Whey Protein Hydrolysate Renovates Age-Related and Scopolamine-Induced Cognitive Impairment
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Determination of Molecular Weight (MW) Distribution and Peptide Sequences in WPH
2.3. Quantification of Peptide GTWY
2.4. Animals and Treatment
2.5. Behavioral Tests
2.5.1. Novel Object Recognition (NOR) Test
2.5.2. Shuttle Box Test
2.5.3. Step-Down Avoidance Test
2.6. Determination of Biochemical Parameters
2.7. 16S rRNA Microbiome Sequencing
2.8. Hematoxylin-Eosin (HE) Staining
2.9. Proteomics Analysis
2.9.1. Total Protein Extraction
2.9.2. Protein Digestion and Tandem Mass Tags (TMT) Labeling
2.9.3. LC-MS/MS Analysis
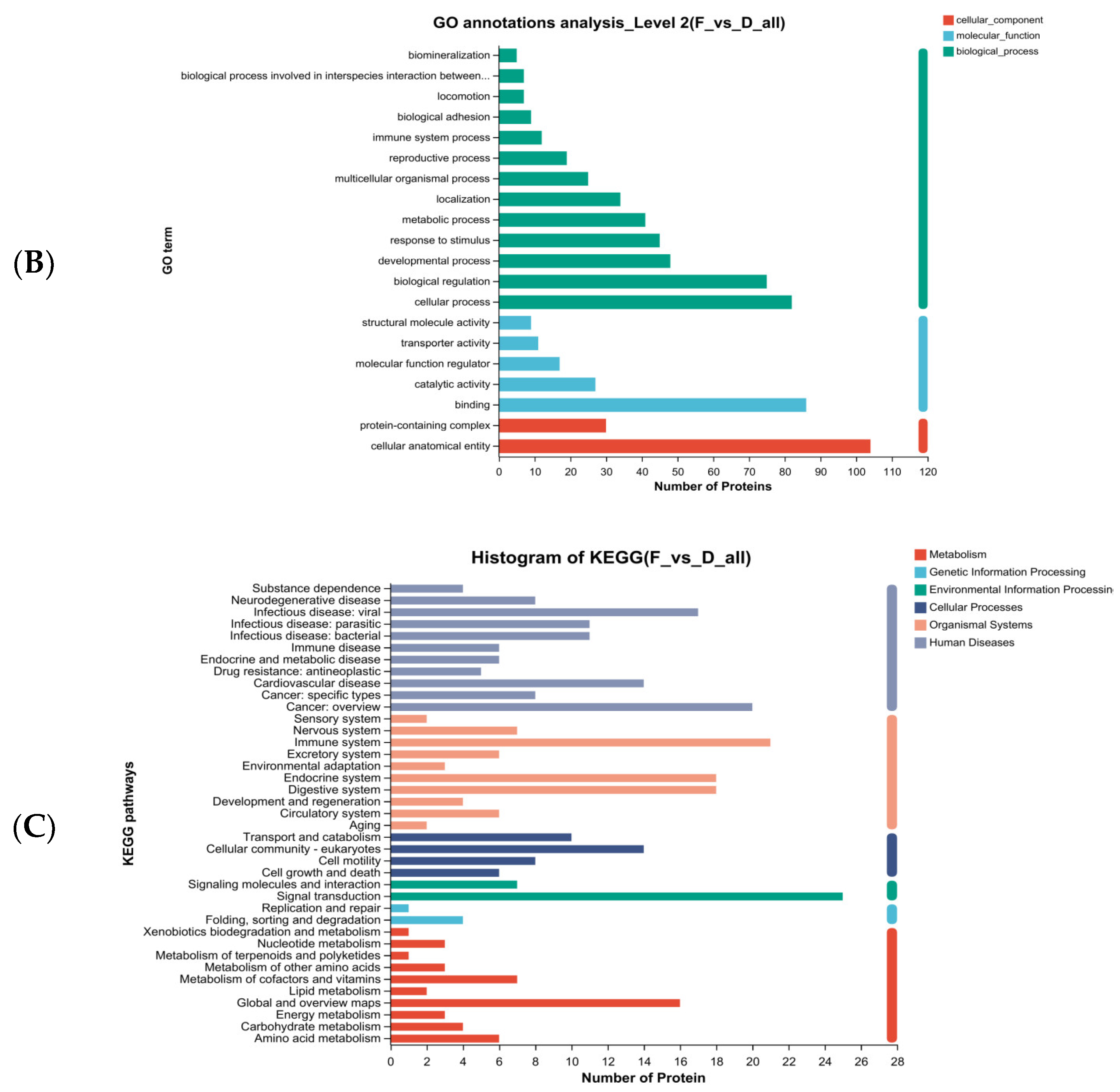
