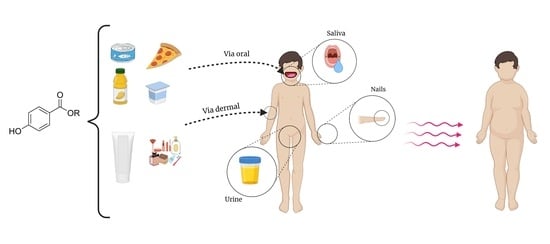
Presence of Parabens in Different Children Biological Matrices and Its Relationship with Body Mass Index
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Settings
2.2. Study Participants
2.3. Data Collection
2.4. Determination of Parabens in Biological Samples
2.4.1. Determination of Parabens in Saliva
2.4.2. Determination of Parabens in Urine
2.4.3. Determination of Parabens in Nails
2.5. Statistical Analysis
3. Results
4. Discussion
Strengths and Weaknesses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Güngör, N.K. Overweight and obesity in children and adolescents. J. Clin. Res. Pediatr. Endocrinol. 2014, 6, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Bayartai, M.E.; Schaer, C.E.; Luomajoki, H.; Tringali, G.; De Micheli, R.; Sartorio, A. Differences in spinal posture and mobility between children/adolescents with obesity and age-matched normal-weight individuals. Sci. Rep. 2022, 12, 15570. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO European Regional Obesity Report; WHO Regional Office for Europe: Copenhagen, Denmark, 2022. [Google Scholar]
- Veiga-Lopez, A.; Pu, Y.; Gingrich, J.; Padmananbhan, V. Obesogenic Endocrine Disrupting Chemicals: Identifying Knowledge gaps. Trends Endocrinol. Metab. 2018, 29, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Grün, F.; Blumberg, B. Environmental Obesogens: Organotins and Endocrine Disruption via Nuclear Receptor Signaling. Endocrinology 2006, 147, S50–S55. [Google Scholar] [CrossRef]
- Nadal, A.; Quesada, I.; Tudurí, E.; Nogueiras, R.; Alonso-Magdalena, P. Endocrine-disrupting chemicals and the regulation of energy balance. Nat. Rev. Endocrinol. 2017, 13, 536–546. [Google Scholar] [CrossRef]
- Fransway, A.F.; Fransway, P.J.; Belsito, D.V.; Warshaw, E.M.; Sasseville, D.; Fowler, J.F., Jr.; DeKoven, J.G.; Pratt, M.D.; Maibach, H.I.; Taylor, J.S.; et al. Parabens. Dermatitis 2019, 30, 3–31. [Google Scholar] [CrossRef]
- Petric, Z.; Ružić, J.; Žuntar, I. The controversies of parabens—An overview nowadays. Acta Pharm. 2021, 71, 17–32. [Google Scholar] [CrossRef]
- Wei, F.; Mortimer, M.; Cheng, H.; Sang, N.; Guo, L.H. Parabens as chemicals of emerging concern in the environment and humans: A review. Sci. Total Environ. 2021, 778, 146150. [Google Scholar] [CrossRef]
- Soni, M.G.; Carabin, I.G.; Burdock, G.A. Safety assessment of esters of p-hydroxybenzoic acid (parabens). Food Chem. Toxicol. 2005, 43, 985–1015. [Google Scholar] [CrossRef]
- Nowak, K.; Ratajczak-Wrona, W.; Górska, M.; Jabłońska, E. Parabens and their effects on the endocrine system. Mol. Cell. Endocrinol. 2018, 474, 238–251. [Google Scholar] [CrossRef]
- EU Commission. Commission Regulation (UE) No 1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council establishing a Union list of food additives; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- Gálvez-Ontiveros, Y.; Moscoso-Ruiz, I.; Rodrigo, L.; Aguilera, M.; Rivas, A.; Zafra-Gómez, A. Presence of parabens and bisphenols in food commonly consumed in Spain. Foods 2021, 10, 92. [Google Scholar] [CrossRef]
- Monneret, C. What is an endocrine disruptor? C. R. Biol. 2017, 340, 403–405. [Google Scholar] [CrossRef]
- Karwacka, A.; Zamkowska, D.; Radwan, M.; Jurewicz, J. Exposure to modern, widespread environmental endocrine disrupting chemicals and their effect on the reproductive potential of women: An overview of current epidemiological evidence. Hum. Fertil. 2019, 22, 2–25. [Google Scholar] [CrossRef]
- Monteagudo, C.; Robles-Aguilera, V.; Salcedo-Bellido, I.; Gálvez-Ontiveros, Y.; Samaniego-Sánchez, C.; Aguilera, M.; Zafra-Gómez, A.; Martínez-Burgos, M.A.; Rivas, A. Dietary exposure to parabens and body mass index in an adolescent Spanish population. Environ. Res. 2021, 201, 111548. [Google Scholar] [CrossRef] [PubMed]
