Lactobacillus johnsonii L531 Ameliorates Salmonella enterica Serovar Typhimurium Diarrhea by Modulating Iron Homeostasis and Oxidative Stress via the IRP2 Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Chemicals and Antibodies
2.3. Bacterial Strains and Growth Conditions
2.4. Cell Culture
2.5. S. Typhimurium Infection In Vitro
2.6. S. Typhimurium Infection In Vivo
2.7. Assessment of Diarrhea Degree
2.8. Cell Viability Assay
2.9. Enumeration of Intracellular Bacteria
2.10. Analysis of Intracellular S. Typhimurium Localization
2.11. Free Cellular Divalent Iron Content Assay
2.12. Iron Measurement in the Serum and Tissue
2.13. Assessment of the Antioxidant Status in Tissue
2.14. Iron Staining
2.15. Assessment of Intracellular ROS Generation
2.16. Dihydroethidium Staining
2.17. siRNA and Transfection Experiments
2.18. Western Blotting
2.19. Immunofluorescence
2.20. RNA Extraction and qRT-PCR
2.21. Statistical Analysis
3. Results
3.1. L. johnsonii L531 Reverses the Disruption of Iron Metabolism Caused by S. Typhimurium in IPEC-J2 Cells
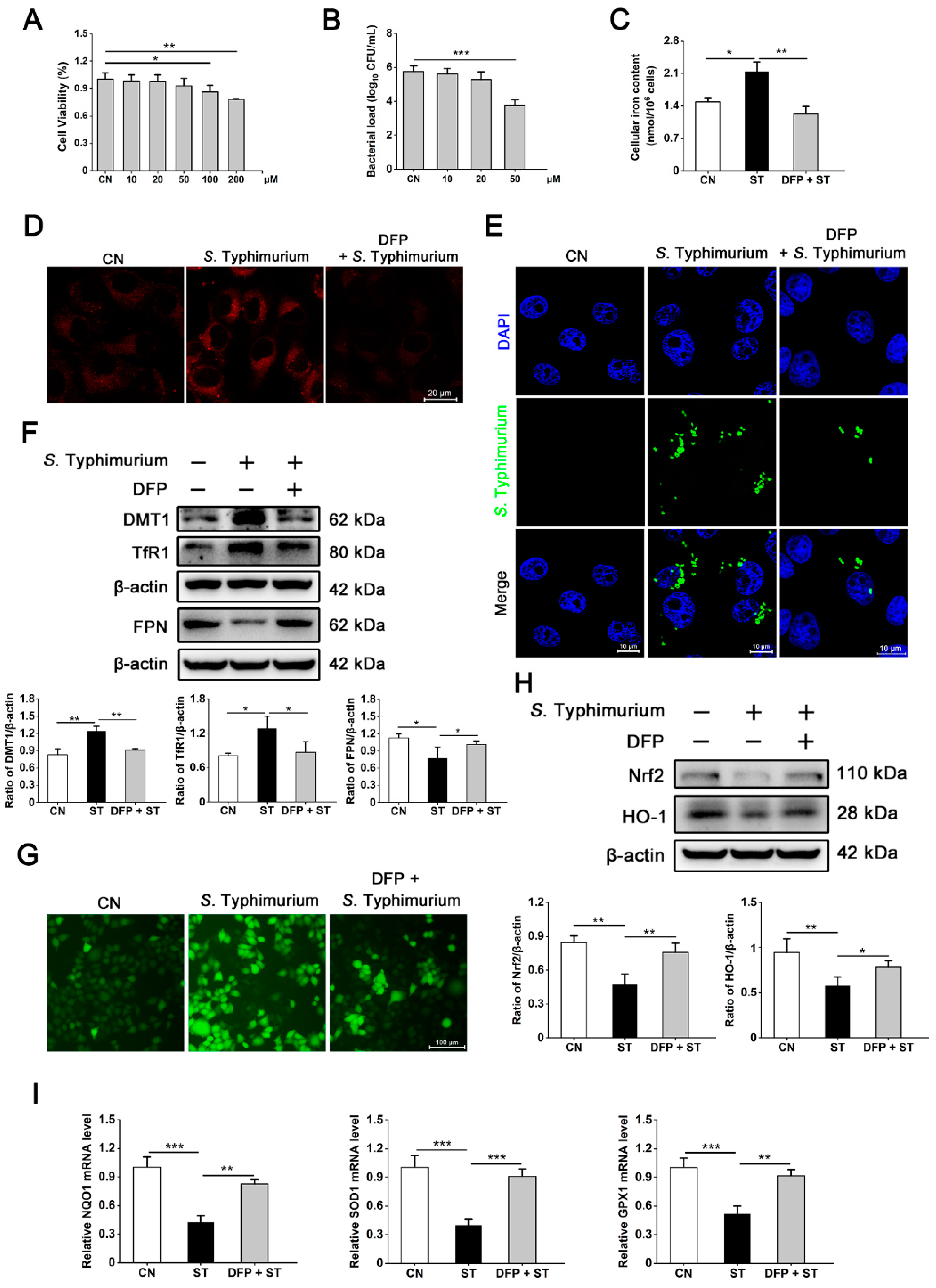
3.2. S. Typhimurium Promotes Its Growth and Triggers Oxidative Stress by Inducing the Disturbance of Iron Homeostasis in IPEC-J2 Cells
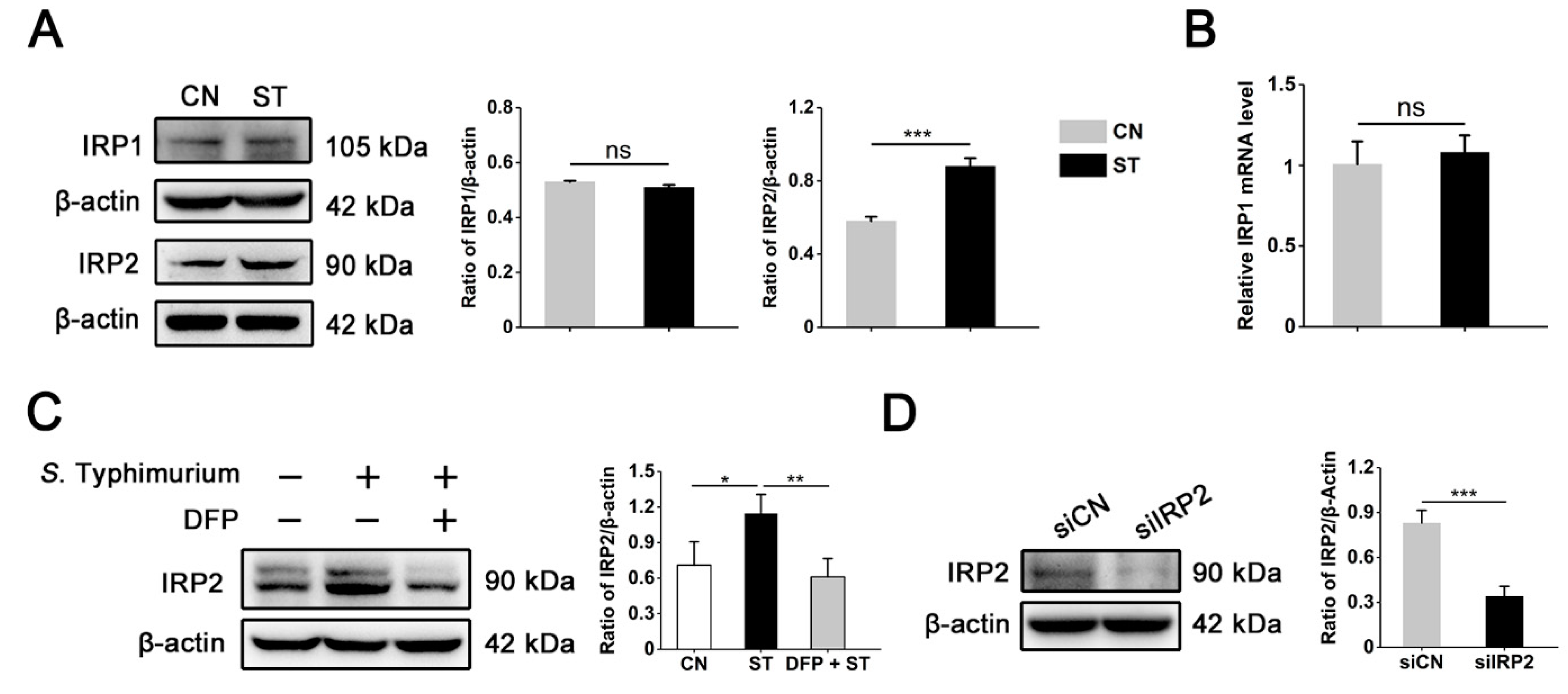
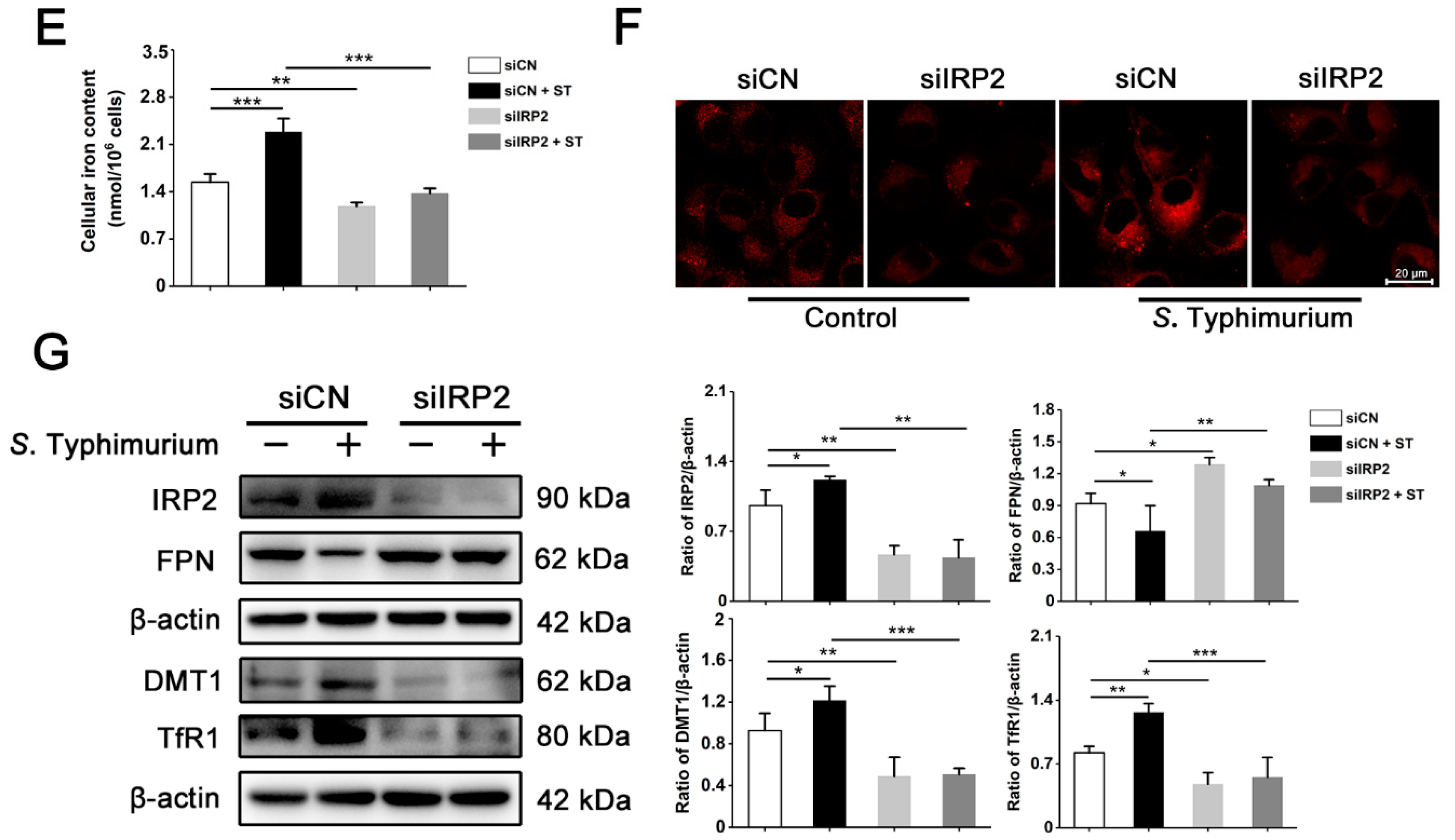
3.3. IRP2, but Not IRP1, Is Involved in Regulating the Dysregulation of Iron Metabolism Induced by S. Typhimurium in IPEC-J2 Cells
3.4. S. Typhimurium Promotes Its Growth and Induces Oxidative Stress via the IRP2 Pathway in IPEC-J2 Cells
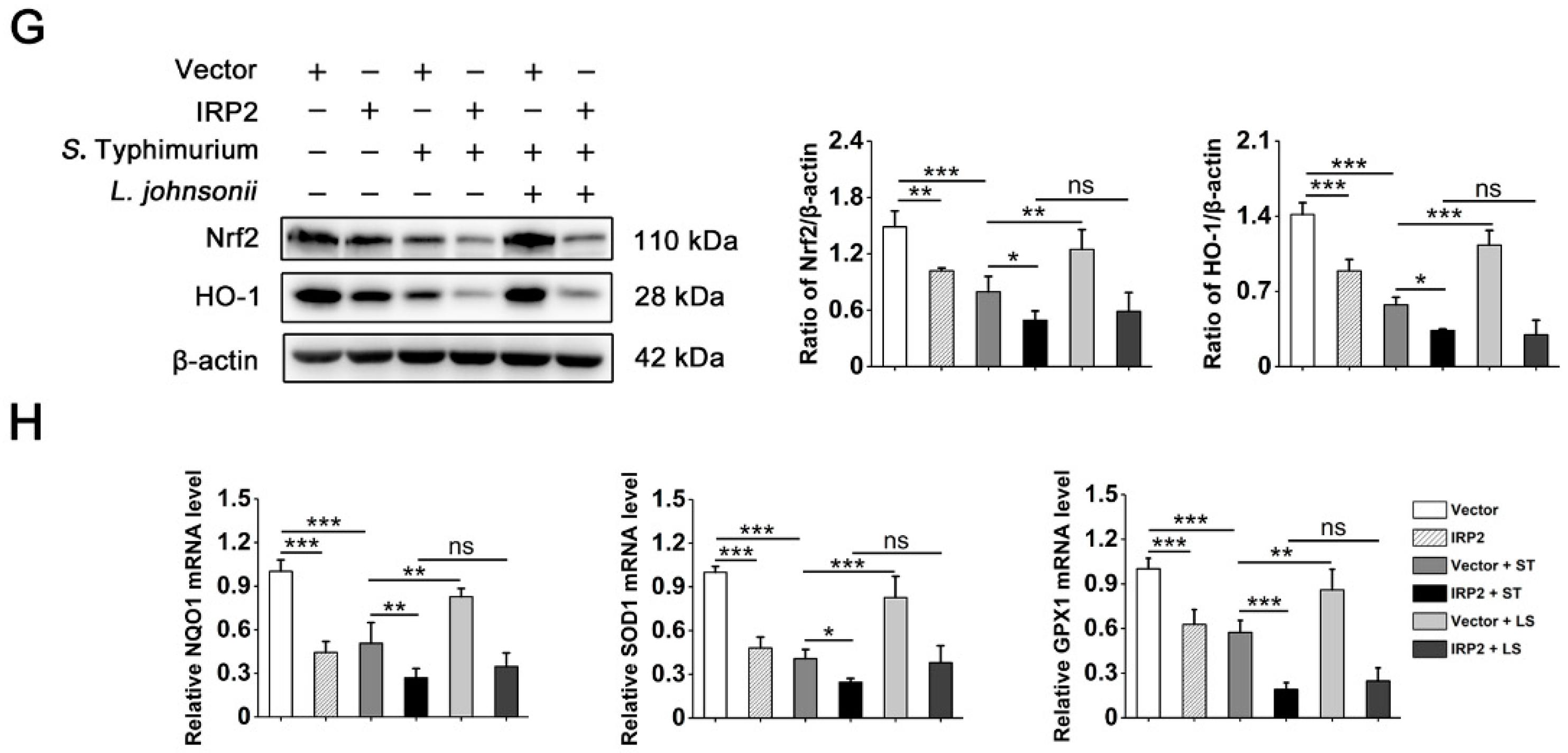
3.5. L. johnsonii L531 Suppresses the High Expression of IRP2 and Alleviates Oxidative Stress Caused by S. Typhimurium
3.6. L. johnsonii L531 Relieves Dysregulation of Iron Homeostasis and Oxidative Stress Caused by S. Typhimurium via IRP2 in Hela Cells
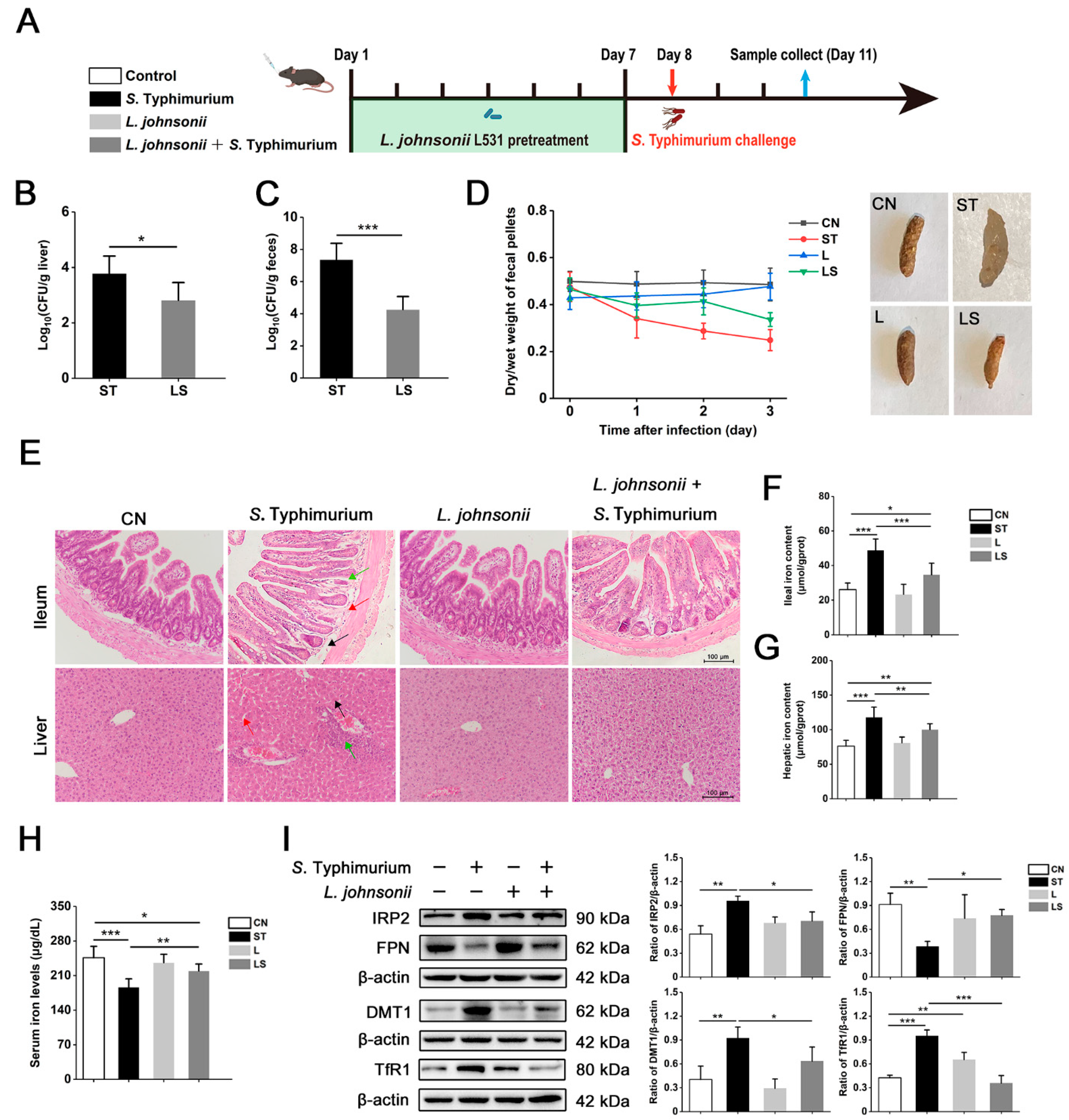
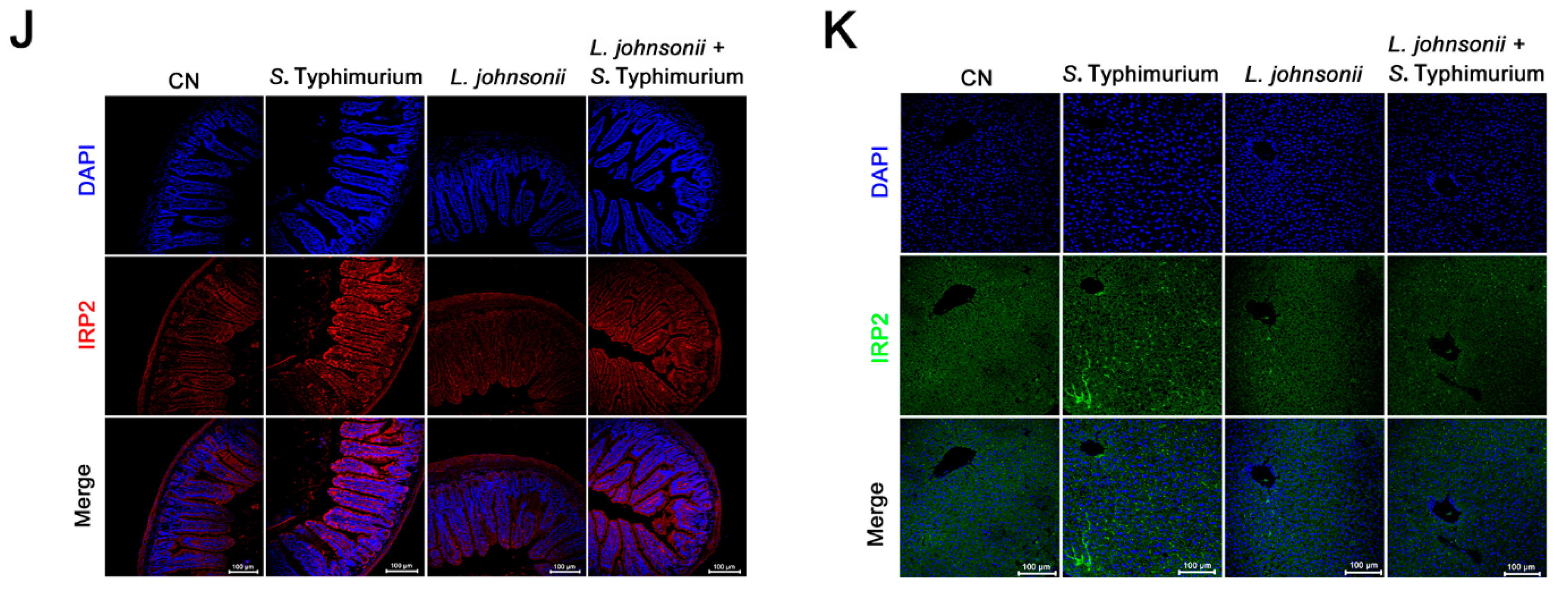
