Abstract
The global market for nutritional supplements (NS) is growing rapidly, and the use of L-arginine (Arg), L-citrulline (Cit), and citrulline malate (CitMal) supplements has been shown to enhance cardiovascular health and athletic performance. Over the past decade, Arg, Cit, and CitMal supplements have received considerable attention from researchers in the field of exercise nutrition, who have investigated their potential effects on hemodynamic function, endothelial function, aerobic and anaerobic capacity, strength, power, and endurance. Previous studies were reviewed to determine the potential impact of Arg, Cit, and CitMal supplements on cardiovascular health and exercise performance. By synthesizing the existing literature, the study aimed to provide insight into the possible uses and limitations of these supplements for these purposes. The results showed that both recreational and trained athletes did not see improved physical performance or increased nitric oxide (NO) synthesis with 0.075 g or 6 g doses of Arg supplement per body weight. However, 2.4 to 6 g of Cit per day for 7 to 16 days of various NSs had a positive impact, increasing NO synthesis, enhancing athletic performance indicators, and reducing feelings of exertion. The effects of an 8 g acute dose of CitMal supplement were inconsistent, and more research is needed to determine its impact on muscle endurance performance. Based on the positive effects reported in previous studies, further testing is warranted in various populations that may benefit from nutritional supplements, including aerobic and anaerobic athletes, resistance-trained individuals, elderly people, and clinical populations, to determine the impact of different doses, timing of ingestion, and long-term and acute effects of Arg, Cit, and CitMal supplements on cardiovascular health and athletic performance.
1. Introduction
L-Arginine (Arg) and L-Citrulline (Cit) are amino acids (AA) that play important roles in the body, including in the production of nitric oxide (NO) and the removal of waste products during exercise [1]. The use of nutritional supplements (NS) for health promotion and athletic performance has become increasingly popular, and Arg and Cit are among the most commonly used supplements in these areas [1]. The global market for NS has shown continuous steady sales growth, with an estimated value of about $101.38 billion in 2018, rising to nearly $220.3 billion in 2020 and projected to reach $327.4 billion in 2030 [2,3]. Over half of adults consume NS daily, and industrial market regulations must be strengthened [4,5].
Functional drinks, and supplements that claim to enhance athletic performance, are popular among both recreational and elite athletes [6,7]. However, athletes should only consume products that are scientifically proven to be effective. Supplements with false or insufficient scientific evidence can negatively affect health and athletic performance, leading to doping problems for athletes [6,8]. Nevertheless, athletes continue to seek out convenient supplements to achieve outstanding performance in international and domestic competitions through athletic performance improvement [9].
The development and use of NS, which offers benefits such as disease prevention, improved performance, and recovery for athletes, has risen rapidly [10]. These products are often formulated in convenient forms, such as gels, bars, protein powders, pills, and beverages. Functional NS are a simple and effective means of quickly replenishing glucose, energy, and electrolytes during physical activity [11]. Existing functional NS have been scientifically verified for their benefits, such as those containing dietary fiber, vitamins, and probiotics [1]. Research on the effects of AA supplements is still ongoing [1].
This study used a narrative review method to evaluate the available literature on the effects of Arg and Cit supplementation on cardiovascular health and athletic performance. The researchers systematically searched multiple electronic databases, including PubMed, MEDLINE, and Scopus, for relevant studies published in English from 1 January 2010, to 10 November 2022. The search terms included “arginine”, “citrulline”, “citrulline malate”, “supplementation”, “cardiovascular health”, “hemodynamic function”, “endothelial function”, “anaerobic capacity”, “aerobic capacity”, “muscular strength”, “power”, “endurance performance”, and “athletic performance.” The inclusion criteria for the review were randomized controlled trials, systematic reviews, and meta-analyses that evaluated the effects of Arg and Cit supplementation on cardiovascular health and athletic performance in humans. The researchers analyzed and synthesized the findings from the selected studies to provide an overview of the effects of Arg and Cit supplementation on cardiovascular health and athletic performance. The review covered a range of outcomes, including endothelial function, blood pressure (BP), athletic performance, and muscle soreness. This narrative review method allowed the researchers to provide a comprehensive and descriptive summary of the available evidence on the effects of Arg and Cit supplementation on cardiovascular health and athletic performance.
2. Physiological Role of Arginine and Citrulline in Human
Arg has been shown to enhance physical performance [12,13,14,15], vascular endothelium function [16,17,18,19], and sexual function [20,21]. Additionally, it is a conditionally essential AA that plays a role in various physiological processes. Therefore, a substantial amount of Arg is required to perform various physiological functions, and a significant amount of ingested Arg (~40%) is decomposed in the intestine while the remainder is transported to the liver where it is metabolized into urea [22,23,24]. Arg increases NO production and draws attention from athletes as having a potential ergogenic advantage [25], since Arg might be able to generate NO through nitric oxide synthase (NOS) [26]. The endogenous synthesis of NO occurs through a process in which Arg is metabolized into Cit by NOS [23]. Arg is also an important precursor for the production of NO. Argininosuccinate lyase and argininosuccinate synthase in the liver recycles Cit into Arg to produce a Cit-Arg cycle that produces NO [23]. The pathways of NOS include neural nitric oxide synthase (nNOS), cytokine-induced nitric oxide synthase (iNOS), and endothelial nitric oxide synthase (eNOS) [27]. In skeletal muscle, nNOS is the predominant isoform and regulates blood flow and glucose uptake during exercise. eNOS is primarily expressed in endothelial cells and produces nitric oxide in response to various stimuli, including physical activity. Details of NOS through the Arg pathway are shown in Figure 1.
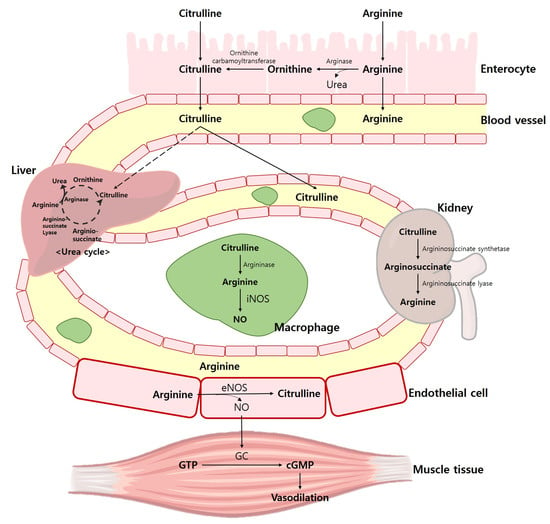
Figure 1.
NOS through the Arg and Cit pathway. iNOS, cytokine-induced nitric oxide synthase; eNOS, endothelial nitric oxide synthase; NO, nitric oxide; GC, guanylate cyclase; GTP, guanosine triphosphate; cGMP, cyclic guanosine monophosphate.
NO acts on a variety of physiological processes, such as vasodilation, mitochondrial respiration, glucose absorption, and muscle contraction [28,29,30]. The roles NO plays in the human body are related to the improvement of exercise performance [30,31,32,33]. Therefore, nitrites and nitrates are generally used to quantify plasma NO concentrations, and it is most important to maintain physiologically appropriate NO concentrations for skeletal muscle health [34]. Figure 2 illustrates the Arg and Cit pathway in exercise performance.
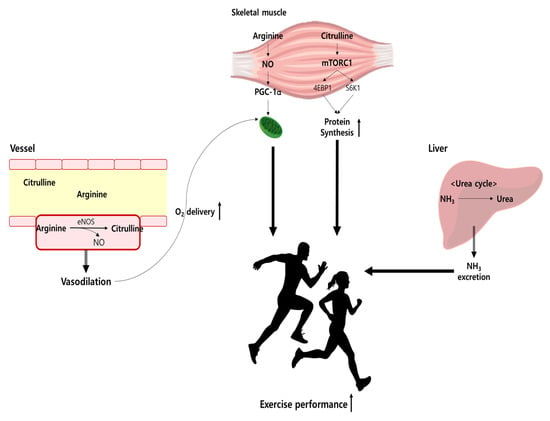
Figure 2.
Arg and Cit pathway in exercise performance. eNOS, endothelial nitric oxide synthase; NO, nitric oxide.
Intake of Arginine and Citrulline Supplements
Previous studies have reported that consuming three daily doses of 6 g/day of Arg along with small amounts of vitamins and branched-chain amino acids (BCAA), increased nitrite and plasma nitrate levels [32]. In addition, consuming Arg and other AA-rich NS, including Cit, BCAA, and fructose, decreased oxygen intake during moderate-intensity exercise, reduced plasma lactate production during exercise, and improved exercise tolerance during high-intensity exercise [32,35]. Taking an acute dose of 0.04 g/kg of Arg along with BCAA improved the sprint performance in handball athletes. However, consuming 6 g/day of Arg for three days did not improve intermittent anaerobic athletic performance in athletes [36]. Consuming 6 g of Arg for four weeks showed no change in hormone or metabolic parameters compared to exercise alone [37]. Both acute and chronic consumption of 5 g of Arg and two daily doses for 13 days were ineffective in improving cycling exercise performance in healthy young men [38]. Previous studies suggest that supplementing with Arg alone has limited impact on athletic performance, but combining it with other ingredients can improve exercise performance [39].
Cit is one of the non-essential AAs that can bypass liver metabolism to enhance Arg synthesis and improve NO bioavailability, as demonstrated in various studies [22,23,40,41]. Combined administration of Arg and Cit increases Arg concentration due to two AA synergies and improves NO biological availability [40,42]. The combination of Arg and Cit intake has been found to reduce energy consumption and enhance athletic performance more effectively than either Arg or Cit alone [42,43]. A study on elite taekwondo athletes who ingested Arg and Cit reported a reduction in exercise-induced central fatigue [44].
