Association between Dietary Habits and Pancreatitis among Individuals of European Ancestry: A Two-Sample Mendelian Randomization Study
Abstract
1. Introduction
2. Materials and Methods
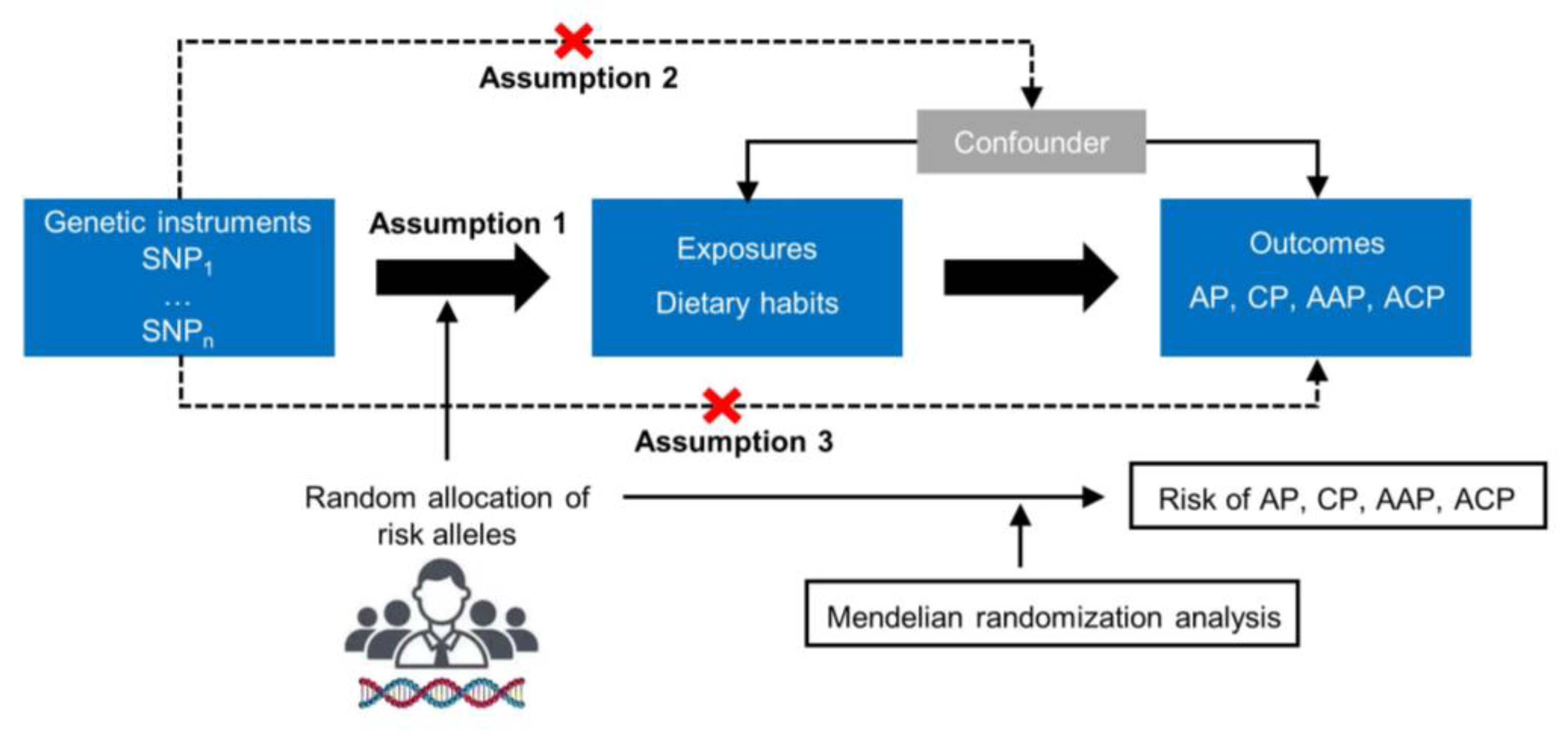
2.1. Study Design
2.2. GWAS Summary-Level Data of Dietary Habits and Pancreatitis
2.3. Genetic Instrument Selection
2.4. Univariate and Multivariate MR Analysis
2.5. Statistical Analyses
3. Results
3.1. Genetic Instruments for Eighteen Dietary Habits
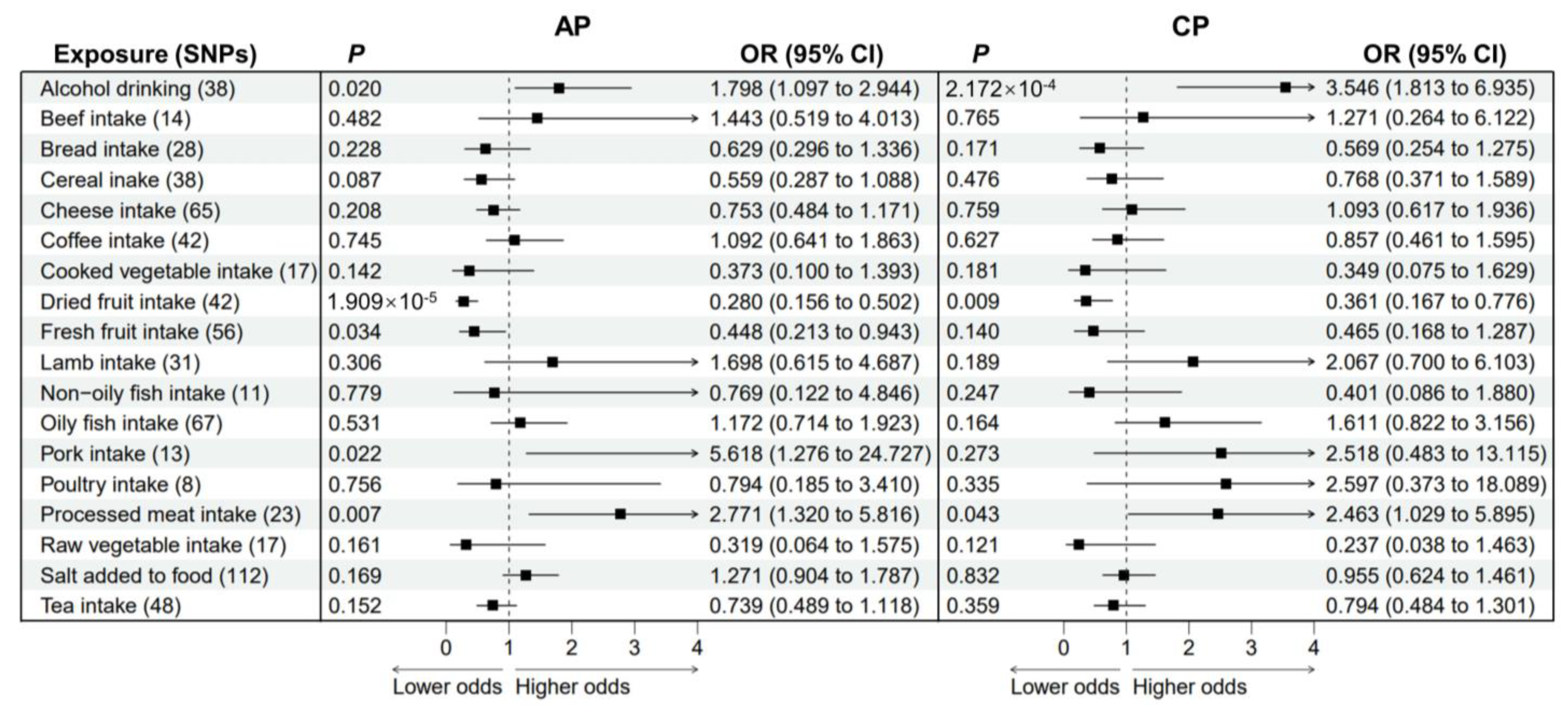
3.2. Causal Effects of Dietary Habits on AP and CP
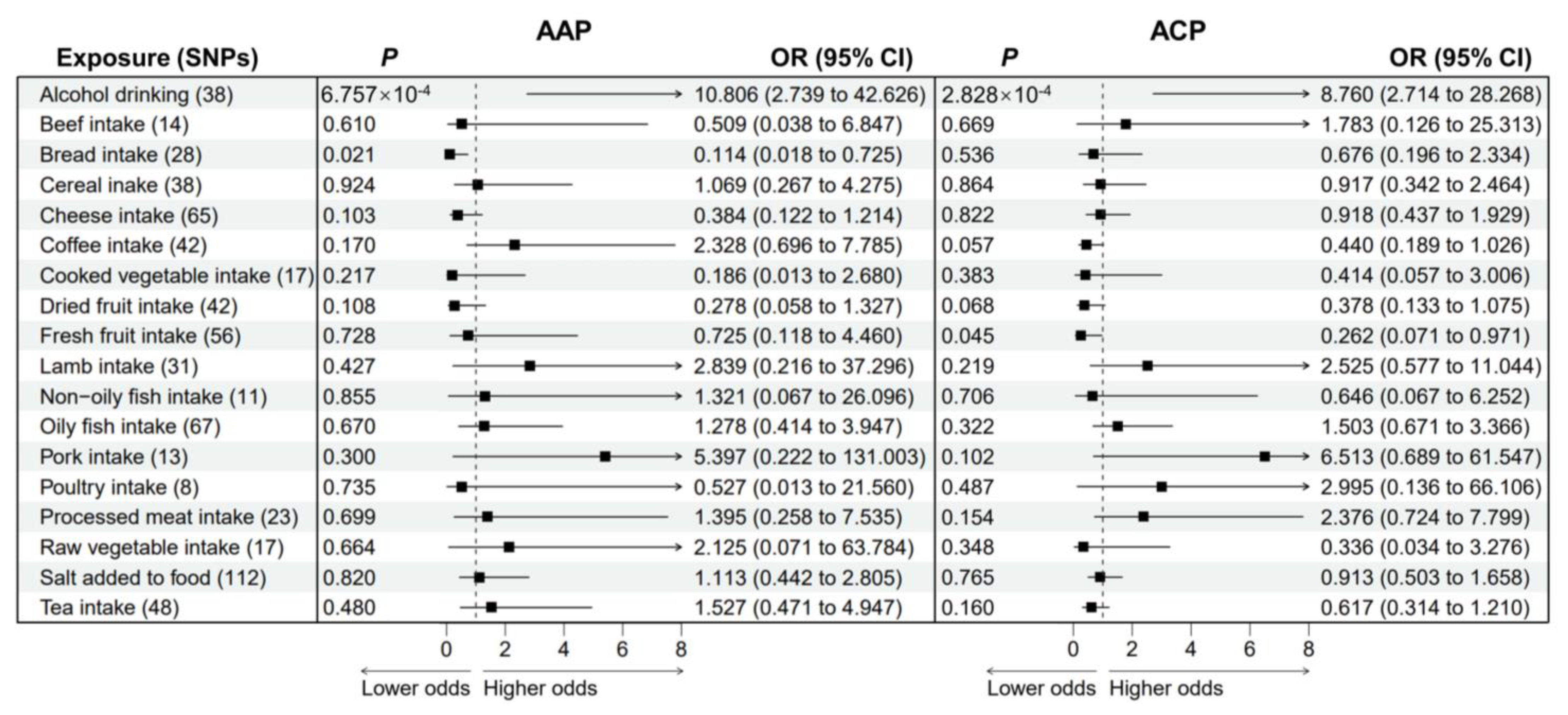
3.3. Causal Effects of Dietary Habits on AAP and ACP
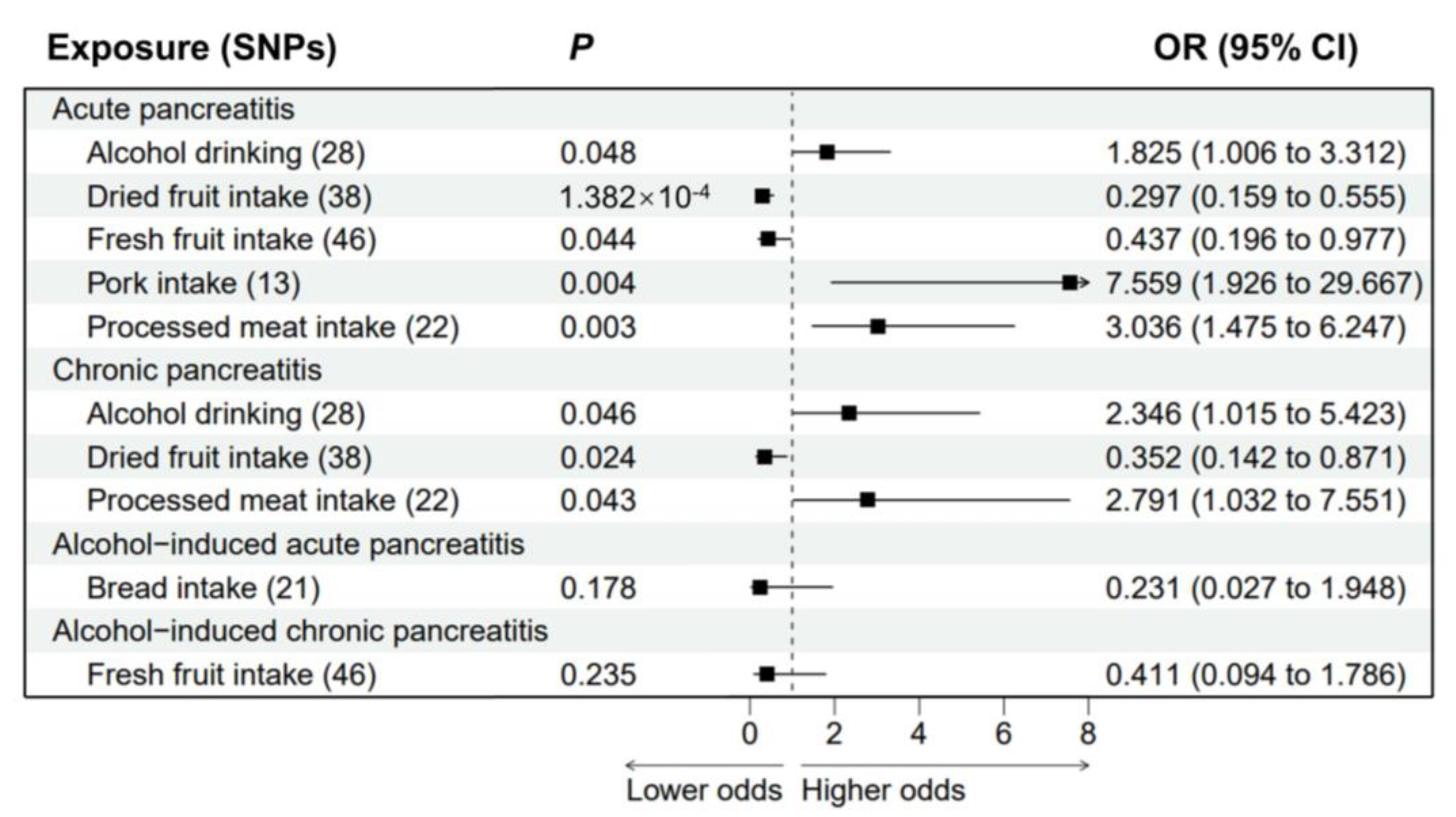
3.4. Multivariable MR Analysis of Pancreatitis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, A.Y.; Tan, M.L.; Wu, L.M.; Asrani, V.M.; Windsor, J.A.; Yadav, D.; Petrov, M.S. Global incidence and mortality of pancreatic diseases: A systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol. Hepatol. 2016, 1, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Boxhoorn, L.; Voermans, R.P.; Bouwense, S.A.; Bruno, M.J.; Verdonk, R.C.; Boermeester, M.A.; van Santvoort, H.C.; Besselink, M.G. Acute pancreatitis. Lancet 2020, 396, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, S.J.; Xiao, A.Y.; Wu, L.M.; Windsor, J.A.; Forsmark, C.E.; Petrov, M.S. Frequency of progression from acute to chronic pancreatitis and risk factors: A meta-analysis. Gastroenterology 2015, 149, 1490–1500.e1. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Ali, U.; Issa, Y.; Hagenaars, J.C.; Bakker, O.J.; van Goor, H.; Nieuwenhuijs, V.B.; Bollen, T.L.; van Ramshorst, B.; Witteman, B.J.; Brink, M.A.; et al. Risk of Recurrent Pancreatitis and Progression to Chronic Pancreatitis After a First Episode of Acute Pancreatitis. Clin. Gastroenterol. Hepatol. 2016, 14, 738–746. [Google Scholar] [CrossRef]