- Darbre, P.D. Chapter 15—Endocrine Disruption and Disorders of Energy Metabolism, Endocrine Disruption and Human Health, 2nd ed; Academic Press: Cambridge, MA, USA, 2022; pp. 321–339. ISBN 9780128219850. [Google Scholar] [CrossRef]
- Darbre, P.D. Endocrine disruptors and obesity. Curr. Obes. Rep. 2017, 6, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.I.; Kwon, H.Y.; Han, X.; Men, X.; Choi, Y.E.; Jang, G.W.; Park, K.T.; Han, J.; Lee, O.H. Environmental obesogens (bisphenols, phthalates and parabens) and their impacts on adipogenic transcription factors in the absence of dexamethasone in 3T3-L1 cells. J. Steroid Biochem. Mol. Biol. 2021, 214, 105994. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wu, C.; Lu, D.; Jiang, S.; Liang, W.; Chang, X.; Xu, H.; Wang, G.; Zhou, Z. Urinary paraben concentrations and their associations with anthropometric measures of children aged 3 years. Environ. Pollut. 2017, 222, 307–314. [Google Scholar] [CrossRef]
- Berger, K.; Hyland, C.; Ames, J.L.; Mora, A.M.; Huen, K.; Eskenazi, B.; Holland, N.; Harley, K.G. Prenatal exposure to mixtures of phthalates, parabens, and other phenols and obesity in five-year-olds in the CHAMACOS Cohort. Int. J. Environ. Res. Public Health 2021, 18, 1796. [Google Scholar] [CrossRef]
- Kang, H.S.; Kyung, M.S.; Ko, A.; Park, J.H.; Hwang, M.S.; Kwon, J.E.; Suh, J.H.; Lee, H.S.; Moon, G.I.; Hong, J.H.; et al. Urinary concentrations of parabens and their association with demographic factors: A population-based cross-sectional study. Environ. Res. 2016, 146, 245–251. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Li, Y.; Zhang, B.; Huo, W.; Zhu, Y.; Wan, Y.; Zheng, T.; Zhou, A.; Chen, Z.; et al. Association between urinary parabens and gestational diabetes mellitus across prepregnancy body mass index categories. Environ. Res. 2019, 170, 151–159. [Google Scholar] [CrossRef]
- Lee, I.; Park, Y.J.; Kim, M.J.; Kim, S.; Choi, S.; Park, J.; Cho, Y.H.; Hong, S.; Yoo, J.; Park, H.; et al. Associations of urinary concentrations of phthalate metabolites, bisphenol A, and parabens with obesity and diabetes mellitus in a Korean adult population: Korean National Environmental Health Survey (KoNEHS) 2015-2017. Environ. Int. 2021, 146, 106227. [Google Scholar] [CrossRef] [PubMed]
- Quirós-Alcalá, L.; Buckley, J.P.; Boyle, M. Parabens and measures of adiposity among adults and children from the U.S. general population: NHANES 2007–2014. Int. J. Hyg. Environ. Health 2018, 221, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Feizabadi, K.G.; Hajizadeh, Y.; Feizi, A.; Ebrahimpour, K. Urinary concentrations of parabens amongst Iranian adults and their associations with socio-demographic factors. J. Environ. Health Sci. Eng. 2020, 18, 1227–1238. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chevrier, J. Exposure to parabens and prevalence of obesity and metabolic syndrome: An analysis of the Canadian Health Measures Survey. Sci. Total Environ. 2020, 713, 135116. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Hu, Y.; Zhu, Q.; Liao, C.; Jiang, G. Several typical endocrine-disrupting chemicals in human urine from general population in China: Regional and demographic-related differences in exposure risk. J. Hazard. Mater. 2022, 424, 127489. [Google Scholar] [CrossRef] [PubMed]
- Güil-Oumrait, N.; Cano-Sancho, G.; Montazeri, P.; Stratakis, N.; Warembourg, C.; Lopez-Espinosa, M.J.; Vioque, J.; Santa-Marina, L.; Jimeno-Romero, A.; Ventura, R.; et al. Prenatal exposure to mixtures of phthalates and phenols and body mass index and blood pressure in Spanish preadolescents. Environ. Int. 2022, 169, 107527, advance online publication. [Google Scholar] [CrossRef]