3.7. L. johnsonii L531 Ameliorates Disturbance of Iron Metabolism and Diarrhea Induced by S. Typhimurium In Vivo
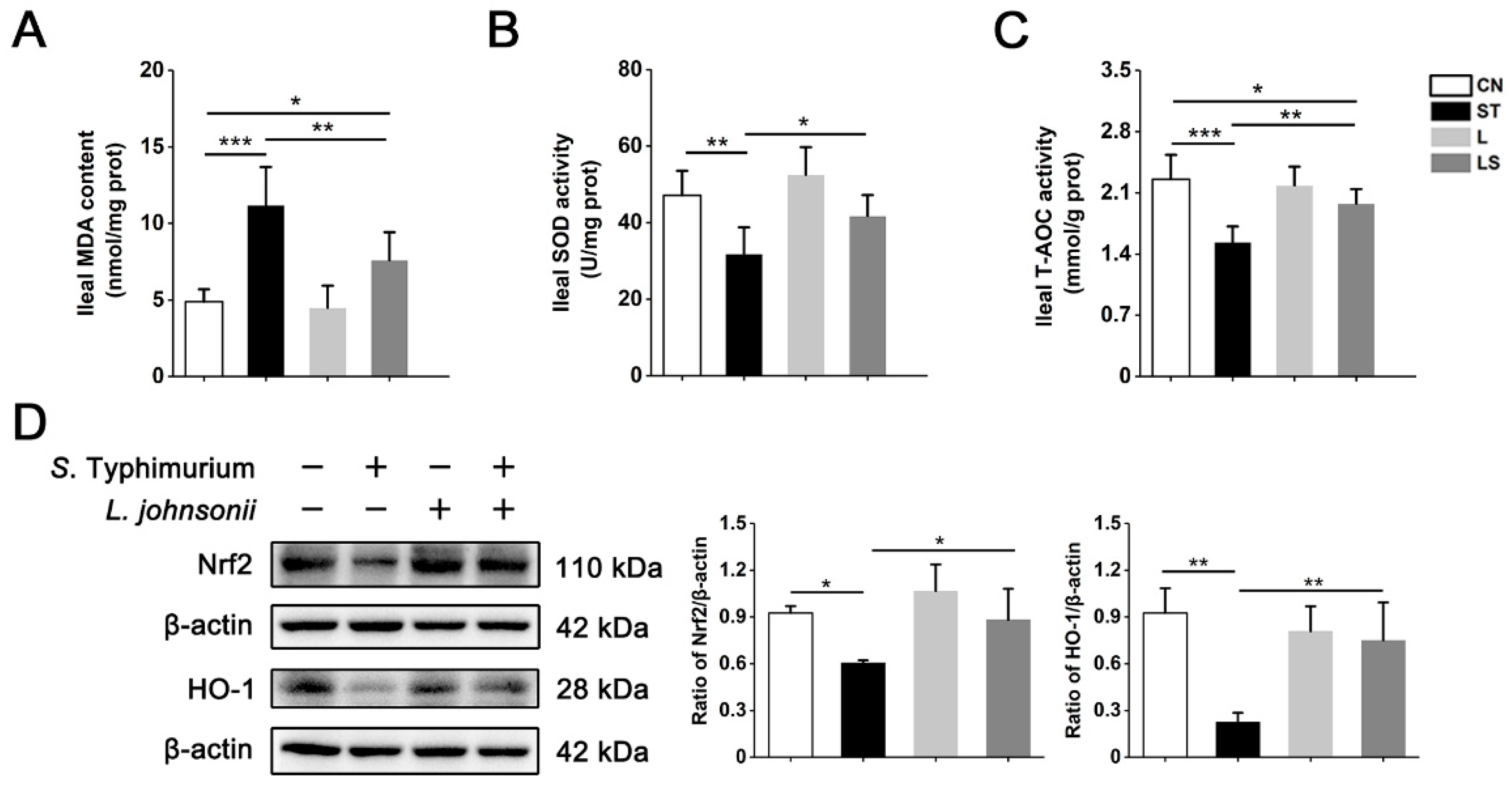
3.8. L. johnsonii L531 Relieves S. Typhimurium-Induced Oxidative Stress In Vivo
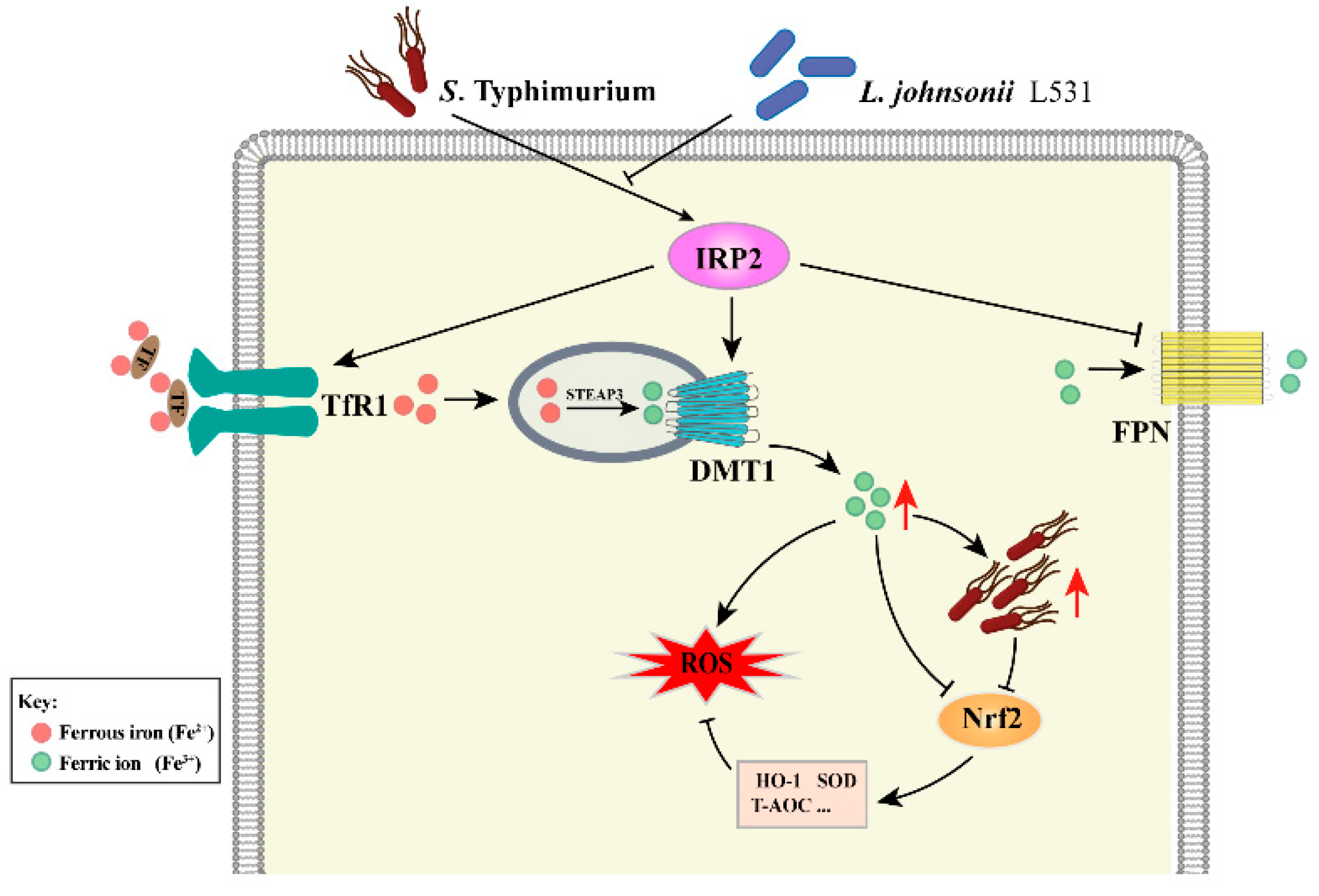
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferrari, R.G.; Rosario, D.; Cunha-Neto, A.; Mano, S.B.; Figueiredo, E.; Conte-Junior, C.A. Worldwide Epidemiology of Salmonella Serovars in Animal-Based Foods: A Meta-analysis. Appl. Environ. Microbiol. 2019, 85, e00591-19. [Google Scholar] [CrossRef]
- Winter, S.E.; Thiennimitr, P.; Winter, M.G.; Butler, B.P.; Huseby, D.L.; Crawford, R.W.; Russell, J.M.; Bevins, C.L.; Adams, L.G.; Tsolis, R.M.; et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature 2010, 467, 426–429. [Google Scholar] [CrossRef]
- Xia, B.; Yu, J.; He, T.; Liu, X.; Su, J.; Wang, M.; Wang, J.; Zhu, Y. Lactobacillus johnsonii L531 ameliorates enteritis via elimination of damaged mitochondria and suppression of SQSTM1-dependent mitophagy in a Salmonella infantis model of piglet diarrhea. FASEB. J. 2020, 34, 2821–2839. [Google Scholar] [CrossRef]
- Liu, X.; Xia, B.; He, T.; Li, D.; Su, J.; Guo, L.; Wang, J.; Zhu, Y. Oral Administration of a Select Mixture of Lactobacillus and Bacillus Alleviates Inflammation and Maintains Mucosal Barrier Integrity in the Ileum of Pigs Challenged with Salmonella Infantis. Microorganisms 2019, 7, 135. [Google Scholar] [CrossRef]
- Seyoum, Y.; Baye, K.; Humblot, C. Iron homeostasis in host and gut bacteria—A complex interrelationship. Gut Microbes 2021, 13, 1–19. [Google Scholar] [CrossRef]
- Yang, S.; Deng, Q.; Sun, L.; Dong, K.; Li, Y.; Wu, S.; Huang, R. Salmonella effector SpvB interferes with intracellular iron homeostasis via regulation of transcription factor NRF2. FASEB J. 2019, 33, 13450–13464. [Google Scholar] [CrossRef]