- Beyer, G.; Habtezion, A.; Werner, J.; Lerch, M.M.; Mayerle, J. Chronic pancreatitis. Lancet 2020, 396, 499–512. [Google Scholar] [CrossRef]
- Yadav, D.; Lowenfels, A.B. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013, 144, 1252–1261. [Google Scholar] [CrossRef]
- Oskarsson, V.; Sadr-Azodi, O.; Orsini, N.; Andrén-Sandberg, Å.; Wolk, A. Vegetables, fruit and risk of non-gallstone-related acute pancreatitis: A population-based prospective cohort study. Gut 2013, 62, 1187–1192. [Google Scholar] [CrossRef]
- Oskarsson, V.; Sadr-Azodi, O.; Orsini, N.; Andrén-Sandberg, Å.; Wolk, A. High dietary glycemic load increases the risk of non-gallstone-related acute pancreatitis: A prospective cohort study. Clin. Gastroenterol. Hepatol. 2014, 12, 676–682. [Google Scholar] [CrossRef]
- Oskarsson, V.; Orsini, N.; Sadr-Azodi, O.; Wolk, A. Fish consumption and risk of non-gallstone-related acute pancreatitis: A prospective cohort study. Am. J. Clin. Nutr. 2015, 101, 72–78. [Google Scholar] [CrossRef]
- Setiawan, V.W.; Pandol, S.J.; Porcel, J.; Wei, P.C.; Wilkens, L.R.; Le Marchand, L.; Pike, M.C.; Monroe, K.R. Dietary Factors Reduce Risk of Acute Pancreatitis in a Large Multiethnic Cohort. Clin. Gastroenterol. Hepatol. 2017, 15, 257–265.e3. [Google Scholar] [CrossRef]
