Evaluation of the Multifunctionality of Soybean Proteins and Peptides in Immune Cell Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Lunasin-Enriched Soybean Extract (LES)
2.3. Simulated Gastrointestinal Digestion of Lunasin-Enriched Soybean Extract (LES)
2.4. Characterization of the Lunasin-Enriched Soybean Extract (LES) and Its Digests
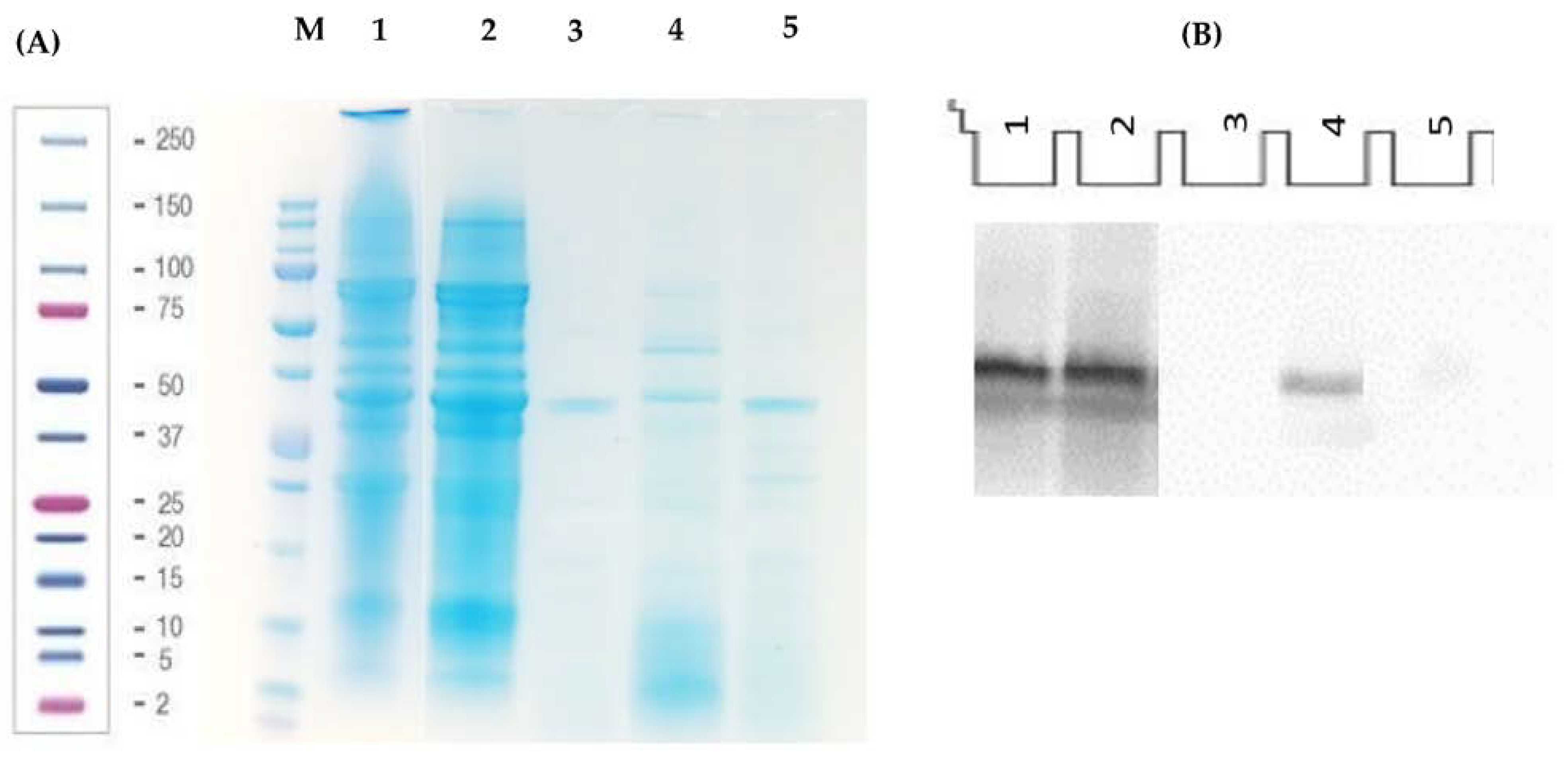
2.4.1. Gel Electrophoresis (SDS-PAGE)
2.4.2. Western Blot
2.5. Assessment of the Antioxidant Activity of the Lunasin-Enriched Soybean Extract (LES) with Biochemical Assays
2.6. Modulatory Effects in RAW264.7 and EL4 Cell Models
2.6.1. Cell Culture
2.6.2. Effects on Cell Viability and Cell Proliferation
2.6.3. Effects on Reactive Oxygen Species (ROS) Generation
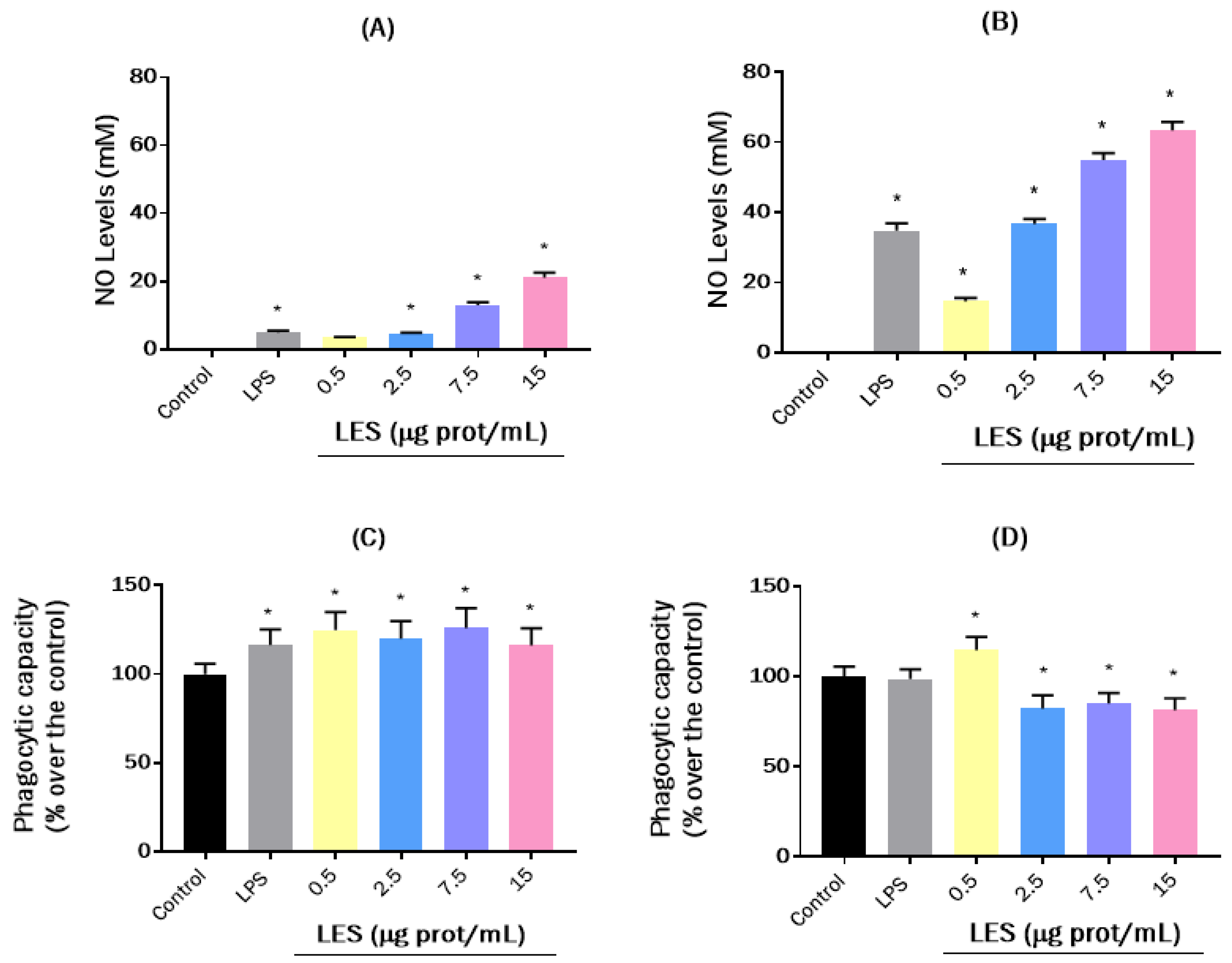
2.6.4. Effects on Nitric Oxide (NO) Levels
2.6.5. Effects on the Phagocytic Capacity of Macrophages
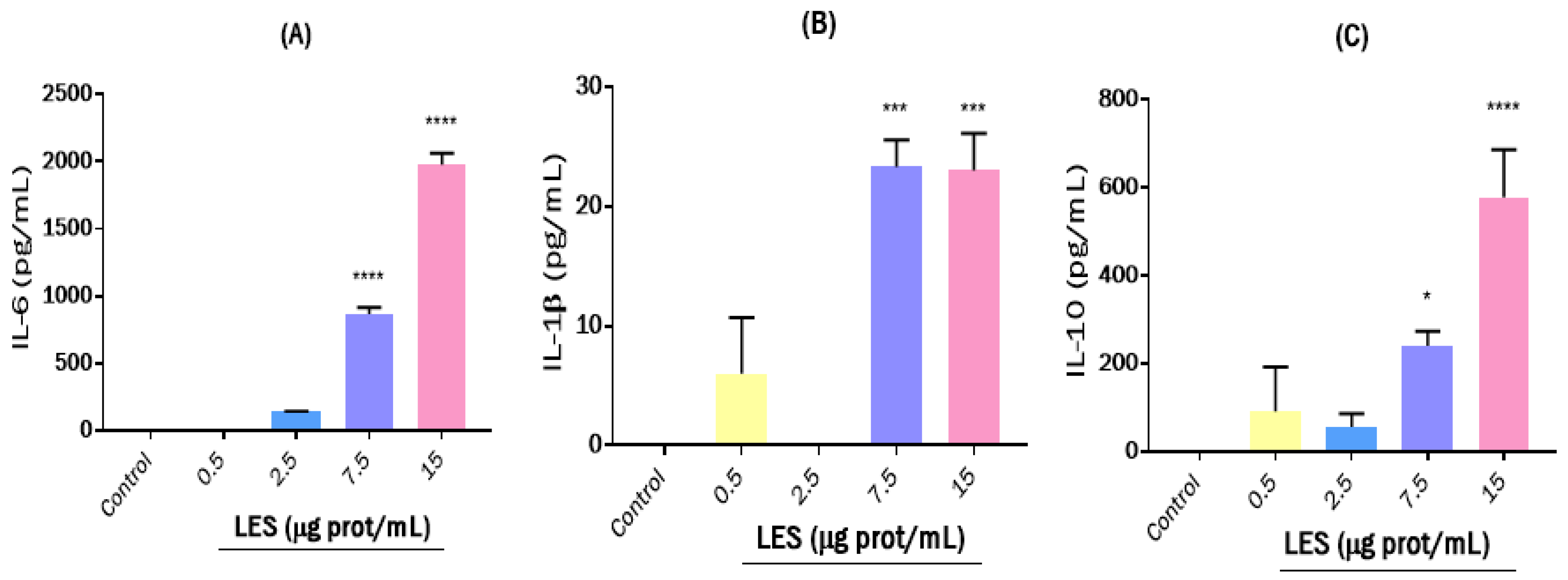
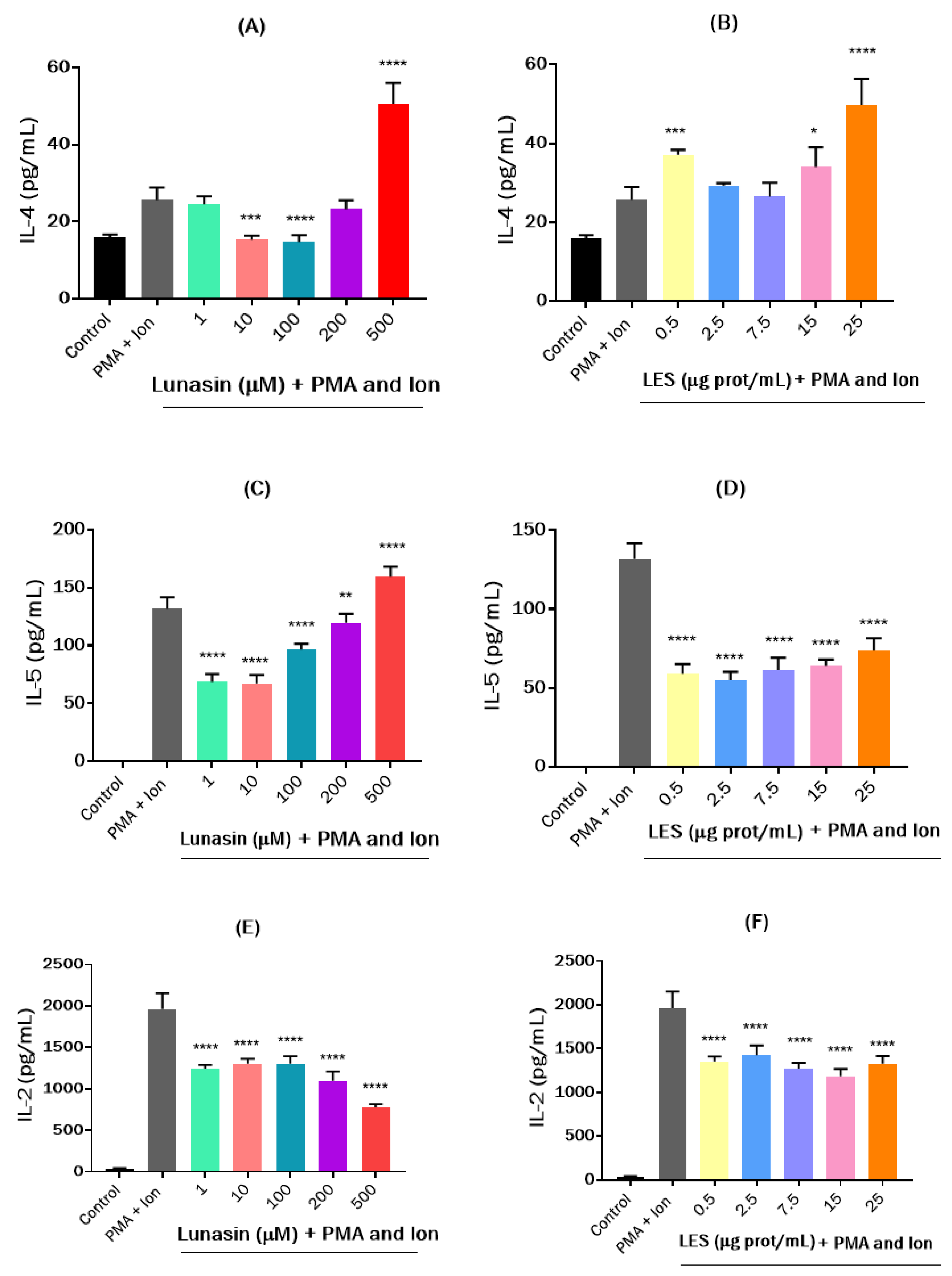
2.6.6. Effects on the Cytokine Release
2.7. Statistical Analysis
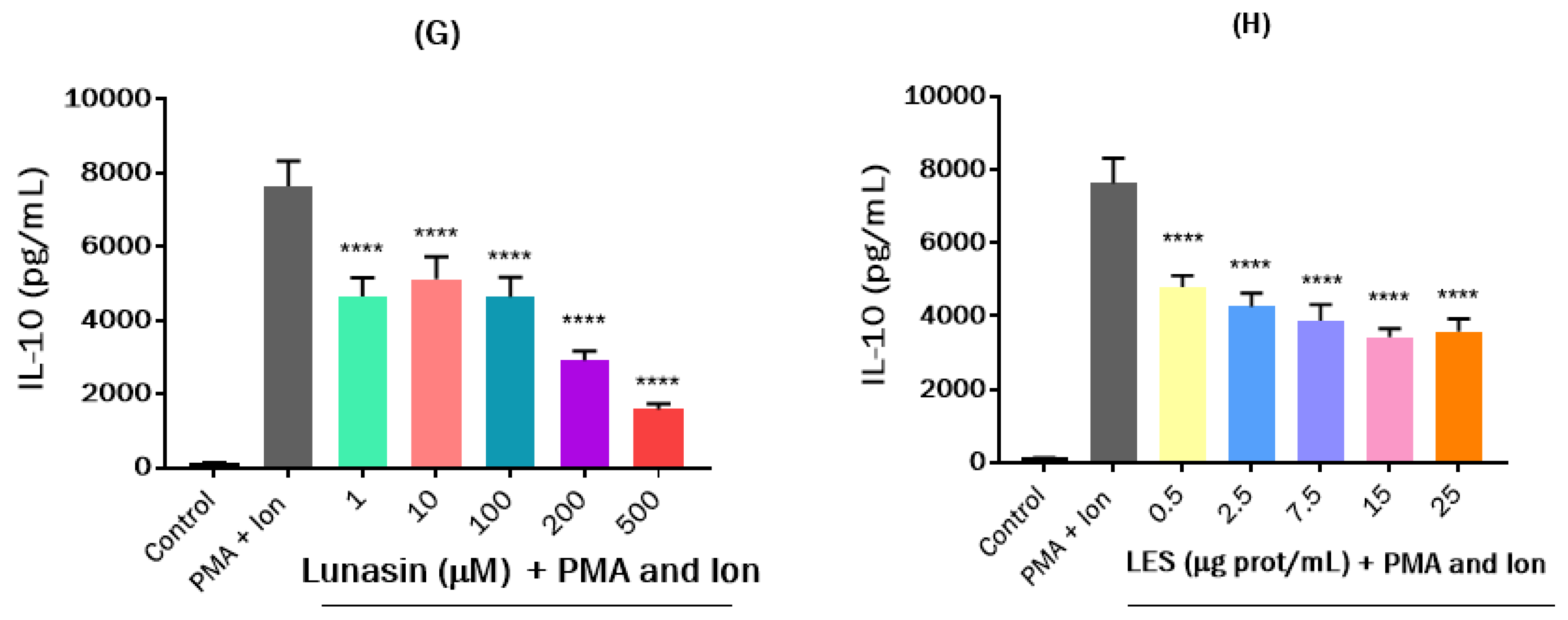
3. Results and Discussion
3.1. Characterization of Lunasin-Enriched Soybean Extract (LES) and Behavior under Simulated Gastrointestinal Digestion
3.2. Antioxidant Activity of Lunasin-Enriched Soybean Extract (LES) and Its Digests
3.3. Effects of Lunasin-Enriched Soybean Extract (LES) in RAW264.7 Macrophages and EL4 Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Jahandideh, F.; Wu, J. Food-Derived Bioactive Peptides on Inflammation and Oxidative Stress. BioMed Res. Int. 2014, 2014, 608979. [Google Scholar] [CrossRef] [PubMed]
- Priya, S. Therapeutic Perspectives of Food Bioactive Peptides: A Mini Review. Protein Pept. Lett. 2019, 26, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Majumder, K.; Mine, Y.; Wu, J. The potential of food protein-derived anti-inflammatory peptides against various chronic inflammatory diseases. J. Sci. Food Agric. 2015, 96, 2303–2311. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Liu, H.; Sun, Y.; Zhang, J.; Chen, X.; Chen, N. Lunasin: A promising polypeptide for the prevention and treatment of cancer. Oncol. Lett. 2017, 13, 3997–4001. [Google Scholar] [CrossRef]
