Eating Vegetables First Regardless of Eating Speed Has a Significant Reducing Effect on Postprandial Blood Glucose and Insulin in Young Healthy Women: Randomized Controlled Cross-Over Study
Abstract
1. Introduction
2. Methods
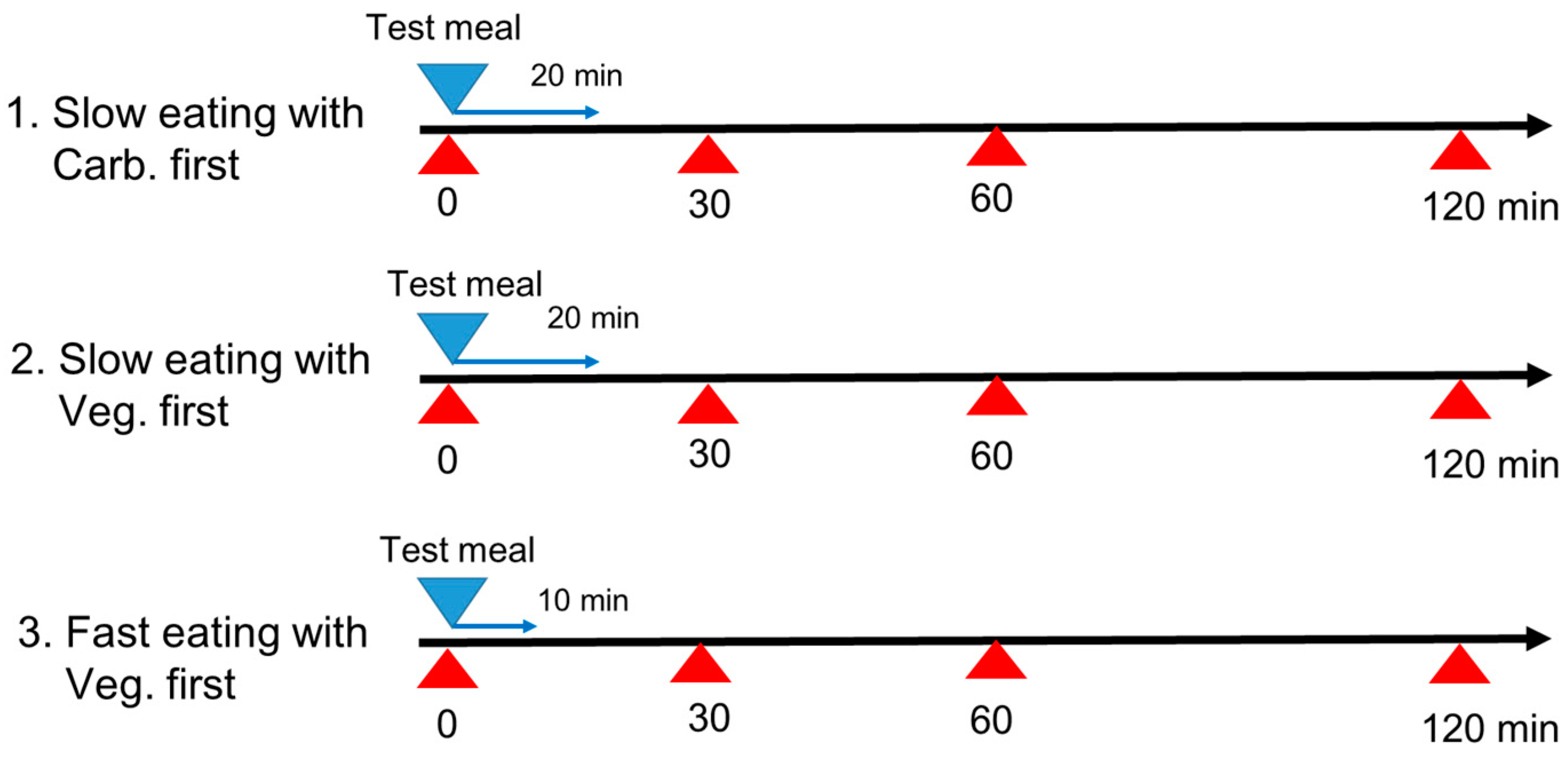
2.1. Study Design
- Carbohydrate first with slow eating speed: carbohydrate (boiled white rice) first for 6 min, and then protein (fried fish) for 7 min, and then vegetables (tomato and broccoli with sesame oil) for 7 min, for a total eating time of 20 min.