- Lim, D.; Kim, K.S.; Jeong, J.H.; Marques, O.; Kim, H.J.; Song, M.; Lee, T.H.; Kim, J.I.; Choi, H.S.; Min, J.J.; et al. The hepcidin-ferroportin axis controls the iron content of Salmonella-containing vacuoles in macrophages. Nat. Commun. 2018, 9, 2091. [Google Scholar] [CrossRef]
- van Zyl, W.F.; Deane, S.M.; Dicks, L. Molecular insights into probiotic mechanisms of action employed against intestinal pathogenic bacteria. Gut Microbes 2020, 12, 1831339. [Google Scholar] [CrossRef]
- Lin, F.; Wu, H.; Zeng, M.; Yu, G.; Dong, S.; Yang, H. Probiotic/prebiotic correction for adverse effects of iron fortification on intestinal resistance to Salmonella infection in weaning mice. Food Funct. 2018, 9, 1070–1078. [Google Scholar] [CrossRef]
- Chu, B.; Zhu, Y.; Su, J.; Xia, B.; Zou, Y.; Nie, J.; Zhang, W.; Wang, J. Butyrate-mediated autophagy inhibition limits cytosolic Salmonella Infantis replication in the colon of pigs treated with a mixture of Lactobacillus and Bacillus. Vet. Res. 2020, 51, 99. [Google Scholar] [CrossRef]
- Yang, G.; Xia, B.; Su, J.; He, T.; Liu, X.; Guo, L.; Zhang, S.; Zhu, Y.; Wang, J. Anti-inflammatory effects of Lactobacillus johnsonii L531 in a pig model of Salmonella Infantis infection involves modulation of CCR6+ T cell responses and ER stress. Vet. Res. 2020, 51, 26. [Google Scholar] [CrossRef]
- Yang, L.; Wang, J.; Liu, N.; Wang, X.; Wang, J.; Yang, G.; Yang, G.; Zhu, Y. Lactobacillus johnsonii L531 Protects against Salmonella Infantis-Induced Intestinal Damage by Regulating the NOD Activation, Endoplasmic Reticulum Stress, and Autophagy. Int. J. Mol. Sci. 2022, 23, 10395. [Google Scholar] [CrossRef]
- Rusu, I.G.; Suharoschi, R.; Vodnar, D.C.; Pop, C.R.; Socaci, S.A.; Vulturar, R.; Istrati, M.; Morosan, I.; Farcas, A.C.; Kerezsi, A.D.; et al. Iron Supplementation Influence on the Gut Microbiota and Probiotic Intake Effect in Iron Deficiency-A Literature-Based Review. Nutrients 2020, 12, 1993. [Google Scholar] [CrossRef]
- Anderson, G.J.; Frazer, D.M. Current understanding of iron homeostasis. Am J Clin Nutr. 2017, 106, 1559S–1566S. [Google Scholar] [CrossRef]