Interestingly, supplementation of Cit has been found to be more effective than Arg in increasing plasma Arg concentration [40,45,46]. Cit supplements can delay fatigue during high-intensity exercise by promoting ammonia removal and suppressing lactic acid accumulation in the blood through the urea cycle pathway [47]. Cit also improves the aerobic pathway by maintaining low plasma lactate concentrations [48]. However, the relationship between improved athletic performance and increased NO production in response to Cit supplementation is unclear [30]. Furthermore, previous studies that reported improved athletic performance with Cit supplementation used it in combination with malates and other components [30,49,50,51,52]. Most studies have reported that supplementation of Cit and malate is done in combination due to their synergistic coupling at the intramuscular level [52,53,54]. Malate, an intermediate product of the tricarboxylic acid cycle, can inhibit lactic acid production and increase energy production [52,55]. The efficacy of supplementing with citrulline malate (CitMal) cannot be defined solely as being related to Cit [1]. Further research is needed to determine the effectiveness of Cit independent of malate [56]. Previous studies evaluating the efficacy of Arg and Cit on NO biomarkers and athletic performance included BCAA or malate [39,53,56,57,58].
3. Effect of Cardiovascular Health
3.1. Hemodynamic Function
Arg is a substrate for numerous enzyme pathways including immune activation, vascular tone, and cell growth and is an AA obtained from dietary sources or produced endogenously [59]. In endothelial cells, Arg is metabolized to NO and Cit by NOS [59]. The positive effect of Arg supplementation on cardiometabolic markers, especially BP, insulin resistance, adiposity, and microvascular endothelial-dependent dilatation, has been well documented [60,61,62,63]. Studies have investigated the effect of Arg intake on resting BP in both healthy participants and mild hypertensive patients. The results showed that Arg-rich diets decreased systolic blood pressure (SBP) and diastolic blood pressure (DBP) [64]. In addition, daily supplementation with 3 to 12 g of Arg has been shown to prevent hypertension by reducing both SBP and DBP in mild hypertensive patients and in women with preeclampsia [65,66]. A meta-analysis showed decreased SBP and DBP in participants receiving 4–24 g/day (median: 9 g) over 2–24 weeks (median: 4 weeks) [67]. However, previous studies reported that Arg supplementation did not significantly change BP in mild chronic hypertensive pregnant women [68] and healthy young people [69]. This discrepancy may be due to various factors, including differences in participants, dosages, duration of intake, and BP ranges.
Cit is known to be an effective derivative of Arg that affects NO and cyclic guanosine monophosphate (cGMP) levels, but its effect on tissue perfusion in healthy subjects is not apparent [70,71,72,73]. Acute nitrate-Cit supplementation and placebo showed no difference in the post-ischemic vascular response measured by near-infrared spectroscopy in healthy young men [74]. However, other studies have reported an increase in muscle blood flow in healthy young participants after short-term (7 day) Cit supplementation during moderate-intensity exercise [73,75,76]. In healthy subjects, surrogate measurements of blood flow and endothelial function have not shown significant improvement, which may be due to physiological limitations of vascular compliance [72]. In addition, sympathetic nerve activation during exercise increases NO production in an eNOS-dependent manner in vascular endothelial cells, leading to local prioritization of systemic vasoconstriction in the arteries that supply active muscles [77]. These autoregulatory mechanisms may not be impacted by Cit supplementation in healthy individuals due to the intactness of the physiological process [78].
Cit, which is abundant in watermelons, is a non-essential AA [79] that can be metabolized to Arg. Arg is an essential AA that produces NO [79,80], which is responsible for its cardiac protective roles: from smooth muscle relaxation of blood vessels, induced by the NO-cGTP pathway to playing a crucial role in regulating BP [81]. Previous studies have reported that Cit supplementation increases the concentration of Arg in the human body and improves the biological availability of plasma Arg as a substrate for NO [54]. In a 6-week intervention study, supplementation with 6 g per day of watermelon extract containing both Cit and Arg led to a decrease in brachial and ankle SBP and DBP in pre-obese and hypertensive men, as well as a decrease in carotid augmentation index (AIx) [82]. A short-term intervention study (7–14 days) with 5.6 g of Cit supplementation per day reported a decrease in arterial stiffness in healthy, overweight middle-aged men [83,84]. A 6-week intervention study with 6 g per day of watermelon extract Cit supplementation in postmenopausal obese women receiving hypertension treatment, resulted in a decrease in arterial stiffness and aortic SBP, as well as a decrease in BP pulse wave reflection [85]. Watermelon-extract supplementation (Cit: 2.7 g/Arg: 1.3 g) was done for 6 weeks in middle-aged men and women with pre-hypertension, which led to a significant decrease in aortic SBP compared to the placebo controls, as well as a decrease in AIx and pulse wave velocity (PWV) [86]. In addition, previous studies have reported that Cit supplementation positively affects vascular wall stiffness measured by PWV and hypertension response to cold [82,83,84]. As some studies have proven the effect of reducing BP and arterial stiffness after Cit supplementation, but other studies have not demonstrated any effect, the impact of Cit supplementation on BP and arterial stiffness remains unclear [82,83,84,85,86,87,88,89,90,91]. The effects of Arg and Cit supplementation on hemodynamic function are summarized in detail in Table 1.

Table 1.
The effects of Arg and Cit supplementation on cardiovascular health.
3.2. Endothelial Function
Cit indirectly increases NO biosynthesis by increasing Arg synthesis, which can improve the endothelial-mediated vasodilation function [71,72,94]. A decrease in the synthesis of NO has been linked to an essential role in endothelial dysfunction related to cardiometabolic diseases, menopause, and aging [95,96,97]. Previous studies in rodent models showed that reducing the biological availability of Arg resulted in increased microcirculation blood flow and NO synthesis when supplementing with Cit rather than Arg [98].
Arg, cGMP activity, and nitrate/nitrite (NOx) were improved in healthy young individuals when supplementing with Cit [70,71,99]. However, despite significant increases in Arg bioavailability and urinary NOx, there was no improvement in endothelial function as measured by brachial artery flow mediated dilatation (FMD) with acute or short-term supplementation (≤7 days) [71,72,94]. In the previous study, superficial femoral artery FMD was improved as a result of taking 10 g of Cit supplement daily for 4 weeks in hypertensive postmenopausal women [93]. In addition, intake of 10 g of Cit supplements daily for 4 weeks improved brachial artery FMD in hypertensive postmenopausal women [16]. Studies that supplemented 10 g of Cit in healthy young individuals showed increases in Arg and NO synthesis [72], indicating that the benefits of Cit supplementation may depend on the acute time period and the participants’ health status [78].
Endothelial dysfunction due to aging is associated with a decrease in NO synthesis and Arg bioavailability [95,97,100]. A study supplementing 10 g of Cit acutely in older adults with heart failure reported increased de novo Arg and NO synthesis, but at a lower synthesis rate compared to younger individuals [72]. Previous studies in elderly male subjects reported no changes in plasma NOx or limb blood flow after rest and exercise and supplementing with Cit combined with whey protein alone or whey protein with other non-essential AA [94]. Even in healthy young participants, the intervention period may be an essential factor in determining the effect of FMD improvement from Cit supplementation. Previous studies found that eight weeks of supplementation with 800 mg of Cit per day was required to increase plasma Arg levels and improve FMD [101]. Additionally, 800 mg of Cit supplementation per day for eight weeks improves Arg/asymmetrical dimethylarginine levels and FMD in patients with vasospastic angina [101]. Studies on Arg supplementation and changes in FMD in response to Cit supplementation have shown similar results [16,93,102]. The variability in measurements in this field of study may be due to several factors, such as the relationship between the increase in the Arg/asymmetric dimethylarginine ratio and the improvement of FMD [78], or due to the reduction in NO production with increasing asymmetric dimethylarginine levels and aging-related endothelial dysfunction. Therefore, Cit or Arg replenishment may not improve endothelial function in the elderly [103].
Endothelial dysfunction caused by obesity-related insulin resistance is often mentioned as a major factor in the development of cardiovascular disease [96]. Different environmental factors such as fat cell-derived factors, high fat/high cholesterol diets, and aging contribute to the development of cardiovascular disease by exacerbating endothelial dysfunction and promoting low-grade inflammation [96,104,105,106,107]. The effects of supplementation of Arg and Cit on endothelial function are summarized in detail in Table 1.
4. Effect of Athletic Performance
4.1. Anaerobic and Aerobic Capacity
The supplementation of Arg has garnered attention for its potential to increase NO synthesis and improve athletic performance [58]. In earlier studies, male cyclists with training received either 0.075 g of Arg or a placebo per kg 60 min prior to completing a submaximal cycling exercise protocol [108]. Plasma metabolites were analyzed at various time points, including pre-supplementation (0 min), start of exercise (60 min), end of exercise (120 min), and end of rest (180 min) [108]. Results showed that the plasma Arg concentration of the supplement group significantly increased at all points from the start of exercise after Arg supplementation [108]. However, no significant difference was observed in plasma NOx concentration in the Arg supplement group [108]. In a different study, elite male judo athletes were given 6 g per day of supplemental Arg for 3 days, which resulted in a significant increase in plasma Arg concentration after 60 min compared to baseline. However, there was no significant difference in plasma NOx concentration compared to the placebo group after 60 min [36]. A study using an acute 6 g of Arg supplementation in resistance-trained physical education students reported no significant change in plasma nitrate concentration from pre-supplementation values till 60 min after exercise [109]. Other previous studies reported a tendency for plasma nitrite concentration to increase after acute intake of Arg supplementation at the same time points of 0 to 90 min, but no significant change was observed [110].
