturated Fatty Acids, Induce the Expression of Cyclooxygenase-2 Mediated through Toll-like Receptor 4. J. Biol. Chem. 2001, 276, 16683–16689. [Google Scholar] [CrossRef]
- Bannenberg, G.; Serhan, C.N. Specialized Pro-Resolving Lipid Mediators in the Inflammatory Response: An Update. Biochim. Biophys. Acta BBA—Mol. Cell Biol. Lipids 2010, 1801, 1260–1273. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis; Borenstein, M., Ed.; John Wiley & Sons: Chichester, UK, 2009; ISBN 978-0-470-05724-7. [Google Scholar]
- Hartung, J. An Alternative Method for Meta-Analysis. Biom. J. 1999, 41, 901–916. [Google Scholar] [CrossRef]
- Aert, R.C.M.; Jackson, D. A New Justification of the Hartung-Knapp Method for Random-effects Meta-analysis Based on Weighted Least Squares Regression. Res. Syn. Meth. 2019, 10, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Uno, H.; Furukawa, K.; Suzuki, D.; Shimizu, H.; Ohtsuka, M.; Kato, A.; Yoshitomi, H.; Miyazaki, M. Immunonutrition Suppresses Acute Inflammatory Responses through Modulation of Resolvin E1 in Patients Undergoing Major Hepatobiliary Resection. Surgery 2016, 160, 228–236. [Google Scholar] [CrossRef]
- Mikagi, K.; Kawahara, R.; Kinoshita, H.; Aoyagi, S. Effect of Preoperative Immunonutrition in Patients Undergoing Hepatectomy; A Randomized Controlled Trial. Kurume Med. J. 2011, 58, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Healy, L.A.; Ryan, A.; Doyle, S.L.; Ní Bhuachalla, É.B.; Cushen, S.; Segurado, R.; Murphy, T.; Ravi, N.; Donohoe, C.L.; Reynolds, J.V. Does Prolonged Enteral Feeding With Supplemental Omega-3 Fatty Acids Impact on Recovery Post-Esophagectomy: Results of a Randomized Double-Blind Trial. Ann. Surg. 2017, 266, 720–728. [Google Scholar] [CrossRef]
- Braga, M.; Gianotti, L.; Vignali, A.; Carlo, V.D. Preoperative Oral Arginine and N-3 Fatty Acid Supplementation Improves the Immunometabolic Host Response and Outcome after Colorectal Resection for Cancer. Surgery 2002, 132, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Ashida, R.; Okamura, Y.; Wakabayashi-Nakao, K.; Mizuno, T.; Aoki, S.; Uesaka, K. The Impact of Preoperative Enteral Nutrition Enriched with Eicosapentaenoic Acid on Postoperative Hypercytokinemia after Pancreatoduodenectomy: The Results of a Double-Blinded Randomized Controlled Trial. Dig. Surg. 2019, 36, 348–356. [Google Scholar] [CrossRef]
- Aida, T.; Furukawa, K.; Suzuki, D.; Shimizu, H.; Yoshidome, H.; Ohtsuka, M.; Kato, A.; Yoshitomi, H.; Miyazaki, M. Preoperative Immunonutrition Decreases Postoperative Complications by Modulating Prostaglandin E2 Production and T-Cell Differentiation in Patients Undergoing Pancreatoduodenectomy. Surgery 2014, 155, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.M.; Reynolds, J.V.; Healy, L.; Byrne, M.; Moore, J.; Brannelly, N.; McHugh, A.; McCormack, D.; Flood, P. Enteral Nutrition Enriched With Eicosapentaenoic Acid (EPA) Preserves Lean Body Mass Following Esophageal Cancer Surgery: Results of a Double-Blinded Randomized Controlled Trial. Ann. Surg. 2009, 249, 355–363. [Google Scholar] [CrossRef]
- Weiss, G.; Meyer, F.; Matthies, B.; Pross, M.; Koenig, W.; Lippert, H. Immunomodulation by Perioperative Administration of n -3 Fatty Acids. Br. J. Nutr. 2002, 87, S89–S94. [Google Scholar] [CrossRef]
- Ma, C.-J.; Wu, J.-M.; Tsai, H.-L.; Huang, C.-W.; Lu, C.-Y.; Sun, L.-C.; Shih, Y.-L.; Chen, C.-W.; Chuang, J.-F.; Wu, M.-H.; et al. Prospective Double-Blind Randomized Study on the Efficacy and Safety of an n-3 Fatty Acid Enriched Intravenous Fat Emulsion in Postsurgical Gastric and Colorectal Cancer Patients. Nutr. J. 2015, 14, 9. [Google Scholar] [CrossRef]