- Zhou, B.; Zhang, J.Y.; Liu, X.S.; Chen, H.Z.; Ai, Y.L.; Cheng, K.; Sun, R.Y.; Zhou, D.; Han, J.; Wu, Q. Tom20 senses iron-activated ROS signaling to promote melanoma cell pyroptosis. Cell Res. 2018, 28, 1171–1185. [Google Scholar] [CrossRef]
- Mittler, R.; Darash-Yahana, M.; Sohn, Y.S.; Bai, F.; Song, L.; Cabantchik, I.Z.; Jennings, P.A.; Onuchic, J.N.; Nechushtai, R. NEET Proteins: A New Link Between Iron Metabolism, Reactive Oxygen Species, and Cancer. Antioxid. Redox Signal. 2019, 30, 1083–1095. [Google Scholar] [CrossRef]
- Deng, Q.; Yang, S.; Sun, L.; Huang, K.; Dong, K.; Zhu, Y.; Cao, Y.; Li, Y.; Wu, S.; Huang, R. A detrimental role of NLRP6 in host iron metabolism during Salmonella infection. Redox Biol. 2022, 49, 102217. [Google Scholar] [CrossRef]
- Niture, S.K.; Khatri, R.; Jaiswal, A.K. Regulation of Nrf2-an update. Free Radic. Biol. Med. 2014, 66, 36–44. [Google Scholar] [CrossRef]
- Zhao, W.; Deng, Z.; Barkema, H.W.; Xu, M.; Gao, J.; Liu, G.; Lin, Y.; Kastelic, J.P.; Han, B. Nrf2 and NF-κB/NLRP3 inflammasome pathways are involved in Prototheca bovis infections of mouse mammary gland tissue and mammary epithelial cells. Free Radical. Bio. Med. 2022, 184, 148–157. [Google Scholar] [CrossRef]
- Sun, N.; Yang, T.; Tang, Y.; Zhao, Y.; Wang, H.; Zhao, S.; Tan, H.; Li, L.; Fan, H. Lycopene Alleviates Chronic Stress-Induced Liver Injury by Inhibiting Oxidative Stress-Mediated Endoplasmic Reticulum Stress Pathway Apoptosis in Rats. J. Agr. Food Chem. 2022, 70, 14414–14426. [Google Scholar] [CrossRef]
- Su, J.H.; Zhu, Y.H.; Ren, T.Y.; Guo, L.; Yang, G.Y.; Jiao, L.G.; Wang, J.F. Distribution and Antimicrobial Resistance of Salmonella Isolated from Pigs with Diarrhea in China. Microorganisms 2018, 6, 117. [Google Scholar] [CrossRef]