- Ghassabian, A.; Trasande, L. Disruption in thyroid signaling pathway: A mechanism for the effect of endocrine-disrupting chemicals on child neurodevelopment. Front. Endocrinol. 2018, 9, 204. [Google Scholar] [CrossRef]
- Moscoso-Ruiz, I.; Gálvez-Ontiveros, Y.; Cantarero-Malagón, S.; Rivas, A.; Zafra-Gómez, A. Optimization of an ultrasound-assisted extraction method for the determination of parabens and bisphenol homologues in human saliva by liquid chromatography-tandem mass spectrometry. Michrochem. J. 2022, 175, 107122. [Google Scholar] [CrossRef]
- Martín-Pozo, L.; Cantarero-Malagón, S.; Hidalgo, F.; Navalón, A.; Zafra-Gómez, A. Determination of endocrine disrupting chemicals in human nails using an alkaline digestion prior to ultra-high performance liquid chromatography-tandem mass spectrometry. Talanta 2020, 208, 120429. [Google Scholar] [CrossRef]
- Barbosa, F., Jr.; Corrêa Rodrigues, M.H.; Buzalaf, M.R.; Krug, M.J.; Gerlach, R.F.; Tanus-Santos, J.E. Evaluation of the use of salivary lead levels as a surrogate of blood lead or plasma lead levels in lead exposed subjects. Arch. Toxicol. 2006, 80, 633–637. [Google Scholar] [CrossRef]
- Nobile, M.; Arioli, F.; Pavlovic, R.; Ceriani, F.; Lin, S.K.; Panseri, S.; Villa, R.; Chiesa, L.M. Presence of emerging contaminants in baby food. Food Addit. Contam. Part A 2020, 37, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Maher, H.M.; Alzoman, N.Z.; Almeshal, M.A.; Alotaibi, H.A.; Alotaibi, N.N.; Al-Showiman, H. Quantitative screening of parabens in Ready-to-eat foodstuffs available in the Saudi market using high performance liquid chromatography with photodiode array detection. Arab. J. Chem. 2020, 13, 2897–2911. [Google Scholar] [CrossRef]
- Al-Halaseh, L.K.; Al-Adaileh, S.; Mbaideen, A.; Hajleh, M.; Al-Samydai, A.; Zakaraya, Z.Z.; Dayyih, W.A. Implication of parabens in cosmetics and cosmeceuticals: Advantages and limitations. J. Cosmet. Dermatol. 2022, 21, 3265–3271. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Bellizzi, M.C.; Flegal, K.M.; Dietz, W.H. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ 2000, 320, 1240–1243. [Google Scholar] [CrossRef]
- Cole, T.J.; Flegal, K.M.; Nicholls, D.; Jackson, A.A. Body mass index cut offs to define thinness in children and adolescents: International survey. BMJ 2007, 335, 194–197. [Google Scholar] [CrossRef]
- International Labour Office. International Standard Classification of Occupations. ISCO-08. Volume 1. Structure, Group Definitions and Correspondence Tables. 2012. Available online: https://www.ilo.org/wcmsp5/groups/public/---dgreports/---dcomm/---publ/documents/publication/wcms_172572.pdf (accessed on 23 February 2023).
- Peake, M.; Whiting, M. Measurement of Serum Creatinine—Current Status and Future Goals. Clin. Biochem. Rev. 2006, 27, 173–184. [Google Scholar]
- Weber, J.A.; Van Zanten, A.P. Interferences in current methods for measurements of creatinine. Clin. Chem. 1991, 37, 695–700. [Google Scholar] [CrossRef]
- Moscoso-Ruiz, I.; Gálvez-Ontiveros, Y.; Giles-Mancilla, M.; Del Carmen Gómez-Regalado, M.; Rivas, A.; Zafra-Gómez, A. Improved method for the determination of endocrine-disrupting chemicals in urine of school-age children using microliquid-liquid extraction and UHPLC-MS/MS. Anal. Bioanal. Chem. 2022, 414, 6681–6694. [Google Scholar] [CrossRef]
- CDC. Fourth National Report on Human Exposure to Environmental Chemicals Updated Tables. 2015. Available online: https://www.cdc.gov/biomonitoring/pdf/fourthreport_updatedtables_feb2015.pdf (accessed on 23 February 2023).