A study of 6 g of acute Arg supplementation in healthy male participants reported no significant difference in plasma NO markers at all points [111]. Similarly, studies of oral administration of 6 g of Arg or placebo daily for 1 month in healthy postmenopausal women reported no significant change in plasma NO synthesis [112]. The findings of previous studies indicate that a 6 g dose of Arg supplementation did not effectively increase NO levels. Recent meta-analysis studies have suggested that higher Arg supplement doses may be more effective [58], although a study of 10 g of Arg supplementation in male non-professional participants reported no significant change in plasma NOx concentrations [113]. In a study examining plasma Arg levels in active young men, both low and high doses of acute Arg supplementation reported similar effects and no effect on NO synthesis [114]. Studies of continuous 6 g of Arg supplementation daily for 4 weeks in trained runners reported insufficient evidence of significant increase in NO synthesis [37]. The ability to improve and maintain NO synthesis, which plays a vital role in vasodilation, is necessary for increased oxygen uptake in skeletal muscle [115]. The limited effect of Arg supplementation on NO synthesis may be associated with Arg metabolism. Depleted plasma Arg levels may fail to maintain NO synthesis [1]. Approximately 15% of Arg is metabolized in the liver and 60% in the gastrointestinal tract, and increased NO production through oral ingestion of Arg may be impaired [116]. Some previous studies have suggested that sheer vascular stress is considered a major stimulus for endothelial NO synthesis during exercise in healthy participants, and therefore Arg supplementation may be unnecessary [111]. However, Arg supplementation may be beneficial for those with endothelial dysfunction, which affects NO synthesis in participants with atherosclerosis risk factors [111]. Other previous studies reported that taking Arg supplements may not continuously improve muscle blood flow and endothelial function during exercise [69]. On the other hand, some studies have reported positive results, such as improved cardiac performance in moderate congestive heart failure patients [117].
The results of previous studies on the effects of Arg supplementation on athletic performance are mixed. In a study of wrestling elite athletes, one dose of 1.5 g of Arg supplement or placebo per 10 kg body weight was found to increase the time exhausted during incremental cycle ergometer testing compared to the placebo group, but there were no significant differences in heart rate and oxygen consumption [118]. Contrastingly, a study of male soccer players who consumed 2 g of Arg supplements per kg of body weight for 45 days reported an improvement in maximum oxygen consumption [92]. Curiously, previous studies that reported significant improvements did not measure plasma concentrations of Arg, NOx, and mechanism studies that reported improvements in athletic performance were not clear [1].
Additionally, studies have shown that combining Arg supplementation with BCAA, aspartic acid, or other AA may improve aerobic capacity in healthy participants [32,119,120]. Yet, in a study of aerobically trained cyclists consuming 0.075 g of Arg supplement per kg of body weight 60 min before submaximal cycling exercise, there was no significant difference in oxygen consumption, heart rate, SBP, and DBP, which were measured at the start and end of the 60 min cycling protocol [108]. Other previous studies reported no significant change in the time to exercise duration and the steady-state pulmonary oxygen uptake during moderate-intensity exercise after ingesting a 6 g of Arg supplement beverage [110]. In addition, chronic intake of Arg supplements did not result in improved athletic performance in well-trained endurance athletes [121]. Furthermore, a study of healthy young men taking 5 g of Arg or 5.5 g of dextrin twice a day for 13 days found no significant difference in mean power output during cycling performance [38]. Therefore, the available evidence suggests that oral Arg supplementation in healthy participants is ineffective in improving the physiological response associated with improving aerobic capacity and increasing NO synthesis.
Citrulline supplements are commonly available in three different forms: standalone Cit, CitMal, and watermelon juice [53]. Oral Cit supplementation increases NO synthesis by elevating plasma Arg concentration [122], and if Arg availability is limited, Cit supplementation can restore NO production [71]. Clinical studies examining the effects of acute or chronic Cit supplementation in several chronic patients, including those with heart failure, obesity, and arteriosclerosis, reported positive effects on NOx levels [123,124], however, inconsistent results were found in healthy participants [58]. In previous studies, the increase in blood NOx concentration following Cit supplement ingestion varied based on the dose and duration of intake [73,101]. In a study where recreationally active participants ingested 3.4 g of Cit per day from watermelon juice for 16 days, there was a significant increase in plasma nitrite and plasma Arg [73]. In a study of male collegiate track athletes who took 3 g of Cit supplements daily for seven days, plasma NOx concentrations were higher compared to baseline [125]. However, in most previous studies, Cit supplements were ineffective in changing NO biomarkers. A study comparing the effect on NO biomarkers after taking 6 g of Cit, Arg or placebo supplements per day for seven days in recreationally active male participants [75] showed that the plasma Cit and Arg concentrations were significantly increased after Cit supplementation, but plasma nitrite concentrations were not significantly increased [75]. In a study of 2.4 g of Cit supplementation per day for eight days in healthy trained men, plasma Arg concentration increased significantly following a cycling exercise protocol, but there was no difference in plasma NOx concentration [126]. Additionally, there was no significant difference in plasma NO observed in a study of swimmers who took 8 g of Cit, Arg or placebo supplements per day for eight days [127]. In a study conducted with 3 or 9 g of Cit supplementation and submaximal exercise protocols in recreationally active participants, plasma NOx concentrations were reported to be significantly reduced [128].
Ingestion of 6 g of Cit before completing a graded exercise test on a treadmill showed no significant change in maximum oxygen consumption or exhaustion time [129]. Acute intake of 6 g of Cit supplements in healthy and trained athletes may be insufficient to improve aerobic or anaerobic performance [53,129]. In addition, a study of 8 g of Cit supplementation for eight days in swimmers did not result in a reduction of 100 m or 200 m swimming trial times [127]. However, long-term supplementation may be more effective than acute intake of Cit supplements [39]. In a study where 3.4 g of Cit was consumed daily for 16 days in the form of watermelon juice concentrate, there was no difference in exhaustion time during a high-intensity exercise test despite an increase in plasma nitrite concentration [73].
A cycling performance evaluation in a study of 6 g of Cit supplementation per day for seven days in trained cyclists resulted in improved time trial performance, average power output, heart rate, and rate of perceived exertion (RPE) [130]. In a study of 6 g of Cit supplementation per day in participants of recreational activities, the mean arterial pressure and oxygen consumption mean response time were reduced, and total workload and tolerance to high-intensity of exercise were significantly improved [75]. As an AA intermediate product, Cit is known to play an essential role in the urea cycle, reducing ammonia toxicity in muscles [1]. In a study of male collegiate track athletes taking 3 g of Cit supplements daily for 7 days, RPE decreased, and oxygen consumption and average power output increased after performing intermittent short-time high-intensity protocols [125]. In a study of healthy trained men supplementing with 2.4 g of Cit per day for eight days, cycle ergometer trial completion times were reduced, average power output was increased, and subjective concentration and fatigue were also improved [126]. Cit supplements improve the aerobic pathway, lower plasma lactic acid concentrations during half marathon races, and alleviate post-race muscle soreness [48]. Decreased glycogen use in muscles during exercise leads to decreased plasma lactic acid and less reliance on anaerobic glycolysis in the energy metabolic system [47]. Supplementation with Cit enhances oxidative production of adenosine triphosphate (ATP) by inhibiting ammonia levels and glycolysis and preventing activation of phosphofructokinase, an important indicator of anaerobic glycolysis [48]. Detailed summaries of previous studies on the effects of Arg, Cit, and CitMal supplementation on anaerobic and aerobic capacity can be found in Table 2.

Table 2.
The effects of Arg, Cit, and CitMal supplementation on athletic performance.
4.2. Muscular Strength, Power, and Endurance Performances
The intake of standalone Arg supplements by well-trained athletes or healthy recreational participants does not appear to improve strength [39]. Forbes et al., conducted a study in which strength-trained males ingested 0.075 g of Arg supplements or placebo per kg of body weight 60 min prior to performing a resistance exercise protocol. The results showed an increase in growth hormone response over time, but no difference in RPE [131]. Additionally, researchers found no significant difference in isokinetic knee extension performance or bench press when resistance-trained physical education students consumed either 6 g of Arg supplement or placebo [109]. However, a study reported a significant reduction in peak torque for elbow flexion and extension after resistance exercise following the ingestion of 3 g of Arg supplementation by physically active male and female participants [136]. The ineffectiveness of Arg supplements may be related to the phenomenon of blunted growth hormone responses after resistance exercise, which resistance exercise alone can stimulate [140,141]. Arg supplements can inhibit endogenous hormones that inhibit growth hormone release and increase hormones that promote growth hormone secretion and insulin-like growth factor-1 [142,143]. However, oral administration of Arg supplements has not been shown to increase exercise-induced growth hormones [144]. In addition, a study of male bodybuilders reported a decrease in growth hormone response with specific AA intake [145]. However, a study of trained runners who took Arg supplements continuously for 4 weeks suggested an increase in growth hormone production [37]. A recent meta-analysis study reported that long-term intake of approximately 1.5 to 2 g of Arg per day can improve aerobic and anaerobic performance [58]. A study of long-term Arg supplementation in intensity-trained men found that it could improve maximal bench presses [13]. It is important to note that previous studies reporting the effectiveness of long-term Arg supplement intake also included other active ingredients, such as alpha-ketoglutaric acid, ornithine, and aspartic acid [119,121,143,146]. Most of the studies that reported significant improvement after taking Arg supplements contained additional compounds [147]. In addition, studies that investigated the consumption 6 g of Arg supplementation per day for acute or prolonged periods reported no benefit for muscle strength, endurance, or maximum number of repetitions [148].
Cit supplements may help alleviate fatigue when taken at a dose of 3 to 4 g 60 min prior to exercise [57]. A recent meta-analysis suggested that acute Cit supplementation can enhance power performance and high-intensity strength [56]. However, most previous studies have focused on the impact of combining Cit with malate [149]. Additionally, many studies have only investigated the effect of Cit supplementation on resistance exercise performance in individuals who meet pre-determined inclusion criteria [1]. In a study where athletes completed one repetition maximum (1RM), taking 6 g of Cit supplements and a sucrose mixture 60 and 120 min before performing a graded exercise test on a treadmill did not significantly improve exercise performance or change the number of repetitions during a chest press test [129]. Acute Cit supplementation alone has also been shown to not improve high-intensity exercise performance in previous studies [54]. A study that administered 2 g of Cit per day for 8 weeks along with strength training programs to resistance-trained male participants did not result in a significant improvement in bench press performance [123]. In the previous study, leg curl strength was improved as a result of taking 10 g of Cit supplement daily for 4 weeks and slow velocity low-intensity resistance training in hypertensive postmenopausal women [93]. In addition, intake of 10 g of Cit supplements daily for 12 weeks and high-intensity interval training improved upper limbs muscle strength and walking speed in dynapenic-obese elderly [139]. This lack of improvement may be due to the fact that Cit is not as effective on its own as when ingested with malate [1]. Malate, which helps reduce lactic acid production and increases ATP production during high-intensity anaerobic exercise, may improve athletic performance [51].