- He, T.; Zhu, Y.; Yu, J.; Xia, B.; Liu, X.; Yang, G.; Su, J.; Guo, L.; Wang, M.; Wang, J. Lactobacillus johnsonii L531 reduces pathogen load and helps maintain short-chain fatty acid levels in the intestines of pigs challenged with Salmonella enterica Infantis. Vet. Microbiol. 2019, 230, 187–194. [Google Scholar] [CrossRef]
- Chu, B.; Li, Y.; Liu, N.; Yuan, L.; Chen, S.; Zhu, Y.; Wang, J. Salmonella Infantis Delays the Death of Infected Epithelial Cells to Aggravate Bacterial Load by Intermittent Phosphorylation of Akt with SopB. Front. Immunol. 2021, 12, 757909. [Google Scholar] [CrossRef]
- Drakesmith, H.; Prentice, A. Viral infection and iron metabolism. Nat. Rev. Microbiol. 2008, 6, 541–552. [Google Scholar] [CrossRef]
- Kim, D.; Jeong, J.; Lee, J.; Kim, K.S.; Park, S.; Kim, Y.D.; Koh, M.; Shin, M.; Jung, Y.S.; Kim, H.; et al. Inverse agonist of estrogen-related receptor γ controls Salmonella typhimurium infection by modulating host iron homeostasis. Nat. Med. 2014, 20, 419–424. [Google Scholar] [CrossRef]
- Deng, Q.; Yang, S.; Sun, L.; Dong, K.; Li, Y.; Wu, S.; Huang, R. Salmonella effector SpvB aggravates dysregulation of systemic iron metabolism via modulating the hepcidin-ferroportin axis. Gut Microbes 2021, 13, 1–18. [Google Scholar] [CrossRef]
- Das, N.K.; Schwartz, A.J.; Barthel, G.; Inohara, N.; Liu, Q.; Sankar, A.; Hill, D.R.; Ma, X.; Lamberg, O.; Schnizlein, M.K.; et al. Microbial Metabolite Signaling Is Required for Systemic Iron Homeostasis. Cell Metab. 2020, 31, 115–130.e6. [Google Scholar] [CrossRef]
- McKie, A.T.; Barrow, D.; Latunde-Dada, G.O.; Rolfs, A.; Sager, G.; Mudaly, E.; Mudaly, M.; Richardson, C.; Barlow, D.; Bomford, A.; et al. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science 2001, 291, 1755–1759. [Google Scholar] [CrossRef]
- Levy, J.E.; Jin, O.; Fujiwara, Y.; Kuo, F.; Andrews, N.C. Transferrin receptor is necessary for development of erythrocytes and the nervous system. Nat. Genet. 1999, 21, 396–399. [Google Scholar] [CrossRef]
