The Association of the Hypothalamic-Pituitary-Adrenal Axis with Appetite Regulation in Children with Fetal Alcohol Spectrum Disorders (FASDs)
Abstract
1. Introduction
2. Materials and Methods
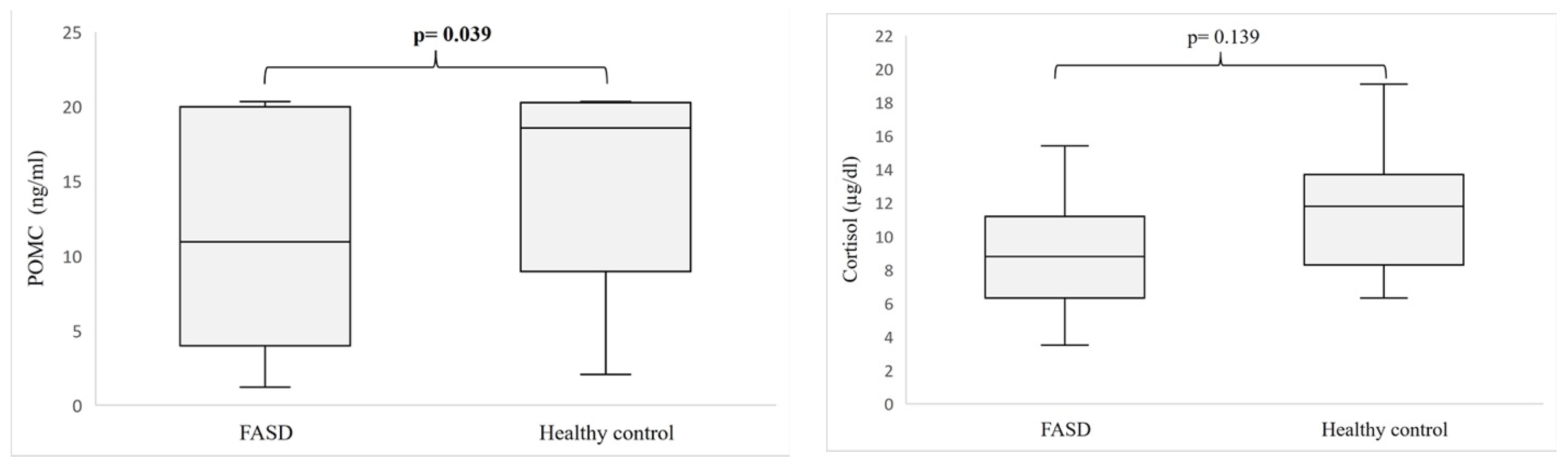
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lange, S.; Probst, C.; Gmel, G.; Rehm, J.; Burd, L.; Popova, S. Global Prevalence of Fetal Alcohol Spectrum Disorder Among Children and Youth: A Systematic Review and Meta-Analysis. JAMA Pediatr. 2017, 171, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.L.; Smith, D.W.; Ulleland, C.N.; Streissguth, P. Pattern of Malformation in Offspring of Chronic Alcoholic Mothers. Lancet 1973, 1, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, L.M.; Kearney, P.M.; McCarthy, F.P.; Khashan, A.S.; Greene, R.A.; North, R.A.; Poston, L.; McCowan, L.M.E.; Baker, P.N.; Dekker, G.A.; et al. Prevalence and Predictors of Alcohol Use during Pregnancy: Findings from International Multicentre Cohort Studies. BMJ Open 2015, 5, e006323. [Google Scholar] [CrossRef] [PubMed]
- Popova, S.; Lange, S.; Probst, C.; Gmel, G.; Rehm, J. Estimation of National, Regional, and Global Prevalence of Alcohol Use during Pregnancy and Fetal Alcohol Syndrome: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2017, 5, e290–e299. [Google Scholar] [CrossRef] [PubMed]
- Chasnoff, I.J.; Wells, A.M.; King, L. Misdiagnosis and Missed Diagnoses in Foster and Adopted Children with Prenatal Alcohol Exposure. Pediatrics 2015, 135, 264–270. [Google Scholar] [CrossRef]
- Hoyme, H.E.; Kalberg, W.O.; Elliott, A.J.; Blankenship, J.; Buckley, D.; Marais, A.-S.; Manning, M.A.; Robinson, L.K.; Adam, M.P.; Abdul-Rahman, O.; et al. Updated Clinical Guidelines for Diagnosing Fetal Alcohol Spectrum Disorders. Pediatrics 2016, 138, e20154256. [Google Scholar] [CrossRef]
- Astley, S.J. Validation of THE fetal alcohol spectrum Disorder (FASD) 4-digit diagnostic code. J. Popul. Ther. Clin. Pharmacol. 2013, 20, e416–e467. [Google Scholar]
- Cook, J.L.; Green, C.R.; Lilley, C.M.; Anderson, S.M.; Baldwin, M.E.; Chudley, A.E.; Conry, J.L.; LeBlanc, N.; Loock, C.A.; Lutke, J.; et al. Fetal Alcohol Spectrum Disorder: A Guideline for Diagnosis across the Lifespan. CMAJ 2016, 188, 191–197. [Google Scholar] [CrossRef]
- Bertrand, J.; Floyd, R.L.; Weber, M.K. Guidelines for Identifying and Referring Persons with Fetal Alcohol Syndrome. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2005, 54, 1-CE-4. [Google Scholar]
- Werts, R.L.; Van Calcar, S.C.; Wargowski, D.S.; Smith, S.M. Inappropriate Feeding Behaviors and Dietary Intakes in Children with Fetal Alcohol Spectrum Disorder or Probable Prenatal Alcohol Exposure. Alcohol Clin. Exp. Res. 2014, 38, 871–878. [Google Scholar] [CrossRef]