2.9.4. Bioinformatics Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Composition and MW Distribution of WPH
3.2. Animal Metrics
3.3. WPH Promoted the Spatial Memory and Cognitive Ability of Mice

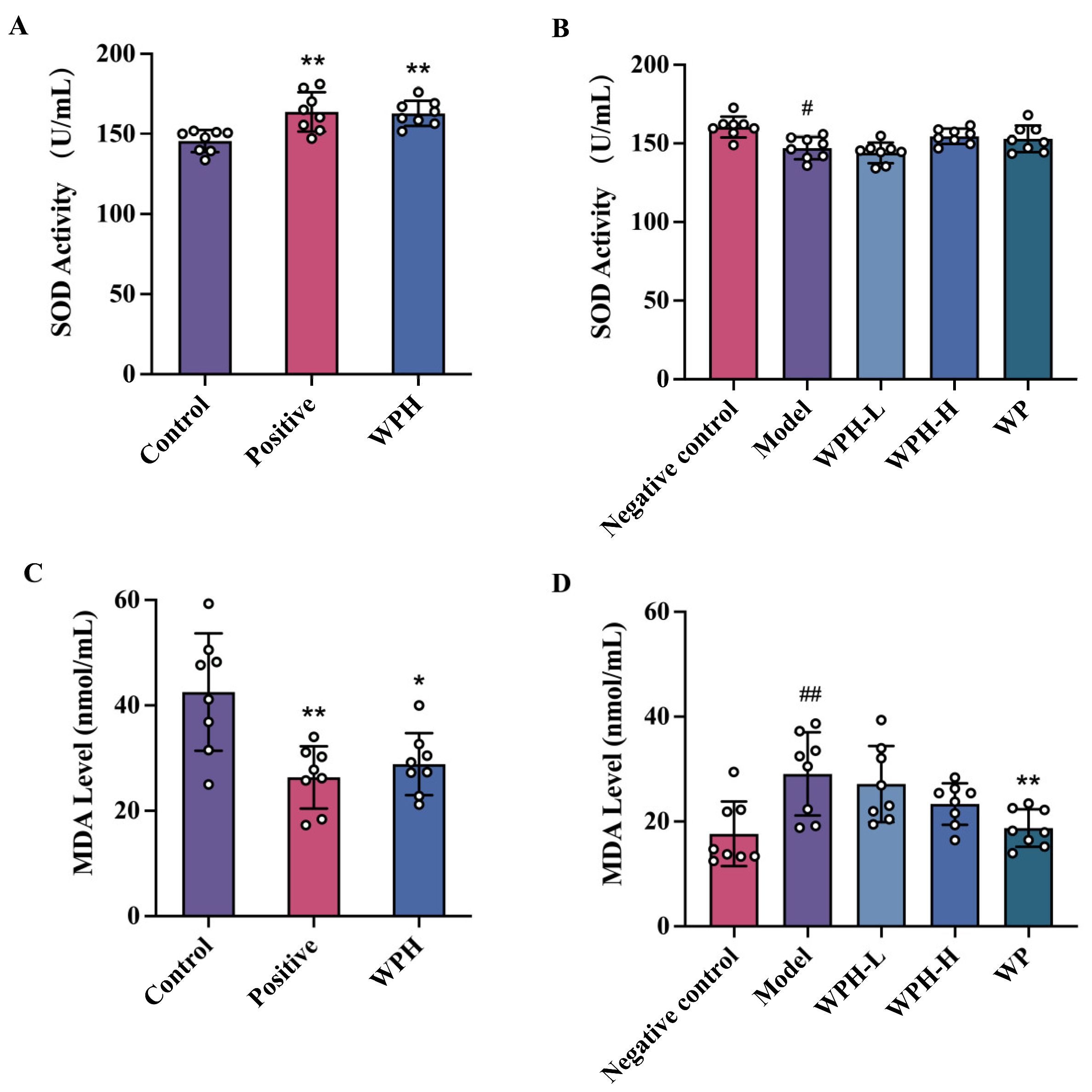
3.4. WPH Reduces the Oxidative Damage Stress in Mice with Memory Decline
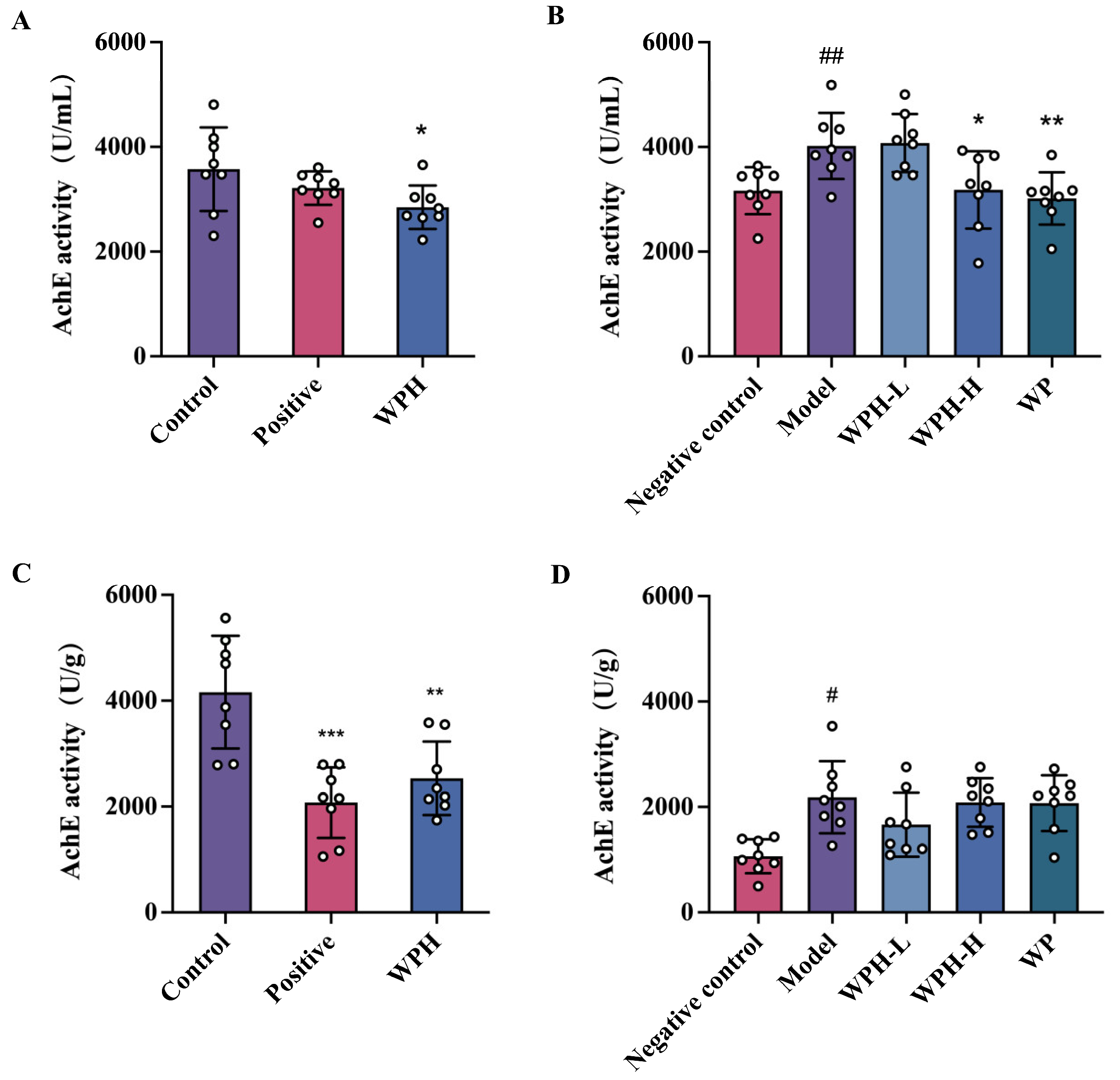
3.5. Effect of WPH on AchE Level in Mice
3.6. Effect of WPH on Aβ1-42 Level in Mice
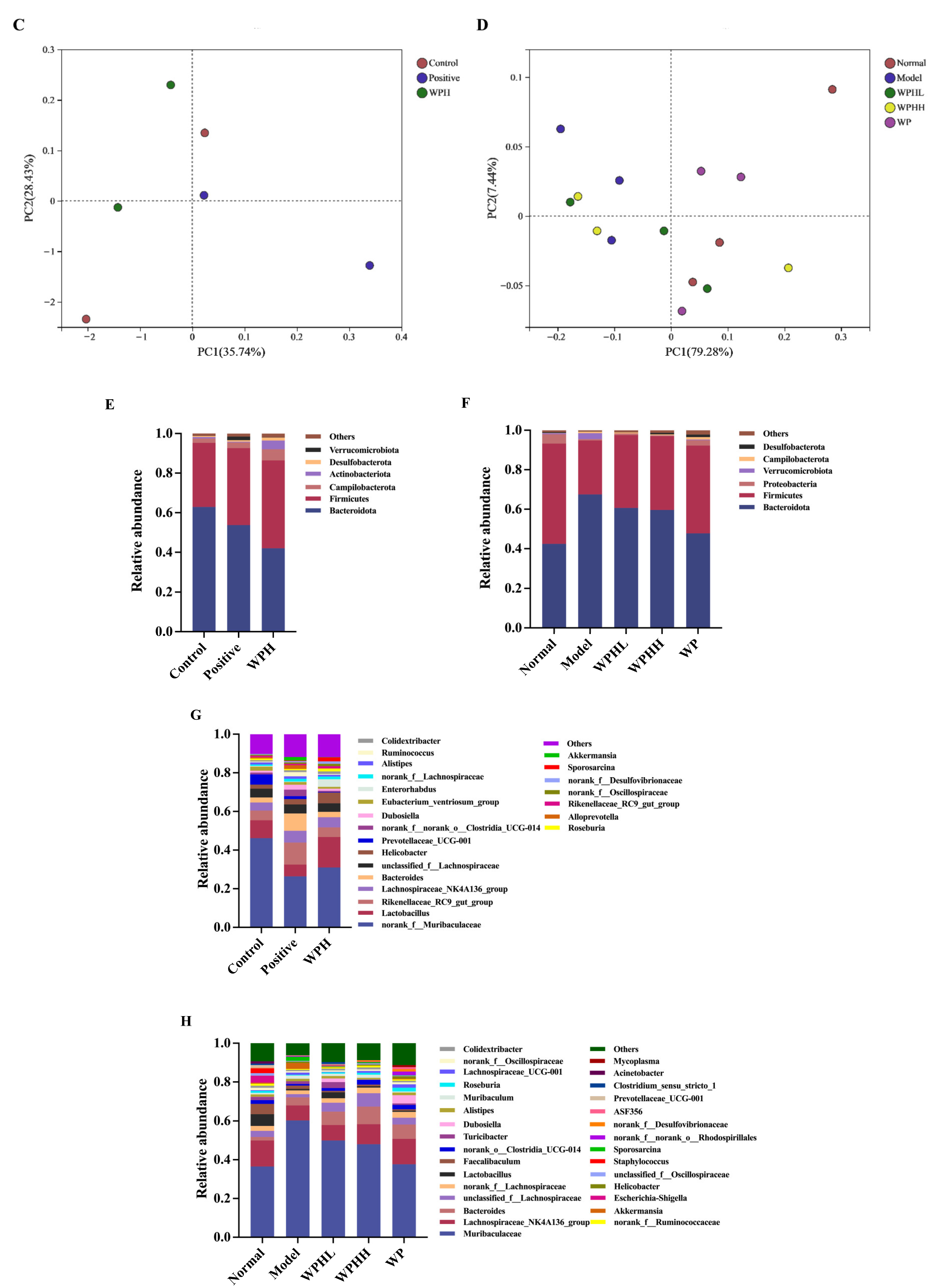
3.7. WPH Regulates the Gut Microbiome Relative to AD

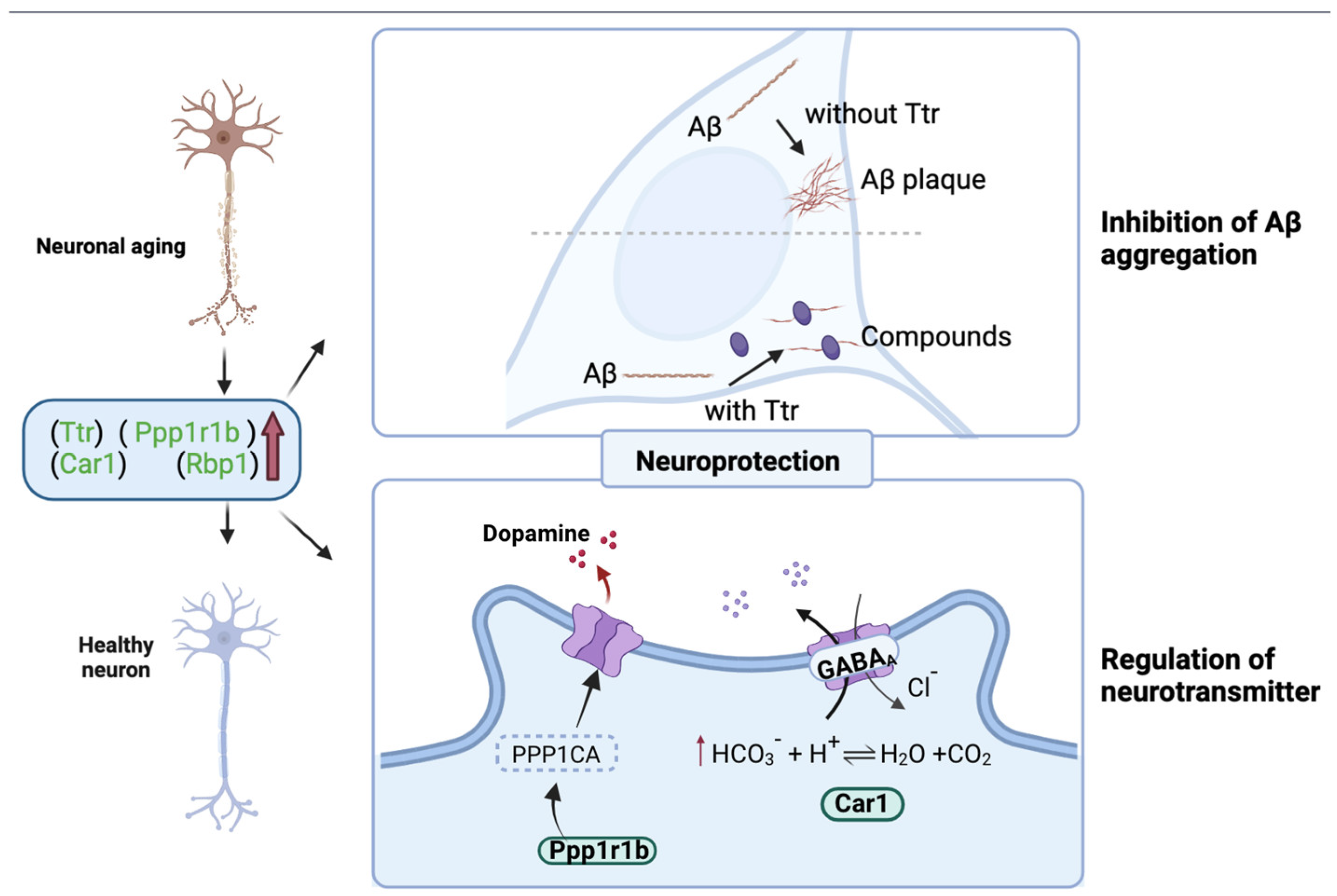
3.8. Effect of WPH on the Hippocampus