- Galvez, A.; Revilleza, M.R.J.; de Lumen, B.O. A novel methionine-rich protein from soybean cotyledon: Cloning and char-acterization of a cDNA (Accession No. AF005030) Plant Gene Register #PGR97-103. Plant Physiol. 1997, 114, 1567–1597. [Google Scholar]
- Solanki, D. Food Derived Bioactive Peptides and its Application on Health Benefits. Int. J. Fermented Foods 2018, 7, 21–30. [Google Scholar] [CrossRef]
- Fernández-Tomé, S.; Sanchón, J.; Recio, I.; Hernández-Ledesma, B. Transepithelial transport of lunasin and derived peptides: Inhibitory effects on the gastrointestinal cancer cells viability. J. Food Compos. Anal. 2018, 68, 101–110. [Google Scholar] [CrossRef]
- Fernández-Tomé, S.; Hernández-Ledesma, B. Gastrointestinal Digestion of Food Proteins under the Effects of Released Bioactive Peptides on Digestive Health. Mol. Nutr. Food Res. 2020, 64, 2000401. [Google Scholar] [CrossRef]
- Fernández-Tomé, S.; Hernández-Ledesma, B. Current state of art after twenty years of the discovery of bioactive peptide lunasin. Food Res. Int. 2018, 116, 71–78. [Google Scholar] [CrossRef]
- Fernández-Tomé, S.; Indiano-Romacho, P.; Mora-Gutiérrez, I.; Pérez-Rodríguez, L.; Moreno, L.O.; Marin, A.C.; Baldán-Martín, M.; Moreno-Monteagudo, J.A.; Santander, C.; Chaparro, M.; et al. Lunasin Peptide is a Modulator of the Immune Response in the Human Gastrointestinal Tract. Mol. Nutr. Food Res. 2021, 65, e2001034. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Hsieh, C.-C.; de Lumen, B.O. Antioxidant and anti-inflammatory properties of cancer preventive peptide lunasin in RAW 264.7 macrophages. Biochem. Biophys. Res. Commun. 2009, 390, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ledesma, B.; Dávalos, A.; Bartolomé, B.; Amigo, L. Preparation of Antioxidant Enzymatic Hydrolysates from α-Lactalbumin and β-Lactoglobulin. Identification of Active Peptides by HPLC-MS/MS. J. Agric. Food Chem. 2005, 53, 588–593. [Google Scholar] [CrossRef]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Paterson, S.; Fernández-Tomé, S.; Hernández-Ledesma, B. Modulatory Effects of a Lunasin-Enriched Soybean Extract on Immune Response and Oxidative Stress-Associated Biomarkers. Biol. Life Sci. Forum 2022, 12, 10. [Google Scholar] [CrossRef]