- Amos-Kroohs, R.M.; Fink, B.A.; Smith, C.J.; Chin, L.; Van Calcar, S.C.; Wozniak, J.R.; Smith, S.M. Abnormal Eating Behaviors Are Common in Children with Fetal Alcohol Spectrum Disorder. J. Pediatr. 2016, 169, 194–200.e1. [Google Scholar] [CrossRef]
- Sampson, P.D.; Bookstein, F.L.; Barr, H.M.; Streissguth, A.P. Prenatal Alcohol Exposure, Birthweight, and Measures of Child Size from Birth to Age 14 Years. Am. J. Public Health 1994, 84, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.C.; Jacobson, J.L.; Sokol, R.J.; Avison, M.J.; Jacobson, S.W. Fetal Alcohol-Related Growth Restriction from Birth through Young Adulthood and Moderating Effects of Maternal Prepregnancy Weight. Alcohol Clin. Exp. Res. 2013, 37, 452–462. [Google Scholar] [CrossRef]
- Day, N.L.; Leech, S.L.; Richardson, G.A.; Cornelius, M.D.; Robles, N.; Larkby, C. Prenatal Alcohol Exposure Predicts Continued Deficits in Offspring Size at 14 Years of Age. Alcohol. Clin. Exp. Res. 2002, 26, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Fuglestad, A.J.; Boys, C.J.; Chang, P.-N.; Miller, B.S.; Eckerle, J.K.; Deling, L.; Fink, B.A.; Hoecker, H.L.; Hickey, M.K.; Jimenez-Vega, J.M.; et al. Overweight and Obesity Among Children and Adolescents with Fetal Alcohol Spectrum Disorders. Alcohol. Clin. Exp. Res. 2014, 38, 2502–2508. [Google Scholar] [CrossRef] [PubMed]
- Druce, M. The Regulation of Appetite. Arch. Dis. Child. 2005, 91, 183–187. [Google Scholar] [CrossRef]
- Biebermann, H.; Kühnen, P.; Kleinau, G.; Krude, H. The Neuroendocrine Circuitry Controlled by POMC, MSH, and AGRP. In Appetite Control; Joost, H.-G., Ed.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 47–75. ISBN 978-3-642-24716-3. [Google Scholar]
- Wynne, K.; Stanley, S.; McGowan, B.; Bloom, S. Appetite Control. J. Endocrinol. 2005, 184, 291–318. [Google Scholar] [CrossRef] [PubMed]
- Adam, E.K.; Quinn, M.E.; Tavernier, R.; McQuillan, M.T.; Dahlke, K.A.; Gilbert, K.E. Diurnal Cortisol Slopes and Mental and Physical Health Outcomes: A Systematic Review and Meta-Analysis. Psychoneuroendocrinology 2017, 83, 25–41. [Google Scholar] [CrossRef]
- Okulicz-Kozaryn, K.; Maryniak, A.; Borkowska, M.; Śmigiel, R.; Dylag, K.A. Diagnosis of Fetal Alcohol Spectrum Disorders (FASDs): Guidelines of Interdisciplinary Group of Polish Professionals. Int. J. Environ. Res. Public. Health 2021, 18, 7526. [Google Scholar] [CrossRef]
- Wardlaw, S.L. Hypothalamic Proopiomelanocortin Processing and the Regulation of Energy Balance. Eur. J. Pharmacol. 2011, 660, 213–219. [Google Scholar] [CrossRef]