- Barr, D.B.; Wilder, L.C.; Caudill, S.P.; Gonzalez, A.J.; Needham, L.L.; Pirkle, J.L. Urinary creatinine concentrations in the U.S. population: Implications for urinary biologic monitoring measurements. Environ. Health. Perspect. 2005, 113, 192–200. [Google Scholar] [CrossRef]
- Hu, P.; Chen, X.; Whitener, R.J.; Boder, E.T.; Jones, J.O.; Porollo, A.; Chen, J.; Zhao, L. Effects of parabens on adipocyte differentiation. Toxicol. Sci. 2013, 131, 56–70. [Google Scholar] [CrossRef]
- Leppert, B.; Strunz, S.; Seiwert, B.; Schlittenbauer, L.; Schilchting, R.; Pfeiffer, C.; Röder, S.; Bauer, M.; Borte, M.; Stangl, G.I.; et al. Maternal paraben exposure triggers childhood overweight development. Nat. Commun. 2020, 11, 561. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Overby, H.; Heal, E.; Wang, S.; Chen, J.; Shen, C.; Zhao, L. Methylparaben and butylparaben alter multipotent mesenchumal stem cell fates towards adipocyte lineage. Toxicol. Appl. Pharmacol. 2017, 329, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Boberg, J.; Metzdorff, S.; Wortziger, R.; Axelstad, M.; Brokken, L.; Vinggaard, A.M.; Dalgaard, M.; Nellemann, C. Impact of diisobutyl phthalate and other PPAR agonists on steroidogenesis and plasma insulin and leptin levels in fetal rats. Toxicology 2008, 250, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Jala, A.; Varghese, B.; Dutta, R.; Adela, R.; Borkar, R.M. Levels of parabens and bisphenols in personal care products and urinary concentrations in Indian young adult women: Implications for human exposure and health risk assessment. Chemosphere 2022, 297, 134028. [Google Scholar] [CrossRef]
- Hajizadeh, Y.; Kiani Feizabadi, G.; Ebrahimpour, K.; Shoshtari-Yeganeh, B.; Fadaei, S.; Darvishmotevalli, M.; Karimi, H. Urinary paraben concentrations and their implications for human exposure in Iranian pregnant women. ESPR 2020, 27, 14723–14734. [Google Scholar] [CrossRef]
- Deierlein, A.L.; Wolff, M.S.; Pajak, A.; Pinney, S.M.; Windham, G.C.; Galvez, M.P.; Rybak, M.; Calafat, A.M.; Kushi, L.H.; Biro, F.M.; et al. Phenol concentrations during childhood and subsequent measures of adiposity among young girls. Am. J. Epidemiol. 2017, 186, 581–592. [Google Scholar] [CrossRef]
- van der Meer, T.P.; Artacho-Cordón, F.; Swaab, D.F.; Struik, D.; Makris, K.C.; Wolffenbuttel, B.; Frederiksen, H.; van Vliet-Ostaptchouk, J.V. Distribution of non-persistent endocrine disruptors in two different regions of the human brain. Int. J. Environ. Res. Public Health 2017, 14, 1059. [Google Scholar] [CrossRef]
- Reimann, B.; Vrijens, K.; Roels, H.A.; Wang, C.; Cosemans, C.; Van Overmeire, I.; Nawrot, T.S.; Plusquin, M. In utero exposure to parabens and early childhood BMI z-scores—Associations between placental ethyl paraben, longitudinal BMI trajectories and cord blood metabolic biomarkers. Environ. Int. 2021, 157, 106845. [Google Scholar] [CrossRef]