- Murphy, P. Soybeans, Soybean Proteins; AOCS Press: Urbana, IL, USA, 2008; pp. 229–267. [Google Scholar] [CrossRef]
- de Mejia, E.G.; Castañeda-Reyes, E.; Mojica, L.; Dia, V.; Wang, H.; Wang, T.; Johnson, L. Potential Health Benefits Associated with Lunasin Concentration in Dietary Supplements and Lunasin-Enriched Soy Extract. Nutrients 2021, 13, 1618. [Google Scholar] [CrossRef]
- Cavazos, A.; Morales, E.; Dia, V.P.; De Mejia, E.G. Analysis of Lunasin in Commercial and Pilot Plant Produced Soybean Products and an Improved Method of Lunasin Purification. J. Food Sci. 2012, 77, C539–C545. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ledesma, B.; Hsieh, C.-C.; de Lumen, B.O. Lunasin and Bowman-Birk protease inhibitor (BBI) in US commercial soy foods. Food Chem. 2009, 115, 574–580. [Google Scholar] [CrossRef]
- Indiano-Romacho, P.; Fernández-Tomé, S.; Amigo, L.; Hernández-Ledesma, B. Multifunctionality of lunasin and peptides released during its simulated gastrointestinal digestion. Food Res. Int. 2019, 125, 108513. [Google Scholar] [CrossRef]
- Dia, V.P.; Torres, S.; De Lumen, B.O.; Erdman, J.W., Jr.; De Mejia, E.G. Presence of Lunasin in Plasma of Men after Soy Protein Consumption. J. Agric. Food Chem. 2009, 57, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-M.; Muramoto, K.; Yamauchi, F. Structural Analysis of Antioxidative Peptides from Soybean.beta.-Conglycinin. J. Agric. Food Chem. 1995, 43, 574–578. [Google Scholar] [CrossRef]
- Gibbs, B.F.; Zougman, A.; Masse, R.; Mulligan, C. Production and characterization of bioactive peptides from soy hydrolysate and soy-fermented food. Food Res. Int. 2004, 37, 123–131. [Google Scholar] [CrossRef]
- Farzamirad, V.; Aluko, R.E. Angiotensin-converting enzyme inhibition and free-radical scavenging properties of cationic peptides derived from soybean protein hydrolysates. Int. J. Food Sci. Nutr. 2008, 59, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Król-Grzymała, A.; Amarowicz, R. Phenolic Compounds of Soybean Seeds from Two European Countries and Their Antioxidant Properties. Molecules 2020, 25, 2075. [Google Scholar] [CrossRef]