According to a review study, taking a single acute dose of 8 g of CitMal 1 h prior to exercise in resistance-trained men and women may improve dynamic muscle endurance and strength performance [53]. In a study involving resistance-trained men, the iteration of failure was improved in all sets of barbell bench presses (except for the first two sets) after ingestion of 8 g of CitMal 1 h before exercise [50]. Similar results were observed in a study of resistance-trained females, with improved performance responses during six sets of plate-loaded leg press and bench press exercises at 80% 1RM [132]. In addition, previous studies have also reported that taking 8 g of CitMal 1 h before exercise led to an increase in the number of repetitions to failure during bodyweight exercises, hack squats, and leg press at 60% 1RM [51,52]. The effect size of these results was insignificant, suggesting that CitMal supplementation effectiveness may be weaker in these scenarios. Nevertheless, small improvements in effect over multiple sessions could enhance training adaptation through training programs [149].
The efficacy of CitMal supplementation on resistance exercise was assessed through the implementation of the German volume training (GVT) protocol, which involved consuming 8 g of CitMal 1 h before exercise [137,150]. Results indicated that CitMal supplementation did not affect the number of repetitions to failures during isokinetic dynamometer leg curls or barbell curls. Furthermore, there was no significant change in maximal isometric, eccentric and concentric force [137]. The lack of performance improvement may be attributed to disproportionate ratios of Cit and malate in the CitMal compounds used, as well as the training methods and dosages employed. Some previous studies have failed to find a positive effect of CitMal supplementation on isokinetic or dynamic muscular power indices [56,133,135,138]. A study of resistance-trained males who consumed 8 g of CitMal prior to a 40-min resistance exercise session (five sets of up to 15 repetitions of barbell bench press exercises at 75% 1RM) reported no change in mean power, peak power, fatigue index, or total repetitions performed [133]. Another previous study found that 8 g of CitMal taken 2 h before exercise had no effect on peak torque, average torque, total work, or metabolic efficiency, blood flow, or lactic acid clearance during five sets of concentric leg extensions [56].
Studies reporting the potential to support the adaptation characteristics and muscle function recovery of human muscle structures following the intake of CitMal supplements remain limited [123,134]. A study examining the effects of 6 g of CitMal supplementation before exercise found no significant impact on muscle damage markers, lower extremity muscle endurance recovery, or electromyography activation at 24, 48, and 72 h after exercise [134]. Long-term supplementation of one of 2 g CitMal, 2 g Cit, 200 mg glutathione, and a placebo over 8 weeks had no effect on maximal muscle strength but showed a large effect on reducing fat mass [123]. The limited impact on performance and adaptation may be due to the reduced dose of CitMal used. However, CitMal supplementation has shown promise in reducing fat mass and helping to manage athlete body composition. Further research is needed to determine the long-term effects of CitMal intake, as well as to identify optimal dosages, timing, and duration for enhancing athletic performance and body composition. The effects of supplementation of Arg, Cit and CitMal on muscular strength, power, and endurance performance found in previous studies are summarized in detail in Table 2.
5. Conclusions and Future Prospects
Taken together, the results of studies conducted on both recreational athletes and trained athletes show that supplementing with 0.075 g or 6 g of Arg per kg body weight did not enhance physical performance and perceptual feeling of exercise or increase NO synthesis. In addition, consuming 2.4 to 6 g of Cit per day for 7 to 16 days of various NSs increased NO synthesis, improved athletic performance, and reduced feelings of exertion. The results of acute supplementation with 8 g of CitMal are inconsistent but may increase muscle endurance, warranting further investigation. Given the positive outcomes reported in prior studies, it is recommended to conduct additional testing on the impact of various forms of Arg, Cit, and CitMal supplementation, including acute and long-term or loading doses, and varying timing of ingestion, on cardiovascular health and athletic performance in various populations (i.e., aerobic and anaerobic athletes, resistance-trained individuals, the elderly, and clinical populations) that may benefit from NS. Furthermore, studies comparing the effects of Cit and CitMal are limited. It is unclear whether malate, Cit, or both, are responsible for the effects observed in studies on CitMal supplementation. Recent research has shown that exercise can have a positive effect on the gut microbiota [151]. For example, studies have found that regular exercise can increase the diversity and abundance of beneficial bacteria in the gut, while also reducing the number of harmful bacteria. This can lead to improved gut health, a stronger immune system, and better overall health outcomes. Future studies should continue to investigate the potential synergistic effects of Arg, Cit, and CitMal supplements on various factors such as body composition, cardiovascular function, cognitive function, and muscle quality.
Author Contributions
Conceptualization, H.-Y.P., S.-W.K. and Y.P.J.; investigation, S.-W.K., J.S., H.K., A.-J.K. and S.K.; writing—original draft preparation S.-W.K.; writing—review and editing, H.-Y.P., Y.P.J. and H.K.; visualization, S.-W.K. and J.S.; supervision, K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nyawose, S.; Naidoo, R.; Naumovski, N.; McKune, A.J. The Effects of Consuming Amino Acids L-Arginine, L-Citrulline (and Their Combination) as a Beverage or Powder, on Athletic and Physical Performance: A Systematic Review. Beverages 2022, 8, 48. [Google Scholar] [CrossRef]
- Hys, K. Identification of the reasons why individual consumers purchase dietary supplements. In Perspectives on Consumer Behaviour; Springer: Cham, Switzerland, 2020; pp. 193–209. [Google Scholar]
- Research, G.V. Dietary Supplements Market Size Worth $327.4 Billion By 2030. Available online: https://www.grandviewresearch.com/press-release/global-dietary-supplements-market# (accessed on 15 November 2022).
- Binns, C.W.; Lee, M.K.; Lee, A.H. Problems and Prospects: Public Health Regulation of Dietary Supplements. Annu. Rev. Public Health 2018, 39, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.A.; Bass, S. Injecting Safety into Supplements—Modernizing the Dietary Supplement Law. N. Engl. J. Med. 2019, 381, 2387–2389. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC consensus statement: Dietary supplements and the high-performance athlete. Br. J. Sports Med. 2018, 52, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Rawson, E.S.; Miles, M.P.; Larson-Meyer, D.E. Dietary Supplements for Health, Adaptation, and Recovery in Athletes. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 188–199. [Google Scholar] [CrossRef]
- Begg, P.M.; Wheatley, V.M. Fraud in dietary supplements. In Food Fraud; Academic Press: Cambridge, MA, USA, 2021; pp. 351–360. [Google Scholar]
- Gibson, M.E.; Schultz, J.; Glover, D. To Supplement or Not. Mo. Med. 2016, 113, 305–309. [Google Scholar]
- Collins, J.; Maughan, R.J.; Gleeson, M.; Bilsborough, J.; Jeukendrup, A.; Morton, J.P.; Phillips, S.M.; Armstrong, L.; Burke, L.M.; Close, G.L.; et al. UEFA expert group statement on nutrition in elite football. Current evidence to inform practical recommendations and guide future research. Br. J. Sports Med. 2021, 55, 416. [Google Scholar] [CrossRef] [PubMed]
- Orrù, S.; Imperlini, E.; Nigro, E.; Alfieri, A.; Cevenini, A.; Polito, R.; Daniele, A.; Buono, P.; Mancini, A. Role of Functional Beverages on Sport Performance and Recovery. Nutrients 2018, 10, 1470. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.; Gholamalizadeh, M.; Tabrizi, R.; Nowrouzi-Sohrabi, P.; Rastgoo, S.; Doaei, S. The effect of L-arginine supplementation on maximal oxygen uptake: A systematic review and meta-analysis. Physiol. Rep. 2021, 9, e14739. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.; Roberts, M.; Kerksick, C.; Wilborn, C.; Marcello, B.; Taylor, L.; Nassar, E.; Leutholtz, B.; Bowden, R.; Rasmussen, C.; et al. Pharmacokinetics, safety, and effects on exercise performance of L-arginine alpha-ketoglutarate in trained adult men. Nutrition 2006, 22, 872–881. [Google Scholar] [CrossRef]
- Fricke, O.; Baecker, N.; Heer, M.; Tutlewski, B.; Schoenau, E. The effect of L-arginine administration on muscle force and power in postmenopausal women. Clin. Physiol. Funct. Imaging 2008, 28, 307–311. [Google Scholar] [CrossRef]