- Vegetables first with slow eating speed: vegetables (tomato and broccoli with sesame oil) first for 7 min, and then protein (fried fish) for 7 min, and then carbohydrate (boiled white rice) for 6 min, for a total eating time of 20 min.
- Vegetables first with fast eating speed: vegetables (tomato and broccoli with sesame oil) first for 4 min, and then protein (fried fish) for 3 min, and then carbohydrate (boiled white rice) for 3 min, for a total eating time of 10 min.
2.2. Meals for the Study
2.3. Primary and Secondary Measurements and Statistical Analysis
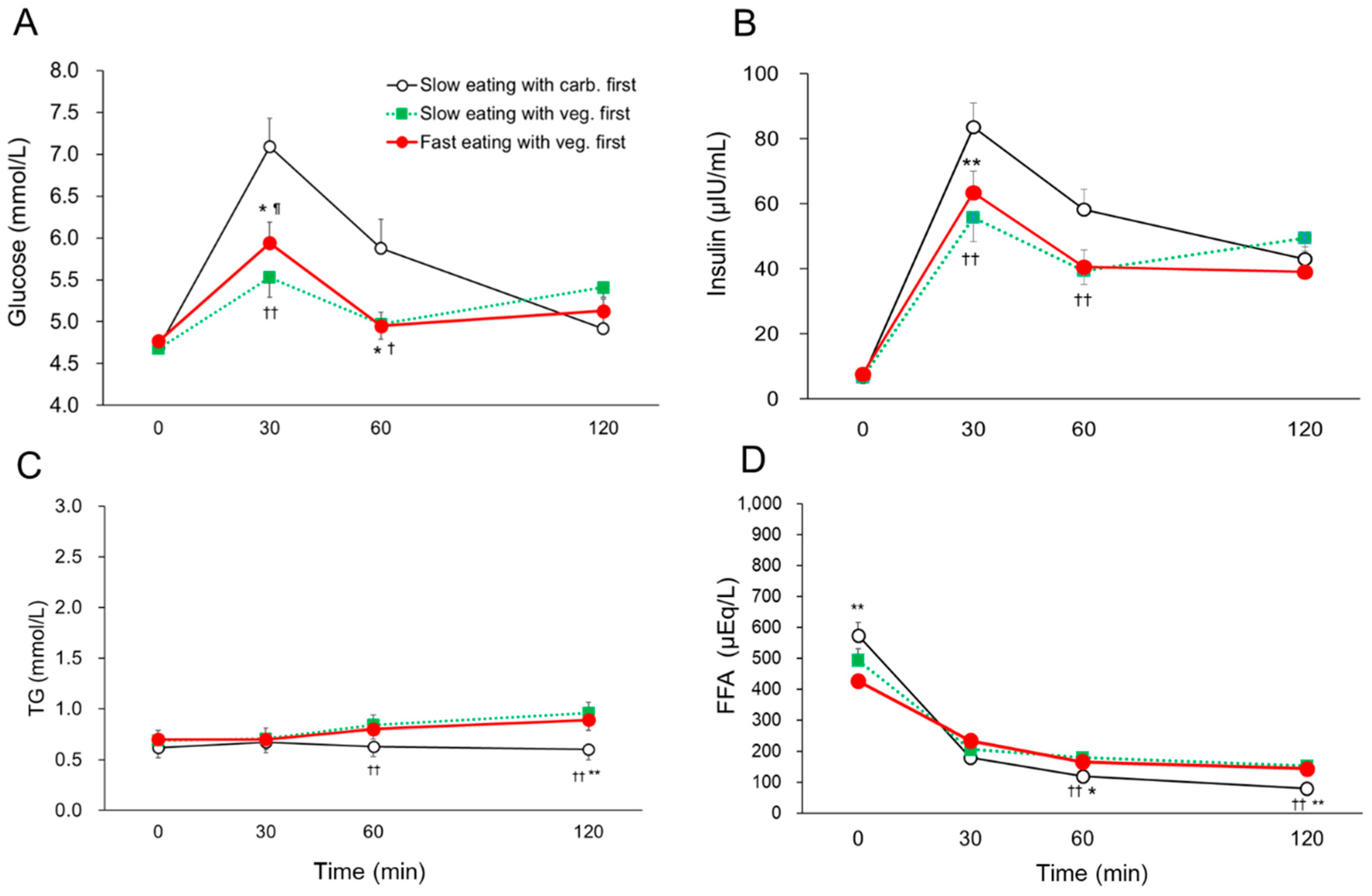
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IDF Diabetes Atlas. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2022; Available online: https://diabetesatlas.org/ (accessed on 5 January 2023).