- Jin, Y.-J.; Pyo, Y.-H. Effect of Monascus-Fermented Soybean Extracts on Antioxidant and Skin Aging-Related Enzymes Inhibitory Activities. Prev. Nutr. Food Sci. 2017, 22, 376–380. [Google Scholar] [CrossRef]
- Coscueta, E.R.; Campos, D.A.; Osório, H.; Nerli, B.B.; Pintado, M. Enzymatic soy protein hydrolysis: A tool for biofunctional food ingredient production. Food Chem. X 2019, 1, 100006. [Google Scholar] [CrossRef]
- Coscueta, E.R.; Malpiedi, L.P.; Pintado, M.M.; Nerli, B.B. Production of soy protein concentrate with the recovery of bioactive compounds: From destruction to valorization. Food Hydrocoll. 2023, 137, 108314. [Google Scholar] [CrossRef]
- Yi, G.; Li, H.; Liu, M.; Ying, Z.; Zhang, J.; Liu, X. Soybean protein-derived peptides inhibit inflammation in LPS-induced RAW264.7 macrophages via the suppression of TLR4-mediated MAPK-JNK and NF-kappa B activation. J. Food Biochem. 2020, 44, e13289. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Diao, P.; Shu, X.; Li, L.; Xiong, L. Quercetin and Quercitrin Attenuates the Inflammatory Response and Oxidative Stress in LPS-Induced RAW264.7 Cells: In Vitro Assessment and a Theoretical Model. BioMed Res. Int. 2019, 2019, 7039802. [Google Scholar] [CrossRef]
- Dávila-Ortiz, G.; Castañeda-Reyes, E.D.; Juárez-Palomo, C.I.; Perea-Flores, M.D.J.; Pérez-Pastén-Borja, R.; Márquez-Flores, Y.K.; de Mejía, E.G. Liposomes Containing Amaranth Unsaponifiable Matter and Soybean Lunasin Suppress ROS Production in Fibroblasts and Reduced Interleukin Production in Macrophages. Int. J. Environ. Res. Public Health 2022, 19, 11678. [Google Scholar] [CrossRef]
- Price, S.J.; Pangloli, P.; Dia, V.P. Pepsin–pancreatin hydrolysis reduced the ability of lunasin-enriched material to inhibit activation of the inflammasomes in THP-1 human macrophages. Food Funct. 2017, 8, 4449–4458. [Google Scholar] [CrossRef] [PubMed]
- García-Nebot, M.J.; Recio, I.; Hernández-Ledesma, B. Antioxidant activity and protective effects of peptide lunasin against oxidative stress in intestinal Caco-2 cells. Food Chem. Toxicol. 2014, 65, 155–161. [Google Scholar] [CrossRef]
- Regueiro González, J.R.; Larrea, C.L.; Rodríguez, S.G.; Naves, E.M. Inmunología, 3rd ed.; Médica Panamericana: Madrid, Spain, 2003; p. 276. [Google Scholar]