- Koppo, K.; Taes, Y.E.; Pottier, A.; Boone, J.; Bouckaert, J.; Derave, W. Dietary arginine supplementation speeds pulmonary VO2 kinetics during cycle exercise. Med. Sci. Sports Exerc. 2009, 41, 1626–1632. [Google Scholar] [CrossRef]
- Maharaj, A.; Fischer, S.M.; Dillon, K.N.; Kang, Y.; Martinez, M.A.; Figueroa, A. Effects of L-Citrulline Supplementation on Endothelial Function and Blood Pressure in Hypertensive Postmenopausal Women. Nutrients 2022, 14, 4396. [Google Scholar] [CrossRef] [PubMed]
- Bode-Böger, S.M.; Muke, J.; Surdacki, A.; Brabant, G.; Böger, R.H.; Frölich, J.C. Oral L-arginine improves endothelial function in healthy individuals older than 70 years. Vasc. Med. 2003, 8, 77–81. [Google Scholar] [CrossRef]
- Lin, C.C.; Tsai, W.C.; Chen, J.Y.; Li, Y.H.; Lin, L.J.; Chen, J.H. Supplements of L-arginine attenuate the effects of high-fat meal on endothelial function and oxidative stress. Int. J. Cardiol. 2008, 127, 337–341. [Google Scholar] [CrossRef]
- Siasos, G.; Tousoulis, D.; Vlachopoulos, C.; Antoniades, C.; Stefanadi, E.; Ioakeimidis, N.; Andreou, I.; Zisimos, K.; Papavassiliou, A.G.; Stefanadis, C. Short-term treatment with L-arginine prevents the smoking-induced impairment of endothelial function and vascular elastic properties in young individuals. Int. J. Cardiol. 2008, 126, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wollman, Y.; Chernichovsky, T.; Iaina, A.; Sofer, M.; Matzkin, H. Effect of oral administration of high-dose nitric oxide donor L-arginine in men with organic erectile dysfunction: Results of a double-blind, randomized, placebo-controlled study. BJU Int. 1999, 83, 269–273. [Google Scholar] [CrossRef]
- Menafra, D.; de Angelis, C.; Garifalos, F.; Mazzella, M.; Galdiero, G.; Piscopo, M.; Castoro, M.; Verde, N.; Pivonello, C.; Simeoli, C.; et al. Long-term high-dose L-arginine supplementation in patients with vasculogenic erectile dysfunction: A multicentre, double-blind, randomized, placebo-controlled clinical trial. J. Endocrinol. Investig. 2022, 45, 941–961. [Google Scholar] [CrossRef]
- van de Poll, M.C.; Soeters, P.B.; Deutz, N.E.; Fearon, K.C.; Dejong, C.H. Renal metabolism of amino acids: Its role in interorgan amino acid exchange. Am. J. Clin. Nutr. 2004, 79, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Curis, E.; Nicolis, I.; Moinard, C.; Osowska, S.; Zerrouk, N.; Bénazeth, S.; Cynober, L. Almost all about citrulline in mammals. Amino Acids 2005, 29, 177–205. [Google Scholar] [CrossRef]
- Hou, X.; Chen, S.; Zhang, P.; Guo, D.; Wang, B. Targeted Arginine Metabolism Therapy: A Dilemma in Glioma Treatment. Front. Oncol. 2022, 12, 938847. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.J.; Williams, S.A.; Canale, R.E.; Farney, T.M.; Kabir, M.M. Acute effect of nitric oxide supplement on blood nitrate/nitrite and hemodynamic variables in resistance trained men. J. Strength Cond. Res. 2010, 24, 2587–2592. [Google Scholar] [CrossRef]
- Lorin, J.; Zeller, M.; Guilland, J.C.; Cottin, Y.; Vergely, C.; Rochette, L. Arginine and nitric oxide synthase: Regulatory mechanisms and cardiovascular aspects. Mol. Nutr. Food Res. 2014, 58, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Szefel, J.; Danielak, A.; Kruszewski, W.J. Metabolic pathways of L-arginine and therapeutic consequences in tumors. Adv. Med. Sci. 2019, 64, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Maté-Muñoz, J.L.; Cuenca, E.; García-Fernández, P.; Mata-Ordoñez, F.; Lozano-Estevan, M.C.; Veiga-Herreros, P.; da Silva, S.F.; Garnacho-Castaño, M.V. Effects of beetroot juice supplementation on intermittent high-intensity exercise efforts. J. Int. Soc. Sport Nutr. 2018, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef]
- Jones, A.M. Dietary nitric oxide precursors and exercise performance. Sports Sci. Exch. 2016, 28, 1–6. [Google Scholar]
- Álvares, T.S.; Meirelles, C.M.; Bhambhani, Y.N.; Paschoalin, V.M.; Gomes, P.S. L-Arginine as a potential ergogenic aid in healthy subjects. Sports Med. 2011, 41, 233–248. [Google Scholar] [CrossRef]
- Bailey, S.J.; Winyard, P.G.; Vanhatalo, A.; Blackwell, J.R.; DiMenna, F.J.; Wilkerson, D.P.; Jones, A.M. Acute L-arginine supplementation reduces the O2 cost of moderate-intensity exercise and enhances high-intensity exercise tolerance. J. Appl. Physiol. 2010, 109, 1394–1403. [Google Scholar] [CrossRef]
- Wylie, L.J.; Mohr, M.; Krustrup, P.; Jackman, S.R.; Ermιdis, G.; Kelly, J.; Black, M.I.; Bailey, S.J.; Vanhatalo, A.; Jones, A.M. Dietary nitrate supplementation improves team sport-specific intense intermittent exercise performance. Eur. J. Appl. Physiol. 2013, 113, 1673–1684. [Google Scholar] [CrossRef]
- Piknova, B.; Park, J.W.; Cassel, K.S.; Gilliard, C.N.; Schechter, A.N. Measuring Nitrite and Nitrate, Metabolites in the Nitric Oxide Pathway, in Biological Materials using the Chemiluminescence Method. J. Vis. Exp. 2016, 118, e54879. [Google Scholar] [CrossRef]
- Schaefer, A.; Piquard, F.; Geny, B.; Doutreleau, S.; Lampert, E.; Mettauer, B.; Lonsdorfer, J. L-arginine reduces exercise-induced increase in plasma lactate and ammonia. Int. J. Sport Med. 2002, 23, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.H.; Wu, C.L.; Chiang, C.W.; Lo, Y.W.; Tseng, H.F.; Chang, C.K. No effect of short-term arginine supplementation on nitric oxide production, metabolism and performance in intermittent exercise in athletes. J. Nutr. Biochem. 2009, 20, 462–468. [Google Scholar] [CrossRef]
- Alvares, T.S.; Conte-Junior, C.A.; Silva, J.T.; Paschoalin, V.M. L-arginine does not improve biochemical and hormonal response in trained runners after 4 weeks of supplementation. Nutr. Res. 2014, 34, 31–39. [Google Scholar] [CrossRef]
- Hiratsu, A.; Tataka, Y.; Namura, S.; Nagayama, C.; Hamada, Y.; Miyashita, M. The effects of acute and chronic oral l-arginine supplementation on exercise-induced ammonia accumulation and exercise performance in healthy young men: A randomised, double-blind, cross-over, placebo-controlled trial. J. Exerc. Sci. Fit. 2022, 20, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M. Dietary nitrate supplementation and exercise performance. Sport Med. 2014, 44 (Suppl. S1), 35–45. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Morita, M.; Hayashi, T.; Kamimura, A. The effects on plasma L-arginine levels of combined oral L-citrulline and L-arginine supplementation in healthy males. Biosci. Biotechnol. Biochem. 2017, 81, 372–375. [Google Scholar] [CrossRef]
- Morita, M.; Hayashi, T.; Ochiai, M.; Maeda, M.; Yamaguchi, T.; Ina, K.; Kuzuya, M. Oral supplementation with a combination of L-citrulline and L-arginine rapidly increases plasma L-arginine concentration and enhances NO bioavailability. Biochem. Biophys. Res. Commun. 2014, 454, 53–57. [Google Scholar] [CrossRef]
- Suzuki, I.; Sakuraba, K.; Horiike, T.; Kishi, T.; Yabe, J.; Suzuki, T.; Morita, M.; Nishimura, A.; Suzuki, Y. A combination of oral L-citrulline and L-arginine improved 10-min full-power cycling test performance in male collegiate soccer players: A randomized crossover trial. Eur. J. Appl. Physiol. 2019, 119, 1075–1084. [Google Scholar] [CrossRef]
- Mor, A.; Atan, T.; Agaoglu, S.A.; Ayyildiz, M. Effect of arginine supplementation on footballers’ anaerobic performance and recovery. Prog. Nutr. 2018, 20, 104–112. [Google Scholar]
- Chen, I.F.; Wu, H.J.; Chen, C.Y.; Chou, K.M.; Chang, C.K. Branched-chain amino acids, arginine, citrulline alleviate central fatigue after 3 simulated matches in taekwondo athletes: A randomized controlled trial. J. Int. Soc. Sport Nutr. 2016, 13, 28. [Google Scholar] [CrossRef]
- Agarwal, U.; Didelija, I.C.; Yuan, Y.; Wang, X.; Marini, J.C. Supplemental Citrulline Is More Efficient Than Arginine in Increasing Systemic Arginine Availability in Mice. J. Nutr. 2017, 147, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Su, Y.T.; Liu, T.Y.; Tsai, C.M.; Chang, C.H.; Yu, H.R. L-Arginine and L-Citrulline Supplementation Have Different Programming Effect on Regulatory T-Cells Function of Infantile Rats. Front. Immunol. 2018, 9, 2911. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Machida, M.; Kohara, A.; Omi, N.; Takemasa, T. Effects of citrulline supplementation on fatigue and exercise performance in mice. J. Nutr. Sci. Vitaminol. 2011, 57, 246–250. [Google Scholar] [CrossRef]
- Martínez-Sánchez, A.; Ramos-Campo, D.J.; Fernández-Lobato, B.; Rubio-Arias, J.A.; Alacid, F.; Aguayo, E. Biochemical, physiological, and performance response of a functional watermelon juice enriched in L-citrulline during a half-marathon race. Food Nutr. Res. 2017, 61, 1330098. [Google Scholar] [CrossRef]
- Glenn, J.M.; Gray, M.; Jensen, A.; Stone, M.S.; Vincenzo, J.L. Acute citrulline-malate supplementation improves maximal strength and anaerobic power in female, masters athletes tennis players. Eur. J. Sport Sci. 2016, 16, 1095–1103. [Google Scholar] [CrossRef]