- Powers, A.C.; Stafford, J.M.; Rickels, M.R. Chapter 398, Diabetes mellitus: Complications. In Harrison's Principles of Internal Medicine, 20th ed.; Jameson, J.L., Fauci, A.S., Kasper, D.L., Hauser, S.L., Longo, D.L., Loscalzo, J., Eds.; McGraw-Hill Education: New York, NY, USA, 2018; pp. 1–17. [Google Scholar]
- Davies, M.J.; D'Alessio, F.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of Hyperglycemia in type 2 diabetes, 2018. A consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of diabetes (EASD). Diabetes Care 2018, 41, 2669–2701. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Control and Complications Trial Research Group; Nathan, D.M.; Genuth, S.; Lachin, J.; Cleary, P.; Crofford, O.; Davis, M.; Rand, L.; Siebert, C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A. The post-prandial state and cardiovascular disease: Relevance to diabetes mellitus. Diabetes Metab. Res. Rev. 2000, 16, 125–132. [Google Scholar] [CrossRef]
- Su, G.; Mi, S.; Tao, H.; Li, Z.; Yang, H.; Zheng, H.; Zhou, Y.; Tian, L. Impact of Admission Glycaemic Variability, Glucose, and Glycosylated Hemoglobin on Major Adverse Cardiac Events After Acute Myocardial Infarction Variability. Diabetes Care 2013, 36, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Delahantry, L.; Simkins, S.W.; Camelon, K. Expanded role of the dietitian in the diabetes Control and Complications Trial: Implications for clinical practice. The DCCT Research Group. Am. Diet. Assoc. 1993, 93, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Hollingsworth, K.G.; Al-Mrabeh, A.; Leah Avery, L.; Benjamin Aribisala, B.; Muriel Caslake, M.; Roy Taylor, R. Very low-calorie diet and 6 months of weight stability in type 2 diabetes: Pathophysiological changes in responders and nonresponders. Diabetes Care 2016, 39, 808–815. [Google Scholar] [CrossRef]
- Tay, J.; Luscombe-Marsh, N.D.; Thompson, C.H.; Noakes, M.; Buckley, J.D.; Wittert, G.A.; Yancy, W.S., Jr.; Brinkworth, G.D. Comparison of low- and high-carbohydrate diets for type 2 diabetes management: A randomized trial. Am. J. Clin. Nutr. 2015, 102, 780–790. [Google Scholar] [CrossRef]
- Chiavaroli, L.; Lee, D.; Ahmed, A.; Cheung, A.; Khan, T.A.; Blanco, S.; Mejia; Mirrahimi, A.; Jenkins, D.J.A.; Livesey, G.; et al. Effect of low glycaemic index or load dietary patterns on glycaemic control and cardiometabolic risk factors in diabetes: Systematic review and meta-analysis of randomised controlled trials. BMJ 2021, 374, n1651. [Google Scholar] [CrossRef]
- Olfert, M.D.; Wattick, R.A. Vegetarian Diets and the Risk of Diabetes. Curr. Diab. Rep. 2018, 18, 101. [Google Scholar] [CrossRef]