- Hsieh, C.-C.; Wang, Y.-F.; Lin, P.-Y.; Peng, S.-H.; Chou, M.-J. Seed peptide lunasin ameliorates obesity-induced inflammation and regulates immune responses in C57BL/6J mice fed high-fat diet. Food Chem. Toxicol. 2021, 147, 111908. [Google Scholar] [CrossRef]
- Ai, W.; Li, H.; Song, N.; Li, L.; Chen, H. Optimal Method to Stimulate Cytokine Production and Its Use in Immunotoxicity Assessment. Int. J. Environ. Res. Public Health 2013, 10, 3834. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-Gil, J.M.; Fernandez-Nieto, M.; Acevedo, N. Understanding the Cellular Sources of the Fractional Exhaled Nitric Oxide (FeNO) and Its Role as a Biomarker of Type 2 Inflammation in Asthma. BioMed Res. Int. 2022, 2022, 5753524. [Google Scholar] [CrossRef]
- MacMicking, J.; Xie, Q.-W.; Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef] [PubMed]
- de Mejia, E.G.; Dia, V.P. Lunasin and lunasin-like peptides inhibit inflammation through suppression of NF-κB pathway in the macrophage. Peptides 2009, 30, 2388–2398. [Google Scholar] [CrossRef]
- Ren, G.; Zhu, Y.; Shi, Z.; Li, J. Detection of lunasin in quinoa (Chenopodium quinoa Willd.) and the in vitro evaluation of its antioxidant and anti-inflammatory activities. J. Sci. Food Agric. 2017, 97, 4110–4116. [Google Scholar] [CrossRef]
- Yao, L.; Yang, P.; Luo, W.; Li, S.; Wu, Y.; Cai, N.; Bi, D.; Li, H.; Han, Q.; Xu, X. Macrophage-stimulating activity of European eel (Anguilla anguilla) peptides in RAW264.7 cells mediated via NF-κB and MAPK signaling pathways. Food Funct. 2020, 11, 10968–10978. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Cai, X.; Huang, M.; Chen, X.; Tian, Y.; Chen, G.; Wang, M.; Wang, S.; Xiao, J. Isolation, Identification, and Immunomodulatory Effect of a Peptide from Pseudostellaria heterophylla Protein Hydrolysate. J. Agric. Food Chem. 2020, 68, 12259–12270. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ahn, D.U.; Paik, H.-D. In Vitro Immune-Enhancing Activity of Ovotransferrin from Egg White via MAPK Signaling Pathways in RAW 264.7 Macrophages. Korean J. Food Sci. Anim. Resour. 2018, 38, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Murray, P.J. Cytokine Signaling Modules in Inflammatory Responses. Immunity 2008, 28, 477–487. [Google Scholar] [CrossRef]