4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Graham, W.V.; Bonito-Oliva, A.; Sakmar, T.P. Update on Alzheimer’s Disease Therapy and Prevention Strategies. Annu. Rev. Med. 2017, 68, 413–430. [Google Scholar] [CrossRef] [PubMed]
- Ismail, Z.; Creese, B.; Aarsland, D.; Kales, H.C.; Lyketsos, C.G.; Sweet, R.A.; Ballard, C. Psychosis in Alzheimer disease—Mechanisms, genetics and therapeutic opportunities. Nat. Rev. Neurol. 2022, 18, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Li, J.; Zhang, R. Current research status of blood biomarkers in Alzheimer’s disease: Diagnosis and prognosis. Ageing Res. Rev. 2021, 72, 101492. [Google Scholar] [CrossRef] [PubMed]
- Winblad, B.; Amouyel, P.; Andrieu, S.; Ballard, C.; Brayne, C.; Brodaty, H.; Cedazo-Minguez, A.; Dubois, B.; Edvardsson, D.; Feldman, H.; et al. Defeating Alzheimer’s disease and other dementias: A priority for European science and society. Lancet Neurol. 2016, 15, 455–532. [Google Scholar] [CrossRef]
- Mortby, M.E.; Black, S.E.; Gauthier, S.; Miller, D.; Porsteinsson, A.; Smith, E.E.; Ismail, Z. Dementia clinical trial implications of mild behavioral impairment. Int. Psychogeriatr. 2018, 30, 171–175. [Google Scholar] [CrossRef]
- Patrizia Cavazzoni. FDA’s Decision to Approve New Treatment for Alzheimer’s Disease. FDA Center for Drug Evaluation and Research. Available online: https://www.fda.gov/drugs/news-events-human-drugs/fdas-decision-approve-new-treatment-alzheimers-disease (accessed on 1 September 2022).