- Albosta, M.; Bakke, J. Intermittent fasting: Is there a role in the treatment of diabetes? A review of the literature and guide for primary care physicians. Clin. Diabetes Endocrinol. 2021, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Schwarzfuchs, D.; Golan, R.; Shai, I. Four-year follow-up after two-year dietary interventions. N. Engl. J. Med. 2012, 367, 1373–1374. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Fukui, M.; Kajiyama, S. Effect of eating vegetables before carbohydrates on glucose excursions in patients with type 2 diabetes. J. Clin. Biochem. Nutr. 2014, 54, 7–11. [Google Scholar] [CrossRef]
- Shukla, A.P.; Dickison, M.; Coughlin, N.; Karan, A.; Mauer, E. The impact of food order on postprandial glycaemic excursions in prediabetes. Diabetes. Obes. Metab. 2019, 21, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Gentilcore, D.; Chaikomin, R.; Jones, K.L.; Russo, A.; Feinle-Bisset, C.; Wishart, J.M.; Rayner, C.K.; Horowitz, M. Effects of fat on gastric emptying of and the glycemic, insulin, and incretin responses to a carbohydrate meal in type 2 diabetes. J. Clin. Endocrinol. Metab. 2006, 91, 2062–2067. [Google Scholar] [CrossRef]
- Ma, J.; Stevens, J.E.; Cukier, K.; Maddox, A.F.; Wishart, J.M.; Jones, K.L.; Clifton, P.M.; Horowitz, M.; Rayner, C.K. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care 2009, 32, 1600–1602. [Google Scholar] [CrossRef] [PubMed]
- Nitta, A.; Imai, S.; Kajiayama, S.; Matsuda, M.; Miyawaki, T.; Matsumoto, S.; Kajiyama, S.; Hashimoto, Y.; Ozasa, N.; Fukui, M. Impact of Dietitian-Led Nutrition Therapy of Food Order on 5-Year Glycemic Control in Outpatients with Type 2 Diabetes at Primary Care Clinic: Retrospective Cohort Study. Nutrients 2022, 14, 2865. [Google Scholar] [CrossRef] [PubMed]
- Yabe, D.; Kuwata, H.; Fujiwara, Y.; Sakaguchi, M.; Moyama, S. Dietary instructions focusing on meal-sequence and nutritional balance for prediabetes subjects: An exploratory, cluster-randomized, prospective, open-label, clinical trial. J. Diabetes Complicat. 2019, 33, 107450. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.L.; Madden, C.; Gray, A.; Waters, D.; Horwath, C.C. Faster self-reported speed of eating is related to higher body mass index in a nationwide survey of middle-aged women. J. Am. Diet. Assoc. 2011, 111, 1192–1197. [Google Scholar] [CrossRef]
- Tanihara, S.; Imatoh, T.; Miyazaki, M.; Babazono, A.; Momose, Y.; Baba, M.; Uryu, Y.; Une, H. Retrospective longitudinal study on the relationship between 8-year weight change and current eating speed. Appetite 2011, 57, 179–183. [Google Scholar] [CrossRef]
- Maruyama, K.; Sato, S.; Ohira, T.; Maeda, K.; Noda, H.; Kubota, Y.; Nishimura, S.; Kitamura, A.; Kiyama, M.; Okada, T.; et al. The joint impact on being overweight of self reported behaviours of eating quickly and eating until full: Cross sectional survey. BMJ 2008, 337, a2002. [Google Scholar] [CrossRef]
- Hurst, Y.; Fukuda, H. Effects of changes in eating speed on obesity in patients with diabetes: A secondary analysis of longitudinal health check-up data. BMJ Open 2018, 8, e019589. [Google Scholar] [CrossRef]