- Tau, G.; Rothman, P.B. Biologic functions of the IFN-gamma receptors. Allergy 1999, 54, 1233–1251. [Google Scholar] [CrossRef]
- Gabay, C. Interleukin-6 and chronic inflammation. Thromb. Haemost. 2006, 8, S3. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef]
- Kaneko, N.; Kurata, M.; Yamamoto, T.; Morikawa, S.; Masumoto, J. The role of interleukin-1 in general pathology. Inflamm. Regen. 2019, 39, 12. [Google Scholar] [CrossRef]
- Zhao, H.-J.; Zhao, X.-H. Effect of the Zn Supplementation on Immuno-Modulatory Activities of Bovine Lactoferrin in the Murine Splenocytes and RAW264.7 Macrophages. Biol. Trace Element Res. 2019, 192, 287–296. [Google Scholar] [CrossRef]
- Hsieh, C.-C.; Chou, M.-J.; Wang, C.-H. Lunasin attenuates obesity-related inflammation in RAW264.7 cells and 3T3-L1 adipocytes by inhibiting inflammatory cytokine production. PLoS ONE 2017, 12, e0171969. [Google Scholar] [CrossRef]
- Iyer, S.S.; Cheng, G. Role of Interleukin 10 Transcriptional Regulation in Inflammation and Autoimmune Disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef] [PubMed]
- Spits, H.; Malefyt, R.D.W. Functional Characterization of Human IL-10. Int. Arch. Allergy Immunol. 1992, 99, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Cheng, D.; Huang, C.; Li, Y.; Lao, C.; Xia, Y.; Liu, W.; Gong, X.; Hu, D.; Li, B.; et al. Improvement of Colonic Immune Function with Soy Isoflavones in High-Fat Diet-Induced Obese Rats. Molecules 2019, 24, 1139. [Google Scholar] [CrossRef]
- Moussa, L.; Bézirard, V.; Salvador-Cartier, C.; Bacquié, V.; Lencina, C.; Lévêque, M.; Braniste, V.; Ménard, S.; Théodorou, V.; Houdeau, E. A Low Dose of Fermented Soy Germ Alleviates Gut Barrier Injury, Hyperalgesia and Faecal Protease Activity in a Rat Model of Inflammatory Bowel Disease. PLoS ONE 2012, 7, e49547. [Google Scholar] [CrossRef] [PubMed]
- Celiberto, L.S.; Bedani, R.; Dejani, N.N.; de Medeiros, A.I.; Zuanon, J.A.S.; Spolidorio, L.C.; Adorno, M.A.T.; Varesche, M.B.A.; Galvão, F.C.; Valentini, S.R.; et al. Effect of a probiotic beverage consumption (Enterococcus faecium CRL 183 and Bifidobacterium longum ATCC 15707) in rats with chemically induced colitis. PLoS ONE 2017, 12, e0175935. [Google Scholar] [CrossRef]