- Cai, F.; Hu, C.; Chen, C.J.; Han, Y.P.; Lin, Z.Q.; Deng, L.H.; Xia, Q. Vitamin D and Pancreatitis: A Narrative Review of Current Evidence. Nutrients 2022, 14, 2113. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.A.; Harbord, R.M.; Sterne, J.A.; Timpson, N.; Davey Smith, G. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat. Med. 2008, 27, 1133–1163. [Google Scholar] [CrossRef] [PubMed]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Timpson, N.J.; Higgins, J.P.T.; Dimou, N.; Langenberg, C.; et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): Explanation and elaboration. BMJ 2021, 375, n2233. [Google Scholar] [CrossRef]
- Hansen, S.E.J.; Madsen, C.M.; Varbo, A.; Tybjærg-Hansen, A.; Nordestgaard, B.G. Genetic variants associated with increased plasma levels of triglycerides, via effects on the lipoprotein lipase pathway, increase risk of acute pancreatitis. Clin. Gastroenterol. Hepatol. 2021, 19, 1652–1660.e6. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Giovannucci, E.L.; Larsson, S.C. Gallstone disease, diabetes, calcium, triglycerides, smoking and alcohol consumption and pancreatitis risk: Mendelian randomization study. NPJ Genom. Med. 2021, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Mi, J.; Liu, Z.; Jiang, L.; Li, M.; Wu, X.; Zhao, N.; Wan, Z.; Bai, X.; Feng, Y. Mendelian randomization in blood metabolites identifies triglycerides and fatty acids saturation level as associated traits linked to pancreatitis risk. Front. Nutr. 2022, 9, 1021942. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Jiang, Y.; Wedow, R.; Li, Y.; Brazel, D.M.; Chen, F.; Datta, G.; Davila-Velderrain, J.; McGuire, D.; Tian, C.; et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat. Genet. 2019, 51, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G.; CRP CHD Genetics Collaboration. Avoiding bias from weak instruments in Mendelian randomization studies. Int. J. Epidemiol. 2011, 40, 755–764. [Google Scholar] [CrossRef]