- Litke, R.; Garcharna, L.C.; Jiwani, S.; Neugroschl, J. Modifiable Risk Factors in Alzheimer Disease and Related Dementias: A Review. Clin. Ther. 2021, 43, 953–965. [Google Scholar] [CrossRef]
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for the Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef]
- Ji, J.; Yi, X.; Zhu, Y.; Yu, H.; Huang, S.; Liu, Z.; Zhang, X.; Xia, G.; Shen, X. Tilapia Head Protein Hydrolysate Attenuates Scopolamine-Induced Cognitive Impairment through the Gut-Brain Axis in Mice. Foods 2021, 10, 3129. [Google Scholar] [CrossRef]
- Camfield, D.A.; Owen, L.; Scholey, A.B.; Pipingas, A.; Stough, C. Dairy constituents and neurocognitive health in aging. Br. J. Nutr. 2011, 106, 159–174. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, C.; Fang, L.; Lu, H.; Wang, J.; Gao, Y.; Gabbianelli, R.; Min, W. Walnut-Derived Peptide Activates PINK1 via the NRF2/KEAP1/HO-1 Pathway, Promotes Mitophagy, and Alleviates Learning and Memory Impairments in a Mice Model. J. Agric. Food Chem. 2021, 69, 2758–2772. [Google Scholar] [CrossRef]
- Shimizu, A.; Mitani, T.; Tanaka, S.; Fujii, H.; Maebuchi, M.; Amiya, Y.; Tanaka, M.; Matsui, T.; Nakamura, S.; Katayama, S. Soybean-Derived Glycine–Arginine Dipeptide Administration Promotes Neurotrophic Factor Expression in the Mouse Brain. J. Agric. Food Chem. 2018, 66, 7935–7941. [Google Scholar] [CrossRef]
- Vasconcelos, Q.D.J.S.; Bachur, T.P.R.; Aragão, G.F. Whey protein supplementation and its potentially adverse effects on health: A systematic review. Appl. Physiol. Nutr. Metab. 2021, 46, 27–33. [Google Scholar] [CrossRef]
- Davies, R.W.; Carson, B.P.; Jakeman, P.M. The Effect of Whey Protein Supplementation on the Temporal Recovery of Muscle Function Following Resistance Training: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 221. [Google Scholar] [CrossRef]
- Li, X.; Feng, C.; Hong, H.; Zhang, Y.; Luo, Z.; Wang, Q.; Luo, Y.; Tan, Y. Novel ACE inhibitory peptides derived from whey protein hydrolysates: Identification and molecular docking analysis. Food Biosci. 2022, 48, 101737. [Google Scholar] [CrossRef]
- Kita, M.; Obara, K.; Kondo, S.; Umeda, S.; Ano, Y. Effect of Supplementation of a Whey Peptide Rich in Tryptophan-Tyrosine-Related Peptides on Cognitive Performance in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2018, 10, 899. [Google Scholar] [CrossRef]
- Wang, K.; Luo, Q.; Hong, H.; Liu, H.; Luo, Y. Novel antioxidant and ACE inhibitory peptide identified from Arthrospira platensis protein and stability against thermal/pH treatments and simulated gastrointestinal digestion. Food Res. Int. 2021, 139, 109908. [Google Scholar] [CrossRef]
- Tanichi, M.; Toda, H.; Shimizu, K.; Koga, M.; Saito, T.; Enomoto, S.; Boku, S.; Asai, F.; Mitsui, Y.; Nagamine, M.; et al. Differential effects of voluntary wheel running and toy rotation on the mRNA expression of neurotrophic factors and FKBP5 in a post-traumatic stress disorder rat model with the shuttle-box task. Biochem. Biophys. Res. Commun. 2018, 501, 307–312. [Google Scholar] [CrossRef]