- Wang, B.; Wu, C. Dietary soy isoflavones alleviate dextran sulfate sodium-induced inflammation and oxidative stress in mice. Exp. Ther. Med. 2017, 14, 276–282. [Google Scholar] [CrossRef]
- Li, J.; Ye, L.; Cook, D.R.; Wang, X.; Liu, J.; Kolson, D.L.; Persidsky, Y.; Ho, W.-Z. Soybean-derived Bowman-Birk inhibitor inhibits neurotoxicity of LPS-activated macrophages. J. Neuroinflammation 2011, 8, 15. [Google Scholar] [CrossRef]
- Gadani, S.P.; Cronk, J.C.; Norris, G.T.; Kipnis, J. IL-4 in the Brain: A Cytokine to Remember. J. Immunol. 2012, 189, 4213–4219. [Google Scholar] [CrossRef]
- Park, J.; Kim, S.H.; Cho, D.; Kim, T.S. Formononetin, a phyto-oestrogen, and its metabolites up-regulate interleukin-4 production in activated T cells via increased AP-1 DNA binding activity. Immunology 2005, 116, 71–81. [Google Scholar] [CrossRef]
- Lee, C.-C.; Kang, J.-J.; Chiang, B.-L.; Wang, C.-N.; Cheng, Y.-W. Shikonin inhibited mitogen-activated IL-4 and IL-5 production on EL-4 cells through downregulation of GATA-3 and c-Maf induction. Life Sci. 2011, 89, 364–370. [Google Scholar] [CrossRef]
- Takatsu, K. Interleukin-5 and IL-5 receptor in health and diseases. Proc. Jpn. Acad. Ser. B 2011, 87, 463–485. [Google Scholar] [CrossRef] [PubMed]
- Kusano, S.; Kukimoto-Niino, M.; Hino, N.; Ohsawa, N.; Ikutani, M.; Takaki, S.; Sakamoto, K.; Hara-Yokoyama, M.; Shirouzu, M.; Takatsu, K.; et al. Structural basis of interleukin-5 dimer recognition by its α receptor. Protein Sci. 2012, 21, 850–864. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-L.; Chen, S.-Y.; Ho, C.-Y.; Yen, G.-C. Citrus flavonoids suppress IL-5 and ROS through distinct pathways in PMA/ionomycin-induced EL-4 cells. Food Funct. 2019, 11, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Wei, C.; Kou, D. Beneficial effects of naringenin and morin on interleukin-5 and reactive oxygen species production in BALB/c mice with ovalbumin-induced asthma. Korean J. Physiol. Pharmacol. 2021, 25, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Ramirez, N.; Woytschak, J.; Boyman, O. Interleukin-2: Biology, Design and Application. Trends Immunol. 2015, 36, 763–777. [Google Scholar] [CrossRef]