- Papadimitriou, N.; Dimou, N.; Tsilidis, K.K.; Banbury, B.; Martin, R.M.; Lewis, S.J.; Kazmi, N.; Robinson, T.M.; Albanes, D.; Aleksandrova, K.; et al. Physical activity and risks of breast and colorectal cancer: A Mendelian randomisation analysis. Nat. Commun. 2020, 11, 597. [Google Scholar] [CrossRef]
- Solovieff, N.; Cotsapas, C.; Lee, P.H.; Purcell, S.M.; Smoller, J.W. Pleiotropy in complex traits: Challenges and strategies. Nat. Rev. Genet. 2013, 14, 483–495. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Verbanck, M.; Chen, C.-Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Baird, D.; Borges, M.C.; Bowden, J.; Hemani, G.; Haycock, P.; Evans, D.M.; Smith, G.D. Recent Developments in Mendelian Randomization Studies. Curr. Epidemiol. Rep. 2017, 4, 330–345. [Google Scholar] [CrossRef] [PubMed]
- Whitcomb, D.C.; Lowe, M.E. Human pancreatic digestive enzymes. Dig. Dis. Sci. 2007, 52, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kinouchi, T.; Koyama, S.; Harada, E.; Yajima, T. Large molecule protein feeding during the suckling period is required for the development of pancreatic digestive functions in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R1268–R1276. [Google Scholar] [CrossRef]
- Swanson, K.C.; Kelly, N.; Salim, H.; Wang, Y.J.; Holligan, S.; Fan, M.Z.; McBride, B.W. Pancreatic mass, cellularity, and alpha-amylase and trypsin activity in feedlot steers fed diets differing in crude protein concentration. J. Anim. Sci. 2008, 86, 909–915. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Glasbrenner, B.; Büchler, M. Role of nutrients in pancreatic adaptation with implication of cholecystokinin release. Scand. J. Gastroenterol. Suppl. 1988, 151, 108–113. [Google Scholar] [CrossRef]