- Artacho-Cordón, F.; Fernández, M.F.; Frederiksen, H.; Iribarne-Durán, L.M.; Jiménez-Díaz, I.; Vela-Soria, F.; Andersson, A.M.; Martin-Olmedo, P.; Peinado, F.M.; Olea, N.; et al. Environmental phenols and parabens in adipose tissue from hospitalized adults in Southern Spain. Environ. Int. 2018, 119, 203–211. [Google Scholar] [CrossRef]
- Aubert, N.; Ameller, T.; Legrand, J.J. Systemic exposure to parabens: Pharmacokinetics, tissue distribution, excretion balance and plasma metabolites of [14C]-methyl-, propyl- and butylparaben in rats after oral, topical or subcutaneous administration. Food Chem. Toxicol. 2012, 50, 445–454. [Google Scholar] [CrossRef]
- Moos, R.K.; Apel, P.; Schröter-Kermani, C.; Kolossa-Gehring, M.; Brüning, T.; Koch, H.M. Daily intake and hazard index of parabens based upon 24h urine samples of the German Environmental Specimen Bank from 1995 to 2012. J. Expo. Sci. Env. Epidemiol. 2017, 27, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Moos, R.K.; Angerer, J.; Dierkes, G.; Brüning, T.; Koch, H.M. Metabolism and elimination of methyl, iso- and n-butyl paraben in human urine after single oral dosage. Arch. Toxicol. 2016, 90, 2699–2709. [Google Scholar] [CrossRef] [PubMed]
- Esteban, M.; Castaño, A. Non-invasive matrices in human biomonitoring: A review. Environ. Int. 2009, 35, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.; Reidy, J.A.; Samandar, E.; Herbert, A.R.; Needham, L.L.; Calafat, A.M. Detection of phthalate metabolites in human saliva. Arch. Toxicol. 2005, 79, 647–652. [Google Scholar] [CrossRef]
- Lum, J.T.; Chan, Y.N.; Leung, K.S. Current applications and future perspectives on elemental analysis of non-invasive samples for human biomonitoring. Talanta 2021, 234, 122683. [Google Scholar] [CrossRef]
- Signes-Pastor, A.J.; Gutiérrez-González, E.; García-Villarino, M.; Rodríguez-Cabrera, F.D.; López-Moreno, J.J.; Varea-Jiménez, E.; Pastor-Barriuso, R.; Pollán, M.; Navas-Acien, A.; Pérez-Gómez, B.; et al. Toenails as a biomarker of exposure to arsenic: A review. Environ. Res. 2021, 195, 110286. [Google Scholar] [CrossRef]
| n | Control (n = 101) | Cases (n = 59) | p | ||
|---|---|---|---|---|---|
| Gender (%) | Male | 84 | 58.8 | 41.2 | 0.505 a |
| Female | 76 | 67.1 | 32.9 | ||
| Age, categorized (%) | 6–10 years | 125 | 64.0 | 36.0 | 0.280 a |
| >10–12 years | 35 | 58.3 | 41.7 | ||
| Weight, kg | Median | 25.5 | 53.3 | <0.001 b | |
| IQR | 12.6 | 21.9 | |||
| Height, cm | Mean | 127.8 | 140.4 | <0.001 c | |
| SD | 20.7 | 12.9 | |||
| BMI, kg m−2 | Mean | 16.14 | 24.45 | <0.001 c | |
| SD | 0.2 | 0.5 | |||
| Energy Intake, kcal day−1 | Mean | 2011.9 | 2001.1 | 0.702 c | |
| SD | 512.4 | 453.0 | |||
| Physical Activity (out-of-school) (%) | No | 58 | 62.1 | 37.9 | 0.995 a |
| Yes | 86 | 59.3 | 40.7 | ||
| Parents’ Level of Education (%) | Primary | 5 | 20.0 | 80.0 | <0.001 a |
| Secondary | 52 | 42.3 | 57.7 | ||
| University | 89 | 74.2 | 25.8 | ||
| Parents’ Smoking Habits (%) | No | 126 | 61.1 | 38.9 | 0.740 a |
| Yes | 32 | 65.6 | 34.4 | ||
| Parents’ Marital Status (%) | Married | 126 | 65.1 | 34.9 | 0.010 a |
| Divorcee | 13 | 23.1 | 76.9 | ||