- Sakurai, M.; Nakamura, K.; Miura, K.; Takamura, T.; Yoshita, K.; Nagasawa, S.-Y.; Morikawa, Y.; Ishizaki, M.; Kido, T.; Naruse, Y.; et al. Self-reported speed of eating and 7-year risk of type 2 diabetes mellitus in middle-aged Japanese men. Metabolism 2012, 61, 1566–1571. [Google Scholar] [CrossRef]
- Nagahama, S.; Kurotani, K.; Pham, N.M.; Nanri, A.; Kuwahara, K.; Dan, M.; Nishiwaki, Y.; Mizoue, T. Self-reported eating rate and metabolic syndrome in Japanese people: Cross-sectional study. BMJ Open 2014, 4, e005241. [Google Scholar] [CrossRef] [PubMed]
- Hamilton-Shield, J.; Goodred, J.; Powell, L.; Thorn, J.; Banks, J.; Hollinghurst, S.; Montgomery, A.; Turner, K.; Sharp, D. Changing Eating Behaviours to Treat Childhood Obesity in the Community Using Mandolean: Te Community Mandolean Randomised Controlled Trial (ComMando)–A Pilot Study. Health Technol. Assess. 2014, 18, 1–75. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Kajiyama, S.; Nitta, A.; Miyawaki, T.; Matsumoto, S.; Ozasa, N.; Kajiyama, S.; Hashimoto, Y.; Fukui, M.; Saeko, I.S. Eating fast has a significant impact on glycemic excursion in healthy women: Randomized controlled cross-over trial. Nutrients 2020, 12, 2767. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Fukui, M.; Ozasa, N.; Ozeki, T.; Kurokawa, M.; Komatsu, T.; Kajiyama, S. Eating vegetables before carbohydrates improves postprandial glucose excursions. Diabet. Med. 2013, 30, 370–372. [Google Scholar] [CrossRef] [PubMed]
- Kuwata, H.; Iwasaki, M.; Shimizu, S.; Minami, K.; Maeda, H.; Seino, S.; Nakada, K.; Nosaka, C.; Murotani, K.; Kurose, T.; et al. Meal sequence and glucose excursion, gastric emptying and incretin secretion in type 2 diabetes: A randomised, controlled crossover, exploratory trial. Diabetologia 2016, 59, 453–461. [Google Scholar] [CrossRef]
- Kim, Y.G.; Hahn, S.; Oh, T.J.; Kwak, S.H.; Park, K.S.; Cho, Y.M. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: A systematic review and meta-analysis. Diabetologia 2013, 56, 696–708. [Google Scholar] [CrossRef]
- Esposito, K.; Nappo, F.; Marfella, R.; Giugliano, G.; Giugliano, F.; Ciotola, M.; Quagliaro, L.; Ceriello, A.; Giugliano, D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans. Circulation 2002, 106, 2067–2072. [Google Scholar] [CrossRef]
- Sędzikowska, A.; Szablewski, L. Insulin and Insulin Resistance in Alzheimer's Disease. Int. J. Mol. Sci. 2021, 22, 9987. [Google Scholar] [CrossRef]
- Imai, S.; Matsuda, M.; Hasegawa, G.; Fukui, M.; Obayashi, H.; Ozasa, N.; Kajiyama, S. A simple meal plan of ‘eating vegetables before carbohydrate’ was more effective for achieving glycaemic control than an exchange–based meal plan in Japanese patients with type 2 diabetes. Asia Pac. J. Clin. Nutr. 2011, 20, 161–168. [Google Scholar]