- Lee, M.; Kim, A.; Chua, S.C.; Obici, S.; Wardlaw, S.L. Transgenic MSH Overexpression Attenuates the Metabolic Effects of a High-Fat Diet. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E121–E131. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, S.L. Clinical Review 127: Obesity as a Neuroendocrine Disease: Lessons to Be Learned from Proopiomelanocortin and Melanocortin Receptor Mutations in Mice and Men. J. Clin. Endocrinol. Metab. 2001, 86, 1442–1446. [Google Scholar] [CrossRef]
- Zhan, C.; Zhou, J.; Feng, Q.; Zhang, J.; Lin, S.; Bao, J.; Wu, P.; Luo, M. Acute and Long-Term Suppression of Feeding Behavior by POMC Neurons in the Brainstem and Hypothalamus, Respectively. J. Neurosci. 2013, 33, 3624–3632. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.A. The Melanocortin System and Energy Balance. Peptides 2006, 27, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Yaswen, L.; Diehl, N.; Brennan, M.B.; Hochgeschwender, U. Obesity in the Mouse Model of Pro-Opiomelanocortin Deficiency Responds to Peripheral Melanocortin. Nat. Med. 1999, 5, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Coll, A.P.; Farooqi, I.S.; Challis, B.G.; Yeo, G.S.H.; O’Rahilly, S. Proopiomelanocortin and Energy Balance: Insights from Human and Murine Genetics. J. Clin. Endocrinol. Metab. 2004, 89, 2557–2562. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Cabrera, M.A.; Boyadjieva, N.I.; Berger, G.; Rousseau, B.; Sarkar, D.K. Alcohol Increases Exosome Release from Microglia to Promote Complement C1q-Induced Cellular Death of Proopiomelanocortin Neurons in the Hypothalamus in a Rat Model of Fetal Alcohol Spectrum Disorders. J. Neurosci. 2020, 40, 7965. [Google Scholar] [CrossRef]
- Bekdash, R.; Zhang, C.; Sarkar, D. Fetal Alcohol Programming of Hypothalamic Proopiomelanocortin System by Epigenetic Mechanisms and Later Life Vulnerability to Stress. Alcohol. Clin. Exp. Res. 2014, 38, 2323–2330. [Google Scholar] [CrossRef] [PubMed]
- Govorko, D.; Bekdash, R.A.; Zhang, C.; Sarkar, D.K. Male Germline Transmits Fetal Alcohol Adverse Effect on Hypothalamic Proopiomelanocortin Gene across Generations. Biol. Psychiatry 2012, 72, 378–388. [Google Scholar] [CrossRef]
- Galiniak, S.; Podgórski, R.; Rachel, M.; Mazur, A. Serum Kisspeptin and Proopiomelanocortin in Cystic Fibrosis: A Single Study. Sci. Rep. 2022, 12, 17669. [Google Scholar] [CrossRef]
- Escelsior, A.; Cogorno, L.; Sukkar, S.G.; Amerio, A.; Donini, L.M.; Bellomo, M.; Iervasi, E.; Amore, M.; Saverino, D. Anti-Hypothalamus Autoantibodies in Anorexia Nervosa: A Possible New Mechanism in Neuro-Physiological Derangement? Eat. Weight Disord.-Stud. Anorex. Bulim. Obes. 2022, 27, 2481–2496. [Google Scholar] [CrossRef] [PubMed]