| Single | 7 | 57.1 | 42.9 | ||
| Urinary Creatinine Levels, g L−1 | Median | 0.9 | 0.9 | 0.439 b | |
| IQR | 0.6 | 0.8 |
| NAILS (ng g−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control (n = 52) | Cases (n = 22) | ||||||||
| % Detection | Median | P25 | P75 | % Detection | Median | P25 | P75 | p * | |
| MetPB | 100.0 | >500 | >500 | >500 | 100 | >500 | >500 | >500 | - |
| EthPB | 98.1 | 25.70 | 9.44 | 77.51 | 100 | 15.93 | 10 | 169.42 | 0.692 |
| PropPB | 73.1 | 14.70 | <LOD | 134.6 | 68.2 | 47.85 | <LOD | 197.97 | 0.624 |
| ButPB | 9.6 | <LOD | <LOD | <LOD | 13.6 | <LOD | <LOD | <LOD | 0.622 |
| Paraben total | 100.0 | 47.76 | 16.47 | 225.71 | 100.0 | 210.91 | 16.04 | 1085.19 | 0.214 |
| URINE (ng mL−1) | |||||||||
| Control (n = 97) | Cases (n = 52) | ||||||||
| % Detection | Median | P25 | P75 | % Detection | Median | P25 | P75 | p * | |
| MetPB | 91.8 | 4 | 2.17 | 8.93 | 90.4 | 4.93 | 2.63 | 22.24 | 0.174 |
| EthPB | 26.8 | 0.21 | <LOD | 0.13 | 23.1 | 0.02 | <LOD | <LOD | 0.533 |
| PropPB | 24.7 | 0.02 | <LOD | 0.26 | 40.4 | 0.02 | <LOD | 1.73 | 0.044 |
| ButPB | 9.3 | <LOD | <LOD | <LOD | 5.8 | <LOD | <LOD | <LOD | 0.468 |
| Paraben total | 100.00 | 4.88 | 2.37 | 11.49 | 98.1 | 7.24 | 2.98 | 27.52 | 0.033 |
| SALIVA (ng g−1) | |||||||||
| Control (n = 58) | Cases (n = 31) | ||||||||
| % Detection | Median | P25 | P75 | % Detection | Median | P25 | P75 | p * | |
| MetPB | 91.4 | 0.71 | 0.71 | 6.65 | 87.1 | 0.71 | 0.71 | 8.05 | 0.904 |
| EthPB | 94.8 | 7.30 | 3.35 | 9.45 | 87.1 | 6.00 | 1.15 | 7.85 | 0.113 |
| PropPB | 87.9 | 0.71 | 0.71 | 1.35 | 77.4 | 0.71 | 0.71 | 1.1 | 0.446 |
| ButPB | 22.4 | <LOD | <LOD | <LOD | 22.6 | <LOD | <LOD | <LOD | 0.943 |
| Paraben total | 100.0 | 11.79 | 8.53 | 19.32 | 100.0 | 12.23 | 8.14 | 16.62 | 0.993 |
| Crude | Adjusted * | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| MetPB (Ref. MetPB concentration ≤ median) | - | - | - | - | - | - |
| EthPB (Ref. EthPB concentration ≤ median) | 0.77 | 0.28–2.10 | 0.611 | 0.77 | 0.28–2.09 | 0.601 |
| PropPB (Ref. PropPB concentration ≤ median) | 1.69 | 0.61–4.63 | 0.311 | 1.41 | 0.47–4.21 | 0.541 |
| Paraben total (Ref. Paraben total concentration ≤ median) | 2.21 | 0.79–6.16 | 0.131 | 2.18 | 0.67–6.96 | 0.195 |
| Crude | Adjusted * | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| MetPB (Ref. MetPB concentration ≤ median) | 0.73 | 0.37–1.46 | 0.376 | 1.14 | 0.46–2.82 | 0.775 |
| EthPB (Ref. EthPB concentration ≤ LOD) | 1.22 | 0.55–2.68 | 0.619 | 2.63 | 0.88–7.84 | 0.082 |
| PropPB (Ref. PropPB concentration ≤ LOD) | 0.49 | 0.24–1.01 | 0.054 | 1.45 | 0.44–4.71 | 0.540 |
| Paraben total (Ref. Paraben total concentration ≤ median) | 0.77 | 0.39–1.52 | 0.455 | 1.06 | 0.40–2.82 | 0.903 |
| Crude | Adjusted * | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| MetPB (Ref. MetPB concentration ≤ median) | 0.90 | 0.37–2.23 | 0.826 | 0.61 | 0.21–1.76 | 0.359 |
| EthPB (Ref. EthPB concentration ≤ median) | 0.45 | 0.18–1.12 | 0.087 | 0.41 | 0.15–1.12 | 0.082 |