- Nakamura, K.; Kariyazono, H.; Komokata, T.; Hamada, N.; Sakata, R.; Yamada, K. Influence of Preoperative Administration of ω-3 Fatty Acid-Enriched Supplement on Inflammatory and Immune Responses in Patients Undergoing Major Surgery for Cancer. Nutrition 2005, 21, 639–649. [Google Scholar] [CrossRef]
- Sultan, J.; Griffin, S.M.; Di Franco, F.; Kirby, J.A.; Shenton, B.K.; Seal, C.J.; Davis, P.; Viswanath, Y.K.S.; Preston, S.R.; Hayes, N. Randomized Clinical Trial of Omega-3 Fatty Acid-Supplemented Enteral Nutrition versus Standard Enteral Nutrition in Patients Undergoing Oesophagogastric Cancer Surgery. Br. J. Surg. 2012, 99, 346–355. [Google Scholar] [CrossRef]
- Ruiz-Tovar, J.; Blanca, M.; Garcia, A.; Gonzalez, J.; Gutierrez, S.; Paniagua, A.; Prieto, M.J.; Ramallo, L.; Llanos, L.; Duran, M. Preoperative Administration of Omega-3 Fatty Acids on Postoperative Pain and Acute-Phase Reactants in Patients Undergoing Roux-En-Y Gastric Bypass: A Randomized Clinical Trial. Clin. Nutr. 2019, 38, 1588–1593. [Google Scholar] [CrossRef] [PubMed]
- Helminen, H.; Raitanen, M.; Kellosalo, J. Immunonutrition in Elective Gastrointestinal Surgery Patients. Scand. J. Surg. 2007, 96, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Desborough, J.P. The Stress Response to Trauma and Surgery. Br. J. Anaesth. 2000, 85, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Shenkin, A.; Schweinlin, A.; Amrein, K.; Augsburger, M.; Biesalski, H.-K.; Bischoff, S.C.; Casaer, M.P.; Gundogan, K.; Lepp, H.-L.; et al. ESPEN Micronutrient Guideline. Clin. Nutr. 2022, 41, 1357–1424. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Jiang, W.; Spinetti, T.; Tardivel, A.; Castillo, R.; Bourquin, C.; Guarda, G.; Tian, Z.; Tschopp, J.; Zhou, R. Omega-3 Fatty Acids Prevent Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome Activation. Immunity 2013, 38, 1154–1163. [Google Scholar] [CrossRef]
- Ali, M.; Heyob, K.M.; Velten, M.; Tipple, T.E.; Rogers, L.K. DHA Suppresses Chronic Apoptosis in the Lung Caused by Perinatal Inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L441–L448. [Google Scholar] [CrossRef]
- Bae, H.J.; Lee, G.Y.; Seong, J.-M.; Gwak, H.S. Outcomes with Perioperative Fat Emulsions Containing Omega-3 Fatty Acid: A Meta-Analysis of Randomized Controlled Trials. Am. J. Health-Syst. Pharm. 2017, 74, 904–918. [Google Scholar] [CrossRef]
- Gao, B.; Luo, J.; Liu, Y.; Zhong, F.; Yang, X.; Gan, Y.; Su, S.; Li, B. Clinical Efficacy of Perioperative Immunonutrition Containing Omega-3-Fatty Acids in Patients Undergoing Hepatectomy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Ann. Nutr. Metab. 2020, 76, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Langlois, P.L.; Hardy, G.; Manzanares, W. Omega-3 Polyunsaturated Fatty Acids in Cardiac Surgery Patients: An Updated Systematic Review and Meta-Analysis. Clin. Nutr. 2017, 36, 737–746. [Google Scholar] [CrossRef]
- Li, N.-N.; Zhou, Y.; Qin, X.-P.; Chen, Y.; He, D.; Feng, J.-Y.; Wu, X.-T. Does Intravenous Fish Oil Benefit Patients Post-Surgery? A Meta-Analysis of Randomised Controlled Trials. Clin. Nutr. 2014, 33, 226–239. [Google Scholar] [CrossRef]
- Wei, C.; Hua, J.; Bin, C.; Klassen, K. Impact of Lipid Emulsion Containing Fish Oil on Outcomes of Surgical Patients: Systematic Review of Randomized Controlled Trials from Europe and Asia. Nutrition 2010, 26, 474–481. [Google Scholar] [CrossRef]