- Pérez-Guisado, J.; Jakeman, P.M. Citrulline malate enhances athletic anaerobic performance and relieves muscle soreness. J. Strength Cond. Res. 2010, 24, 1215–1222. [Google Scholar] [CrossRef]
- Wax, B.; Kavazis, A.N.; Luckett, W. Effects of Supplemental Citrulline-Malate Ingestion on Blood Lactate, Cardiovascular Dynamics, and Resistance Exercise Performance in Trained Males. J. Diet. Suppl. 2016, 13, 269–282. [Google Scholar] [CrossRef]
- Wax, B.; Kavazis, A.N.; Weldon, K.; Sperlak, J. Effects of supplemental citrulline malate ingestion during repeated bouts of lower-body exercise in advanced weightlifters. J. Strength Cond. Res. 2015, 29, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.M.; Trexler, E.T. Effects of Citrulline Supplementation on Exercise Performance in Humans: A Review of the Current Literature. J. Strength Cond. Res. 2020, 34, 1480–1495. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Wong, A.; Jaime, S.J.; Gonzales, J.U. Influence of L-citrulline and watermelon supplementation on vascular function and exercise performance. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Bendahan, D.; Mattei, J.P.; Ghattas, B.; Confort-Gouny, S.; Le Guern, M.E.; Cozzone, P.J. Citrulline/malate promotes aerobic energy production in human exercising muscle. Br. J. Sports Med. 2002, 36, 282–289. [Google Scholar] [CrossRef]
- Trexler, E.T.; Persky, A.M.; Ryan, E.D.; Schwartz, T.A.; Stoner, L.; Smith-Ryan, A.E. Acute Effects of Citrulline Supplementation on High-Intensity Strength and Power Performance: A Systematic Review and Meta-Analysis. Sport Med. 2019, 49, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Rhim, H.C.; Kim, S.J.; Park, J.; Jang, K.M. Effect of citrulline on post-exercise rating of perceived exertion, muscle soreness, and blood lactate levels: A systematic review and meta-analysis. J. Sport Health Sci. 2020, 9, 553–561. [Google Scholar] [CrossRef]
- Viribay, A.; Burgos, J.; Fernández-Landa, J.; Seco-Calvo, J.; Mielgo-Ayuso, J. Effects of Arginine Supplementation on Athletic Performance Based on Energy Metabolism: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1300. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, D.; Krüger, M.; Wehland, M.; Infanger, M.; Grimm, D. The Effects of Oral L-Arginine and L-Citrulline Supplementation on Blood Pressure. Nutrients 2019, 11, 1679. [Google Scholar] [CrossRef]
- McNeal, C.J.; Meininger, C.J.; Reddy, D.; Wilborn, C.D.; Wu, G. Safety and Effectiveness of Arginine in Adults. J. Nutr. 2016, 146, S2587–S2593. [Google Scholar] [CrossRef]
- Ástvaldsdóttir, Á.; Naimi-Akbar, A.; Davidson, T.; Brolund, A.; Lintamo, L.; Granath, A.A.; Tranæus, S.; Östlund, P. Arginine and Caries Prevention: A Systematic Review. Caries Res. 2016, 50, 383–393. [Google Scholar] [CrossRef]
- Melik, Z.; Zaletel, P.; Virtic, T.; Cankar, K. L-Arginine as dietary supplement for improving microvascular function. Clin. Hemorheol. Microcirc. 2017, 65, 205–217. [Google Scholar] [CrossRef]
- Zarezadeh, M.; Emami, M.R.; Kord-Varkane, H.; Mousavi, S.M.; Alizadeh, H.; Asbaghi, O.; Olang, B.; Khorshidi, M. The effect of oral L-arginine supplementation on asymmetric dimethylarginine levels: A systematic review and meta-analysis of randomized clinical trials. Adv. Integr. Med. 2020, 7, 61–66. [Google Scholar] [CrossRef]
- Siani, A.; Pagano, E.; Iacone, R.; Iacoviello, L.; Scopacasa, F.; Strazzullo, P. Blood pressure and metabolic changes during dietary L-arginine supplementation in humans. Am. J. Hypertens. 2000, 13, 547–551. [Google Scholar] [CrossRef]
- Ast, J.; Jablecka, A.; Bogdanski, P.; Smolarek, I.; Krauss, H.; Chmara, E. Evaluation of the antihypertensive effect of L-arginine supplementation in patients with mild hypertension assessed with ambulatory blood pressure monitoring. Med. Sci. Monit. 2010, 16, CR266–CR271. [Google Scholar] [CrossRef] [PubMed]
- Rytlewski, K.; Olszanecki, R.; Korbut, R.; Zdebski, Z. Effects of prolonged oral supplementation with l-arginine on blood pressure and nitric oxide synthesis in preeclampsia. Eur. J. Clin. Investig. 2005, 35, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Y.; Qin, L.Q.; Zhang, Z.; Zhao, Y.; Wang, J.; Arigoni, F.; Zhang, W. Effect of oral L-arginine supplementation on blood pressure: A meta-analysis of randomized, double-blind, placebo-controlled trials. Am. Heart J. 2011, 162, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Neri, I.; Monari, F.; Sgarbi, L.; Berardi, A.; Masellis, G.; Facchinetti, F. L-arginine supplementation in women with chronic hypertension: Impact on blood pressure and maternal and neonatal complications. J. Matern. Neonatal Med. 2010, 23, 1456–1460. [Google Scholar] [CrossRef] [PubMed]
- Chin-Dusting, J.P.; Alexander, C.T.; Arnold, P.J.; Hodgson, W.C.; Lux, A.S.; Jennings, G.L. Effects of in vivo and in vitro L-arginine supplementation on healthy human vessels. J. Cardiovasc. Pharmacol. 1996, 28, 158–166. [Google Scholar] [CrossRef]
- Moinard, C.; Nicolis, I.; Neveux, N.; Darquy, S.; Bénazeth, S.; Cynober, L. Dose-ranging effects of citrulline administration on plasma amino acids and hormonal patterns in healthy subjects: The Citrudose pharmacokinetic study. Br. J. Nutr. 2008, 99, 855–862. [Google Scholar] [CrossRef]
- Schwedhelm, E.; Maas, R.; Freese, R.; Jung, D.; Lukacs, Z.; Jambrecina, A.; Spickler, W.; Schulze, F.; Böger, R.H. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: Impact on nitric oxide metabolism. Br. J. Clin. Pharmacol. 2008, 65, 51–59. [Google Scholar] [CrossRef]
- Kim, I.Y.; Schutzler, S.E.; Schrader, A.; Spencer, H.J.; Azhar, G.; Deutz, N.E.; Wolfe, R.R. Acute ingestion of citrulline stimulates nitric oxide synthesis but does not increase blood flow in healthy young and older adults with heart failure. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E915–E924. [Google Scholar] [CrossRef]
- Bailey, S.J.; Blackwell, J.R.; Williams, E.; Vanhatalo, A.; Wylie, L.J.; Winyard, P.G.; Jones, A.M. Two weeks of watermelon juice supplementation improves nitric oxide bioavailability but not endurance exercise performance in humans. Nitric Oxide 2016, 59, 10–20. [Google Scholar] [CrossRef]
- Le Roux-Mallouf, T.; Vibert, F.; Doutreleau, S.; Verges, S. Effect of acute nitrate and citrulline supplementation on muscle microvascular response to ischemia-reperfusion in healthy humans. Appl. Physiol. Nutr. Metab. 2017, 42, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.J.; Blackwell, J.R.; Lord, T.; Vanhatalo, A.; Winyard, P.G.; Jones, A.M. l-Citrulline supplementation improves O2 uptake kinetics and high-intensity exercise performance in humans. J. Appl. Physiol. 2015, 119, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sánchez, A.; Alacid, F.; Rubio-Arias, J.A.; Fernández-Lobato, B.; Ramos-Campo, D.J.; Aguayo, E. Consumption of Watermelon Juice Enriched in l-Citrulline and Pomegranate Ellagitannins Enhanced Metabolism during Physical Exercise. J. Agric. Food Chem. 2017, 65, 4395–4404. [Google Scholar] [CrossRef] [PubMed]
- Shabeeh, H.; Seddon, M.; Brett, S.; Melikian, N.; Casadei, B.; Shah, A.M.; Chowienczyk, P. Sympathetic activation increases NO release from eNOS but neither eNOS nor nNOS play an essential role in exercise hyperemia in the human forearm. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1225–H1230. [Google Scholar] [CrossRef]
- Allerton, T.D.; Proctor, D.N.; Stephens, J.M.; Dugas, T.R.; Spielmann, G.; Irving, B.A. l-Citrulline Supplementation: Impact on Cardiometabolic Health. Nutrients 2018, 10, 921. [Google Scholar] [CrossRef]
- Collins, J.K.; Wu, G.; Perkins-Veazie, P.; Spears, K.; Claypool, P.L.; Baker, R.A.; Clevidence, B.A. Watermelon consumption increases plasma arginine concentrations in adults. Nutrition 2007, 23, 261–266. [Google Scholar] [CrossRef]
- Tarazona-Díaz, M.P.; Martínez-Sánchez, A.; Aguayo, E. Preservation of bioactive compounds and quality parameters of watermelon juice enriched with L-Citrulline through short thermal treatment. J. Food Qual. 2017, 2017, 3283054. [Google Scholar] [CrossRef]
- Thoonen, R.; Sips, P.Y.; Bloch, K.D.; Buys, E.S. Pathophysiology of hypertension in the absence of nitric oxide/cyclic GMP signaling. Curr. Hypertens. Rep. 2013, 15, 47–58. [Google Scholar] [CrossRef]
- Figueroa, A.; Sanchez-Gonzalez, M.A.; Wong, A.; Arjmandi, B.H. Watermelon extract supplementation reduces ankle blood pressure and carotid augmentation index in obese adults with prehypertension or hypertension. Am. J. Hypertens. 2012, 25, 640–643. [Google Scholar] [CrossRef]
- Ochiai, M.; Hayashi, T.; Morita, M.; Ina, K.; Maeda, M.; Watanabe, F.; Morishita, K. Short-term effects of L-citrulline supplementation on arterial stiffness in middle-aged men. Int. J. Cardiol. 2012, 155, 257–261. [Google Scholar] [CrossRef]