- Abbas, A.K. The Surprising Story of IL-2. Am. J. Pathol. 2020, 190, 1776–1781. [Google Scholar] [CrossRef] [PubMed]
- Yea, S.S.; Jeong, H.-S.; Choi, C.Y.; Park, K.-R.; Oh, S.; Shin, J.-G.; Yun, C.-H. Inhibitory effect of anethole on T-lymphocyte proliferation and interleukin-2 production through down-regulation of the NF-AT and AP-1. Toxicol. Vitr. 2006, 20, 1098–1105. [Google Scholar] [CrossRef]

| Condition | Viable Cells (% over Control) | ROS (% over Control) | ||
|---|---|---|---|---|
| 8 h | 24 h | 8 h | 24 h | |
| Control | 100.0 ± 5.6 | 100.0 ± 4.2 | 100.0 ± 10.0 | 100.0 ± 6.2 |
| LPS a | 107.7 ± 9.2 | 76.2 ± 8.4 **** | 124.9 ± 12.6 **** | 115.2 ± 7.4 * |
| LES 0.5 µg protein/mL | 116.4 ± 12.6 **** | 105.4 ± 6.3 | 74.4 ± 6.1 **** | 81.6 ± 6.9 ** |
| LES 2.5 µg protein/mL | 118.5 ± 12.9 **** | 83.6 ± 5.4 ** | 73.5 ± 8.6 **** | 77.8 ± 6.2 *** |
| LES 7.5 µg protein/mL | 118.4 ± 12.7 **** | 65.2 ± 7.7 **** | 74.5 ± 0.9 **** | 76.5 ± 5.3 *** |
| LES 15 µg protein/mL | 119.2 ± 6.5 **** | 53.0 ± 7.2 **** | 105.0 ± 8.9 | 136.1 ± 12.5 **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paterson, S.; Fernández-Tomé, S.; Galvez, A.; Hernández-Ledesma, B. Evaluation of the Multifunctionality of Soybean Proteins and Peptides in Immune Cell Models. Nutrients 2023, 15, 1220. https://doi.org/10.3390/nu15051220
Paterson S, Fernández-Tomé S, Galvez A, Hernández-Ledesma B. Evaluation of the Multifunctionality of Soybean Proteins and Peptides in Immune Cell Models. Nutrients. 2023; 15(5):1220. https://doi.org/10.3390/nu15051220
Chicago/Turabian StylePaterson, Samuel, Samuel Fernández-Tomé, Alfredo Galvez, and Blanca Hernández-Ledesma. 2023. "Evaluation of the Multifunctionality of Soybean Proteins and Peptides in Immune Cell Models" Nutrients 15, no. 5: 1220. https://doi.org/10.3390/nu15051220
APA StylePaterson, S., Fernández-Tomé, S., Galvez, A., & Hernández-Ledesma, B. (2023). Evaluation of the Multifunctionality of Soybean Proteins and Peptides in Immune Cell Models. Nutrients, 15(5), 1220. https://doi.org/10.3390/nu15051220