- Page-Wilson, G.; Freda, P.U.; Jacobs, T.P.; Khandji, A.G.; Bruce, J.N.; Foo, S.T.; Meece, K.; White, A.; Wardlaw, S.L. Clinical Utility of Plasma POMC and AgRP Measurements in the Differential Diagnosis of ACTH-Dependent Cushing’s Syndrome. J. Clin. Endocrinol. Metab. 2014, 99, E1838–E1845. [Google Scholar] [CrossRef]
- Page-Wilson, G.; Meece, K.; White, A.; Rosenbaum, M.; Leibel, R.L.; Smiley, R.; Wardlaw, S.L. Proopiomelanocortin, Agouti-Related Protein, and Leptin in Human Cerebrospinal Fluid: Correlations with Body Weight and Adiposity. Am. J. Physiol.-Endocrinol. Metab. 2015, 309, E458–E465. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.K.; Kuhn, P.; Marano, J.; Chen, C.; Boyadjieva, N. Alcohol Exposure during the Developmental Period Induces Beta-Endorphin Neuronal Death and Causes Alteration in the Opioid Control of Stress Axis Function. Endocrinology 2007, 148, 2828–2834. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, L.; Fish, E.W.; O’Leary-Moore, S.K.; Parnell, S.E.; Sulik, K.K. Hypothalamic-Pituitary-Adrenal Axis and Behavioral Dysfunction Following Early Binge-like Prenatal Alcohol Exposure in Mice. Alcohol Fayettev. N 2015, 49, 207–217. [Google Scholar] [CrossRef]
- Gangisetty, O.; Bekdash, R.; Maglakelidze, G.; Sarkar, D.K. Fetal Alcohol Exposure Alters Proopiomelanocortin Gene Expression and Hypothalamic-Pituitary-Adrenal Axis Function via Increasing MeCP2 Expression in the Hypothalamus. PLoS ONE 2014, 9, e113228. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, A.; Priftis, K.N. Regulation of the Hypothalamic-Pituitary-Adrenal Axis. Neuroimmunomodulation 2009, 16, 265–271. [Google Scholar] [CrossRef]
- de Kloet, E.R.; Joëls, M.; Holsboer, F. Stress and the Brain: From Adaptation to Disease. Nat. Rev. Neurosci. 2005, 6, 463–475. [Google Scholar] [CrossRef]
- Podgórski, R.P.; Aebisher, D.; Stompor, M.; Podgórska, D.; Mazur, A. Congenital Adrenal Hyperplasia: Clinical Symptoms and Diagnostic Methods. Acta Biochim. Pol. 2018, 65, 25–33. [Google Scholar] [CrossRef]
- Roy, M.P.; Kirschbaum, C.; Steptoe, A. Psychological, Cardiovascular, and Metabolic Correlates of Individual Differences in Cortisol Stress Recovery in Young Men. Psychoneuroendocrinology 2001, 26, 375–391. [Google Scholar] [CrossRef]
- Riad, M.; Mogos, M.; Thangathurai, D.; Lumb, P.D. Steroids. Curr. Opin. Crit. Care 2002, 8, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Jirikowic, T.; Chen, M.; Nash, J.; Gendler, B.; Carmichael Olson, H. Regulatory Behaviors and Stress Reactivity among Infants at High Risk for Fetal Alcohol Spectrum Disorders: An Exploratory Study. J. Ment. Health Res. Intellect. Disabil. 2016, 9, 171–188. [Google Scholar] [CrossRef]