- Rodrigues, K.D.C.; de Oliveira, R.L.; Chaves, J.D.S.; da Rocha, V.M.E.; dos Santos, B.F.; Fronza, M.G.; Domingues, N.L.D.C.; Savegnago, L.; Wilhelm, E.A.; Luchese, C. A new arylsulfanyl-benzo-2,1,3-thiadiazoles derivative produces an anti-amnesic effect in mice by modulating acetylcholinesterase activity. Chem. -Biol. Interact. 2022, 351, 109736. [Google Scholar] [CrossRef]
- John, J.A.; Ghosh, B.C. Production of whey protein hydrolyzates and its incorporation into milk. Food Prod. Process. Nutr. 2021, 3, 9. [Google Scholar] [CrossRef]
- Yamamoto, K.; Sato, Y.; Hagihara, K.; Kirikihira, K.; Jotaki, A.; Michihara, A.; Miyake, Y. Effects of Rikkunshi-To, a Japanese kampo medicine, on donepezil-induced gastrointestinal side effects in mice. J. Pharmacol. Sci. 2022, 150, 123–133. [Google Scholar] [CrossRef]
- Ni, L.; Zhuge, F.; Yang, S.; Ma, L.; Zheng, A.; Zhao, Y.; Hu, L.; Fu, Z.; Ni, Y. Hydrolyzed Chicken Meat Extract Attenuates Neuroinflammation and Cognitive Impairment in Middle-Aged Mouse by Regulating M1/M2 Microglial Polarization. J. Agric. Food Chem. 2021, 69, 9800–9812. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-C.; Li, Z.; Liu, X.-R.; Hu, J.-N.; Liu, R.; Zhu, N.; Li, Y. The Antioxidant Effects of Whey Protein Peptide on Learning and Memory Improvement in Aging Mice Models. Nutrients 2021, 13, 2100. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Schrag, M.; Mueller, C.; Zabel, M.; Crofton, A.; Kirsch, W.; Ghribi, O.; Squitti, R.; Perry, G. Oxidative stress in blood in Alzheimer’s disease and mild cognitive impairment: A meta-analysis. Neurobiol. Dis. 2013, 59, 100–110. [Google Scholar] [CrossRef]
- Zhang, T.; Kim, M.J.; Kim, M.J.; Wu, X.; Yang, H.J.; Yuan, H.; Huang, S.; Yoon, S.M.; Kim, K.-N.; Park, S. Long-Term Effect of Porcine Brain Enzyme Hydrolysate Intake on Scopolamine-Induced Memory Impairment in Rats. Int. J. Mol. Sci. 2022, 23, 63361. [Google Scholar] [CrossRef]
- Zhang, H.; Peng, Y.; Zhuo, L.; Wang, Y.; Zeng, G.; Wang, S.; Long, L.; Li, X.; Wang, Z. Recent advance on pleiotropic cholinesterase inhibitors bearing amyloid modulation efficacy. Eur. J. Med. Chem. 2022, 242, 114695. [Google Scholar] [CrossRef]
- Lucena, P.B.; Vanherle, S.; Lodder, C.; de Ravé, M.G.; Stancu, I.-C.; Lambrichts, I.; Vangheluwe, R.; Bruffaerts, R.; Dewachter, I. Blood-based Aβ42 increases in the earliest pre-pathological stage before decreasing with progressive amyloid pathology in preclinical models and human subjects: Opening new avenues for prevention. Acta Neuropathol. 2022, 144, 489–508. [Google Scholar] [CrossRef]
- Kwon, M.-J.; Lee, J.-W.; Kim, K.-S.; Chen, H.; Cui, C.-B.; Lee, G.W.; Cho, Y.H. The Influence of Tyrosol-Enriched Rhodiola sachalinensis Extracts Bioconverted by the Mycelium of Bovista plumbe on Scopolamine-Induced Cognitive, Behavioral, and Physiological Responses in Mice. Molecules 2022, 27, 4455. [Google Scholar] [CrossRef]
- Arendash, G.W.; Mori, T.; Cao, C.; Mamcarz, M.; Runfeldt, M.; Dickson, A.; Rezai-Zadeh, K.; Tan, J.; Citron, B.A.; Lin, X.; et al. Caffeine Reverses Cognitive Impairment and Decreases Brain Amyloid-β Levels in Aged Alzheimer’s Disease Mice. J. Alzheimer’s Dis. 2009, 17, 661–680. [Google Scholar] [CrossRef]