- Sanchez-Gonzalez, M.A.; Koutnik, A.P.; Ramirez, K.; Wong, A.; Figueroa, A. The effects of short term L-citrulline supplementation on wave reflection responses to cold exposure with concurrent isometric exercise. Am. J. Hypertens. 2013, 26, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Wong, A.; Hooshmand, S.; Sanchez-Gonzalez, M.A. Effects of watermelon supplementation on arterial stiffness and wave reflection amplitude in postmenopausal women. Menopause 2013, 20, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Sanchez-Gonzalez, M.A.; Perkins-Veazie, P.M.; Arjmandi, B.H. Effects of watermelon supplementation on aortic blood pressure and wave reflection in individuals with prehypertension: A pilot study. Am. J. Hypertens. 2011, 24, 40–44. [Google Scholar] [CrossRef]
- Massa, N.M.; Silva, A.S.; Toscano, L.T.; Silva, J.D.; Persuhn, D.C.; Gonçalves, M.D.C.R. Watermelon extract reduces blood pressure but does not change sympathovagal balance in prehypertensive and hypertensive subjects. Blood Press 2016, 25, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Gutiérrez, J.J.; Castillo-Martínez, L.; Orea-Tejeda, A.; Vázquez-Díaz, O.; Valdespino-Trejo, A.; Narváez-David, R.; Keirns-Davis, C.; Carrasco-Ortiz, O.; Navarro-Navarro, A.; Sánchez-Santillán, R. Effect of L-arginine or L-citrulline oral supplementation on blood pressure and right ventricular function in heart failure patients with preserved ejection fraction. Cardiol. J. 2010, 17, 612–618. [Google Scholar]
- Wong, A.; Alvarez-Alvarado, S.; Jaime, S.J.; Kinsey, A.W.; Spicer, M.T.; Madzima, T.A.; Figueroa, A. Combined whole-body vibration training and l-citrulline supplementation improves pressure wave reflection in obese postmenopausal women. Appl. Physiol. Nutr. Metab. 2016, 41, 292–297. [Google Scholar] [CrossRef]
- Figueroa, A.; Alvarez-Alvarado, S.; Jaime, S.J.; Kalfon, R. l-Citrulline supplementation attenuates blood pressure, wave reflection and arterial stiffness responses to metaboreflex and cold stress in overweight men. Br. J. Nutr. 2016, 116, 279–285. [Google Scholar] [CrossRef]
- Figueroa, A.; Trivino, J.A.; Sanchez-Gonzalez, M.A.; Vicil, F. Oral L-citrulline supplementation attenuates blood pressure response to cold pressor test in young men. Am. J. Hypertens. 2010, 23, 12–16. [Google Scholar] [CrossRef]
- Pahlavani, N.; Entezari, M.H.; Nasiri, M.; Miri, A.; Rezaie, M.; Bagheri-Bidakhavidi, M.; Sadeghi, O. The effect of l-arginine supplementation on body composition and performance in male athletes: A double-blinded randomized clinical trial. Eur. J. Clin. Nutr. 2017, 71, 544–548. [Google Scholar] [CrossRef]
- Kang, Y.; Dillon, K.N.; Martinez, M.A.; Maharaj, A.; Fischer, S.M.; Figueroa, A. Combined L-Citrulline Supplementation and Slow Velocity Low-Intensity Resistance Training Improves Leg Endothelial Function, Lean Mass, and Strength in Hypertensive Postmenopausal Women. Nutrients 2022, 15, 74. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Cotie, L.M.; MacDonald, M.J.; Mitchell, C.J.; Prior, T.; Baker, S.K.; Phillips, S.M. Citrulline does not enhance blood flow, microvascular circulation, or myofibrillar protein synthesis in elderly men at rest or following exercise. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E71–E83. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.P.; Fleming, I.; Busse, R. Endothelial aging. Cardiovasc. Res. 2005, 66, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Gori, T.; Keaney, J.F., Jr.; Maack, C.; Daiber, A. Pathophysiological role of oxidative stress in systolic and diastolic heart failure and its therapeutic implications. Eur. Heart J. 2015, 36, 2555–2564. [Google Scholar] [CrossRef]
- Klawitter, J.; Hildreth, K.L.; Christians, U.; Kohrt, W.M.; Moreau, K.L. A relative L-arginine deficiency contributes to endothelial dysfunction across the stages of the menopausal transition. Physiol. Rep. 2017, 5, e13409. [Google Scholar] [CrossRef]
- Wijnands, K.A.; Meesters, D.M.; van Barneveld, K.W.; Visschers, R.G.; Briedé, J.J.; Vandendriessche, B.; van Eijk, H.M.; Bessems, B.A.; van den Hoven, N.; von Wintersdorff, C.J.; et al. Citrulline Supplementation Improves Organ Perfusion and Arginine Availability under Conditions with Enhanced Arginase Activity. Nutrients 2015, 7, 5217–5238. [Google Scholar] [CrossRef] [PubMed]
- Cynober, L. Pharmacokinetics of arginine and related amino acids. J. Nutr. 2007, 137, S1646–S1649. [Google Scholar] [CrossRef]
- Hecker, M.; Sessa, W.C.; Harris, H.J.; Anggård, E.E.; Vane, J.R. The metabolism of L-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: Cultured endothelial cells recycle L-citrulline to L-arginine. Proc. Natl. Acad. Sci. USA 1990, 87, 8612–8616. [Google Scholar] [CrossRef]
- Morita, M.; Sakurada, M.; Watanabe, F.; Yamasaki, T.; Doi, H.; Ezaki, H.; Morishita, K.; Miyakex, T. Effects of Oral L-Citrulline Supplementation on Lipoprotein Oxidation and Endothelial Dysfunction in Humans with Vasospastic Angina. Immunol. Endocr. Metab. Agents Med. Chem. 2013, 13, 214–220. [Google Scholar] [CrossRef]
- Bai, Y.; Sun, L.; Yang, T.; Sun, K.; Chen, J.; Hui, R. Increase in fasting vascular endothelial function after short-term oral L-arginine is effective when baseline flow-mediated dilation is low: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2009, 89, 77–84. [Google Scholar] [CrossRef]
- Gates, P.E.; Boucher, M.L.; Silver, A.E.; Monahan, K.D.; Seals, D.R. Impaired flow-mediated dilation with age is not explained by L-arginine bioavailability or endothelial asymmetric dimethylarginine protein expression. J. Appl. Physiol. 2007, 102, 63–71. [Google Scholar] [CrossRef]
- Baker, P.R., II; Boyle, K.E.; Koves, T.R.; Ilkayeva, O.R.; Muoio, D.M.; Houmard, J.A.; Friedman, J.E. Metabolomic analysis reveals altered skeletal muscle amino acid and fatty acid handling in obese humans. Obesity 2015, 23, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Donato, A.J.; Eskurza, I.; Silver, A.E.; Levy, A.S.; Pierce, G.L.; Gates, P.E.; Seals, D.R. Direct evidence of endothelial oxidative stress with aging in humans: Relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ. Res. 2007, 100, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation and cardiovascular disease mechanisms. Am. J. Clin. Nutr. 2006, 83, 456S–460S. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Mañas, L.; El-Assar, M.; Vallejo, S.; López-Dóriga, P.; Solís, J.; Petidier, R.; Montes, M.; Nevado, J.; Castro, M.; Gómez-Guerrero, C.; et al. Endothelial dysfunction in aged humans is related with oxidative stress and vascular inflammation. Aging Cell 2009, 8, 226–238. [Google Scholar] [CrossRef]
- Forbes, S.C.; Harber, V.; Bell, G.J. The acute effects of L-arginine on hormonal and metabolic responses during submaximal exercise in trained cyclists. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 369–377. [Google Scholar] [CrossRef]
- Meirelles, C.M.; Matsuura, C. Acute supplementation of L-arginine affects neither strength performance nor nitric oxide production. J. Sport Med. Phys. Fit. 2018, 58, 216–220. [Google Scholar] [CrossRef]
- Vanhatalo, A.; Bailey, S.J.; DiMenna, F.J.; Blackwell, J.R.; Wallis, G.A.; Jones, A.M. No effect of acute L-arginine supplementation on O2 cost or exercise tolerance. Eur. J. Appl. Physiol. 2013, 113, 1805–1819. [Google Scholar] [CrossRef]
- Alvares, T.S.; Conte-Junior, C.A.; Silva, J.T.; Paschoalin, V.M. Acute L-Arginine supplementation does not increase nitric oxide production in healthy subjects. Nutr. Metab. 2012, 9, 54. [Google Scholar] [CrossRef]
- Blum, A.; Hathaway, L.; Mincemoyer, R.; Schenke, W.H.; Kirby, M.; Csako, G.; Waclawiw, M.A.; Panza, J.A.; Cannon, R.O., 3rd. Effects of oral L-arginine on endothelium-dependent vasodilation and markers of inflammation in healthy postmenopausal women. J. Am. Coll. Cardiol. 2000, 35, 271–276. [Google Scholar] [CrossRef]
- Tang, J.E.; Lysecki, P.J.; Manolakos, J.J.; MacDonald, M.J.; Tarnopolsky, M.A.; Phillips, S.M. Bolus arginine supplementation affects neither muscle blood flow nor muscle protein synthesis in young men at rest or after resistance exercise. J. Nutr. 2011, 141, 195–200. [Google Scholar] [CrossRef]
- Forbes, S.C.; Bell, G.J. The acute effects of a low and high dose of oral L-arginine supplementation in young active males at rest. Appl. Physiol. Nutr. Metab. 2011, 36, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Maiorana, A.; O’Driscoll, G.; Taylor, R.; Green, D. Exercise and the nitric oxide vasodilator system. Sports Med. 2003, 33, 1013–1035. [Google Scholar] [CrossRef]
- O’Sullivan, D.; Brosnan, J.T.; Brosnan, M.E. Catabolism of arginine and ornithine in the perfused rat liver: Effect of dietary protein and of glucagon. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E516–E521. [Google Scholar] [CrossRef] [PubMed]
- Koifman, B.; Wollman, Y.; Bogomolny, N.; Chernichowsky, T.; Finkelstein, A.; Peer, G.; Scherez, J.; Blum, M.; Laniado, S.; Iaina, A.; et al. Improvement of cardiac performance by intravenous infusion of L-arginine in patients with moderate congestive heart failure. J. Am. Coll. Cardiol. 1995, 26, 1251–1256. [Google Scholar] [CrossRef]