- Keiver, K.; Bertram, C.P.; Orr, A.P.; Clarren, S. Salivary Cortisol Levels Are Elevated in the Afternoon and at Bedtime in Children with Prenatal Alcohol Exposure. Alcohol 2015, 49, 79–87. [Google Scholar] [CrossRef]
- Ramsay, D.S.; Bendersky, M.I.; Lewis, M. Effect of Prenatal Alcohol and Cigarette Exposure on Two- and Six-Month-Old Infants’ Adrenocortical Reactivity to Stress1. J. Pediatr. Psychol. 1996, 21, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Hanlon-Dearman, A.; Chen, M.L.; Olson, H.C. Understanding and Managing Sleep Disruption in Children with Fetal Alcohol Spectrum Disorder. Biochem. Cell Biol. 2018, 96, 267–274. [Google Scholar] [CrossRef]
- Schuetze, P.; Lopez, F.A.; Granger, D.A.; Eiden, R.D. The Association between Prenatal Exposure to Cigarettes and Cortisol Reactivity and Regulation in 7-Month-Old Infants. Dev. Psychobiol. 2008, 50, 819–834. [Google Scholar] [CrossRef]
- Keller, J.; Flores, B.; Gomez, R.G.; Solvason, H.B.; Kenna, H.; Williams, G.H.; Schatzberg, A.F. Cortisol Circadian Rhythm Alterations in Psychotic Major Depression. Biol. Psychiatry 2006, 60, 275–281. [Google Scholar] [CrossRef]
- Tataranni, P.A.; Larson, D.E.; Snitker, S.; Young, J.B.; Flatt, J.P.; Ravussin, E. Effects of Glucocorticoids on Energy Metabolism and Food Intake in Humans. Am. J. Physiol. 1996, 271, E317–E325. [Google Scholar] [CrossRef]
- Epel, E.; Lapidus, R.; McEwen, B.; Brownell, K. Stress May Add Bite to Appetite in Women: A Laboratory Study of Stress-Induced Cortisol and Eating Behavior. Psychoneuroendocrinology 2001, 26, 37–49. [Google Scholar] [CrossRef]
- Jéquier, E. Leptin Signaling, Adiposity, and Energy Balance. Ann. N. Y. Acad. Sci. 2002, 967, 379–388. [Google Scholar] [CrossRef]
- Sinha, R.; Jastreboff, A.M. Stress as a Common Risk Factor for Obesity and Addiction. Biol. Psychiatry 2013, 73, 827–835. [Google Scholar] [CrossRef]
- Newell-Price, J.; Bertagna, X.; Grossman, A.B.; Nieman, L.K. Cushing’s Syndrome. Lancet 2006, 367, 1605–1617. [Google Scholar] [CrossRef]
- Rosmond, R.; Dallman, M.F.; Björntorp, P. Stress-Related Cortisol Secretion in Men: Relationships with Abdominal Obesity and Endocrine, Metabolic and Hemodynamic Abnormalities. J. Clin. Endocrinol. Metab. 