- Saji, N.; Niida, S.; Murotani, K.; Hisada, T.; Tsuduki, T.; Sugimoto, T.; Kimura, A.; Toba, K.; Sakurai, T. Analysis of the relationship between the gut microbiome and dementia: A cross-sectional study conducted in Japan. Sci. Rep. 2019, 9, 1008. [Google Scholar] [CrossRef]
- John, S.K.; Chandrapragasam, V.; Dey, P. Impact of Gut Microbiome Lactobacillus spp. in Brain Function and its Medicament towards Alzheimer’s Disease Pathogenesis. J. Pure Appl. Microbiol. 2021, 15, 1029–1042. [Google Scholar] [CrossRef]
- Kaiyrlykyzy, A.; Kozhakhmetov, S.; Babenko, D.; Zholdasbekova, G.; Alzhanova, D.; Olzhayev, F.; Baibulatova, A.; Kushugulova, A.R.; Askarova, S. Study of gut microbiota alterations in Alzheimer’s dementia patients from Kazakhstan. Sci. Rep. 2022, 12, 15115. [Google Scholar] [CrossRef]
- Almasi, F.; Mohammadipanah, F.; Adhami, H.-R.; Hamedi, J. Introduction of marine-derived Streptomyces sp. UTMC 1334 as a source of pyrrole derivatives with anti-acetylcholinesterase activity. J. Appl. Microbiol. 2018, 125, 1370–1382. [Google Scholar] [CrossRef]
- Murray, E.; Smith, K.B.; Stoby, K.S.; Thomas, B.J.; Swenson, M.J.; Arber, L.A.; Frenette, E.; Ismail, N. Pubertal probiotic blocks LPS-induced anxiety and the associated neurochemical and microbial outcomes, in a sex dependent manner. Psychoneuroendocrinology 2020, 112, 104481. [Google Scholar] [CrossRef]
- Barichella, M.; Severgnini, M.; Cilia, R.; Cassani, E.; Bolliri, C.; Caronni, S.; Ferri, V.; Cancello, R.; Ceccarani, C.; Faierman, S.; et al. Unraveling gut microbiota in Parkinson’s disease and atypical parkinsonism. Mov. Disord. 2019, 34, 396–405. [Google Scholar] [CrossRef]
- Zhao, L.-N.; Ma, S.-W.; Xiao, J.; Yang, L.-J.; Xu, S.-X.; Zhao, L. Bone marrow mesenchymal stem cell therapy regulates gut microbiota to improve post-stroke neurological function recovery in rats. World J. Stem Cells 2021, 13, 1905–1917. [Google Scholar] [CrossRef]
- Lazarov, O.; Hollands, C. Hippocampal neurogenesis: Learning to remember. Prog. Neurobiol. 2016, 138–140, 1–18. [Google Scholar] [CrossRef]
- Moser, E.I.; Kropff, E.; Moser, M.-B. Place cells, grid cells, and the brain’s spatial representation system. Annu. Rev. Neurosci. 2008, 31, 69–89. [Google Scholar] [CrossRef]
- Silva, C.S.; Eira, J.; Ribeiro, C.A.; Oliveira, Â.; Sousa, M.; Cardoso, I.; Liz, M.A. Transthyretin neuroprotection in Alzheimer’s disease is dependent on proteolysis. Neurobiol. Aging 2017, 59, 10–14. [Google Scholar] [CrossRef]
- Serot, J.-M.; Christmann, D.; Dubost, T.; Couturier, M. Cerebrospinal fluid transthyretin: Aging and late onset Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 1997, 63, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Cheong, L.-Z.; Sun, T.; Li, Y.; Zhou, J.; Lu, C.; Li, Y.; Huang, Z.; Su, X. Dietary krill oil enhances neurocognitive functions and modulates proteomic changes in brain tissues of d-galactose induced aging mice. Food Funct. 2017, 8, 2038–2045. [Google Scholar] [CrossRef] [PubMed]
- Rühl, R.; Krzyżosiak, A.; Niewiadomska-Cimicka, A.; Rochel, N.; Szeles, L.; Vaz, B.; Wietrzych-Schindler, M.; Álvarez, S.; Szklenar, M.; Nagy, L.; et al. 9-cis-13,14-Dihydroretinoic Acid Is an Endogenous Retinoid Acting as RXR Ligand in Mice. PLoS Genet. 2015, 11, e1005213. [Google Scholar] [CrossRef] [PubMed]