- Hegyi, E.; Sahin-Tóth, M. Genetic Risk in Chronic Pancreatitis: The Trypsin-Dependent Pathway. Dig. Dis. Sci. 2017, 62, 1692–1701. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.; Wen, N.; Nie, G.; Peng, D.; Xiong, X.; Cheng, N.; Li, B. The role of diet and nutrition related indicators in biliary diseases: An umbrella review of systematic review and meta-analysis. Nutr. Metab. 2022, 19, 51. [Google Scholar] [CrossRef] [PubMed]
- Ribichini, E.; Stigliano, S.; Rossi, S.; Zaccari, P.; Sacchi, M.C.; Bruno, G.; Badiali, D.; Severi, C. Role of Fibre in Nutritional Management of Pancreatic Diseases. Nutrients 2019, 11, 2219. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, L.; Shi, Y.H.; Sui, G.T.; Wu, Y.F.; Lu, X.Q.; Li, M.Y.; Xia, Q.; Bian, X.X.; Li, H.H.; et al. Risk factors of acute pancreatitis in the elderly Chinese population: A population-based cross-sectional study. J. Dig. Dis. 2014, 15, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Mah, L.; Barreto, S.G. Systematic review of diet in the pathogenesis of acute pancreatitis: A tale of too much or too little? Saudi. J. Gastroenterol. 2012, 18, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Sánchez, N.; Zamora-Valdés, D.; Chávez-Tapia, N.C.; Uribe, M. Role of diet in cholesterol gallstone formation. Clin. Chim. Acta. 2007, 376, 1–8. [Google Scholar] [CrossRef]
- Cuevas, A.; Miquel, J.F.; Reyes, M.S.; Zanlungo, S.; Nervi, F. Diet as a risk factor for cholesterol gallstone disease. J. Am. Coll. Nutr. 2004, 23, 187–196. [Google Scholar] [CrossRef]