- Lu, S.; Yang, Z.; Tang, H.; Sun, X.; Wang, B.; Qu, J.; Wang, Y.; Yang, P.; Rao, B. Associations between Omega-3 Polyunsaturated Fatty Acids Supplementation and Surgical Prognosis in Patients with Gastrointestinal Cancer: A Systematic Review and Meta-Analysis. Food Chem. Mol. Sci. 2022, 4, 100099. [Google Scholar] [CrossRef]
- Xiao, F.; Han, W.; Yue, Q.; Ke, J.; Jia, B.; Fu, X. Perioperative Omega-3 Fatty Acids for Liver Surgery: A Meta-Analysis of Randomized Controlled Trials. Medicine 2021, 100, e25743. [Google Scholar] [CrossRef] [PubMed]
- Slim, K.; Badon, F.; Vacheron, C.H.; Occean, B.V.; Dziri, C.; Chambrier, C. Umbrella Review of the Efficacy of Perioperative Immunonutrition in Visceral Surgery. Clin. Nutr. ESPEN 2022, 48, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Akintoye, E.; Sethi, P.; Harris, W.S.; Thompson, P.A.; Marchioli, R.; Tavazzi, L.; Latini, R.; Pretorius, M.; Brown, N.J.; Libby, P.; et al. Fish Oil and Perioperative Bleeding: Insights From the OPERA Randomized Trial. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e004584. [Google Scholar] [CrossRef]
- Calder, P.C. Mechanisms of Action of (n-3) Fatty Acids. J. Nutr. 2012, 142, 592S–599S. [Google Scholar] [CrossRef]
- Ryan, J. Fish Oil and Postoperative Atrial Fibrillation: The Omega-3 Fatty Acids for Prevention of Post-Operative Atrial Fibrillation (OPERA) Randomized Trial. J. Emerg. Med. 2013, 44, 730. [Google Scholar] [CrossRef]
- Nishizaki, Y.; Daida, H. Optimal Dose of N-3 Polyunsaturated Fatty Acids for Cardiovascular Event Prevention. Circ. Rep. 2020, 2, 260–264. [Google Scholar] [CrossRef]
- Katan, M.B.; Deslypere, J.P.; Van Birgelen, A.P.; Penders, M.; Zegwaard, M. Kinetics of the Incorporation of Dietary Fatty Acids into Serum Cholesteryl Esters, Erythrocyte Membranes, and Adipose Tissue: An 18-Month Controlled Study. J. Lipid Res. 1997, 38, 2012–2022. [Google Scholar] [CrossRef] [PubMed]
- Browning, L.M.; Walker, C.G.; Mander, A.P.; West, A.L.; Madden, J.; Gambell, J.M.; Young, S.; Wang, L.; Jebb, S.A.; Calder, P.C. Incorporation of Eicosapentaenoic and Docosahexaenoic Acids into Lipid Pools When given as Supplements Providing Doses Equivalent to Typical Intakes of Oily Fish. Am. J. Clin. Nutr. 2012, 96, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, A. WebPlotDigitizer, Version: 4.5. 2021. Available online: https://Automeris.Io/WebPlotDigitizer (accessed on 30 September 2022).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohsen, G.; Stroemer, A.; Mayr, A.; Kunsorg, A.; Stoppe, C.; Wittmann, M.; Velten, M. Effects of Omega-3 Fatty Acids on Postoperative Inflammatory Response: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 3414. https://doi.org/10.3390/nu15153414
Mohsen G, Stroemer A, Mayr A, Kunsorg A, Stoppe C, Wittmann M, Velten M. Effects of Omega-3 Fatty Acids on Postoperative Inflammatory Response: A Systematic Review and Meta-Analysis. Nutrients. 2023; 15(15):3414. https://doi.org/10.3390/nu15153414
Chicago/Turabian StyleMohsen, Ghaith, Annika Stroemer, Andreas Mayr, Andrea Kunsorg, Christian Stoppe, Maria Wittmann, and Markus Velten. 2023. "Effects of Omega-3 Fatty Acids on Postoperative Inflammatory Response: A Systematic Review and Meta-Analysis" Nutrients 15, no. 15: 3414. https://doi.org/10.3390/nu15153414
APA StyleMohsen, G., Stroemer, A., Mayr, A., Kunsorg, A., Stoppe, C., Wittmann, M., & Velten, M. (2023). Effects of Omega-3 Fatty Acids on Postoperative Inflammatory Response: A Systematic Review and Meta-Analysis. Nutrients, 15(15), 3414. https://doi.org/10.3390/nu15153414