1998, 83, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Poggioli, R.; Vergoni, A.V.; Bertolini, A. ACTH-(1-24) and Alpha-MSH Antagonize Feeding Behavior Stimulated by Kappa Opiate Agonists. Peptides 1986, 7, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Rivier, C. Alcohol Stimulates ACTH Secretion in the Rat: Mechanisms of Action and Interactions with Other Stimuli. Alcohol. Clin. Exp. Res. 1996, 20, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Fehm, H.L.; Smolnik, R.; Kern, W.; McGregor, G.P.; Bickel, U.; Born, J. The Melanocortin Melanocyte-Stimulating Hormone/Adrenocorticotropin(4-10) Decreases Body Fat in Humans. J. Clin. Endocrinol. Metab. 2001, 86, 1144–1148. [Google Scholar] [CrossRef]
- Krude, H.; Biebermann, H.; Schnabel, D.; Tansek, M.Z.; Theunissen, P.; Mullis, P.E.; Grüters, A. Obesity Due to Proopiomelanocortin Deficiency: Three New Cases and Treatment Trials with Thyroid Hormone and ACTH4-10. J. Clin. Endocrinol. Metab. 2003, 88, 4633–4640. [Google Scholar] [CrossRef]
- McLachlan, K.; Rasmussen, C.; Oberlander, T.F.; Loock, C.; Pei, J.; Andrew, G.; Reynolds, J.; Weinberg, J. Dysregulation of the Cortisol Diurnal Rhythm Following Prenatal Alcohol Exposure and Early Life Adversity. Alcohol 2016, 53, 9–18. [Google Scholar] [CrossRef]
- Belgardt, B.F.; Okamura, T.; Brüning, J.C. Hormone and Glucose Signalling in POMC and AgRP Neurons. J. Physiol. 2009, 587, 5305–5314. [Google Scholar] [CrossRef]
- Calixto, C.; Martinez, F.E.; Jorge, S.M.; Moreira, A.C.; Martinelli, C.E. Correlation between Plasma and Salivary Cortisol Levels in Preterm Infants. J. Pediatr. 2002, 140, 116–118. [Google Scholar] [CrossRef]
- Gallo-Payet, N. 60 YEARS OF POMC: Adrenal and Extra-Adrenal Functions of ACTH. J. Mol. Endocrinol. 2016, 56, T135–T156. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.M.; Clark, P. The Short Synacthen Test: Is Less Best? Clin. Endocrinol. 1999, 51, 151–152. [Google Scholar] [CrossRef]
- Knutsson, U.; Dahlgren, J.; Marcus, C.; Rosberg, S.; Brönnegård, M.; Stierna, P.; Albertsson-Wikland, K. Circadian Cortisol Rhythms in Healthy Boys and Girls: Relationship with Age, Growth, Body Composition, and Pubertal Development*. J. Clin. Endocrinol. Metab. 1997, 82, 536–540. [Google Scholar] [CrossRef]
- Dahl, R.E.; Siegel, S.F.; Williamson, D.E.; Lee, P.A.; Perel, J.; Birmaher, B.; Ryan, N.D. Corticotropin Releasing Hormone Stimulation Test and Nocturnal Cortisol Levels in Normal Children. Pediatr. Res. 1992, 32, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Martens, A.; Duran, B.; Vanbesien, J.; Verheyden, S.; Rutteman, B.; Staels, W.; Anckaert, E.; Gies, I.; De Schepper, J. Clinical and Biological Correlates of Morning Serum Cortisol in Children and Adolescents with Overweight and Obesity. PLoS ONE 2021, 16, e0258653. [Google Scholar] [CrossRef]