- Mitta, N.; Barreto, S.G.; Rodrigues, J. Dietary risk factors for acute pancreatitis: A case-control study. Surg. Chron. 2011, 16, 186–187. [Google Scholar]
- Oskarsson, V.; Sadr-Azodi, O.; Orsini, N.; Wolk, A. A prospective cohort study on the association between coffee drinking and risk of non-gallstone-related acute pancreatitis. Br. J. Nutr. 2016, 115, 1830–1834. [Google Scholar] [CrossRef]
- Morton, C.; Klatsky, A.L.; Udaltsova, N. Smoking, coffee, and pancreatitis. Am. J. Gastroenterol. 2004, 99, 731–738. [Google Scholar] [CrossRef]
- Burgess, S.; Davies, N.M.; Thompson, S.G. Bias due to participant overlap in two-sample Mendelian randomization. Genet. Epidemiol. 2016, 40, 597–608. [Google Scholar] [CrossRef]
| Exposures | SNPs (Number) | Unit | Sample (n) | Ancestry | R2 (%) | F | Consortium |
|---|---|---|---|---|---|---|---|
| Alcohol drinking | 39 | SD | 335,394 | European | 0.54 | 46.69 | GSCAN |
| Beef intake | 17 | SD | 461,053 | European | 0.15 | 40.74 | UK biobank |
| Bread intake | 34 | SD | 452,236 | European | 0.31 | 41.36 | UK biobank |
| Cereal intake | 44 | SD | 441,640 | European | 0.45 | 45.37 | UK biobank |
| Cheese intake | 73 | SD | 451,486 | European | 0.62 | 38.58 | UK biobank |
| Coffee intake | 44 | SD | 428,860 | European | 0.73 | 71.67 | UK biobank |
| Cooked vegetable intake | 17 | SD | 448,651 | European | 0.14 | 37.00 | UK biobank |
| Dried fruit intake | 46 | SD | 421,764 | European | 0.45 | 41.44 | UK biobank |
| Fresh fruit intake | 58 | SD | 446,462 | European | 0.59 | 45.68 | UK biobank |
| Lamb intake | 33 | SD | 460,006 | European | 0.29 | 40.54 | UK biobank |
| Non-oily fish intake | 12 | SD | 460,880 | European | 0.11 | 42.29 | UK biobank |