- Donovan, A.; Lima, C.A.; Pinkus, J.L.; Pinkus, G.S.; Zon, L.I.; Robine, S.; Andrews, N.C. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005, 1, 191–200. [Google Scholar] [CrossRef]
- Willemetz, A.; Beatty, S.; Richer, E.; Rubio, A.; Auriac, A.; Milkereit, R.J.; Thibaudeau, O.; Vaulont, S.; Malo, D.; Canonne-Hergaux, F. Iron- and Hepcidin-Independent Downregulation of the Iron Exporter Ferroportin in Macrophages during Salmonella Infection. Front. Immunol. 2017, 8, 498. [Google Scholar] [CrossRef]
- Grander, M.; Hoffmann, A.; Seifert, M.; Demetz, E.; Grubwieser, P.; Pfeifhofer-Obermair, C.; Haschka, D.; Weiss, G. DMT1 Protects Macrophages from Salmonella Infection by Controlling Cellular Iron Turnover and Lipocalin 2 Expression. Int. J. Mol. Sci. 2022, 23, 6789. [Google Scholar] [CrossRef]
- Nairz, M.; Theurl, I.; Ludwiczek, S.; Theurl, M.; Mair, S.M.; Fritsche, G.; Weiss, G. The co-ordinated regulation of iron homeostasis in murine macrophages limits the availability of iron for intracellular Salmonella typhimurium. Cell. Microbiol. 2007, 9, 2126–2140. [Google Scholar] [CrossRef]
- Hoffmann, A.; Haschka, D.; Valente De Souza, L.; Tymoszuk, P.; Seifert, M.; von Raffay, L.; Hilbe, R.; Petzer, V.; Moser, P.L.; Nairz, M.; et al. Baseline iron status and presence of anaemia determine the course of systemic Salmonella infection following oral iron supplementation in mice. Ebiomedicine 2021, 71, 103568. [Google Scholar] [CrossRef]
- Song, C.; Pantopoulos, K.; Chen, G.; Zhong, C.; Zhao, T.; Zhang, D.; Luo, Z. Iron increases lipid deposition via oxidative stress-mediated mitochondrial dysfunction and the HIF1α-PPARγ pathway. Cell. Mol. Life Sci. 2022, 79, 394. [Google Scholar] [CrossRef]
- Feng, T.; Wang, J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: A systematic review. Gut Microbes 2020, 12, 1801944. [Google Scholar] [CrossRef]
- Chandra, K.; Roy Chowdhury, A.; Chatterjee, R.; Chakravortty, D. GH18 family glycoside hydrolase Chitinase A of Salmonella enhances virulence by facilitating invasion and modulating host immune responses. PLoS Pathog. 2022, 18, e1010407. [Google Scholar] [CrossRef]