- Jonetz-Mentzel, L.; Wiedemann, G. Establishment of Reference Ranges for Cortisol in Neonates, Infants, Children and Adolescents. Eur. J. Clin. Chem. Clin. Biochem. J. Forum Eur. Clin. Chem. Soc. 1993, 31, 525–529. [Google Scholar] [CrossRef]
- Kiess, W.; Meidert, A.; Dressendörfer, R.A.; Schriever, K.; Kessler, U.; König, A.; Schwarz, H.P.; Strasburger, C.J. Salivary Cortisol Levels throughout Childhood and Adolescence: Relation with Age, Pubertal Stage, and Weight. Pediatr. Res. 1995, 37, 502–506. [Google Scholar] [CrossRef]

| FASD | Healthy Controls | p-Value | ||
|---|---|---|---|---|
| Sex (F/M) | 31/31 | 7/16 | ||
| Age (years) | mean ± SD | 7.52 ± 4.16 | 7.45 ± 5.12 | 0.847 |
| range | 0.42–16.5 | 0.42–17 | ||
| BMI percentile | mean ± SD | 32.38 ± 31.24 | 60.71 ± 27.03 | 0.035 |
| range | 0.1–99.9 | 12.0–99 | ||
| Clinical laboratory markers | ||||
| Cholesterol (mg/dL) Norm < 190 | median | 150 | 155 | 0.971 |
| range | 76–244 | 126–191 | ||
| LDL (mg/dL) Norm < 135 | median | 90 | 95 | 0.827 |
| range | 31–163 | 72–104 | ||
| HDL (mg/dL) Norm > 40 | median | 53 | 53 | 0.856 |
| range | 24–108 | 42–59 | ||
| Triglycerides (mg/dL) Norm < 150 | median | 74 | 65 | 0.753 |
| range | 30–241 | 38–141 | ||
| Glucose (mg/dL) Norm (70–99) | median | 84 | 87 | 0.669 |
| range | 72–99 | 68–94 | ||
| Insulin (mIU/mL) Norm < 15 | median | 4.85 | 2.05 | 0.167 |
| range | 1.25–17 | 1.0–9.03 | ||
| HbA1c (%) Normal range (4–6) | median | 5.36 | 5.41 | 0.774 |
| range | 4.71–5.86 | 5.26–5.55 | ||
| HOMA-IR Norm < 2.5 | median | 0.96 | - | - |
| range | 0.23–3.62 | - | ||
| FAS | ND-PAE | FASD Risk | p-Value | p-Value * | ||
|---|---|---|---|---|---|---|
| Sex (F/M) | 14/12 | 15/16 | 2/3 | |||
| Age (years) | mean ± SD | 7.91 ± 4.77 | 8.13 ± 3.32 | 2.25 ± 1.26 | 0.004 | 0.843 |
| range | 0.42–16.5 | 2.08–13.5 | 1.17–4.42 | |||
| BMI percentile | mean ± SD | 22.12 ± 27.51 | 42.04 ± 33.02 | 27.33 ± 13.87 | 0.053 | 0.020 |
| range | 0.1–78 | 0.1–99.9 | 12–39 | |||
| Clinical laboratory markers | ||||||
| Cholesterol (mg/dL) Norm < 190 | median | 154.5 | 161 | 141 | 0.277 | 0.110 |
| range | 76–238 | 114–244 | 104–185 | |||
| LDL (mg/dL) Norm < 135 | median | 86 | 75 | 84 | 0.598 | 0.365 |
| range | 31–143 | 114–244 | 33–119 | |||
| HDL (mg/dL) Norm > 40 | median | 49.5 | 53 | 46 | 0.515 | 0.382 |
| range | 33–80 | 24–108 | 33–71 | |||
| Triglycerides (mg/dL) Norm < 150 | median | 64 | 75 | 75 | 0.590 | 0.607 |
| range | 30.0–229 | 34–241 | 55–99 | |||
| Glucose (mg/dL) Norm (70–99) | median | 82 | 87 | 80 | 0.111 | 0.211 |
| range | 72–99 | 74–99 | 76–91 | |||
| Insulin (mIU/mL) Norm < 15 mIU/mL | median | 5.10 | 4.22 | 3.22 | 0.596 | 0.623 |
| range | 1.41–16.46 | 1.56–13.97 | 1.25–17 | |||