- McIntosh, M.; Miller, C. A diet containing food rich in soluble and insoluble fiber improves glycaemic control and reduces hyperlipidemia among patients with type 2 diabetes mellitus. Nutr. Rev. 2001, 59, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Matsuda, M.; Miyatani, S.; Hasegawa, G.; Fukui, M.; Kajiyama, S. Crossover Study of the Effect of "Vegetables Before Carbohydrates" on the Reduction of the Postprandial Glucose and Insulin Levels in Japanese Patients with Type 2 Diabetes Mellitus. J. Jpn. Diabetes. Soc. 2010, 53, 112–115. [Google Scholar]
- Alsalim, W.; Ahrén, B. Insulin and incretin hormone responses to rapid versus slow ingestion of a standardized solid breakfast in healthy subjects. Endocrinol. Diab. Metab. 2019, 2, e00056. [Google Scholar] [CrossRef] [PubMed]
- Kamiko, K.; Aoki, K.; Kamiyama, H.; Taguri, M.; Terauchi, Y. Comparison of Plasma Glucose and Gut Hormone Levels between Drinking Enteral Formula over a Period of 5 and 20 Minutes in Japanese Patients with Type 2 Diabetes: A Pilot Study. J. Clin. Med. Res. 2016, 8, 749–752. [Google Scholar] [CrossRef]
- Schwingshack, L.; Hoffmann, G.; Lampousi, A.M.; Knüppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 363–375. [Google Scholar] [CrossRef]
- Robinson, E.; Almiron-Roig, E.; Rutters, F.; de Graaf, C.; Forde, C.G.; Smith, T.C.; Nolan, S.J.; Jebb, S.A. A systematic review and meta-analysis examining the effect of eating rate on energy intake and hunger. Am. J. Clin. Nutr. 2014, 100, 123–151. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; Jenkins, D.J. Carbohydrate digestibility and metabolic effects. J. Nutr. 2007, 137, S2539–S2546. [Google Scholar] [CrossRef]
- Japanese National Health and Nutrition Survey in 2016. Available online: https://www.mhlw.go.jp/file/04-Houdouhappyou-10904750-Kenkoukyoku-Gantaisakukenkouzoushinka/kekkagaiyou_7.pdf (accessed on 6 January 2023).
- Casagrande, S.S.; Wang, Y.; Anderson, C.; Gary, T.L. Have Americans increased their fruit and vegetable intake? The trends between 1988 and 2002. Am. J. Prev. Med. 2007, 32, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Nitta, A.; Imai, S.; Kajiyama, S.; Miyawaki, T.; Ozasa, N.; Kajiyama, S.; Hashimoto, Y.; Fukui, M. Tomato juice preload has a significant impact on postprandial glucose concentration in healthy women: A randomized cross-over trial. Asis Pac. J. Clin. Nutr. 2020, 29, 491–497. [Google Scholar]
- Iehara, R.; Imai, S.; Kajiyama, S.; Yamamoto, M.; Miyawaki, T.; Matsumoto, S.; Hashimoto, Y.; Ozasa, N.; Kajiyama, S.; Fukui, M. Vegetable juice preload ameliorates postprandial glucose concentration in healthy women: A randomized cross-over trial. J. Food Sci. Kyoto Women’s Univ. 2022, 77, 1–6. [Google Scholar]
- Livesey, G.; Taylor, R.; Livesey, H.F.; Buyken, A.E.; Jenkins, D.J.A.; Augustin, L.S.; Sievenpiper, J.; Barclay, A.W.; Liu, S.; Wolever, T.M.S.; et al. Dietary Glycemic Index and Load and the Risk of Type 2 Diabetes: Assessment of Causal Relations. Nutrients 2019, 11, 1436. [Google Scholar] [CrossRef] [PubMed]
- Naci, H.; Lehman, R.; Wouters, O.J.; Goldacre, B.; Yudkin, J.S. Rethinking the appraisal and approval of drugs for type 2 diabetes. BMJ 2015, 51, h5260. [Google Scholar] [CrossRef]