| PropPB (Ref. PropPB concentration ≤ median) | 0.85 | 0.35–2.10 | 0.729 | 0.94 | 0.37–2.40 | 0.892 |
| Paraben total (Ref. Paraben total concentration ≤ median) | 1.14 | 0.48–2.74 | 0.764 | 1.00 | 0.38–2.62 | 0.995 |
| Reference | Matrix | N | Age | Country | Tendency/Relationship with BMI | |||
|---|---|---|---|---|---|---|---|---|
| MethPB | EthPB | PropPB | ButPB | |||||
| Xu et al., 2022 [28] | Urine | 300 | 2–60 | China | - | Inverse | - | - |
| Lee et al., 2021 [24] | Urine | 3782 | 19–86 | Korea | - | Direct | ||
| Feizabadi et al., 2020 [26] | Urine | 178 | 21–>50 | Iran | Inverse * | - | * | - |
| Hajizadeh et al., 2020 [50] | Urine | 95 (w) | 34.2 (m) | Iran | - | Inverse | - | - |
| Jala et al., 2022 [49] | Urine | 52 | 18–31 | India | - | - | - | - |
| Li et al., 2019 [23] | Urine | 696 | NR | China | - | - | Direct | - |
| Guo et al., 2017 [20] | Urine | 436 | 3 | China | Direct | - | - | |
| Kang et al., 2016 [22] | Urine | 2541 | 3–69 | Korea | Direct * | - | Direct * | - |
| Berger et al., 2021 [21] | Urine | 309 | 5 | USA | - | - | Direct | - |
| Quirós-Alcalá et al., 2018 [25] | Urine | 1324 | 6–19 | USA | Inverse | - | - | - |
| Deierlein et al., 2017 [51] | Urine | 1017 (w) | 6–8 | USA | - | - | - | |
| Kim and Chevrier, 2020 [27] | Urine | 2564 | 3–79 | Canada | - | Inverse (woman) | - | - |
| Güil-Oumrait et al., 2022 [29] | Urine | 1015 | 11 (m) | Spain | - | - | - | - |
| Artacho-Cordón et al., 2018 [54] | Adipose tissue | 144 | +16 | Spain | - | - | - | - |
| van der Meer et al., 2017 [52] | Brain | 25 | 74 (m) | Netherlands | - | ND | ND | ND |
| Reimann et al., 2021 [53] | Placenta | 229 | 30 (m) | Belgium | - | Direct | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moscoso-Ruiz, I.; Gálvez-Ontiveros, Y.; Samaniego-Sánchez, C.; Almazán Fernández de Bobadilla, V.; Monteagudo, C.; Zafra-Gómez, A.; Rivas, A. Presence of Parabens in Different Children Biological Matrices and Its Relationship with Body Mass Index. Nutrients 2023, 15, 1154. https://doi.org/10.3390/nu15051154
Moscoso-Ruiz I, Gálvez-Ontiveros Y, Samaniego-Sánchez C, Almazán Fernández de Bobadilla V, Monteagudo C, Zafra-Gómez A, Rivas A. Presence of Parabens in Different Children Biological Matrices and Its Relationship with Body Mass Index. Nutrients. 2023; 15(5):1154. https://doi.org/10.3390/nu15051154
Chicago/Turabian StyleMoscoso-Ruiz, Inmaculada, Yolanda Gálvez-Ontiveros, Cristina Samaniego-Sánchez, Vega Almazán Fernández de Bobadilla, Celia Monteagudo, Alberto Zafra-Gómez, and Ana Rivas. 2023. "Presence of Parabens in Different Children Biological Matrices and Its Relationship with Body Mass Index" Nutrients 15, no. 5: 1154. https://doi.org/10.3390/nu15051154
APA StyleMoscoso-Ruiz, I., Gálvez-Ontiveros, Y., Samaniego-Sánchez, C., Almazán Fernández de Bobadilla, V., Monteagudo, C., Zafra-Gómez, A., & Rivas, A. (2023). Presence of Parabens in Different Children Biological Matrices and Its Relationship with Body Mass Index. Nutrients, 15(5), 1154. https://doi.org/10.3390/nu15051154