| Oily fish intake | 73 | SD | 460,443 | European | 0.69 | 43.82 | UK biobank |
| Pork intake | 14 | SD | 460,162 | European | 0.12 | 39.49 | UK biobank |
| Poultry intake | 9 | SD | 461,900 | European | 0.06 | 30.81 | UK biobank |
| Processed meat intake | 24 | SD | 461,981 | European | 0.20 | 38.57 | UK biobank |
| Raw vegetable intake | 21 | SD | 435,435 | European | 0.18 | 37.39 | UK biobank |
| Salt added to food | 124 | SD | 462,630 | European | 1.30 | 49.13 | UK biobank |
| Tea intake | 50 | SD | 447,485 | European | 0.63 | 56.73 | UK biobank |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, X.; Huang, C.; Wang, Y.; Mao, S.; Li, Z.; Zou, W.; Liao, Z. Association between Dietary Habits and Pancreatitis among Individuals of European Ancestry: A Two-Sample Mendelian Randomization Study. Nutrients 2023, 15, 1153. https://doi.org/10.3390/nu15051153
Mao X, Huang C, Wang Y, Mao S, Li Z, Zou W, Liao Z. Association between Dietary Habits and Pancreatitis among Individuals of European Ancestry: A Two-Sample Mendelian Randomization Study. Nutrients. 2023; 15(5):1153. https://doi.org/10.3390/nu15051153
Chicago/Turabian StyleMao, Xiaotong, Chunyou Huang, Yuanchen Wang, Shenghan Mao, Zhaoshen Li, Wenbin Zou, and Zhuan Liao. 2023. "Association between Dietary Habits and Pancreatitis among Individuals of European Ancestry: A Two-Sample Mendelian Randomization Study" Nutrients 15, no. 5: 1153. https://doi.org/10.3390/nu15051153
APA StyleMao, X., Huang, C., Wang, Y., Mao, S., Li, Z., Zou, W., & Liao, Z. (2023). Association between Dietary Habits and Pancreatitis among Individuals of European Ancestry: A Two-Sample Mendelian Randomization Study. Nutrients, 15(5), 1153. https://doi.org/10.3390/nu15051153