- Poggetti, V.; Salerno, S.; Baglini, E.; Barresi, E.; Da Settimo, F.; Taliani, S. Carbonic Anhydrase Activators for Neurodegeneration: An Overview. Molecules 2022, 27, 2544. [Google Scholar] [CrossRef]
- Morawski, M.; Filippov, M.; Tzinia, A.; Tsilibary, E.; Vargova, L. ECM in brain aging and dementia. In Progress in Brain Research; Dityatev, A., Wehrle-Haller, B., Pitkänen, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 207–227. [Google Scholar]
- Caltagarone, J.; Jing, Z.; Bowser, R. Focal adhesions regulate Aβ signaling and cell death in Alzheimer’s disease. Biochim. Biophys. Acta (BBA) -Mol. Basis Dis. 2007, 1772, 438–445. [Google Scholar] [CrossRef]
- Chen, M.; Xia, W. Proteomic Profiling of Plasma and Brain Tissue from Alzheimer’s Disease Patients Reveals Candidate Network of Plasma Biomarkers. J. Alzheimer’s Dis. 2020, 76, 349–368. [Google Scholar] [CrossRef]

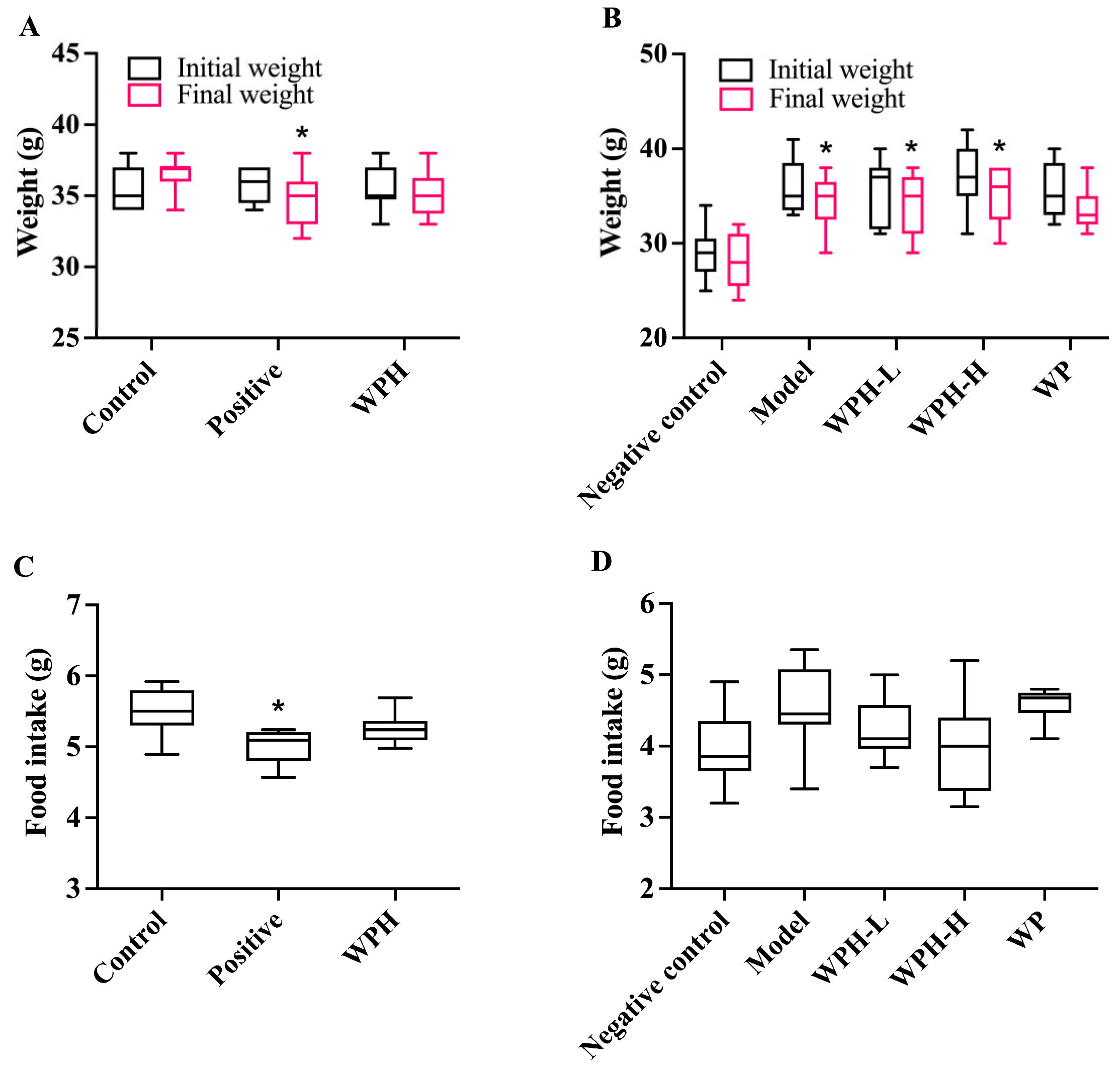

| Scopolamine-Induced ICR Mice | Aged C57BL/6J Mice | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Positive | WPH | Negative Control | Model | WPH-L | WPH-H | WP | ||
| Food intake (g/d) | 5.50 ± 0.36 | 5.02 ± 0.24 * | 5.25 ± 0.21 | 3.97 ± 0.53 | 4.56 ± 0.59 | 4.24 ± 0.43 | 3.95 ± 0.67 | 4.58 ± 0.25 | |
| Energy intake (kcal/d) | 17.66 ± 1.14 | 16.11 ± 0.78 | 16.85 ± 0.66 | 12.74 ± 1.69 | 14.64 ± 1.90 | 13.61 ± 1.39 | 12.68 ± 2.15 | 14.70 ± 0.79 | |
| Organic coefficient (%) | Brain | 1.25 ± 0.14 | 1.16 ± 0.11 | 1.17 ± 0.04 | 1.39 ± 0.18 | 1.09 ± 0.08 ## | 1.13 ± 0.12 | 1.18 ± 0.14 | 1.18 ± 0.14 |
| Liver | 4.42 ± 0.51 | 4.59 ± 0.10 | 4.50 ± 0.24 | 4.28 ± 0.29 | 4.52 ± 0.33 | 4.31 ± 0.33 | 4.71 ± 1.18 | 4.29 ± 0.63 | |
| Heart | 0.56 ± 0.09 | 0.48 ± 0.03 | 0.51 ± 0.05 | 0.61 ± 0.09 | 0.60 ± 0.09 | 0.54 ± 0.05 | 0.52 ± 0.04 | 0.61 ± 0.06 | |
| Kidney | 1.50 ± 0.19 | 1.47 ± 0.06 | 1.51 ± 0.11 | 1.37 ± 0.19 | 1.38 ± 0.21 | 1.33 ± 0.13 | 1.31 ± 0.15 | 1.27 ± 0.12 | |
| Spleen | 0.34 ± 0.04 | 0.35 ± 0.04 | 0.33 ± 0.04 | 0.29 ± 0.09 | 0.25 ± 0.05 | 0.22 ± 0.04 | 0.22 ± 0.11 | 0.27 ± 0.05 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, N.; Meng, H.; Wu, C.; Yokoyama, W.; Hong, H.; Luo, Y.; Tan, Y. Whey Protein Hydrolysate Renovates Age-Related and Scopolamine-Induced Cognitive Impairment. Nutrients 2023, 15, 1228. https://doi.org/10.3390/nu15051228
Ding N, Meng H, Wu C, Yokoyama W, Hong H, Luo Y, Tan Y. Whey Protein Hydrolysate Renovates Age-Related and Scopolamine-Induced Cognitive Impairment. Nutrients. 2023; 15(5):1228. https://doi.org/10.3390/nu15051228
Chicago/Turabian StyleDing, Ning, Hanxiu Meng, Chao Wu, Wallace Yokoyama, Hui Hong, Yongkang Luo, and Yuqing Tan. 2023. "Whey Protein Hydrolysate Renovates Age-Related and Scopolamine-Induced Cognitive Impairment" Nutrients 15, no. 5: 1228. https://doi.org/10.3390/nu15051228
APA StyleDing, N., Meng, H., Wu, C., Yokoyama, W., Hong, H., Luo, Y., & Tan, Y. (2023). Whey Protein Hydrolysate Renovates Age-Related and Scopolamine-Induced Cognitive Impairment. Nutrients, 15(5), 1228. https://doi.org/10.3390/nu15051228