| Amount | Energy | Protein | Fat | Carbohydrate | Dietary Fiber | Salt | |
|---|---|---|---|---|---|---|---|
| (g) | (kcal) | (g) | (g) | (g) | (g) | (g) | |
| Boiled while rice | 200 | 336 | 5 | 0.6 | 74.2 | 3 | 0 |
| Tomato | 150 | 29 | 1.1 | 0.2 | 7.1 | 1.5 | 0 |
| Broccoli | 70 | 19 | 2.5 | 0.3 | 3 | 2.6 | 0 |
| Sesame oil | 10 | 92 | 0 | 10 | 0 | 0 | 0 |
| Frozen box of fried fish | 237 | 195 | 15.9 | 5.2 | 21.1 | 0 | 2.2 |
| Total | 667 | 671 | 24.5 | 16.3 | 105.4 | 7.1 | 2.2 |
| Slow Eating with Carb. First (n = 18) | Slow Eating with Veg. First (n = 18) | Fast Eating with Veg. First (n = 18) | |
|---|---|---|---|
| MBG (mmoll/L) | 5.65 ± 0.21 | 5.15 ± 0.11 | 5.20 ± 0.12 |
| SD for BG (mmoll/L) | 1.06 ± 0.11 *† | 0.58 ± 0.06 | 0.61 ± 0.05 |
| MAX BG (mmoll/L) | 7.13 ± 0.33 | 5.92 ± 0.19 | 6.10 ± 0.19 |
| LAGE (mmoll/L) | 2.71 ± 0.26 *† | 1.50 ± 0.14 | 1.60 ± 0.14 |
| IAUC 120 min for BG (mmoll/L × min) | 140 ± 27 *† | 68 ± 12 | 67 ± 12 |
| Mean insulin (μIU/mL) | 47.9 ± 3.7 | 37.9 ± 3.6 | 37.7 ± 3.7 |
| SD for insulin (μIU/mL) | 29.7 ± 2.3 **†† | 21.0 ± 2.1 | 21.8 ± 2.3 |
| MAX insulin (μIU/mL) | 85.6 ± 7.7 **† | 61.8 ± 5.7 | 65.8 ± 6.9 |
| Large amplitude of insulin excursion (μIU/mL) | 78.8 ± 7.3 **†† | 55.0 ± 5.2 | 58.2 ± 6.3 |
| IAUC 120 min for insulin (μU/mL × min) | 5702 ± 486 *†† | 4222 ± 458 | 4113 ± 402 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imai, S.; Kajiyama, S.; Kitta, K.; Miyawaki, T.; Matsumoto, S.; Ozasa, N.; Kajiyama, S.; Hashimoto, Y.; Fukui, M. Eating Vegetables First Regardless of Eating Speed Has a Significant Reducing Effect on Postprandial Blood Glucose and Insulin in Young Healthy Women: Randomized Controlled Cross-Over Study. Nutrients 2023, 15, 1174. https://doi.org/10.3390/nu15051174
Imai S, Kajiyama S, Kitta K, Miyawaki T, Matsumoto S, Ozasa N, Kajiyama S, Hashimoto Y, Fukui M. Eating Vegetables First Regardless of Eating Speed Has a Significant Reducing Effect on Postprandial Blood Glucose and Insulin in Young Healthy Women: Randomized Controlled Cross-Over Study. Nutrients. 2023; 15(5):1174. https://doi.org/10.3390/nu15051174
Chicago/Turabian StyleImai, Saeko, Shizuo Kajiyama, Kaoru Kitta, Takashi Miyawaki, Shinya Matsumoto, Neiko Ozasa, Shintaro Kajiyama, Yoshitaka Hashimoto, and Michiaki Fukui. 2023. "Eating Vegetables First Regardless of Eating Speed Has a Significant Reducing Effect on Postprandial Blood Glucose and Insulin in Young Healthy Women: Randomized Controlled Cross-Over Study" Nutrients 15, no. 5: 1174. https://doi.org/10.3390/nu15051174
APA StyleImai, S., Kajiyama, S., Kitta, K., Miyawaki, T., Matsumoto, S., Ozasa, N., Kajiyama, S., Hashimoto, Y., & Fukui, M. (2023). Eating Vegetables First Regardless of Eating Speed Has a Significant Reducing Effect on Postprandial Blood Glucose and Insulin in Young Healthy Women: Randomized Controlled Cross-Over Study. Nutrients, 15(5), 1174. https://doi.org/10.3390/nu15051174