| HbA1c (%) Normal range (4–6) | median | 5.24 | 5.45 | 5.35 | 0.076 | 0.039 |
| range | 4.81–5.86 | 4.89–5.85 | 4.71–5.53 | |||
| HOMA-IR Norm < 2.5 | median | 1.07 | 1.29 | 1.73 | 0.591 | 0.790 |
| range | 0.27–3.62 | 0.31–3.53 | 0.23–3.36 | |||
| Hormone | FAS | ND-PAE | FASD Risk | p-Value | p-Value * | |
|---|---|---|---|---|---|---|
| POMC (ng/mL) | median | 9.35 | 15.93 | 11.22 | 0.725 | 0.479 |
| range | 1.22–20.32 | 1.51–20.32 | 3.92–16.84 | |||
| ACTH (pg/mL) | median | 24.9 | 17.7 | 14.3 | 0.942 | 0.401 |
| range | 12.3–38.6 | 5.0–37.6 | 12.5–66.1 | |||
| Cortisol (µg/dL) | median | 9.9 | 7.95 | 9.0 | 0.649 | 0.370 |
| range | 4.30–18.9 | 3.5–26.0 | 6.1–23.2 |
| Age | BMI Percentile | Cortisol | ACTH | Cholesterol | LDL | HDL | TGL | Glucose | Insulin | HOMA-IR | HbA1c | POMC | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| POMC | R | 0.370 | 0.285 | 0.015 | 0.386 | 0.193 | 0.126 | 0.154 | 0.177 | 0.292 | 0.475 | 0.473 | 0.076 | |
| p | 0.003 | 0.030 | 0.912 | 0.020 | 0.137 | 0.333 | 0.236 | 0.173 | 0.026 | <0.001 | <0.001 | 0.587 | ||
| ACTH | R | 0.304 | 0.306 | 0.607 | 0.335 | 0.262 | 0.208 | 0.175 | 0.152 | 0.183 | 0.189 | 0.045 | 0.386 | |
| p | 0.071 | 0.083 | <0.001 | 0.046 | 0.141 | 0.245 | 0.33 | 0.398 | 0.307 | 0.292 | 0.805 | 0.020 | ||
| Cortisol | R | 0.071 | −0.093 | 0.607 | 0.106 | 0.122 | −0.024 | 0.188 | −0.158 | −0.089 | −0.127 | −0.116 | 0.015 | |
| p | 0.592 | 0.497 | <0.001 | 0.444 | 0.379 | 0.866 | 0.172 | 0.268 | 0.543 | 0.386 | 0.424 | 0.912 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podgórski, R.; Galiniak, S.; Mazur, A.; Domin, A. The Association of the Hypothalamic-Pituitary-Adrenal Axis with Appetite Regulation in Children with Fetal Alcohol Spectrum Disorders (FASDs). Nutrients 2023, 15, 1366. https://doi.org/10.3390/nu15061366
Podgórski R, Galiniak S, Mazur A, Domin A. The Association of the Hypothalamic-Pituitary-Adrenal Axis with Appetite Regulation in Children with Fetal Alcohol Spectrum Disorders (FASDs). Nutrients. 2023; 15(6):1366. https://doi.org/10.3390/nu15061366
Chicago/Turabian StylePodgórski, Rafał, Sabina Galiniak, Artur Mazur, and Agnieszka Domin. 2023. "The Association of the Hypothalamic-Pituitary-Adrenal Axis with Appetite Regulation in Children with Fetal Alcohol Spectrum Disorders (FASDs)" Nutrients 15, no. 6: 1366. https://doi.org/10.3390/nu15061366
APA StylePodgórski, R., Galiniak, S., Mazur, A., & Domin, A. (2023). The Association of the Hypothalamic-Pituitary-Adrenal Axis with Appetite Regulation in Children with Fetal Alcohol Spectrum Disorders (FASDs). Nutrients, 15(6), 1366. https://doi.org/10.3390/nu15061366