- Yavuz, H.U.; Turnagol, H.; Demirel, A.H. Pre-exercise arginine supplementation increases time to exhaustion in elite male wrestlers. Biol. Sport 2014, 31, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, M.; Brunner, F.; Faulhaber, M.; Hotter, B.; Likar, R. The prolonged intake of L-arginine-L-aspartate reduces blood lactate accumulation and oxygen consumption during submaximal exercise. J. Sport Sci. Med. 2005, 4, 314–322. [Google Scholar]
- Chang, C.K.; Chien, K.M.C.; Chang, J.H.; Huang, M.H.; Liang, Y.C.; Liu, T.H. Branched-chain amino acids and arginine improve performance in two consecutive days of simulated handball games in male and female athletes: A randomized trial. PLoS ONE 2015, 10, e0121866. [Google Scholar] [CrossRef]
- Abel, T.; Knechtle, B.; Perret, C.; Eser, P.; von Arx, P.; Knecht, H. Influence of chronic supplementation of arginine aspartate in endurance athletes on performance and substrate metabolism—A randomized, double-blind, placebo-controlled study. Int. J. Sport Med. 2005, 26, 344–349. [Google Scholar] [CrossRef]
- Kaore, S.N.; Amane, H.S.; Kaore, N.M. Citrulline: Pharmacological perspectives and its role as an emerging biomarker in future. Fundam. Clin. Pharmacol. 2013, 27, 35–50. [Google Scholar] [CrossRef]
- Hwang, P.; Marroquín, F.E.M.; Gann, J.; Andre, T.; McKinley-Barnard, S.; Kim, C.; Morita, M.; Willoughby, D.S. Eight weeks of resistance training in conjunction with glutathione and L-Citrulline supplementation increases lean mass and has no adverse effects on blood clinical safety markers in resistance-trained males. J. Int. Soc. Sport Nutr. 2018, 15, 30. [Google Scholar] [CrossRef]
- Barkhidarian, B.; Khorshidi, M.; Shab-Bidar, S.; Hashemi, B. Effects of L-citrulline supplementation on blood pressure: A systematic review and meta-analysis. Avicenna J. Phytomed. 2019, 9, 10–20. [Google Scholar] [PubMed]
- Terasawa, N.; Nakada, K. Effect of l-citrulline intake on intermittent short-time high-intensity exercise performance in male collegiate track athletes. J. Phys. Fit. Sport Med. 2019, 8, 147–157. [Google Scholar] [CrossRef]
- Suzuki, T.; Morita, M.; Kobayashi, Y.; Kamimura, A. Oral L-citrulline supplementation enhances cycling time trial performance in healthy trained men: Double-blind randomized placebo-controlled 2-way crossover study. J. Int. Soc. Sport Nutr. 2016, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Esen, O.; Eser, M.C.; Abdioglu, M.; Benesova, D.; Gabrys, T.; Karayigit, R. Eight Days of L-Citrulline or L-Arginine Supplementation Did Not Improve 200-m and 100-m Swimming Time Trials. Int. J. Environ. Res. Public Health 2022, 19, 4462. [Google Scholar] [CrossRef] [PubMed]
- Hickner, R.C.; Tanner, C.J.; Evans, C.A.; Clark, P.D.; Haddock, A.; Fortune, C.; Geddis, H.; Waugh, W.; McCammon, M. L-citrulline reduces time to exhaustion and insulin response to a graded exercise test. Med. Sci. Sport Exerc. 2006, 38, 660–666. [Google Scholar] [CrossRef]
- Cutrufello, P.T.; Gadomski, S.J.; Zavorsky, G.S. The effect of l-citrulline and watermelon juice supplementation on anaerobic and aerobic exercise performance. J. Sport Sci. 2015, 33, 1459–1466. [Google Scholar] [CrossRef]
- Stanelle, S.T.; McLaughlin, K.L.; Crouse, S.F. One Week of L-Citrulline Supplementation Improves Performance in Trained Cyclists. J. Strength Cond. Res. 2020, 34, 647–652. [Google Scholar] [CrossRef]
- Forbes, S.C.; Harber, V.; Bell, G.J. Oral L-arginine before resistance exercise blunts growth hormone in strength trained males. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 236–244. [Google Scholar] [CrossRef]
- Glenn, J.M.; Gray, M.; Wethington, L.N.; Stone, M.S.; Stewart, R.W., Jr.; Moyen, N.E. Acute citrulline malate supplementation improves upper- and lower-body submaximal weightlifting exercise performance in resistance-trained females. Eur. J. Nutr. 2017, 56, 775–784. [Google Scholar] [CrossRef]
- Gonzalez, A.M.; Spitz, R.W.; Ghigiarelli, J.J.; Sell, K.M.; Mangine, G.T. Acute Effect of Citrulline Malate Supplementation on Upper-Body Resistance Exercise Performance in Recreationally Resistance-Trained Men. J. Strength Cond. Res. 2018, 32, 3088–3094. [Google Scholar] [CrossRef]
- da Silva, D.K.; Jacinto, J.L.; de Andrade, W.B.; Roveratti, M.C.; Estoche, J.M.; Balvedi, M.C.W.; de Oliveira, D.B.; da Silva, R.A.; Aguiar, A.F. Citrulline Malate Does Not Improve Muscle Recovery after Resistance Exercise in Untrained Young Adult Men. Nutrients 2017, 9, 1132. [Google Scholar] [CrossRef]
- Trexler, E.T.; Keith, D.S.; Schwartz, T.A.; Ryan, E.D.; Stoner, L.; Persky, A.M.; Smith-Ryan, A.E. Effects of Citrulline Malate and Beetroot Juice Supplementation on Blood Flow, Energy Metabolism, and Performance During Maximum Effort Leg Extension Exercise. J. Strength Cond. Res. 2019, 33, 2321–2329. [Google Scholar] [CrossRef] [PubMed]
- Streeter, D.M.; Trautman, K.A.; Bennett, T.W.; McIntosh, L.E.; Grier, J.W.; Stastny, S.N.; Hackney, K.J. Endothelial, Cardiovascular, and Performance Responses to L-Arginine Intake and Resistance Exercise. Int. J. Exerc. Sci. 2019, 12, 701–713. [Google Scholar]
- Chappell, A.J.; Allwood, D.M.; Johns, R.; Brown, S.; Sultana, K.; Anand, A.; Simper, T. Citrulline malate supplementation does not improve German Volume Training performance or reduce muscle soreness in moderately trained males and females. J. Int. Soc. Sport Nutr. 2018, 15, 42. [Google Scholar] [CrossRef]
- Farney, T.M.; Bliss, M.V.; Hearon, C.M.; Salazar, D.A. The Effect of Citrulline Malate Supplementation on Muscle Fatigue Among Healthy Participants. J. Strength Cond. Res. 2019, 33, 2464–2470. [Google Scholar] [CrossRef] [PubMed]
- Buckinx, F.; Gouspillou, G.; Carvalho, L.P.; Marcangeli, V.; El Hajj Boutros, G.; Dulac, M.; Noirez, P.; Morais, J.A.; Gaudreau, P.; Aubertin-Leheudre, M. Effect of High-Intensity Interval Training Combined with L-Citrulline Supplementation on Functional Capacities and Muscle Function in Dynapenic-Obese Older Adults. J. Clin. Med. 2018, 7, 561. [Google Scholar] [CrossRef] [PubMed]
- Kanaley, J.A. Growth hormone, arginine and exercise. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 50–54. [Google Scholar] [CrossRef]
- Manini, T.M.; Yarrow, J.F.; Buford, T.W.; Clark, B.C.; Conover, C.F.; Borst, S.E. Growth hormone responses to acute resistance exercise with vascular restriction in young and old men. Growth Horm. IGF Res. 2012, 22, 167–172. [Google Scholar] [CrossRef]
- Ghigo, E.; Arvat, E.; Valente, F.; Nicolosi, M.; Boffano, G.M.; Procopio, M.; Bellone, J.; Maccario, M.; Mazza, E.; Camanni, F. Arginine reinstates the somatotrope responsiveness to intermittent growth hormone-releasing hormone administration in normal adults. Neuroendocrinology 1991, 54, 291–294. [Google Scholar] [CrossRef]
- Zajac, A.; Poprzecki, S.; Zebrowska, A.; Chalimoniuk, M.; Langfort, J. Arginine and ornithine supplementation increases growth hormone and insulin-like growth factor-1 serum levels after heavy-resistance exercise in strength-trained athletes. J. Strength Cond. Res. 2010, 24, 1082–1090. [Google Scholar] [CrossRef]
- Chromiak, J.A.; Antonio, J. Use of amino acids as growth hormone-releasing agents by athletes. Nutrition 2002, 18, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.I.; Hefer, J.A.; Millar, R.P.; Macfarlane, P.W. Failure of commercial oral amino acid supplements to increase serum growth hormone concentrations in male body-builders. Int. J. Sport Nutr. 1993, 3, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Wax, B.; Kavazis, A.N.; Webb, H.E.; Brown, S.P. Acute L-arginine alpha ketoglutarate supplementation fails to improve muscular performance in resistance trained and untrained men. J. Int. Soc. Sport Nutr. 2012, 9, 17. [Google Scholar] [CrossRef]
- Hurst, H.T.; Sinclair, J.K.; Beenham, M. Influence of Absolute versus relative L-arginine Dosage on 1 km and 16.1 km time trial performance in trained cyclists. J. Sci. Cycl. (JSC) 2014, 3, 2–8. [Google Scholar]
- Greer, B.K.; Jones, B.T. Acute arginine supplementation fails to improve muscle endurance or affect blood pressure responses to resistance training. J. Strength Cond. Res. 2011, 25, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Gough, L.A.; Sparks, S.A.; McNaughton, L.R.; Higgins, M.F.; Newbury, J.W.; Trexler, E.; Faghy, M.A.; Bridge, C.A. A critical review of citrulline malate supplementation and exercise performance. Eur. J. Appl. Physiol. 2021, 121, 3283–3295. [Google Scholar] [CrossRef] [PubMed]
- Chappell, A.J.; Allwood, D.M.; Simper, T.N. Citrulline Malate Fails to Improve German Volume Training Performance in Healthy Young Men and Women. J. Diet. Suppl. 2020, 17, 249–260. [Google Scholar] [CrossRef]
- Wegierska, A.E.; Charitos, I.A.; Topi, S.; Potenza, M.A.; Montagnani, M.; Santacroce, L. The Connection Between Physical Exercise and Gut Microbiota: Implications for Competitive Sports Athletes. Sports Med. 2022, 52, 2355–2369. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).