- Schürmann, N.; Forrer, P.; Casse, O.; Li, J.; Felmy, B.; Burgener, A.; Ehrenfeuchter, N.; Hardt, W.; Recher, M.; Hess, C.; et al. Myeloperoxidase targets oxidative host attacks to Salmonella and prevents collateral tissue damage. Nat. Microbiol. 2017, 2, 16268. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant Properties of Probiotic Bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Wang, S.; Wang, Y.; Chu, X.; Ji, H. Lactobacillus plantarum Exhibits Antioxidant and Cytoprotective Activities in Porcine Intestinal Epithelial Cells Exposed to Hydrogen Peroxide. Oxid. Med. Cell. Longev. 2021, 2021, 8936907. [Google Scholar] [CrossRef]
- Chen, F.; Chen, J.; Chen, Q.; Yang, L.; Yin, J.; Li, Y.; Huang, X. Lactobacillus delbrueckii Protected Intestinal Integrity, Alleviated Intestinal Oxidative Damage, and Activated Toll-Like Receptor-Bruton’s Tyrosine Kinase-Nuclear Factor Erythroid 2-Related Factor 2 Pathway in Weaned Piglets Challenged with Lipopolysaccharide. Antioxidants 2021, 10, 468. [Google Scholar]
- Wang, L.; Zhao, Z.; Zhao, L.; Zhao, Y.; Yang, G.; Wang, C.; Gao, L.; Niu, C.; Li, S. Lactobacillus plantarum DP189 Reduces α-SYN Aggravation in MPTP-Induced Parkinson’s Disease Mice via Regulating Oxidative Damage, Inflammation, and Gut Microbiota Disorder. J. Agr. Food Chem. 2022, 70, 1163–1173. [Google Scholar] [CrossRef]




| Gene Primer | Direction | Sequence (5′-3′) | GenBank Accession |
|---|---|---|---|
| IRP1 | F | CCGTGCCAATTATCTCGCCTCTC | XM_021078286.1 |
| R | TGCCCGTAGAGTCAGTACCTAAAGG | ||
| SOD1 (Sus scrofa) | F | CTCTCGGGAGACCATTCCATCATTG | NM_001190422.1 |
| R | TTCTTCATTTCCACCTCTGCCCAAG | ||
| GPX1 (Sus scrofa) | F | CACGCTCGGTGTATGCCTTCTC | NM_214201.1 |
| R | GCAGCTCATTCATCTGGGTGTAGT | ||
| NQO1 (Sus scrofa) | F | CGTACAGCATTGGGCACACTCC | NM_001159613.1 |
| R | CAAAGTACAGTGGCGTCTCATCCC | ||
| SOD1 (Homo sapiens) | F | GATGACTTGGGCAAAGGTGGAAATG | NM_000454.5 |
| R | CCAATTACACCACAAGCCAAACGAC | ||
| GPX1 (Homo sapiens) | F | GCAACCAGTTTGGGCATCAGGAG | NM_000581.4 |
| R | CACCGTTCACCTCGCACTTCTC | ||
| NQO1 (Homo sapiens) | F | AAGCCGCAGACCTTGTGATATTCC | NM_000903.3 |
| R | CATGGCAGCGTAAGTGTAAGCAAAC | ||
| GAPDH (Sus scrofa) | F | GCTGCTGAACGGGAAGACAA | NM_001206359.1 |
| R | AGCACCAGCATCACCCCATTTG | ||
| GAPDH (Homo sapiens) | F | GGAGCGAGATCCCTCCAAAAT | NM_001289746.2 |
| R | GGCTGTTGTCATACTTCTCATGG | ||
| IRP2-xhol | F | CCGCTCGAGATGGACGCCCCAAGTGCA | |
| IRP2-BamHI | R | CGCGGATCCCTATGAGAATTTTCGTGCCACAAAGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.; Wang, J.; Guo, L.; Wang, J.; Yang, L.; Hu, T.; Zhao, Y.; Wang, X.; Zhu, Y. Lactobacillus johnsonii L531 Ameliorates Salmonella enterica Serovar Typhimurium Diarrhea by Modulating Iron Homeostasis and Oxidative Stress via the IRP2 Pathway. Nutrients 2023, 15, 1127. https://doi.org/10.3390/nu15051127
Chen K, Wang J, Guo L, Wang J, Yang L, Hu T, Zhao Y, Wang X, Zhu Y. Lactobacillus johnsonii L531 Ameliorates Salmonella enterica Serovar Typhimurium Diarrhea by Modulating Iron Homeostasis and Oxidative Stress via the IRP2 Pathway. Nutrients. 2023; 15(5):1127. https://doi.org/10.3390/nu15051127
Chicago/Turabian StyleChen, Keyuan, Jiufeng Wang, Liang Guo, Jing Wang, Lan Yang, Ting Hu, Yiqing Zhao, Xue Wang, and Yaohong Zhu. 2023. "Lactobacillus johnsonii L531 Ameliorates Salmonella enterica Serovar Typhimurium Diarrhea by Modulating Iron Homeostasis and Oxidative Stress via the IRP2 Pathway" Nutrients 15, no. 5: 1127. https://doi.org/10.3390/nu15051127
APA StyleChen, K., Wang, J., Guo, L., Wang, J., Yang, L., Hu, T., Zhao, Y., Wang, X., & Zhu, Y. (2023). Lactobacillus johnsonii L531 Ameliorates Salmonella enterica Serovar Typhimurium Diarrhea by Modulating Iron Homeostasis and Oxidative Stress via the IRP2 Pathway. Nutrients, 15(5), 1127. https://doi.org/10.3390/nu15051127






